Identification of Soybean Yield QTL in Irrigated and Rain-Fed Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phenotypic Evaluation
2.2. SSR Marker Data Collection
2.3. Linkage Map and Statistical Analysis
3. Results
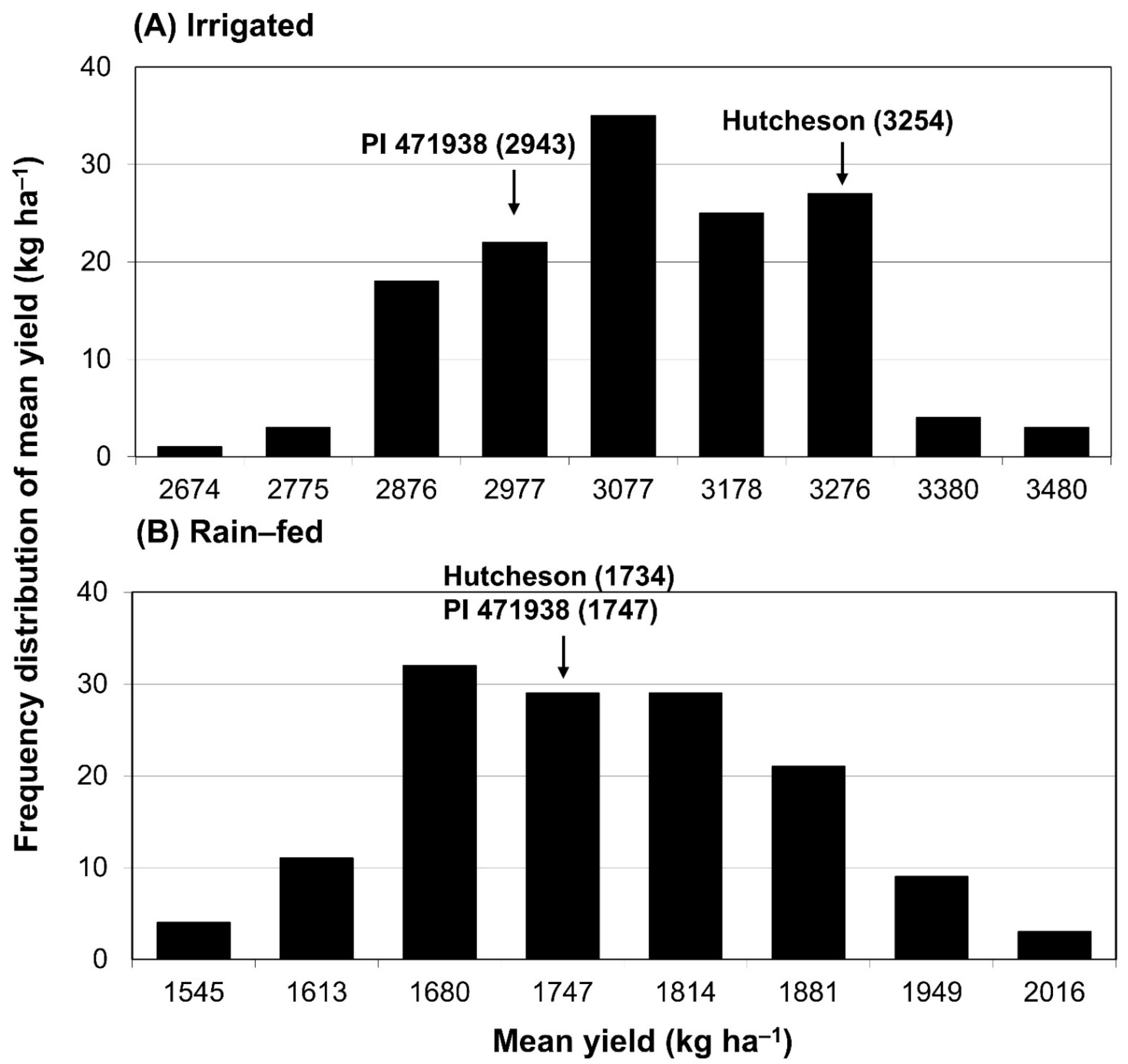
3.1. Frequency Distribution of Seed Yield under Irrigated and Rain-Fed Conditions
3.2. Genetic Map
3.3. QTL Analysis for Yield and Wilting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chapman, A.; Pantalone, V.R.; Ustun, A.; Allen, F.L.; Landau-Ellis, D.; Trigiano, R.N.; Gresshoff, P.M. Quantitative trait loci for agronomic and seed quality traits in an F2 and F4:6 soybean population. Euphytica 2003, 129, 387–393. [Google Scholar] [CrossRef]
- Fang, L.; Wang, Q.; Hu, Y.; Jia, Y.; Chen, J.; Liu, B.; Zhang, Z.; Guan, X.; Chen, S.; Zhou, B.; et al. Genomic analyses in cotton identify signatures of selection and loci associated with fiber quality and yield traits. Nat. Genet. 2017, 49, 1089–1098. [Google Scholar] [CrossRef]
- Mansur, L.M.; Orf, J.H.; Chase, K.; Jarvik, T.; Cregan, P.B.; Lark, K.G. Genetic mapping of agronomic traits using recombinant inbred lines of soybean. Crop Sci. 1996, 36, 1327–1336. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, H.; Du, Q.; Hu, Z.; Yang, Z.; Li, X.; Wang, J.; Huang, F.; Yu, D.; Wang, H.; et al. Genetic dissection of yield-related traits via genome-wide association analysis across multiple environments in wild soybean (Glycine soja Sieb. and Zucc.). Planta 2020, 251, 39. [Google Scholar] [CrossRef]
- Yuan, J.; Njiti, V.N.; Meksem, K.; Iqbal, M.J.; Triwitayakorn, K.; Kassem, M.A.; Davis, G.T.; Schmidt, M.E.; Lightfoot, D.A. Quantitative trait loci in two soybean recombinant inbred line populations segregating for yield and disease resistance. Crop Sci. 2002, 42, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Basal, O.; Szabó, A. The combined effect of drought stress and nitrogen fertilization on soybean. Agronomy 2020, 10, 384. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.C.; Macguidwin, A.; Gratton, C. Soybean aphid and soybean cyst nematode interactions in the field and effects on soybean yield. J. Econ. Entomol. 2011, 104, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Kunert, K.; Vorster, B.J. In search for drought-tolerant soybean: Is the slow-wilting phenotype more than just a curiosity? J. Exp. Bot. 2020, 71, 457–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.J.; Boerma, H.R.; Villagarcia, M.R.; Zhou, X.; Carter, T.E.; Li, Z.; Gibbs, M.O. A major QTL conditioning salt tolerance in S-100 soybean and descendent cultivars. Theor. Appl. Genet. 2004, 109, 1610–1619. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarker, U.; Oba, S. Response of nutrients, minerals, antioxidant leaf pigments, vitamins, polyphenol, flavonoid and antioxidant activity in selected vegetable amaranth under four soil water content. Food Chem. 2018, 252, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Drought stress effects on growth, ROS markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. J. Sci. Food Agric. 2019, 99, 2275–2284. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef]
- Stuber, C.W.; Polacco, M.; Lynn Senior, M. Synergy of empirical breeding, marker-assisted selection, and genomics to increase crop yield potential. Crop Sci. 1999, 39, 1571–1583. [Google Scholar] [CrossRef] [Green Version]
- Xavier, A.; Rainey, K.M. Quantitative genomic dissection of soybean yield components. G3 Genes Genom. Genet. 2020, 10, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Mazur, B.J.; Tingey, S.V. Genetic mapping and introgression of genes of agronomic importance. Curr. Opin. Biotechnol. 1995, 6, 175–182. [Google Scholar] [CrossRef]
- Ribaut, J.M.; Jiang, C.; Gonzalez-De-Leon, D.; Edmeades, G.O.; Hoisington, D.A. Identification of quantitative trait loci under drought conditions in tropical maize. 2. Yield components and marker-assisted selection strategies. Theor. Appl. Genet. 1997, 94, 887–896. [Google Scholar] [CrossRef] [Green Version]
- Orf, J.H.; Chase, K.; Jarvik, T.; Mansur, L.M.; Cregan, P.B.; Adler, F.R.; Lark, K.G. Genetics of soybean agronomic traits: I. Comparison of three related recombinant inbred populations. Crop Sci. 1999, 39, 1642–1651. [Google Scholar] [CrossRef] [Green Version]
- Reyna, N.; Sneller, C.H. Evaluation of marker-assisted introgression of yield QTL alleles into adapted soybean. Crop Sci. 2001, 41, 1317–1321. [Google Scholar] [CrossRef]
- Gizlice, Z.; Carter, T.E., Jr.; Gerig, T.M.; Burton, J.W. Genetic diversity patterns in North American public soybean cultivars based on coefficient of parentage. Crop Sci. 1996, 36, 614–620. [Google Scholar] [CrossRef]
- Devicente, M.C.; Tanksley, S.D. QTL analysis of transgressive segregation in an interspecific tomato cross. Genetics 1993, 134, 585–596. [Google Scholar] [CrossRef]
- Kisha, T.J.; Diers, B.W.; Hoyt, J.M.; Sneller, C.H. Genetic diversity among soybean plant introductions and North American germplasm. Crop Sci. 1998, 38, 1669–1680. [Google Scholar] [CrossRef]
- Keim, P.; Olson, T.C.; Shoemaker, R.C. A rapid protocol for isolating soybean DNA. Soybean Genet. Newsl. 1988, 15, 150–152. [Google Scholar]
- Diwan, N.; Cregan, P.B. Automated sizing of fluorescent-labeled simple sequence repeat (SSR) markers to assay genetic variation in soybean. Theor. Appl. Genet. 1997, 95, 723–733. [Google Scholar] [CrossRef]
- Holloway, J.L.; Knapp, S.J. Gmendel 3.0 Users Guide; Department of Crop and Soil Science, Oregon State University: Corvallis, OR, USA, 1993. [Google Scholar]
- Sas Institute. The SAS System for Windows, Version 8; SAS Inst. Inc.: Cary, NC, USA, 2001. [Google Scholar]
- Manly, K.F.; Cudmore, R.H., Jr.; Meer, J.M. Map Manager QTX, cross-platform software for genetic mapping. Mamm. Genome 2001, 12, 930–932. [Google Scholar] [CrossRef]
- Kosambi, D.D. The estimation of map distances from recombination values. Ann. Eugen. 1943, 12, 172–175. [Google Scholar] [CrossRef]
- Churchill, G.A.; Doerge, R.W. Empirical threshold values for quantitative trait mapping. Genetics 1994, 138, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Eshed, Y.; Zamir, D. An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL. Genetics 1995, 141, 1147–1162. [Google Scholar] [CrossRef]
- Tanksley, S.D.; Mccouch, S.R. Seed banks and molecular maps: Unlocking genetic potential from the wild. Science 1997, 277, 1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmberg, N.; Bülow, L. Improving stress tolerance in plants by gene transfer. Trends Plant Sci. 1998, 3, 61–66. [Google Scholar] [CrossRef]
- Cregan, P.B.; Jarvik, T.; Bush, A.L.; Shoemaker, R.C.; Lark, K.G.; Kahler, A.L.; Kaya, N.; Vantoai, T.T.; Lohnes, D.G.; Chung, J.; et al. An integrated genetic linkage map of the soybean genome. Crop Sci. 1999, 39, 1464–1490. [Google Scholar] [CrossRef] [Green Version]
- Specht, J.E.; Chase, K.; Macrander, M.; Graef, G.L.; Chung, J.; Markwell, J.P.; Germann, M.; Orf, J.H.; Lark, K.G. Soybean response to water: A QTL analysis of drought tolerance. Crop Sci. 2001, 41, 493–509. [Google Scholar] [CrossRef]
- Orf, J.H.; Chase, K.; Adler, F.R.; Mansur, L.M.; Lark, K.G. Genetics of soybean agronomic traits: II. Interactions between yield quantitative trait loci in soybean. Crop Sci. 1999, 39, 1652–1657. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; Hanumantharao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and heat-stress effects on seed filling in food crops: Impacts on functional biochemistry, seed yields, and nutritional quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef] [Green Version]
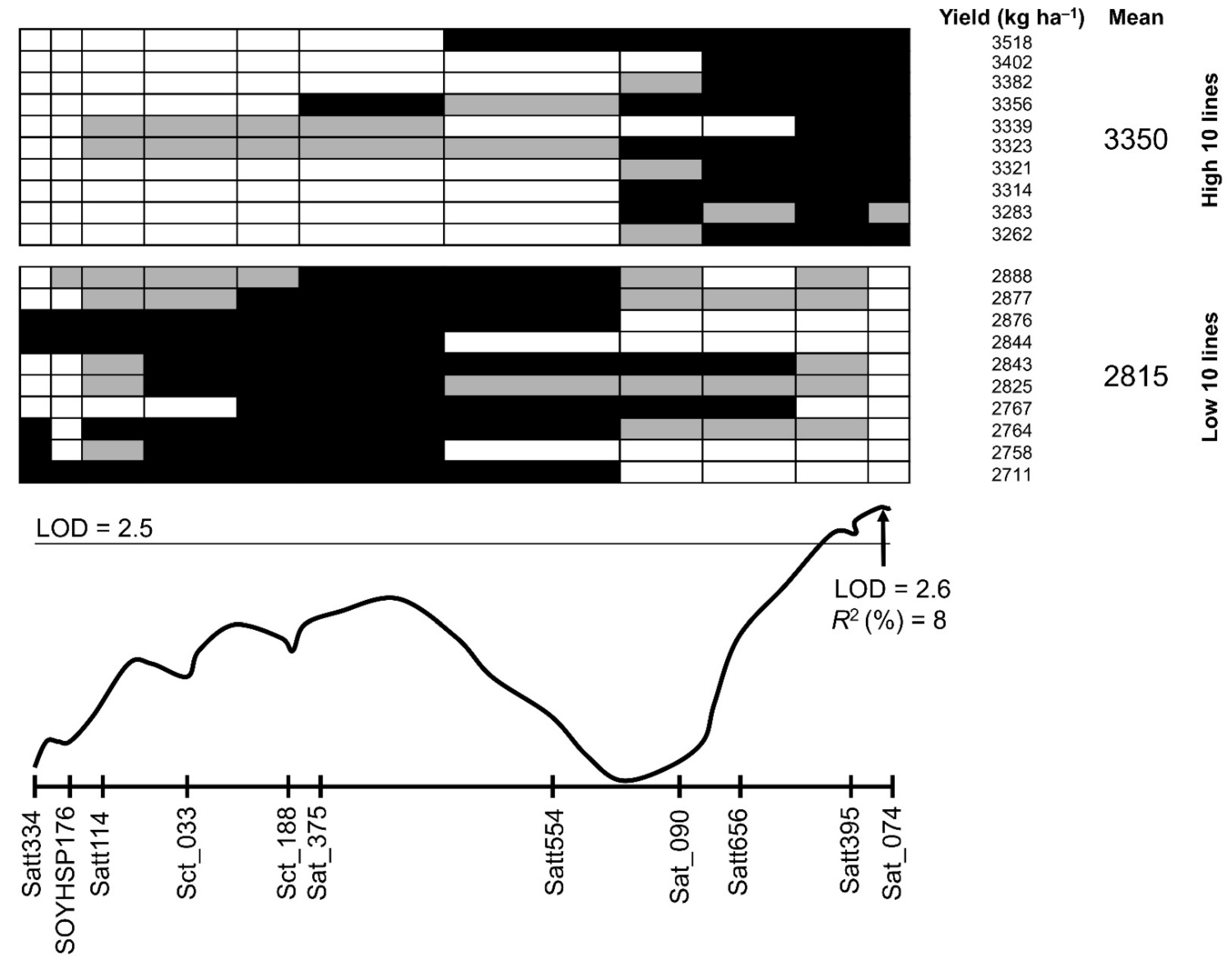


| Chr(LG) | Marker 1 | p-Value | R2 (%) | Additive Effect 2 | Allelic Means (kg ha−1) 3 | ||
|---|---|---|---|---|---|---|---|
| Hut/Hut | Hut/PI | PI/PI | |||||
| 17(D2) | Satt226 | 0.0253 | 5 | 0.59 | 3037 | 3097 | 3104 |
| 13(F) | Sat_375 | 0.0261 | 5 | 0.59 | 3044 | 3071 | 3124 |
| 13(F) | Sat_074 | 0.0029 | 8 | −0.72 | 3124 | 3077 | 3024 |
| 18(G) | Satt217 | 0.0189 | 6 | 0.57 | 3044 | 3098 | 3124 |
| 9(K) | Satt518 | 0.0479 | 4 | 0.57 | 3044 | 3098 | 3118 |
| Chr(LG) | Marker 1 | p-Value | R2 (%) | Additive Effect 2 | Allelic Means (kg ha−1) 3 | ||
|---|---|---|---|---|---|---|---|
| Hut/Hut | Hut/PI | PI/PI | |||||
| 1(D1a) | Satt507 | 0.0390 | 5 | 0.43 | 1801 | 1807 | 1854 |
| 2(D1b) | Satt296 | 0.0143 | 6 | −0.40 | 1868 | 1807 | 1814 |
| 17(D2) | Satt226 | 0.0030 | 8 | 0.54 | 1794 | 1828 | 1868 |
| 13 (F) | Sat_375 | 0.0000 | 16 | 0.69 | 1787 | 1807 | 1888 |
| 9 (K) | Satt137 | 0.0132 | 6 | 0.41 | 1801 | 1814 | 1861 |
| Environment | Marker | Chr (LG) | SF Analysis | R2 (%) from Multiple Regression 1 | Positive Allele | ||
|---|---|---|---|---|---|---|---|
| R2 (%) | p Value | Within Chr | Among Chrs | ||||
| Irrigated | Sat_375 | 13 (F) | 5 | 0.0261 | 4 | 3 | PI 471938 |
| Sat_074 | 13 (F) | 8 | 0.0029 | 9 | 8 | Hutcheson | |
| Satt217 | 18 (G) | 6 | 0.0189 | 7 | 5 | PI 471938 | |
| Sat_094 | 18 (G) | 7 | 0.0110 | 5 | 6 | Hutcheson | |
| Rain-fed | Satt507 | 1 (D1a) | 5 | 0.0390 | 5 | 6 | PI 471938 |
| Satt226 | 17 (D2) | 8 | 0.0030 | 8 | 3 | PI 471938 | |
| Sat_375 | 13 (F) | 16 | 0.0000 | 14 | 15 | PI 471938 | |
| Satt244 | 16 (J) | 6 | 0.0130 | 4 | 6 | PI 471938 | |
| Satt137 | 9 (K) | 6 | 0.0132 | 7 | 4 | PI 471938 | |
| Chr (LG) | Marker 1 | p-Value | R2 (%) | Allelic Means 2 | ||
|---|---|---|---|---|---|---|
| Hut/Hut | Hut/PI | PI/PI | ||||
| visual rating (0 = no stress, 5 = dead) | ||||||
| 4 (C1) | Satt194 | 0.0211 | 6 | 2.4 | 2.3 | 2.3 |
| 1 (D1a) | Satt507 | 0.0274 | 5 | 2.4 | 2.3 | 2.2 |
| 17 (D2) | Sat_365 | 0.0346 | 5 | 2.4 | 2.2 | 2.3 |
| 13 (F) | Sat_375 | 0.0459 | 4 | 2.4 | 2.3 | 2.2 |
| 9 (K) | Sat_087 | 0.0019 | 9 | 2.2 | 2.3 | 2.4 |
| Satt546 (Chr 2) | Satt395 (Chr 13) | Mean | |
|---|---|---|---|
| Hut/Hut 1 | PI/PI 1 | ||
| kg ha−1 | |||
| Hut/Hut | 3118 | 2963 | 3057 |
| PI/PI | 3158 | 3064 | 3097 |
| Mean | 3138 | 3044 | |
| Sat_365 (Chr 17) | Satt298 (Chr 11) | Mean | |
| Hut/Hut | PI/PI | ||
| Hut/Hut | 1747 | 1801 | 1794 |
| PI/PI | 1841 | 1868 | 1861 |
| Mean | 1794 | 1848 | |
| Sat_365 (Chr 17) | Sat_375 (Chr 13) | Mean | |
| Hut/Hut | PI/PI | ||
| Hut/Hut | 1760 | 1875 | 1794 |
| PI/PI | 1774 | 1901 | 1854 |
| Mean | 1794 | 1888 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.-J.; Lee, S.; Carter, T.E., Jr.; Shannon, G.; Boerma, H.R. Identification of Soybean Yield QTL in Irrigated and Rain-Fed Environments. Agronomy 2021, 11, 2207. https://doi.org/10.3390/agronomy11112207
Lee G-J, Lee S, Carter TE Jr., Shannon G, Boerma HR. Identification of Soybean Yield QTL in Irrigated and Rain-Fed Environments. Agronomy. 2021; 11(11):2207. https://doi.org/10.3390/agronomy11112207
Chicago/Turabian StyleLee, Geung-Joo, Sungwoo Lee, Tommy E. Carter, Jr., Grover Shannon, and H. Roger Boerma. 2021. "Identification of Soybean Yield QTL in Irrigated and Rain-Fed Environments" Agronomy 11, no. 11: 2207. https://doi.org/10.3390/agronomy11112207
APA StyleLee, G.-J., Lee, S., Carter, T. E., Jr., Shannon, G., & Boerma, H. R. (2021). Identification of Soybean Yield QTL in Irrigated and Rain-Fed Environments. Agronomy, 11(11), 2207. https://doi.org/10.3390/agronomy11112207







