Potential Role and Involvement of Antioxidants and Other Secondary Metabolites of Wheat in the Infection Process and Resistance to Fusarium spp.
Abstract
:1. Introduction
2. Infection Process
3. Plant Defense
3.1. Mechanisms of Resistance
3.1.1. Active Resistance
3.1.2. Passive Resistance
4. Secondary Metabolites
4.1. Phenolic Compounds/Antioxidants
4.1.1. Phenolic Acids
4.1.2. Anthocyanins and Flavonoids
4.1.3. Alkylresorcinols
4.2. Benzoxazinoids
4.3. Volatile Organic Compounds
4.4. Phytohormones
4.5. Carotenoids
5. Pigmentation of Grains and Fhb Resistance
6. Use of Antioxidants in Breeding for FHB Resistance
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction. Agronomy 2020, 20, 509. [Google Scholar] [CrossRef] [Green Version]
- Zain, M.E. Impact of Mycotoxins on Humans and Animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef] [Green Version]
- Pleadin, J. Mycotoxins in Grains and Feed—Contamination and Toxic Effect in Animals. Biotechnol. Anim. Husb. 2015, 31, 441–456. [Google Scholar] [CrossRef]
- Chrpová, J.; Šíp, V.; Štočková, L.; Milec, Z.; Bobková, L. Resistance of Winter Wheat Varieties Registered in the Czech Republic to Fusarium Head Blight in Relation to the Presence of Specific Rht alleles. Czech J. Genet. Plant Breed. 2010, 46, 122–134. [Google Scholar] [CrossRef] [Green Version]
- Edwards, S. Investigation of Fusarium Mycotoxins in UK Wheat Production-Update. In Society of Feed Technologists, Proceedings of the 2nd International Symposium on Fusarium Head Blight, Orlando, FL, USA, 11–15 December 2004; Society of Feed Technologists, Michigan State University: East Lansing, MI, USA, 2005; pp. 398–400. [Google Scholar]
- Krska, R.; Baumgartner, S.; Josephs, R. The State-of-the-Art in the Analysis of Type-A and -B Trichothecene Mycotoxins in Cereals. Fresenius J. Anal. Chem. 2001, 371, 285–299. [Google Scholar] [CrossRef]
- Spanu, F.; Scherm, B.; Camboni, I.; Pani, G.; Oufensou, S.; Macciotta, N.; Pasquali, M.; Migheli, Q. FcRav2, a Gene with a ROGDI Domain Involved in Fusarium Head Blight and Crown Rot on Durum Wheat Caused by Fusarium culmorum. Mol. Plant Pathol. 2018, 9, 677–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilska, K.I.; Jurczak, S.; Kulik, T.; Ropelewska, E.; Olszewski, J.; Zelechowski, M.; Zapotoczny, P. Species Composition and Trichothecene Genotype Profiling of Fusarium Field Isolates Recovered from Wheat in Poland. Toxins 2018, 10, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilska, K.; Stuper-Szablewska, K.; Kulik, T.; Buśko, M.; Załuski, D.; Jurczak, S.; Perkowski, J. Changes in Phenylpropanoid and Trichothecene Production by Fusarium culmorum and F. graminearum Sensu Stricto via Exposure to Flavonoids. Toxins 2018, 10, 110. [Google Scholar] [CrossRef] [Green Version]
- Aoki, T.; Ward, T.J.; Kistlet, H.C.; O’Donnell, K. Systematics, Phylogeny and Trichothecene Mycotoxin Potential of Fusarium Head Blight Cereal Pathogens. JSM Mycotoxins 2012, 62, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Wagacha, J.M.; Muthomi, J.W. Fusarium culmorum: Infection Process, Mechanisms of Mycotoxin Production and Their Role in Pathogenesis in Wheat. J. Crop Prot. 2007, 26, 877–885. [Google Scholar] [CrossRef]
- D’Mello, J.P.; Placinta, C.M.; Macdonald, A.M. Fusarium Mycotoxins: A Review of Global Implications for Animal Health, Welfare and Productivity. Anim. Feed Sci. Technol. 1999, 80, 183–205. [Google Scholar] [CrossRef] [Green Version]
- Buerstmayr, H.; Stierschneider, M.; Steiner, B.; Lemmens, M.; Griesser, M.; Nevo, E.; Fahima, T. Variation for Resistance to Head Blight Caused by Fusarium graminearum in Wild Emmer (Triticum dicoccoides) Originating from Israel. Euphytica 2003, 130, 17–23. [Google Scholar] [CrossRef]
- Pereyra, S.A.; Dill-Macky, R.; Sims, A.L. Survival and Inoculum Production of Gibberella zeae in Wheat Residue. Plant Dis. 2004, 88, 724–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wang, J.; Xu, J.; Shi, J. FgIlv5 is Required for Branched-Chain Amino Acid Biosynthesis and Full Virulence in Fusarium graminearum. Microbiology 2014, 160, 692–702. [Google Scholar] [CrossRef]
- Sperschneider, J.; Gardiner, D.M.; Thatcher, L.F.; Lyons, R.; Singh, K.B.; Manners, J.M.; Taylor, J.M. Genome-Wide Analysis in Three Fusarium Pathogens Identifies Rapidly Evolving Chromosomes and Genes Associated with Pathogenicity. Genome Biol. Evol. 2015, 7, 1613–1627. [Google Scholar] [CrossRef]
- Dweba, C.C.; Figlan, S.; Shimelis, H.A.; Motaung, T.E.; Sydenham, S.; Mwadzingeni, L.; Tsilo, T.J. Fusarium Head Blight of Wheat: Pathogenesis and Control Strategies. Crop Prot. 2017, 91, 114–122. [Google Scholar] [CrossRef]
- Khaledi, N.; Taheri, P.; Falahati-Rastegar, M. Identification, Virulence Factors Characterization, Pathogenicity and Aggressiveness Analysis of Fusarium spp., Causing Wheat Head Blight in Iran. Eur. J. Plant Pathol. 2017, 147, 897–918. [Google Scholar] [CrossRef]
- Kikot, G.E.; Hours, R.A.; Alconada, T.M. Contribution of Cell Wall Degrading Enzymes to Pathogenesis of Fusarium graminearum: A Review. J. Basic Microbiol. 2009, 49, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wang, G.; Jiang, C.; Xu, J.R.; Wang, C. 1Fgk3 Glycogen Synthase Kinase is Important for Development, Pathogenesis, and Stress Responses in Fusarium graminearum. Sci. Rep. 2015, 5, 8504. [Google Scholar] [CrossRef] [Green Version]
- Wanyoike, W.M.; Kang, Z.; Buchenauer, H. Importance of Cell Wall Degrading Enzymes Produced by Fusarium graminearum During Infection of Wheat Head. Eur. J. Plant Pathol. 2002, 108, 803–810. [Google Scholar]
- Feng, J.; Liu, G.; Selvaraj, G.; Hughes, G.R.; Wei, Y. A secreted Lipase Encoded by LIP1 is Necessary for Efficient Use of Saturated Triglyceride Lipids in Fusarium graminearum. Microbiology 2005, 151, 3911–3921. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Glenn, A.E.; Blacutt, A.A.; Gold, S.E. Fungal Lactamases: Their Occurrence and Function. Front. Microbiol. 2017, 8, E1775. [Google Scholar] [CrossRef]
- Glenn, A.E.; Davis, C.B.; Gao, M.; Gold, S.E.; Mitchell, T.R.; Proctor, R.H.; Stewart, J.E.; Snook, M.E. Two Horizontally Transferred Xenobiotic Resistance Gene Cluster Associated with Detoxification of Benzoxazolinones by Fusarium Species. PLoS ONE 2016, 11, e0147486. [Google Scholar] [CrossRef]
- Luz, C.; Saladino, F.; Luciano, F.; Mañes, J.; Meca, G. Occurrence, Toxicity, Bioaccessibility and Mitigation Strategies of Beauvericin, a Minor Fusarium Mycotoxin. Food Chem. Toxicol. 2017, 107, 430–439. [Google Scholar] [CrossRef]
- Kosová, K.; Chrpová, J.; Šíp, V. Cereal Resistance to Fusarium Head Blight and Possibilities of Its Improvement through Breeding. Czech J. Genet. Plant Breed. 2009, 45, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Bushnell, W.; Perkins-Veazie, P.; Russo, V.; Collins, J.; Seeland, T. Effects of Deoxynivalenol on Content of Chloroplast Pigments in Barley Leaf Tissues. Phytopathology 2010, 100, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cossette, F.; Miller, J.D. Phytotoxic Effect of Deoxynivalenol and Gibberella Ear Rot Resistance of Corn. Nat. Toxins 1995, 3, 383–388. [Google Scholar] [CrossRef]
- Wipfler, R.; Mc Cormick, S.P.; Proctor, R.H.; Teresi, J.M.; Hao, G.; Ward, T.J.; Alexander, N.J.; Vaughan, M.M. Synergistic Phytotoxic Effects of Culmorin and Trichothecene Mycotoxins. Toxins 2019, 11, 555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.; Edwards, M.C. Genome Wide Analysis of Small Secreted Cysteine-Rich Proteins Identifies Candidate Effector Proteins Potentially Involved in Fusarium graminearum—Wheat Interactions. Phytopathology 2016, 106, 166–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabre, F.; Bormann, J.; Urbach, S.; Roche, S.; Langin, T.; Bonhommer, L. Unbalanced Roles of Fungal Aggressiveness and Host Cultivars in the Establishment of the Fusarium Head Blight in Bread Wheat. Front. Microbiol. 2019, 10, 2857. [Google Scholar] [CrossRef] [PubMed]
- Ravensdale, M.; Rocheleau, H.; Wang, L.; Nasmith, C.; Ouellet, T.; Subramaniam, R. Components of Priming Induced Resistance to Fusarium Head Blight in Wheat Revealed by Two Distinct Mutants of Fusarium graminearum. Mol. Plant Pathol. 2014, 15, 948–956. [Google Scholar] [CrossRef]
- Schroeder, H.W.; Christensen, J.J. Factors Affecting Resistance of Wheat to Scab by Gibberella zeae. Phytopathology 1963, 53, 831–838. [Google Scholar]
- Miller, J.D.; Arnison, P.G. Degradation of Deoxynivalenol by Suspension Cultures of the Fusarium Head Blight Resistant Wheat Cultivar Frontana. Can. J. Plant Pathol. 1986, 8, 147–150. [Google Scholar] [CrossRef]
- Spanic, V.; Marcek, T.; Abicic, I.; Sarkanj, B. Effects of Fusarium Head Blight on Wheat Grain and Malt Infected by Fusarium culmorum. Toxins 2018, 10, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesterházy, Á. Types and Components of Resistance to Fusarium Head Blight of Wheat. Plant Breed. 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Mesterházy, Á. Theory and Practice of the Breeding for Fusarium Head Blight Resistance in Wheat. J. Appl. Gen. 2002, 43A, 289–302. [Google Scholar]
- Malinovsky, F.G.; Fangel, J.U.; Willats, G.T. The Role of the Cell Wall in Plant Immunity. Front. Plant Sci. 2014, 5, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miedes, E.; Vanholme, R.; Boerjan, W.; Molina, A. The Role of Secondary Cell Wall in Plant Resistance to Pathogens. Front. Plant Sci. 2014, 5, 358. [Google Scholar] [CrossRef] [Green Version]
- Sekhon, R.S.; Kuldau, G.; Mansfield, M.; Chopra, S. Characterization of Fusarium-Induced Expression of Flavonoids and PR Genes in Maize. Physiol. Mol. Plant Pathol. 2006, 69, 109–117. [Google Scholar] [CrossRef]
- Khaledi, N.; Taheri, P.; Falahati-Rastegar, M. Reactive Oxygen Species and Antioxidant System Responses in Wheat Cultivars during Interaction with Fusarium Species. Austr. Plant Pathol. 2016, 45, 653–670. [Google Scholar] [CrossRef]
- Ellinger, D.; Voigt, C.A. Callose Biosynthesis in Arabidopsis with a Focus on Pathogen Response: What We Have Learned Within the Last Decade. Ann. Bot. 2014, 114, 1349–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taheri, P. Cereal Diseases Caused by Fusarium graminearum: From Biology of the Pathogen to Oxidative Burst-Related Host Defense Responses. Eur. J. Plant Pathol. 2018, 152, 1–20. [Google Scholar] [CrossRef]
- Khaledi, N.; Taheri, P.; Falahati-Rastegar, M. Evaluation of Resistance and the Role of Some Defense Responses in Wheat Cultivars to Fusarium Head Blight. J. Plant Protect. Res. 2018, 57, 205–217. [Google Scholar] [CrossRef] [Green Version]
- Boutigny, A.L.; Richard-Forget, F.; Barreau, C. Natural Mechanisms for Cereal Resistance to the Accumulation of Fusarium Trichothecenes. Eur. J. Plant Pathol. 2008, 121, 411–423. [Google Scholar] [CrossRef]
- Jain, D.; Khurana, J.P. Role of Pathogenesis-Related (PR) Proteins in Plant Defense Mechanism. In Molecular Aspects of Plant-Pathogen Interaction; Singh, A., Singh, I., Eds.; Springer: Singapore, 2018; pp. 265–281. [Google Scholar]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.J.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-Related Proteins and Peptides as Promising Tools for Engineering Plants with Multiple Stress Tolerance. Microbiol. Res. 2018, 212–213, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Sels, J.; Mathys, J.; De Coninck, B.M.; Cammue, B.P.; De Bolle, M.F. Plant Pathogenesis-Related (PR) Proteins: A Focus on PR Peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef]
- Etzerodt, T.; Gislum, R.; Laursen, B.B.; Heinrichson, K.; Gregersen, P.L.; Jørgensen, L.N.; Fomsgaard, I.S. Correlation of Deoxynivalenol Accumulation in Fusarium-Infected Winter and Spring Wheat Cultivars with Secondary Metabolites at Different Growth Stages. J. Agric. Food Chem. 2016, 64, 4545–4555. [Google Scholar] [CrossRef] [PubMed]
- Boba, A.; Kulma, A.; Kostyn, K.; Starzycki, M.; Starzycka, E.; Szopa, J. The Influence of Carotenoid Biosynthesis Modification on the Fusarium culmorum and Fusarium oxysporum Resistance in Flax. Physiol. Mol. Plant Pathol. 2011, 76, 39–47. [Google Scholar] [CrossRef]
- Lachman, J.; Martinek, P.; Kotíková, Z.; Orsák, M.; Šulc, M. Genetics and Chemistry of Pigments in Wheat Grain—A Review. J. Cereal Sci. 2017, 74, 145–154. [Google Scholar] [CrossRef]
- Pani, G.; Scherm, B.; Azara, E.; Balmas, V.; Jahanshiri, Z.; Carta, P.; Fabbri, D.; Dettori, M.A.; Fadda, A.; Dessi, A.; et al. Natural and Natural-Like Phenolic Inhibitors of Type B Trichothecene In Vitro Production by the Wheat (Triticum sp.) Pathogen Fusarium culmorum. J. Agric. Food Chem. 2014, 62, 4969–4978. [Google Scholar] [CrossRef]
- Ziegler, J.U.; Steingass, C.B.; Longin, C.F.; Würschum, T.; Carle, R.; Schweiggert, R.M. Alkylresorcinol Composition Allows the Differentiation of Triticum spp. Having Different Degrees of Ploidy. J. Cereal Sci. 2015, 65, 244–251. [Google Scholar] [CrossRef]
- Landberg, R. Alkylresorcinols as Biomarkers of Whole Grain Wheat and Rye Intake; Acta Universitatis Agriculturae Sueciae: Uppsala, Sweden, 2009. [Google Scholar]
- Bordiga, M.; Locatelli, M.; Travaglia, F.; Arlorio, M.; Reyneri, A.; Blandino, M.; Coisson, J.D. Alkylresorcinol Content in Whole Grains and Pearled Fractions of Wheat and Barley. J. Cereal Sci. 2016, 70, 38–46. [Google Scholar] [CrossRef]
- Righetti, L.; Cirlini, M.; Folloni, S.; Ranieri, R.; Galaverna, G.; Bertuzzi, T.; Dall’Asta, C.; Battilani, P.; Giorni, P. 5-n-Alkylresorcinols but Not Hydroxycinnamic Acids Are Directly Related to a Lower Accumulation of Deoxynivalenol and Its Glucoside in Triticum spp. Genotypes with Different Ploidity Levels. J. Cereal Sci. 2019, 85, 214–220. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.; Hucl, P.; Rabalski, I. Compositional and Antioxidant Properties of Anthocyanin-Rich Products Prepared from Purple Wheat. Food Chem. 2018, 254, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Etzerodt, T.; Maeda, K.; Nakajima, Y.; Laursen, B.; Fomsgaard, I.S.; Kimura, M. 2,4-Dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one (DIMBOA) Inhibits Trichothecene Production by Fusarium graminearum through Suppression of Tri6 Expression. Int. J. Food Microbiol. 2015, 214, 123–128. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S. Do “Ancient” Wheat Species Differ from Modern Bread Wheat in Their Contents of Bioactive Components? J. Cereal Sci. 2015, 65, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Martini, D.; Taddei, F.; Ciccoritti, R.; Pasquini, M.; Nicoletti, I.; Corradini, D.; D´Egidio, M.G. Variation of Total Antioxidant Activity and of Phenolic Acid, Total Phenolics and Yellow Coloured Pigments in Durum Wheat (Triticum turgidum L. var. durum) as a Function of Genotype, Crop Year and Growing Area. J. Cereal Sci. 2015, 65, 175–185. [Google Scholar]
- Ma, D.Y.; Sun, D.X.; Zuo, Y.; Wang, C.Y.; Zhu, Y.J.; Guo, T.C. Diversity of Antioxidant Content and Its Relationship to Grain Color and Morphological Characteristics in Winter Wheat Grains. J. Integr. Agric. 2014, 13, 1258–1267. [Google Scholar] [CrossRef] [Green Version]
- Lachman, J.; Hejtmánková, K.; Kotíková, Z. Tocols and Carotenoids of Einkorn, Emmer and Spring Wheat Varieties: Selection for Breeding and Production. J. Cereal Sci. 2013, 57, 207–214. [Google Scholar] [CrossRef]
- Li, Y.G.; Ma, D.Y.; Sun, D.X.; Wang, C.Y.; Zhang, J.; Xie, Y.X.; Guo, T.C. Total Phenolic, Flavonoid Content, and Antioxidant Activity of Flour, Noodles, and Steamed Bread Made from Different Colored Wheat Grains by Three Milling Methods. J. Crop Prod. 2015, 3, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Buśko, M.; Gòral, T.; Ostrowska, A.; Matysiak, A.; Walentyn-Gòral, D.; Perkowski, J. The Effect of Fusarium Fungicide Application on Concentrations of Flavonoids (Apigenin, Kaempferol, Luteolin, Naringenin, Quercetin, Rutin, Vitexin) in Winter Wheat Cultivars. Am. J. Sci. 2014, 5, 3727–3736. [Google Scholar] [CrossRef] [Green Version]
- Kovács, V.; Gondor, O.K.; Szalai, G.; Darkó, É.; Majláth, I.; Janda, T.; Pál, M. Synthesis and Role of Salicylic Acid in Wheat Varieties with Different Levels of Cadmium Tolerance. J. Hazard. Mater. 2014, 280, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Verma, B.; Hucl, P.; Chibbar, R.N. Phenolic Acid Composition and Antioxidant Capacity of Acid and Alkali Hydrolysed Bran Fractions. Food Chem. 2009, 116, 947–954. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Perkowski, J. Phenolic Acids in Cereals Grain: Occurrence, Metabolism and Role in Living Organisms. Crit. Rev. Food Sci. Nutr. 2019, 59, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Martin, C. Resistance Elements of Small Grain Cereals against Fusarium Head Blight and Contribution of Health Promoting Compounds. Ph.D. Thesis, University of Neuchâtel, Neuchâtel, Switzerland, 2017. [Google Scholar]
- Qi, P.F.; Balcerzak, M.; Rocheleau, H.; Leung, W.; Wei, Y.M.; Zheng, Y.L.; Ouellet, T. Jasmonic Acid and Abscisic Acid Play Important Roles in Host-Pathogen Interaction between Fusarium graminearum and Wheat during the Early Stages of Fusarium Head Blight. Physiol. Mol. Plant Pathol. 2016, 93, 39–48. [Google Scholar] [CrossRef]
- Piesik, D.; Pańka, D.; Delaney, K.J.; Skoczek, A.; Lamparski, R.; Weaver, D.K. Cereal Crop Volatile Organic Compound Induction After Mechanical Injury, Beetle Herbivory (Oulema spp.), or Fungal Infection (Fusarium spp.). J. Plant Physiol. 2011, 168, 878–886. [Google Scholar] [CrossRef]
- Buśko, M.; Gòral, T.; Boczkowska, M.; Perkowski, J. Relationships between Volatile Organic Compounds with Emphasis on Terpene Compounds and Genetic Matrix in Inoculated and Non-inoculated Winter Wheat Cultivars. Chem. Ecol. 2019, 35, 971–986. [Google Scholar] [CrossRef]
- Steiner, B.; Buerstmayr, M.; Michel, S.; Schweiger, W.; Lemmens, M.; Buerstmayr, H. Breeding Strategies and Advances in Line Selection for Fusarium Head Blight Resistance in Wheat. Trop. Plant Pathol. 2017, 42, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.; Farooqi, A.; Foulkes, J.; Sparkes, D.L.; Linforth, R.; Ray, R.V. Canopy and Ear Traits Associated with Avoidance of Fusarium Head Blight in Wheat. Front. Plant Sci. 2018, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Tessmann, E.W.; Van Sanford, D.A. Associations Between Morphological and FHB Traits in a Soft Red Winter Wheat Population. Euphytica 2019, 215, 189. [Google Scholar] [CrossRef]
- McCartney, C.A.; Brûlé-Babel, A.L.; Fedak, G.; Martin, R.A.; McCallum, B.D.; Gilbert, J.; Hiebert, C.W.; Pozniak, C.V. Fusarium Head Blight Resistance QTL in the Spring Wheat Cross Kenyon/86ISMN 2137. Front. Microbiol. 2016, 7, 1542. [Google Scholar] [CrossRef] [Green Version]
- Giancaspro, A.; Giove, S.L.; Zito, D.; Blanco, A.; Gadaleta, A. Mapping QTLs for Fusarium Head Blight Resistance in an Interspecific Wheat Population. Front. Plant Sci. 2016, 7, 1381. [Google Scholar] [CrossRef]
- Haile, J.K.; N’Diaye, A.; Walkowiak, S.; Nilsen, K.T.; Clarke, J.M.; Kutcher, H.R.; Steiner, B.; Buerstmayr, H.; Pozniak, C.J. Fusarium Head Blight in Durum Wheat: Recent Status, Breeding Directions and Future Research Prospects. Phytopathology 2019, 109, 1664–1675. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Buerstmayr, H. The Semidwarfing Alleles Rht D1b and Rht B1b Show Marked Differences in Their Associations with Anther-Retention in Wheat Heads and with Fusarium Head Blight Susceptibility. Phytopathology 2016, 106, 1544–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, D.; Beta, T.; Vanara, F.; Blandino, M. Influence of Agricultural Management on Phytochemicals of Colored Corn Genotypes (Zea mays L.). Part 1: Nitrogen Fertilization. J. Agric. Food Chem. 2018, 66, 4300–4308. [Google Scholar] [CrossRef] [PubMed]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant Secondary Metabolites in Cereals: Potential Involvement in Resistance to Fusarium and Mycotoxin Accumulation. Front. Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef] [Green Version]
- Žilič, S. Phenolic Compounds of Wheat: Their Content, Antioxidant Capacity and Bioaccessibility. MOJ Food Process. Technol. 2016, 2, 85–89. [Google Scholar] [CrossRef]
- Tian, S.; Sun, Y.; Chen, Z.; Zhao, R. Bioavailability and Bioactivity of Alkylresorcinols from Different Cereal Products. J. Food Qual. 2020, 2020, 5781356. [Google Scholar] [CrossRef]
- Niculaes, C.; Abramov, A.; Hannemann, L.; Frey, M. Plant Protection by Benzoazinoids—Recent Insights into Biosynthesis and Function. Agronomy 2018, 8, 143. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, T.; Mori, I.C.; Himi, E.E.; Hirayama, T. Plant Hormone Profiling in Developing Seeds of Common Wheat (Triticum aestivum L.). Breed. Sci. 2019, 69, 601–610. [Google Scholar] [CrossRef] [Green Version]
- Wenda-Piesik, A.; Piesik, D.; Ligor, T.; Buszewski, B. Volatile Organic Compounds (VOCs) from Cereal Plants Infested with Crown Rot: Their Identity and Their Capacity for Inducing Production of VOCs in Uninfested Plants. Int. J. Pest Manag. 2010, 4, 377–383. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Saito, K. Integrated Metabolomics for Abiotic Stress Responses in Plants. Curr. Opin. Plant Biol. 2015, 24, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, S., García-Mateos, R., Palma-Tenango, M., Eds.; IntechOpen: London, UK, 2019; Chapter 3; pp. 35–50. [Google Scholar]
- Paznocht, L.; Kotíková, Z.; Burešová, B.; Lachman, J.; Martinek, P. Phenolic Acids in Kernels of Different Coloured-Grain Wheat Genotypes. Plant Soil Environ. 2020, 66, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Chateigner-Boutin, A.L.; Lapierre, C.; Alvarado, C.; Yoshinaga, A.; Barron, C.; Bouchet, B.; Bakan, B.; Saulnier, L.; Devaux, M.F.; Girousse, C.; et al. Ferulate and Lignin Cross-Links Increase in Cell Walls of Wheat Grain Outer Layers during Late Development. Plant Sci. 2018, 276, 199–207. [Google Scholar] [CrossRef]
- Martinek, P.; Škorpík, M.; Chrpová, J.; Fučík, P.; Schweiger, J. Development of the New Winter Wheat Variety Skorpion with Blue Grain. Czech J. Genet. Plant Breed. 2013, 49, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, L.; Atanasova-Penichon, V.; Chéreau, S.; Richard-Forget, F. Metabolomics to Decipher the Chemical Defense of Cereals against Fusarium graminearum and Deoxynivalenol Accumulation. Int. J. Mol. Sci. 2015, 6, 24839–24872. [Google Scholar] [CrossRef]
- Ponts, N.; Pinson-Gadais, L.; Barreau, C.; Richard-Forget, F.; Quellet, T. Exogenous H2O2 and Catalase Treatments Interfere with Tri Genes Expression in Liquid Cultures of Fusarium graminearum. FEBS Lett. 2007, 581, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöneberg, T.; Kibler, K.; Sulyok, M.; Musa, T.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Foegele, R.T.; Vogelsang, S. Can Plant Phenolic Compounds Reduce Fusarium Growth and Mycotoxin Production in Cereals? Food Addit. Contam. Part A 2018, 35, 2455–2470. [Google Scholar] [CrossRef] [Green Version]
- Siranidou, E.; Kang, Z.; Buchenauer, H. Studies on Symptom Development, Phenolic Compounds and Morphological Defense Responses in Wheat Cultivars Differing in Resistance to Fusarium Head Blight. J. Phytopathol. 2003, 150, 200–208. [Google Scholar] [CrossRef]
- Gauthier, L.; Bonnin-Verdal, M.N.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Richard-Forget, F.; Atanasova-Penichon, V. Fungal Biotransformation of Chlorogenic and Caffeic Acids by Fusarium graminearum: New Insights in the Contribution of Phenolic Acids to Resistance to Deoxynivalenol Accumulation in Cereals. Int. J. Food Microbiol. 2016, 221, 61–68. [Google Scholar] [CrossRef]
- Giordano, D.; Beta, T.; Reyneri, A.; Blandino, M. Changes in the Phenolic Acid Content and Antioxidant Activity during Kernel Development in Corn (Zea mays L.) and Relationship with Mycotoxin Contamination. Cereal Chem. 2017, 94, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Sampietro, D.A.; Fauguel, C.M.; Vattuone, M.A.; Presello, D.A.; Catalán, C.A. Phenylpropanoids from Maize Pericarp: Resistance Factors to Kernel Infection and Fumonisin Accumulation by Fusarium verticillioides. Eur. J. Plant Pathol. 2013, 135, 105–113. [Google Scholar] [CrossRef]
- Erayman, M.; Turktas, M.; Akdogan, G.; Gurkok, T.; Inal, B.; Ishakoglu, E.; Ilhan, E.; Unver, T. Transcriptome Analysis of Wheat Inoculated with Fusarium graminearum. Front. Plant Sci. 2015, 6, 867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocheleau, H.; Al-Harthi, R.; Quellet, T. Degradation of Salicylic Acid by Fusarium graminearum. Fungal Biol. 2019, 123, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Ponts, N.; Couedelo, L.; Pinson-Gadais, L.; Verdal-Bonnin, M.N.; Barreau, C.; Richard-Forget, F. Fusarium Response to by H2O2 is Trichothecene Chemotype-Dependent. FEMS Microbiol. Lett. 2009, 293, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Ferrigo, D.; Raiola, A.; Bogialli, S.; Bortolini, C.; Tapparo, A.; Causin, R. In Vitro Production of Fumonisins by Fusarium verticillioides under Oxidative Stress Induced by H2O2. J. Agric. Food Chem. 2015, 63, 4879–4885. [Google Scholar] [CrossRef] [PubMed]
- Steinkellner, S.; Mammerler, R. Effect of Flavonoids on the Development of Fusarium oxysporum f. sp. lycopersici. J. Plant Interact. 2007, 2, 17–23. [Google Scholar] [CrossRef]
- Jimenez-Garcia, S.N.; Garcia-Mier, L.; Garcia-Trejo, J.F.; Ramirez-Gomez, X.S.; Guevara-Gonzalez, R.G.; Feregrino-Perez, A.A. Chapter 3. Fusarium Mycotoxins and Metabolites that Modulate Their Production. In Fusarium Plant Diseases, Pathogen Diversity, Resistance and Molecular Markers; Askun, T., Ed.; IntechOpen: London, UK, 2018; pp. 23–40. [Google Scholar]
- Stuper-Szablewska, K.; Kurasiak-Popowska, D.; Nawracała, J.; Perkowski, J. Quantitative Profile of Phenolic Acids and Antioxidant Activity of Wheat Grain Exposed to Stress. Eur. Food Res. Technol. 2019, 245, 1595–1603. [Google Scholar] [CrossRef] [Green Version]
- Pervaiz, T.; Songtao, J.; Faghihi, H.; Halder, M.S.; Fang, J. Naturally Occurring Anthocyanin, Structure, Functions and Biosynthetic Pathway in Fruit Plants. J. Plant. Physiol. Biochem. 2017, 5, 187. [Google Scholar] [CrossRef]
- Shoeva, O.Y.; Gordeeva, E.I.; Arbuzova, V.S.; Khlestkina, E.K. Anthocyanins Participate in Protection of Wheat Seedlings from Osmotic Stress. Cereal Res. Commun. 2017, 45, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Eliášová, M.; Kotíková, Z.; Lachman, J.; Orsák, M.; Martinek, P. Influence of Baking on Anthocyanin Content in Coloured-Grain Wheat Bread. Plant Soil Environ. 2020, 66, 381–383. [Google Scholar] [CrossRef]
- Tyl, C.E.; Bunzel, M. Antioxidant Activity-Guided Fractionation of Blue Wheat (UC66049 Triticum aestivum L.). J. Agric. Food Chem. 2012, 60, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol. Res. 2019, 52, E39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Olsen, O.; Knudsen, S. Expression of Dihydroflavonol Reductase Gene in an Anthocyanin-Free Barley Mutant. Hereditas 1993, 119, 67–75. [Google Scholar] [CrossRef]
- Masisi, K.; Beta, T.; Moghadasian, M.H. Antioxidant Properties of Diverse Cereal Grains: A Review on In Vitro and In Vivo studies. Food Chem. 2016, 196, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Bollina, V.; Kushalappa, A.C. In Vitro Inhibition of Trichothecene Biosynthesis in Fusarium graminearum by Resistance-Related Endogenous Metabolites Identified in Barley. Mycology 2011, 2, 291–296. [Google Scholar]
- Gunnaiah, R.; Kushalappa, A.C. Metabolomics Deciphers the Host Resistance Mechanisms in Wheat Cultivar Sumai-3, against Trichothecene Producing and Non-Producing Isolates of Fusarium graminearum. Plant Physiol. Biochem. 2014, 83, 40–50. [Google Scholar] [CrossRef]
- Durazzo, A.; Zaccaria, M.; Polito, A.; Maiani, G.; Carcea, M. Lignan Content in Cereals, Buckwheat and Derived Foods. Foods 2013, 2, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Dwyer, J.; Adlercreutz, H.; Scalbert, A.; Jacques, P.; McCullough, M.L. Dietary Lignans: Physiology and Potential for Cardiovascular Disease risk reduction. Nutr. Rev. 2010, 68, 571–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagniewska-Zadworna, A.; Barakat, A.; Łakomy, P.; Smoliński, D.J.; Zadworny, M. Lignin and Lignans in Plant Defence: Insight from Expression Profiling of Cinnamyl Alcohol Dehydrogenase Genes during Development and Following Fungal Infection in Populus. Plant Sci. J. 2014, 229, 111–121. [Google Scholar] [CrossRef]
- Mattila, P.; Pihlava, J.M.; Hellstrom, J. Contents of Phenolic Acids, Alkyl- and Alkenylresorcinols, and Aventhramides in Commercial Grain Products. J. Agric. Food Chem. 2005, 53, 8290–8295. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kurano, M.; Esumi, Y.; Yamaguchi, I.; Yoshiharu, D. Biosynthesis of 5-alkylresorcinol in Rice: Icorporation of Putative Fatty Acid Unit in the 5-alkylresorcinol Carbon Chain. Bioorg. Chem. 2003, 31, 437–452. [Google Scholar] [CrossRef]
- Ross, A.B.; Kamal-Eldin, A.; Åman, P. Dietary Alkylresorcinols: Absorption, Bioactivities, and Possible Use as Biomarkers of Whole-Grain Wheat- and Rye-Rich Foods. Nutr. Rev. 2004, 3, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, I.; Kowalczyk, M. Determination of benzoxazinoids in Spring and Winter Varieties Using Ultra-Performance Liquid Chromatography Coupled with Mass Spectrometry. Acta Chromatogr. 2019, 31, 179–182. [Google Scholar] [CrossRef]
- Meyer, J.; Murray, S.L.; Berger, D.K. Signals that Stop the Rot: Regulation of Secondary Metabolite Defences in Cereals. Physiol. Mol. Plant Pathol. 2016, 94, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Shao, B.; Shaikh, F.I.; Friedt, W.; Gottwald, S. Wheat Resistances to Fusarium Root Rot and Head Blight Are Both Associated with Deoxynivalenol- and Jasmonate-Related Gene Expression. Phytopathology 2018, 108, 602–616. [Google Scholar] [CrossRef] [Green Version]
- Nazareth, T.M.; Bordin, K.; Manyes, L.; Meca, G.; Mañes, J.; Luciano, F.B. Gaseous Allyl Isothiocyanate to Inhibit the Production of Aflatoxins, Beauvericin and Enniatins by Aspergillus parasiticus and Fusarium poae in Wheat Flour. Food Control 2016, 62, 317–321. [Google Scholar] [CrossRef] [Green Version]
- Small, I.M.; Flett, B.C.; Marasas, W.F.; McLeod, A.; Stander, M.A.; Viljoen, A. Resistance in Maize Inbred Lines to Fusarium verticillioides and Fumonisin Accumulation in South Africa. Plant Dis. 2012, 96, 881–888. [Google Scholar] [CrossRef] [Green Version]
- Trapp, M.A.; De Souza, G.D.; Rodrigues-Filho, E.; Boland, W.; Mithöfer, A. Validated Method for Quantification in Plants. Front. Plant Sci. 2014, 5, 417. [Google Scholar]
- Paznocht, L.; Kotíková, Z.; Šulc, M.; Lachman, J.; Orsák, M.; Eliášová, M.; Martinek, P. Free and Esterified Carotenoids in Pigmented Wheat, Tritordeum and Barley Grains. Food Chem. 2018, 240, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.M.; Sulyok, M.; Jirsa, O.; Spitzer, T.; Krska, R.; Polišenská, I. Relationship between Lutein and Mycotoxin Content in Durum Wheat. Food Addit. Contam. Part A 2014, 31, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Fongaro, L.; Brandolini, A. Colour Screening of Whole Meal Flours and Discrimination of Seven Triticum Subspecies. J. Cereal Sci. 2017, 77, 9–16. [Google Scholar] [CrossRef]
- Colasuonno, P.; Lozito, M.L.; Marcotuli, I.; Nigro, D.; Giancaspro, A.; Mangini, G.; De Vita, P.; Mastrangelo, A.M.; Pecchini, N.; Houston, K.; et al. The Carotenoid Biosynthetic and Catabolic Genes and Their Association with Yellow Pigments. BMC Genet. 2017, 18, 122. [Google Scholar] [CrossRef] [Green Version]
- Golkari, S.; Gilbert, J.; Prashar, S.; Procunier, J.D. Microarray Analysis of Fusarium graminearum-Induced Wheat Genes: Identification of Organ-Specific and Differentially Expressed Genes. Plant Biotechnol. J. 2007, 5, 38–49. [Google Scholar] [CrossRef]
- Gunnaiah, R.; Kushalappa, A.C.; Duggavathi, R.; Fox, S.; Somers, D.J. Integrated Metabolo-Proteomic Approach to Decipher the Mechanisms by Which Wheat QTL (Fhb1) Contributes to Resistance against Fusarium graminearum. PLoS ONE 2012, 7, e40695. [Google Scholar] [CrossRef] [Green Version]
- Zykin, P.A.; Andreeva, E.A.; Lakholay, A.N.; Tsvetkova, N.V.; Voloylokov, A.V. Anthocyanin Composition and Content in Rye Plants with Different Grain Color. Molecules 2018, 23, 948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordeeva, E.I.; Shoeva, O.Y.; Khlestkina, E.K. Marker-Assisted Development of Bread Wheat Near-Isogenic Lines Carrying Various Combinations of Purple Pericarp (Pp) Alleles. Euphytica 2015, 203, 469–476. [Google Scholar] [CrossRef]
- Syed Jaafar, S.N.; Baron, J.; Siebenhandl-Ehn, S.; Rosenau, T.; Böhmdorfer, S.; Grausgruber, H. Increased Anthocyanin Content in Purple Pericarp x Blue Aleurone Wheat Crosses. Plant Breed. 2013, 132, 48–58. [Google Scholar] [CrossRef]
- Francavilla, A.; Joye, I. Anthocyanins in Whole Grain Cereals and Their Potential Effect on Health. Nutrients 2020, 12, 2922. [Google Scholar] [CrossRef]
- Paznocht, L.; Burešová, B.; Kotíková, Z.; Martinek, P. Carotenoid Content of Extruded and Puffed Products Made of Colored-Grain Wheats. Food Chem. 2021, 340, 127951. [Google Scholar] [CrossRef]
- Lachman, J.; Hejtmánková, A.; Orsák, M.; Popov, M.; Martinek, P. Tocotrienols and Tocopherols in Colored-Grain Wheat, Tritordeum and Barley. Food Chem. 2018, 240, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Spanić, V.; Viljevać Vuletić, M.; Abicić, I.; Marček, T. Early Response of Wheat Antioxidant System with Special Reference to Fusarium Head Blight Stress. Plant Physiol. Biochem. 2017, 115, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Schöneberg, T.; Vogelsang, S.; Vincenti, J.; Bertossa, M.; Mauch-Mani, B.; Masher, F. Factors of Wheat Grain Resistance to Fusarium Head Blight. Phytopathol. Mediterr. 2017, 56, 154–166. [Google Scholar]
- Bernardi, J.; Stagnati, L.; Lucini, L.; Rocchetti, G.; Lanubil, A.; Cortellini, C.; De Poli, G.; Busconi, M.; Marocco, A. Phenolic Profile and Susceptibility to Fusarium Infection of Pigmented Maize Cultivars. Front. Plant Sci. 2018, 9, 1189. [Google Scholar] [CrossRef]
- Lorenz-Kukuła, K.; Wróbel-Kwiatkowska, M.; Starzycki, M.; Szopa, J. Engineering Flax with Increased Flavonoid Content and thus Fusarium Resistance. Physiol. Mol. Plant Pathol. 2007, 70, 38–48. [Google Scholar] [CrossRef]
- Long, L.; Liu, J.; Gao, Y.; Xu, F.C.; Zhao, J.R.; Li, B.; Gao, W. Flavonoid Accumulation in Spontaneous Cotton Mutant Results in Red Coloration and Enhanced Disease Resistance. Plant Physiol. Biochem. 2019, 143, 40–49. [Google Scholar] [CrossRef] [PubMed]

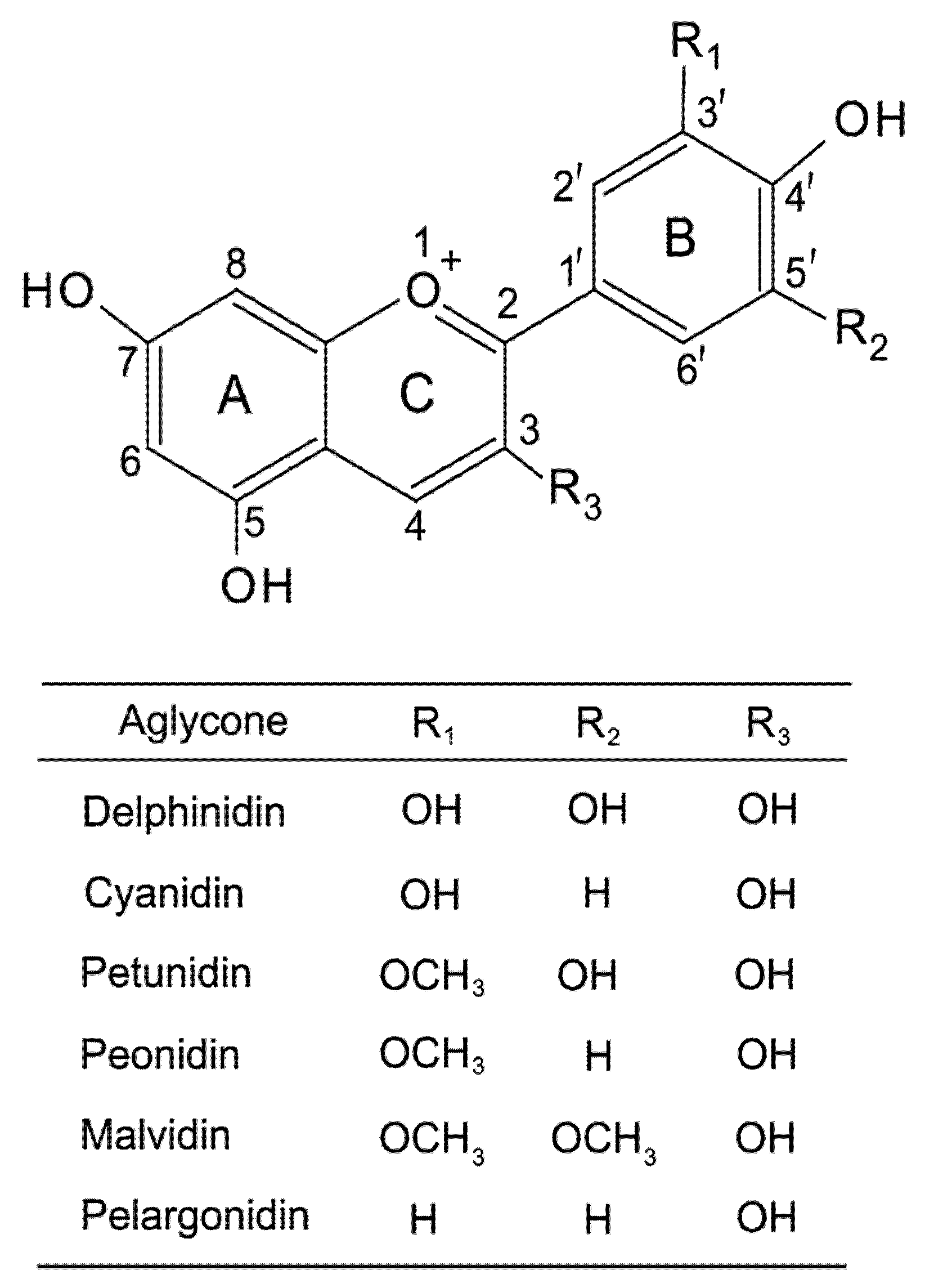

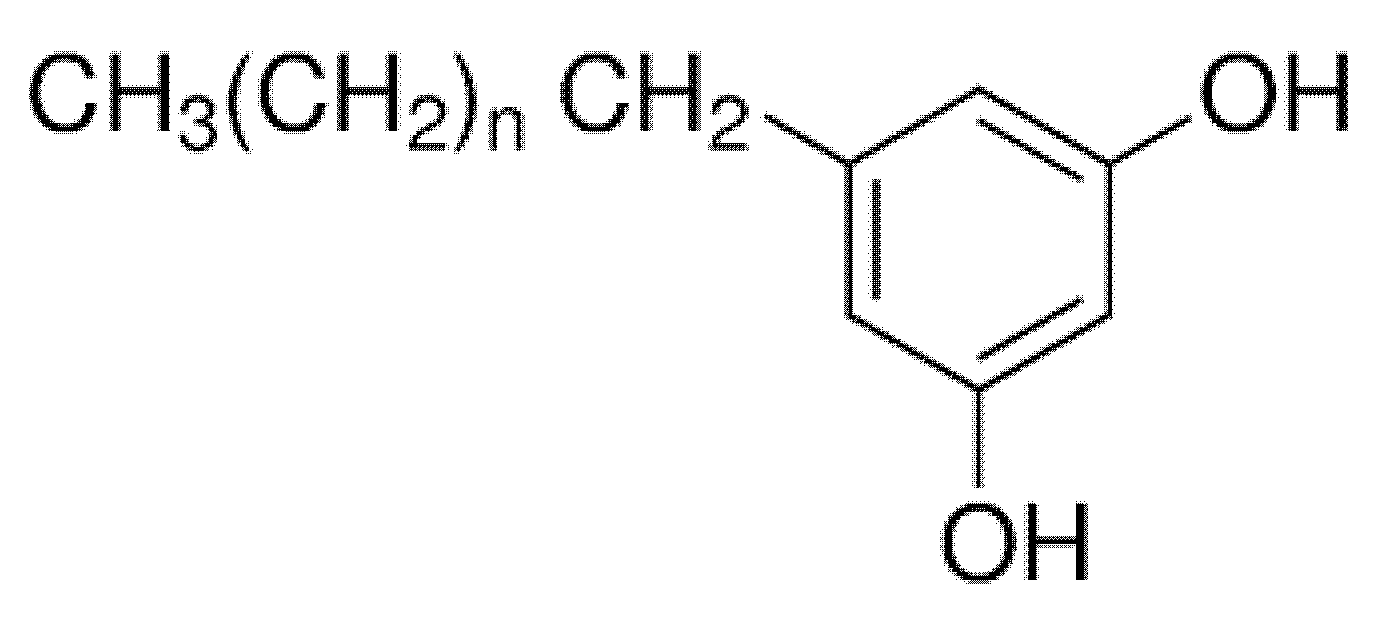
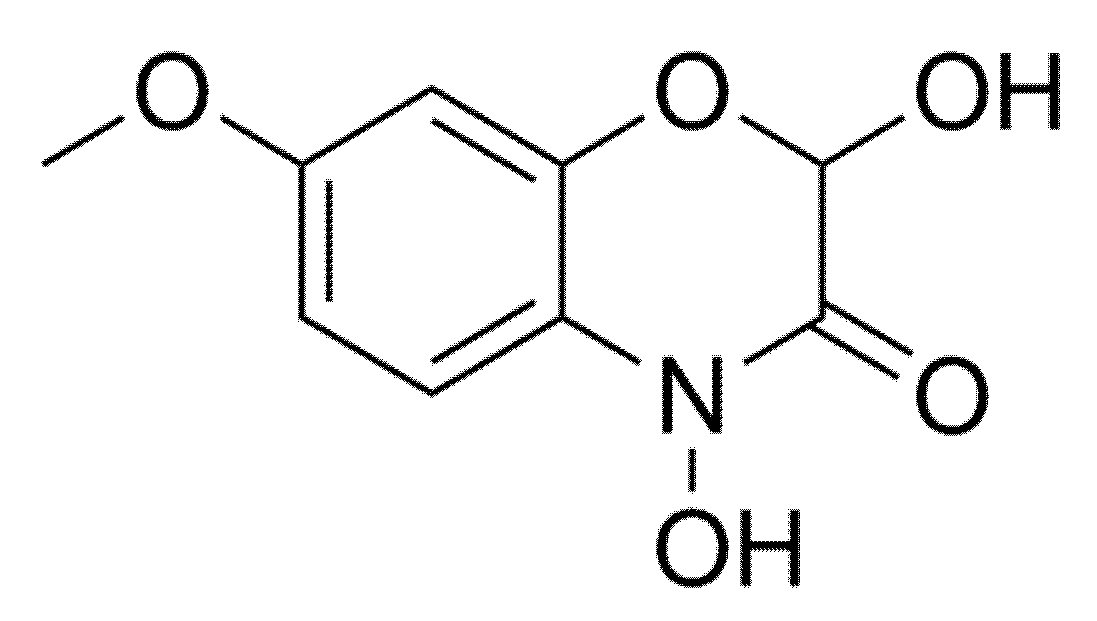
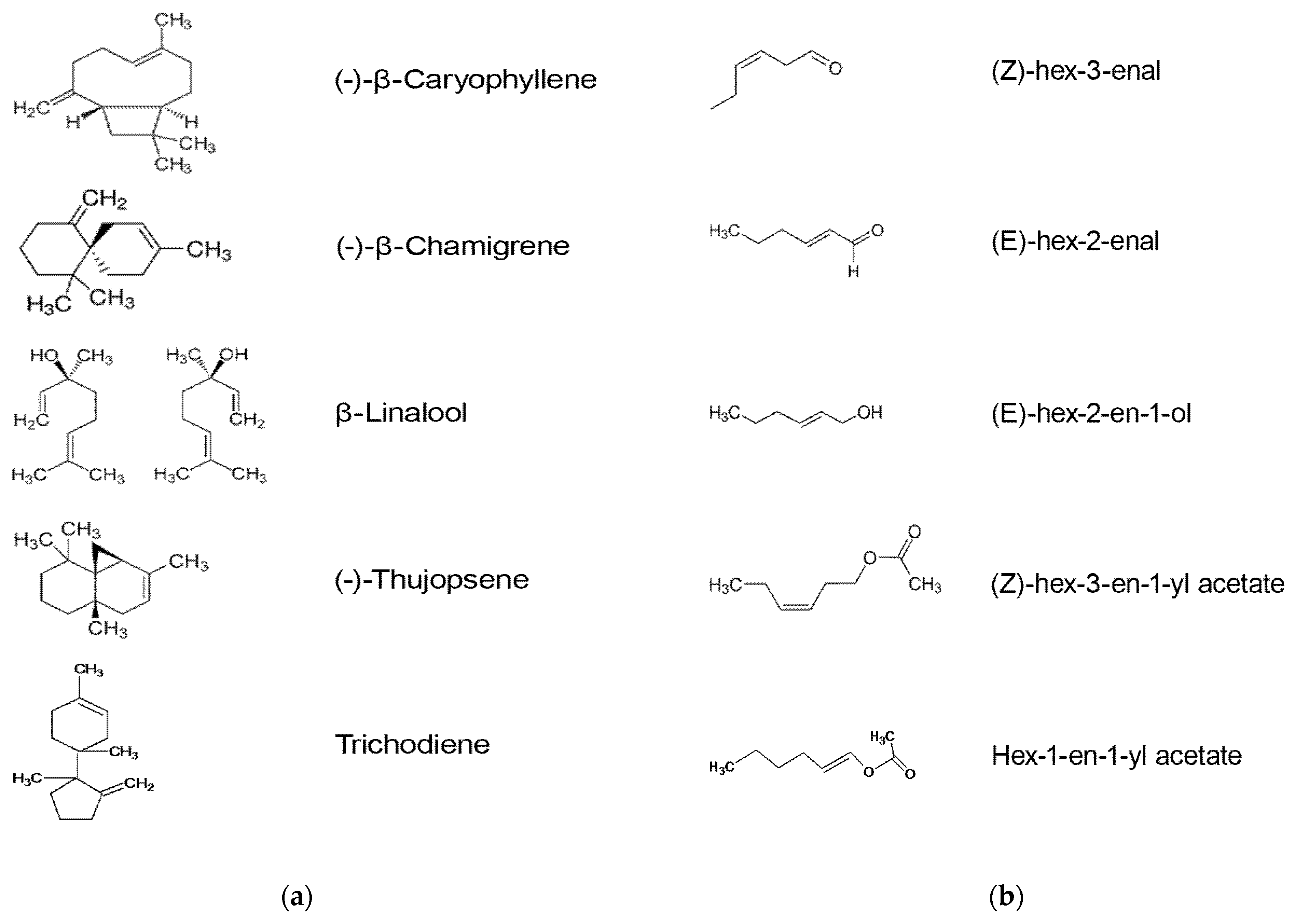


| Compound | Content | Reference |
|---|---|---|
| Total 5-n-alkylresorcinols | 761 bread wheat, 743 spelt, 654 durum, 697 emmer, 737 einkorn 300–943 common wheat, 194–687 durum wheat, 545–654 einkorn wheat, 531–784 emmer wheat, 490–741 spelt wheat | [53,54] |
| 5-n-Heptadecylresorcinol (C17:0) | 32–34 common wheat 1.2 (T. turgidum ssp. dicoccum) 26.0 (T. turgidum ssp. turgidum) | [55,56] |
| 5-n-Nonadecylresorcinol (C19:0) | 250–272 common wheat 20.4 (T. turgidum ssp. dicoccum) 187.9 (T. aestivum) | [55,56] |
| 5-n-Heneicosylresorcinol (C21:0) | 368–474 common wheat 196.5 (T. turgidum ssp. dicoccum) 653.1 (T. aestivum) 164.4 (T. turgidum var. durum) 65.4 (T. aestivum) | [55,56] |
| 5-n-Tricosylresorcinol (C23:0) | 84–108 common wheat | [55] |
| 5-n-Pentacosylresorcinol (C25:0) | 26–33 common wheat | [55] |
| Total anthocyanins | 210 Pp grain; 430 Pp bran 21–157 Ba, R, 78 Pp | [51,57] |
| Cyanidin-3-glucoside | Ba 3.07, Pp 10.34, R 4.02 | |
| Cyanidin-3-rutinoside | 8.42 Ba, Pp | |
| Delphinidin-3-glucoside | 13.68 Ba | |
| Delphinidin-3-rutinoside | 33.44 Ba | |
| Malvidin-3-glucoside | 12.04 Ba, 0.48 Pp, 0.22 R | |
| Peonidin-3-arabinoside | 2.22 Ba, Pp | |
| Peonidin-3-glucoside | 0.88 Pp | |
| Peonidin-3-galactoside | 1.94 Ba, 0.58 Pp, 0.33 R | |
| DIMBOA-glucoside | 18 common wheat | [58] |
| Total carotenoids | 1.63–4.19 einkorn, 4.73–13.64 emmer, 2.69–8.38 durum, 1.62–2.98 spelt, 1.40–4.90 bread wheat 5.47 mg β-carotene kg−1 DM (T. turgidum var. durum) 3.3 < 1.4–6.6 > wheat grains 3.2 < 1.6–4.7 > white wheat grains 3.1 < 1.4–4.1 > red wheat grains 6.0 < 4.7–6.6 > black wheat grains | [59,60,61] |
| α-Carotene | 7.3–13.4 T. monococcum | [62] |
| β-Carotene | 0.116 spring wheat, 0.195 einkorn | |
| Zeaxanthin | 0.144 spring wheat, 0.351 einkorn, 0.138 emmer wheat | |
| Lutein | 1.096 spring wheat, 5.246 einkorn, 0.761 emmer wheat | |
| Total phenolics | 1499; 1545.7 mg FAE kg−1 DM 559.1, 506.5–659.8 mg GAE kg−1 1265.7 < 837.0–2233.7 > wheat grains 1231.7 < 837.0–1759.0 > white grains 1401.8 < 1105.8–1850.9 > red grains 1546.4 < 1122.8–2233.7 > black grains | [60,61,63] |
| Total flavonoids | 270.0, 236.2–319.3 mg RE kg−1 DM 252 < 147–397 > winter wheat grains 241 < 147–351 > white grains 290 < 218–389 > red grains 361 < 321–397 > black grains | [61,63] |
| Apigenin | 2.512 control, 104.565 inoculated with F. culmorum | [64] |
| Kaempferol | 6.009 control, 124.739 inoculated with F. culmorum | |
| Luteolin | 7.117 control, 458.404 inoculated with F. culmorum | |
| Naringenin | 7.115 control, 127.787 inoculated with F. culmorum | |
| Quercetin | 6.958 control, 512.934 inoculated with F. culmorum | |
| Rutin | 13.764 control, 332.44 inoculated with F. culmorum | |
| Vitexin | 6.481 control, 148.256 inoculated with F. culmorum | |
| Total phenolic acids | 987.3; 4061.4 mg kg−1 DM | [60] |
| Salicylic acid | < 0.3–0.8 > free salicylic acid in leaves | [65] |
| Protocatechuic acid | < 6.8–13.3 > bran; 9.2 | [66] |
| Ferulic acid | 270–1446; 3000 bran 194.18 grain at 10 days post-anthesis flower tissues (S) 69.9; (MR) 99.0; (R) 101; developing grains 10 days post-anthesis (S) 97.1, (MR) 122.3, (R) 126.2, 130.1–233 developing grains | [67,68] |
| 4-Hydroxybenzoic acid | 87.3 control, 87.3 infected | [67] |
| Gallic acid | < 1–37 >; control 57, infected 77,3 | |
| Vanillic acid | < 30–70 >; control 26.7, infected 37.0 | |
| Syringic acid | < 1–62 >; control 30.7, infected 23 | |
| t-Cinnamic acid | < 3–83 >; control 127.0, infected 343.3 | |
| p-Coumaric acid | < 1–63 >; bran 90; control 45.7, infected 44.0 | |
| Caffeic acid | < 2–90 >; bran 38; control 40, infected 46.7 | |
| Sinapic acid | < 2–2017 >; bran 200; control 136.0, infected 360.0 | |
| Chlorogenic acid | < 10–69 >; control 38.0, infected 39.0 | |
| Abscisic acid | Increase from 86 to 154 ng g−1 DW after inoculation with F. graminearum | [69] |
| Indol-3-acetic acid | Increase from 83 to 26 328 ng g−1 DW after inoculation with F. graminearum | |
| Jasmonic acid | Increase from 29 to 410 ng g−1 FW after inoculation with F. graminearum | |
| (-)-β-Caryophyllene | Increase from 9 to 104 ng sample−1 after inoculation with F. graminearum | [70] |
| β-Linalool | Increase from 12 to 405 ng sample−1 after inoculation with F. graminearum | |
| (-)-Thujopsene | Increase from 0.005 to 0.018 ratio unit after inoculation with F. culmorum | [71] |
| Trichodiene | Increase from 0.009 to 0.027 ratio unit after inoculation with F. culmorum | |
| (-)-β-Chamigrene | Increase from 0.003 to 0.012 ratio unit after inoculation with F. culmorum | |
| (Z)-hex-3-enal | Increase from 14 to 139 ng sample−1 after inoculation with F. graminearum | [70] |
| (E)-hex-2-enal | Increase from 1 to 709 ng sample−1 after inoculation with F. graminearum | |
| (E)-hex-2-en-1-ol | Increase from 9 to 881 ng sample−1 after inoculation with F. graminearum | |
| (Z)-hex-3-en-1-yl acetate | Increase from 22 to 218 ng sample−1 after inoculation with F. graminearum | |
| Hex-1-en-1-yl acetate | Increase from 3 to 477 ng sample−1 after inoculation with F. graminearum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrpová, J.; Orsák, M.; Martinek, P.; Lachman, J.; Trávníčková, M. Potential Role and Involvement of Antioxidants and Other Secondary Metabolites of Wheat in the Infection Process and Resistance to Fusarium spp. Agronomy 2021, 11, 2235. https://doi.org/10.3390/agronomy11112235
Chrpová J, Orsák M, Martinek P, Lachman J, Trávníčková M. Potential Role and Involvement of Antioxidants and Other Secondary Metabolites of Wheat in the Infection Process and Resistance to Fusarium spp. Agronomy. 2021; 11(11):2235. https://doi.org/10.3390/agronomy11112235
Chicago/Turabian StyleChrpová, Jana, Matyáš Orsák, Petr Martinek, Jaromír Lachman, and Martina Trávníčková. 2021. "Potential Role and Involvement of Antioxidants and Other Secondary Metabolites of Wheat in the Infection Process and Resistance to Fusarium spp." Agronomy 11, no. 11: 2235. https://doi.org/10.3390/agronomy11112235
APA StyleChrpová, J., Orsák, M., Martinek, P., Lachman, J., & Trávníčková, M. (2021). Potential Role and Involvement of Antioxidants and Other Secondary Metabolites of Wheat in the Infection Process and Resistance to Fusarium spp. Agronomy, 11(11), 2235. https://doi.org/10.3390/agronomy11112235





