Genome-Wide Association Study (GWAS) of Mesocotyl Length for Direct Seeding in Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Genomic Data
2.2. Evaluation of Mesocotyl Length
2.3. Population and Genotype Analysis
2.4. GWAS Analysis
2.5. Haplotype Analysis
3. Results
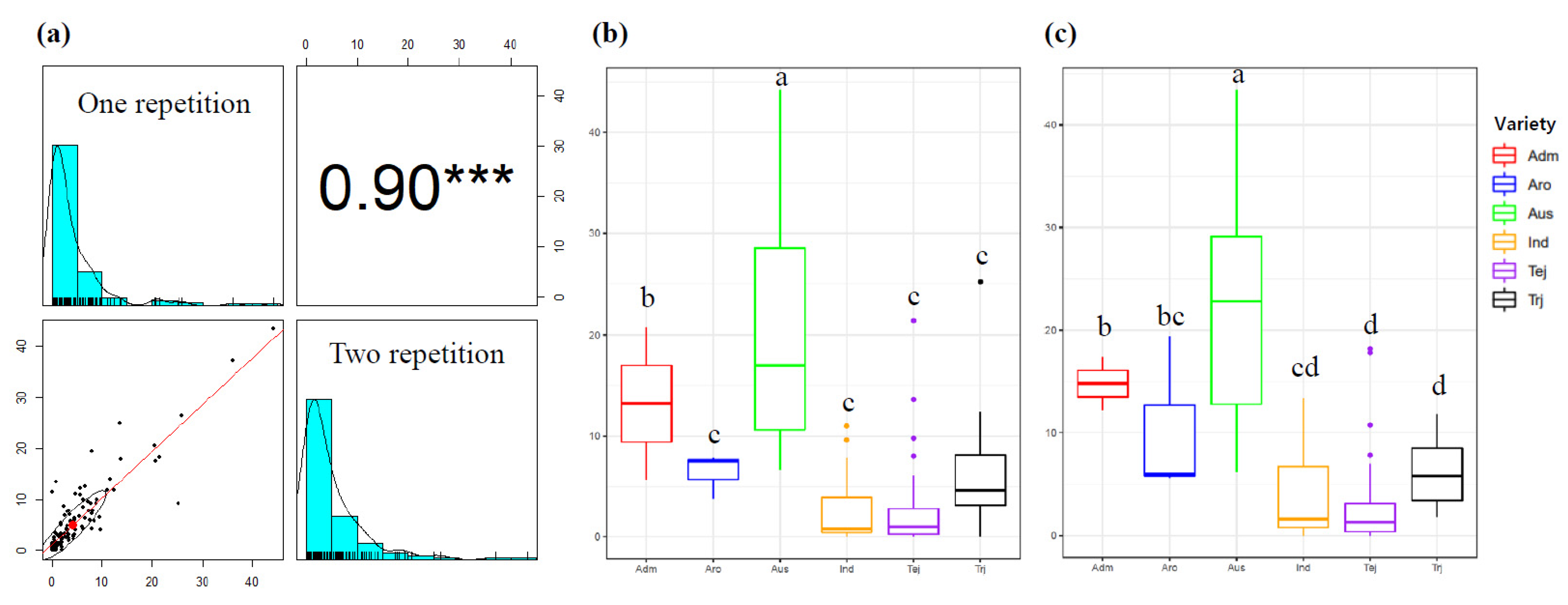
3.1. Phenotypic Variation of Mesocotyl Elongation
3.2. Population Structure and LD Decay Analysis
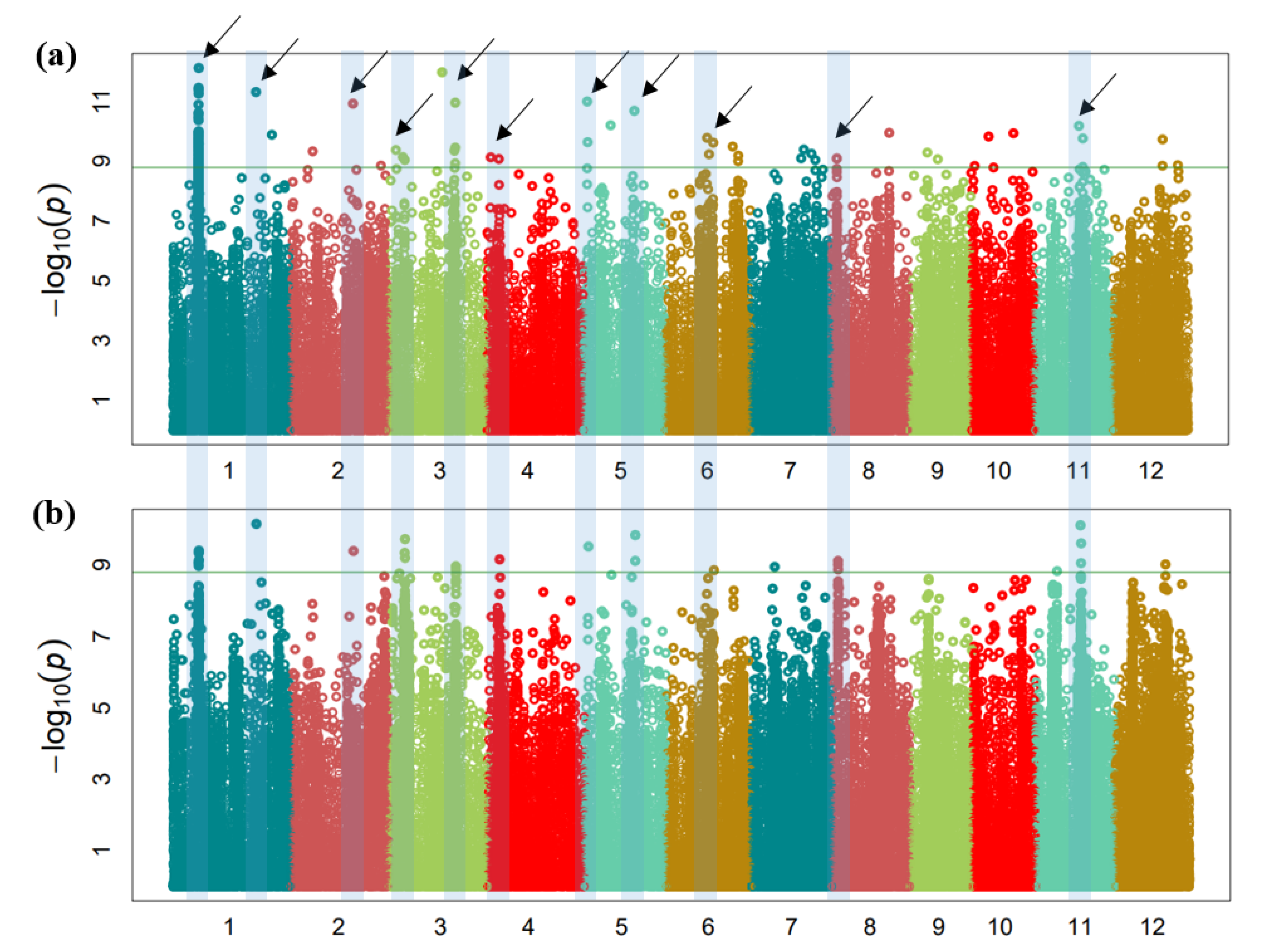
3.3. GWAS for Mesocotyl Length
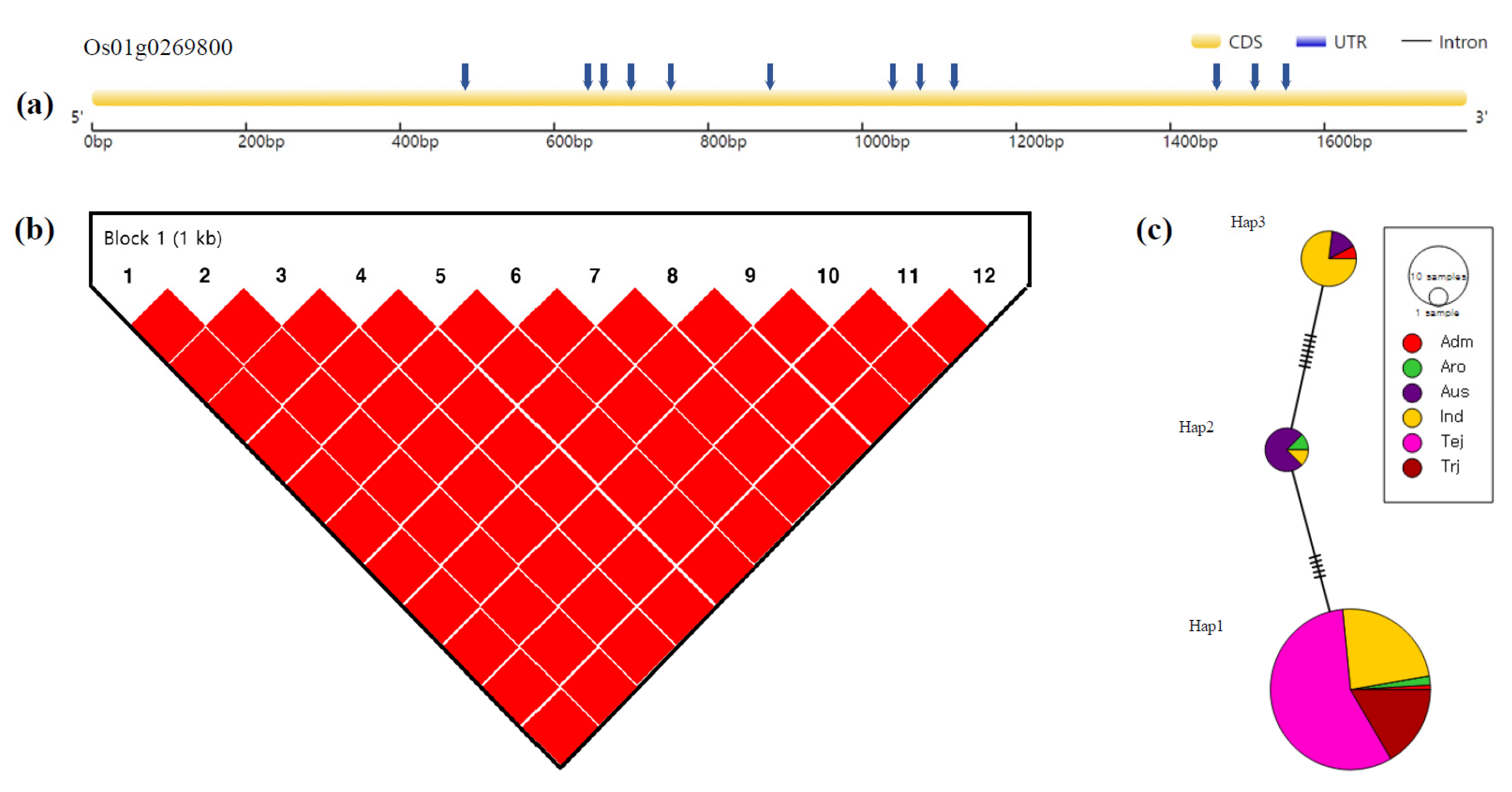
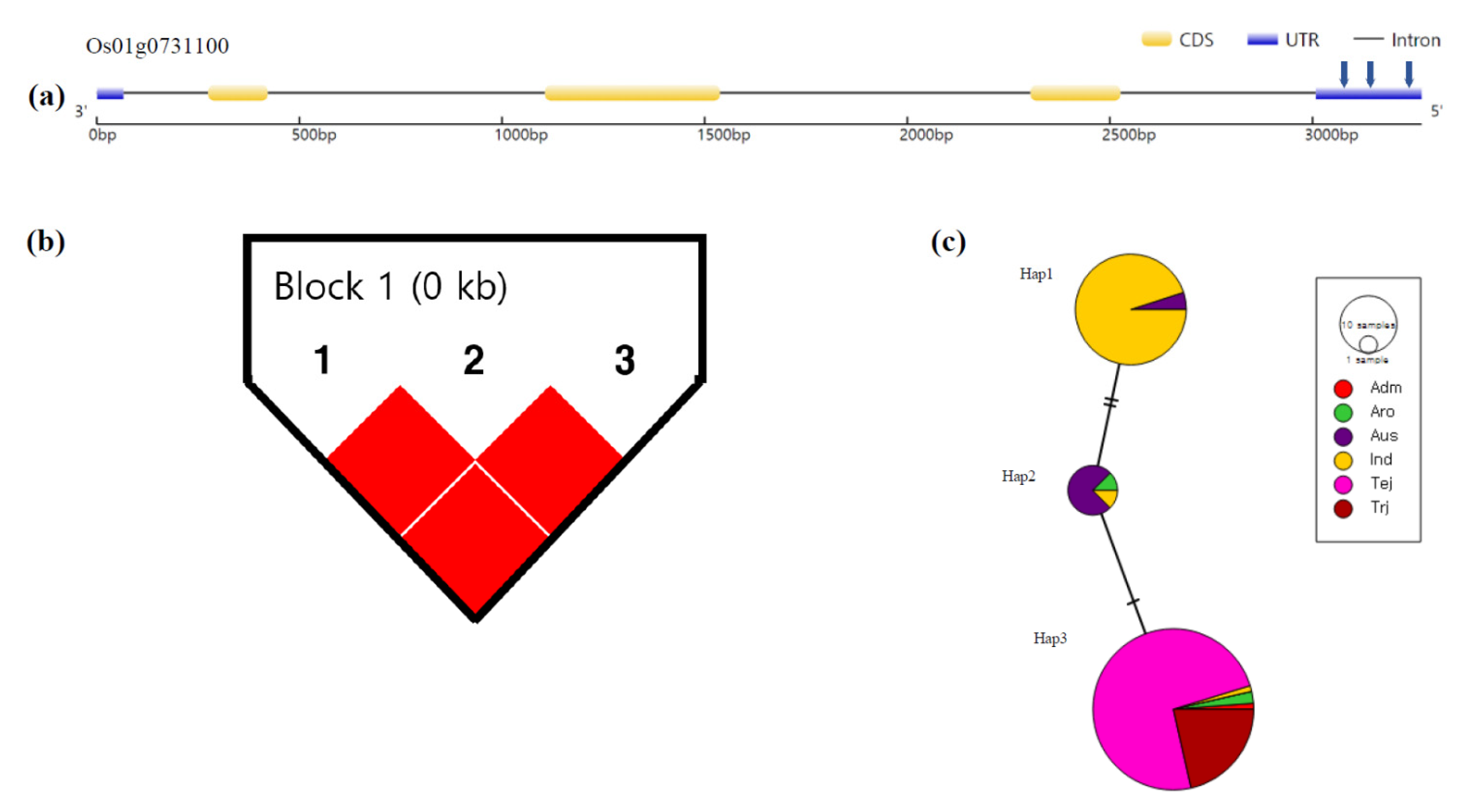
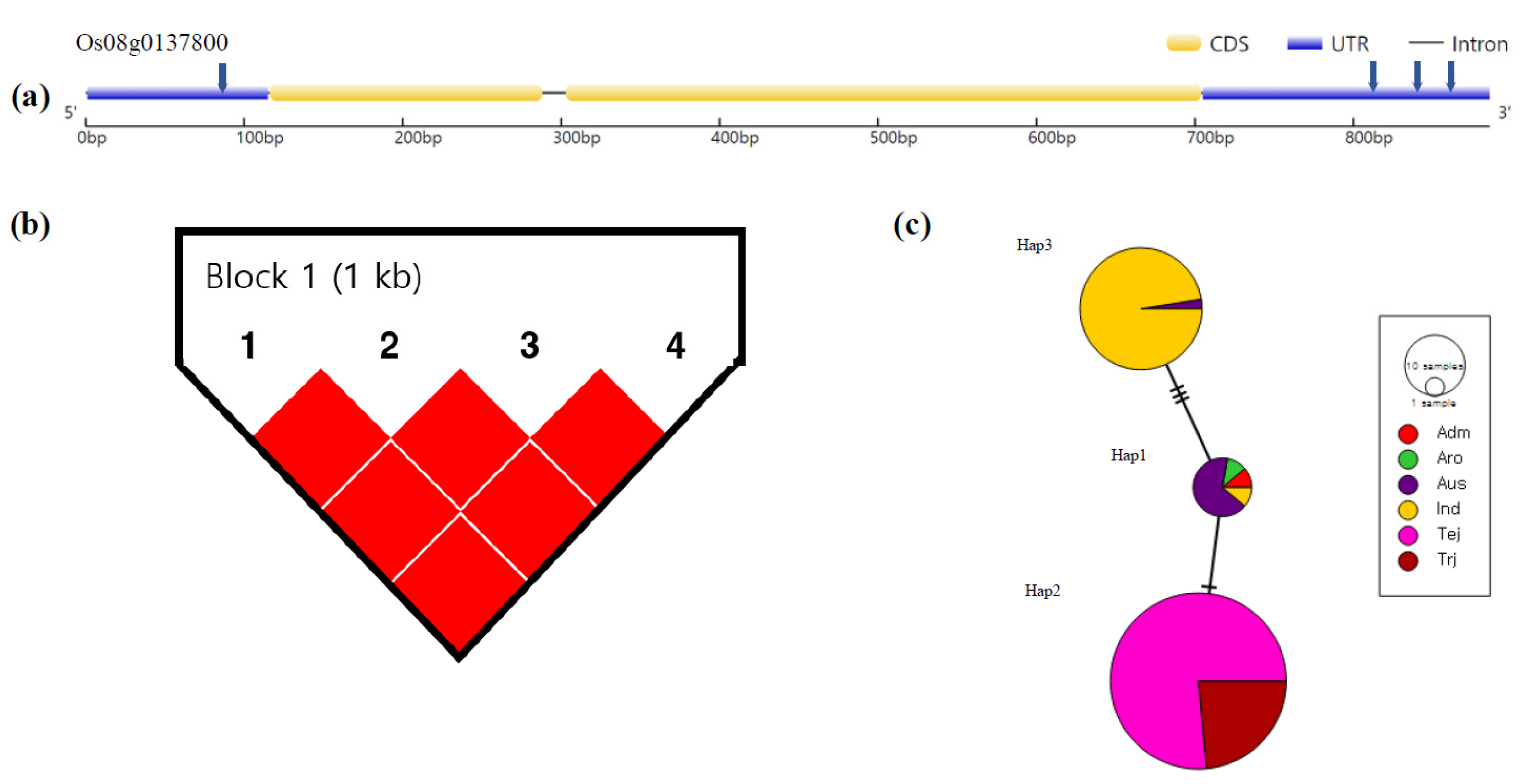
3.4. Haplotype Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, V.; Ladha, J.K. Direct Seeding of Rice: Recent Developments and Future Research Needs. Adv. Agron. 2011, 111, 297–413. [Google Scholar] [CrossRef]
- Farooq, M.; Siddique, K.H.M.; Rehman, H.; Aziz, T.; Lee, D.J.; Wahid, A. Rice direct seeding: Experiences, challenges and opportunities. Soil Till. Res. 2011, 111, 87–98. [Google Scholar] [CrossRef]
- Liu, H.Y.; Hussain, S.; Zheng, M.M.; Peng, S.B.; Huang, J.L.; Cui, K.H.; Nie, L.X. Dry direct-seeded rice as an alternative to transplanted-flooded rice in Central China. Agron. Sustain. Dev. 2015, 35, 285–294. [Google Scholar] [CrossRef]
- Farooq, M.; Barsa, S.M.A.; Wahid, A. Priming of field-sown rice seed enhances germination, seedling establishment, allometry and yield. Plant Growth Regul. 2006, 49, 285–294. [Google Scholar] [CrossRef]
- Lv, Y.S.; Shao, G.N.; Jiao, G.A.; Sheng, Z.H.; Xie, L.H.; Hu, S.K.; Tang, S.Q.; Wei, X.J.; Hu, P.S. Targeted mutagenesis of POLYAMINE OXIDASE 5 that negatively regulates mesocotyl elongation enables the generation of direct-seeding rice with improved grain yield. Mol. Plant 2021, 14, 344–351. [Google Scholar] [CrossRef]
- Lee, H.S.; Sasaki, K.; Kang, J.W.; Sato, T.; Song, W.Y.; Ahn, S.N. Mesocotyl Elongation is Essential for Seedling Emergence Under Deep-Seeding Condition in Rice. Rice 2017, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N. Adaptive Importance of Mesocotyl and Coleoptile Growth in Rice under Different Moisture Regimes. Aust. J. Plant Physiol. 1978, 5, 511–517. [Google Scholar] [CrossRef]
- Cona, A.; Cenci, F.; Cervelli, M.; Federico, R.; Mariottini, P.; Moreno, S.; Angelini, R. Polyamine oxidase, a hydrogen peroxide-producing enzyme, is up-regulated by light and down-regulated by auxin in the outer tissues of the maize mesocotyl. Plant Physiol. 2003, 131, 803–813. [Google Scholar] [CrossRef]
- Gray, W.M.; Ostin, A.; Sandberg, G.; Romano, C.P.; Estelle, M. High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc. Natl. Acad. Sci. USA 1998, 95, 7197–7202. [Google Scholar] [CrossRef]
- Riemann, M.; Muller, A.; Korte, A.; Furuya, M.; Weiler, E.W.; Nick, P. Impaired induction of the jasmonate pathway in the rice mutant hebiba. Plant Physiol. 2003, 133, 1820–1830. [Google Scholar] [CrossRef]
- Saab, I.N.; Sharp, R.E.; Pritchard, J. Effect of Inhibition of Abscisic-Acid Accumulation on the Spatial-Distribution of Elongation in the Primary Root and Mesocotyl of Maize at Low Water Potentials. Plant Physiol. 1992, 99, 26–33. [Google Scholar] [CrossRef]
- Vanderhoef, L.N.; Quail, P.H.; Briggs, W.R. Red Light-Inhibited Mesocotyl Elongation in Maize Seedlings: II. Kinetic and Spectral Studies. Plant Physiol. 1979, 63, 1062–1067. [Google Scholar] [CrossRef]
- Watanabe, H.; Takahashi, K.; Saigusa, M. Morphological and anatomical effects of abscisic acid (ABA) and fluridone (FLU) on the growth of rice mesocotyls. Plant Growth Regul. 2001, 34, 273–275. [Google Scholar] [CrossRef]
- Chang, T.-T.; Vergara, B.S. Varietal Diversity and Morpho-Agronomic Characteristics of Upland Rice; International Rice Research Institute: Manila, Philippines, 1975; pp. 72–90. [Google Scholar]
- Takahashi, K.; Watanabe, H.; Hoshikawa, K. Varietal Differences and Geographical Distributions in the Growth of Mesocotyl and Internodes of Rice (Oryza-sativa L.) Seedlings. Jpn. J. Crop. Sci 1995, 64, 66–72. [Google Scholar] [CrossRef][Green Version]
- Wu, M.-G.; Zhang, G.-H.; Lin, J.-R.; Cheng, S.-H. Screening for rice germplasms with specially-elongated mesocotyl. Rice Sci. 2005, 12, 226–228. [Google Scholar]
- Lee, H.S.; Sasaki, K.; Higashitani, A.; Ahn, S.N.; Sato, T. Mapping and characterization of quantitative trait loci for mesocotyl elongation in rice (Oryza sativa L.). Rice 2012, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Suge, H. Mesocotyl Elongation in Japonica Rice—Effect of High-Temperature Pre-Treatment and Ethylene. Plant Cell Physiol. 1972, 13, 401–405. [Google Scholar]
- Huang, C.; Jiang, S.-K.; Ling-Ling, F.; Zheng-Jin, X.; Wen-Fu, C. Analysis of QTLs for mesocotyl length in rice (Oryza sativa L.). Acta Agron. Sin. 2010, 36, 1108–1113. [Google Scholar] [CrossRef]
- Wu, J.H.; Feng, F.J.; Lian, X.M.; Teng, X.Y.; Wei, H.B.; Yu, H.H.; Xie, W.B.; Yan, M.; Fan, P.Q.; Li, Y.; et al. Genome-wide Association Study (GWAS) of mesocotyl elongation based on re-sequencing approach in rice. Bmc Plant Biol. 2015, 15, 218. [Google Scholar] [CrossRef]
- Yamauchi, M.; Chuong, P.; Chau, N.M. Ecophysiology of Rice-Crop Establishment in Wet Direct Seeding in Vietnam with Emphasis on Anaerobic Seedling Growth; International Rice Research Institute: Manila, Philippines, 1995; pp. 89–95. [Google Scholar]
- Kim, K.W.; Chung, H.K.; Cho, G.T.; Ma, K.H.; Chandrabalan, D.; Gwag, J.G.; Kim, T.S.; Cho, E.G.; Park, Y.J. PowerCore: A program applying the advanced M strategy with a heuristic search for establishing core sets. Bioinformatics 2007, 23, 2155–2162. [Google Scholar] [CrossRef]
- Zhao, W.G.; Cho, G.T.; Ma, K.H.; Chung, J.W.; Gwag, J.G.; Park, Y.J. Development of an allele-mining set in rice using a heuristic algorithm and SSR genotype data with least redundancy for the post-genomic era. Mol. Breed. 2010, 26, 639–651. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.Z.; Zhou, S.G.; et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; He, Q.; Kim, K.W.; Yoon, M.Y.; Ra, W.H.; Li, F.P.; Tong, W.; Yu, J.; Oo, W.H.; Choi, B.; et al. Genome-wide resequencing of KRICE_CORE reveals their potential for future breeding, as well as functional and evolutionary studies in the post-genomic era. BMC Genom. 2016, 17, 408. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Yan, H.F.; Yang, J.H.; Yamaguchi, S.; Maekawa, M.; Takamure, I.; Tsutsumi, N.; Kyozuka, J.; Nakazono, M. Strigolactones Negatively Regulate Mesocotyl Elongation in Rice during Germination and Growth in Darkness. Plant Cell Physiol. 2010, 51, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jang, S.G.; Lee, J.; Kwon, S.W. Evaluation of Mesocotyl Elongation Ability in Korean Rice Landraces (Oryza sativa L.). Korean J. Breed. Sci. 2019, 51, 351–356. [Google Scholar] [CrossRef]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Research. 2009, 19, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statical Computing: Vienna, Austria, 2010; ISBN 3-900051-07-0. [Google Scholar]
- Lipka, A.E.; Tian, F.; Wang, Q.; Peiffer, J.; Li, M.; Bradbury, P.J.; Gore, M.A.; Buckler, E.S.; Zhang, Z. GAPIT: Genome association and prediction integrated tool. Bioinformatics 2012, 28, 2397–2399. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life v2: Online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. 2011, 39, W475–W478. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, S.S.; Xu, J.Y.; He, W.M.; Yang, T.L. PopLDdecay: A fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics 2019, 35, 1786–1788. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Huang, M.; Fan, B.; Buckler, E.S.; Zhang, Z.W. Iterative Usage of Fixed and Random Effect Models for Powerful and Efficient Genome-Wide Association Studies. PLoS Genet. 2016, 12. [Google Scholar] [CrossRef]
- Yang, W.N.; Guo, Z.L.; Huang, C.L.; Duan, L.F.; Chen, G.X.; Jiang, N.; Fang, W.; Feng, H.; Xie, W.B.; Lian, X.M.; et al. Combining high-throughput phenotyping and genome-wide association studies to reveal natural genetic variation in rice. Nat. Commun. 2014, 5, 5087. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.P.; Guo, A.Y.; Zhang, H.; Luo, J.C.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Zhan, J.H.; Lu, X.; Liu, H.Y.; Zhao, Q.Z.; Ye, G.Y. Mesocotyl elongation, an essential trait for dry-seeded rice (Oryza sativa L.): A review of physiological and genetic basis. Planta 2020, 251, 27. [Google Scholar] [CrossRef]
- Mu, P.; Li, Z.C.; Li, C.P.; Zhang, H.L.; Wu, C.M.; Li, C.; Wang, X.K. QTL mapping of the root traits and their correlation analysis with drought resistance using DH lines from paddy and upland rice cross. Chinese Sci. Bull. 2003, 48, 2718–2724. [Google Scholar] [CrossRef]
- Jun, Y.; Qiang, L.; Bing, Y.; Wei-Ya, X.; Li-Jun, L.; Xiong, L.-Z. Identification of quantitative trait loci for ABA sensitivity at seed germination and seedling stages in rice. Acta Genet. Sin. 2006, 33, 532–541. [Google Scholar]
- Cui, K.H.; Peng, S.B.; Xing, Y.Z.; Xu, C.G.; Yu, S.B.; Zhang, Q. Molecular dissection of seedling-vigor and associated physiological traits in rice. Theor. Appl. Genet. 2002, 105, 745–753. [Google Scholar] [CrossRef]
- Miura, K.; Lin, S.Y.; Yano, M.; Nagamine, T. Mapping quantitative trait loci controlling low temperature germinability in rice (Oryza sativa L.). Breed. Sci. 2001, 51, 293–299. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, S.J.; Hou, M.Y.; Tang, J.Y.; Chen, L.M.; Zhai, H.Q.; Wan, J.M. Analysis of QTLs for seed low temperature germinability and anoxia germinability in rice (Oryza sativa L.). Field Crop. Res. 2006, 98, 68–75. [Google Scholar] [CrossRef]
- Guangheng, Z.; Dali, Z.; Shikai, H.; Yan, S.; Latie, A.; Longbiao, G.; Qian, Q. QTL analysis of traits concerned submergence tolerance at seedling stage in rice (Oryza sativa L.). Zuo Wu Xue Bao 2006, 32, 1280–1286. [Google Scholar]
- Cai, H.W.; Morishima, H. QTL clusters reflect character associations in wild and cultivated rice. Theor. Appl. Genet. 2002, 104, 1217–1228. [Google Scholar] [CrossRef]
- Kato, Y.; Katsura, K. Rice Adaptation to Aerobic Soils: Physiological Considerations and Implications for Agronomy. Plant Prod. Sci. 2014, 17, 1–12. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhan, J.H.; Li, J.L.; Lu, X.; Liu, J.D.; Wang, Y.M.; Zhao, Q.Z.; Ye, G.Y. Genome-Wide Association Study (GWAS) for Mesocotyl Elongation in Rice (Oryza sativa L.) under Multiple Culture Conditions. Genes 2020, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, W.P.; Jiang, C.H.; Wang, X.N.; Xiong, H.Y.; Todorovska, E.G.; Yin, Z.G.; Chen, Y.F.; Wang, X.; Xie, J.Y.; et al. Genetic Architecture and Candidate Genes for Deep-Sowing Tolerance in Rice Revealed by Non-syn GWAS. Front. Plant Sci. 2018, 9, 332. [Google Scholar] [CrossRef]
- Yu, Z.M.; Dong, L.X.; Jiang, Z.F.; Yi, K.K.; Zhang, J.H.; Zhang, Z.C.; Zhu, Z.X.; Wu, Y.H.; Xu, M.J.; Ni, J. A semi-dominant mutation in a CC-NB-LRR-type protein leads to a short-root phenotype in rice. Rice 2018, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Qin, G.; Ma, Q.Q.; Wei, M.Y.; Yang, X.H.; Ma, Z.F.; Liang, H.F.; Liu, C.; Li, Z.J.; Liu, F.; et al. Identification of Major Locus Bph35 Resistance to Brown Planthopper in Rice. Rice Sci. 2020, 27, 237–245. [Google Scholar] [CrossRef]
- Colmer, T.D.; Voesenek, L.A.C.J. Flooding tolerance: Suites of plant traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.K.; Tung, C.W. Genetic Mapping of Anaerobic Germination-Associated QTLs Controlling Coleoptile Elongation in Rice. Rice 2015, 8, 38. [Google Scholar] [CrossRef]
- Dixon, K.; Merritt, D.; Flematti, G.; Ghisalberti, E. Karrikinolide—A phytoreactive compound derived from smoke with applications in horticulture, ecological restoration and agriculture. Acta Hortic. 2009, 813, 155–170. [Google Scholar] [CrossRef]
- Jain, N.; Ascough, G.D.; Van Staden, J. A smoke-derived butenolide alleviates HgCl2 and ZnCl2 inhibition of water uptake during germination and subsequent growth of tomato–Possible involvement of aquaporins. J. Plant Physiol. 2008, 165, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Jahangir, M.; Rehman, S.U. Smoke induced physiological, biochemical and molecular changes in germinating rice seeds. Pak. J. Bot 2020, 52, 865–871. [Google Scholar] [CrossRef]
- Dennison, C. Investigating the structure and function of cupredoxins. Coordin. Chem. Rev. 2005, 249, 3025–3054. [Google Scholar] [CrossRef]
- Dong, J.; Kim, S.T.; Lord, E.M. Plantacyanin plays a role in reproduction in Arabidopsis. Plant Physiol. 2005, 138, 778–789. [Google Scholar] [CrossRef]
- De Rienzo, F.; Gabdoulline, R.R.; Menziani, M.C.; Wade, R.C. Blue copper proteins: A comparative analysis of their molecular interaction properties. Protein Sci. 2000, 9, 1439–1454. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Deng, F.L.; Yamaji, N.; Pinson, S.R.M.; Fujii-Kashino, M.; Danku, J.; Douglas, A.; Guerinot, M.L.; Salt, D.E.; Ma, J.F. A heavy metal P-type ATPase OsHMA4 prevents copper accumulation in rice grain. Nat. Commun. 2016, 7, 12138. [Google Scholar] [CrossRef]
- Navarro, B.B.; Del Frari, B.K.; Dias, P.V.D.; Lemainski, L.E.; Mario, R.B.; Ponte, L.R.; Goergen, A.; Tarouco, C.P.; Neves, V.M.; Dressler, V.L.; et al. The copper economy response is partially conserved in rice (Oryza sativa L.). Plant Physiol. Bioch 2021, 158, 113–124. [Google Scholar] [CrossRef]
- Yruela, I. Transition metals in plant photosynthesis. Metallomics 2013, 5, 1090–1109. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.C.; Zhang, J.P.; Yu, Y.; Zhou, Y.F.; Feng, Y.Z.; Yang, Y.W.; Lei, M.Q.; He, H.; Lian, J.P.; et al. Rice UCL8, a plantacyanin gene targeted by miR408, regulates fertility by controlling pollen tube germination and growth. Rice 2018, 11, 60. [Google Scholar] [CrossRef] [PubMed]



| QTLs | Genomic Region (Mbp) | Chromosome | −log10(p) | −log10(p) | Reported QTL | Reference of Previously Reported QTL | |
|---|---|---|---|---|---|---|---|
| (First Replication) | (Second Replication) | QTL Accession | Related Trait | ||||
| qML1-1 | 9.49 | 1 | 11.38 | 9.4 | rfw1b | Drought tolerance | [41] |
| qML1-2 | 30.57 | 1 | 11.31 | 10.15 | qLRC-1 | Drought tolerance | [42] |
| qML2 | 22.97 | 2 | 10.93 | 9.39 | qLRS-2 | Drought tolerance | [42] |
| qML3-1 | 5.97 | 3 | 9.04 | 9.73 | qTAA3-1 | Germination rate and seedling growth | [43] |
| qML3-2 | 24.64 | 3 | 10.96 | 9.86 | qMel-3 | Mesocotyl length | [17] |
| qML4 | 4.25 | 4 | 9.08 | 9.15 | qLTG-4-1 | Low temperature germinability | [44] |
| qML5-1 | 1.38 | 5 | 9.64 | 9.52 | qLTG-5-1 | Low temperature germinability | [45] |
| qML5-2 | 18.55 | 5 | 10.69 | 9.12 | qLTG-5 | Low temperature germinability | [44] |
| qML6 | 15.32 | 6 | 9.79 | 9.03 | qML6 | Mesocotyl length | [19] |
| qML8 | 2.07 | 8 | 9.09 | 9.02 | qLOE-8 | Submergence tolerance | [46] |
| qML11 | 16.48 | 11 | 10.19 | 10.11 | qml11 | Mesocotyl length | [47] |
| ID | SNP1 | SNP2 | SNP3 | SNP4 | SNP5 | SNP6 | SNP7 | SNP8 | SNP9 | SNP10 | SNP11 | SNP12 | Number of Accessions | First Repetition | Second Repetition | ||
| Region | Exon1 | Exon1 | Exon1 | Exon1 | Exon1 | Exon1 | Exon1 | Exon1 | Exon1 | Exon1 | Exon1 | Exon1 | Average Mesocotyl Length (mm) | F-value | Average Mesocotyl Length (mm) | F-value | |
| Position | 9316694 | 9316963 | 9316970 | 9316999 | 9317054 | 9317151 | 9317281 | 9317344 | 9317356 | 9317749 | 9317750 | 9317793 | |||||
| Allele | T/C | T/G | G/A | C/T | A/T | C/G | A/G | A/G | A/G | C/T | A/G | G/T | |||||
| Ref.seq | T | T | G | C | A | C | A | A | A | C | A | G | |||||
| Hap1 | T | T | G | C | A | C | A | A | A | C | A | G | 109 | 3.07 b | 3.27 b | ||
| Hap2 | T | G | G | C | A | C | G | G | G | C | G | G | 8 | 19.73 a | 35.09 *** | 20.85 a | 47.23 *** |
| Hap3 | C | G | A | T | T | G | G | G | G | T | G | T | 13 | 5.36 b | 5.97 b | ||
| ID | SNP1 | SNP2 | SNP3 | Number of accessions | First Repetition | Second Repetition | ||
| Region | 5′UTR | 5′UTR | 5′UTR | Average Mesocotyl Length (mm) | F-value | Average Mesocotyl Length (mm) | F-value | |
| Position | 30513562 | 30513616 | 30513617 | |||||
| Allele | G/C | G/A | T/A | |||||
| Ref.seq | G | G | T | |||||
| Hap1 | C | A | A | 40 | 2.55 b | 3.88 b | ||
| Hap2 | C | G | T | 8 | 20.03 a | 37.1 *** | 22.30 a | 46.76 *** |
| Hap3 | G | G | T | 84 | 3.55 b | 3.66 b | ||
| ID | SNP1 | SNP2 | SNP3 | Number of Accessions | First Repetition | Second Repetition | ||
| Region | Exon1 | Exon1 | Exon1 | Average Mesocotyl Length (mm) | F-value | Average Mesocotyl Length (mm) | F-value | |
| Position | 2063552 | 2063755 | 2063912 | |||||
| Allele | G/A | T/C | C/T | |||||
| Ref.seq | G | T | C | |||||
| Hap1 | A | C | C | 24 | 2.15 b | 4.04 c | ||
| Hap2 | G | T | C | 90 | 3.11 b | 13.66 *** | 3.36 c | 15.84 *** |
| Hap3 | A | C | T | 18 | 11.16 a | 11.89 b | ||
| Hap4 | A | T | T | 2 | 17.10 a | 21.2 a | ||
| ID | SNP1 | SNP2 | SNP3 | SNP4 | Number of Accessions | First Repetition | Second Repetition | ||
| Region | 5′UTR | 3′UTR | 3′UTR | 3′UTR | Average Mesocotyl Length (mm) | F-value | Average Mesocotyl Length (mm) | F-value | |
| Position | 2129695 | 2130465 | 2130532 | 2130566 | |||||
| Allele | G/A | T/C | A/G | G/T | |||||
| Ref.seq | G | T | A | G | |||||
| Hap1 | G | C | A | G | 9 | 20.67 a | 21.78 a | ||
| Hap2 | G | T | A | G | 83 | 3.31 b | 52.43 *** | 3.51 b | 53.26 *** |
| Hap3 | A | C | G | T | 39 | 2.39 b | 3.95 b | ||
| ID | SNP1 | SNP2 | SNP3 | SNP4 | Number of Accessions | First Repetition | Second Repetition | ||
| Region | Exon1 | Exon1 | 3′UTR | 3′UTR | Average Mesocotyl Length (mm) | F-value | Average Mesocotyl Length (mm) | F-value | |
| Position | 2134167 | 2134171 | 2134402 | 2134431 | |||||
| Allele | T/C | G/A | G/T | T/C | |||||
| Ref.seq | T | G | G | T | |||||
| Hap1 | C | A | G | C | 31 | 6.2 b | 3.95 b | ||
| Hap2 | C | G | G | C | 13 | 10.29 a | 9.96 *** | 11.32 a | 9.46 *** |
| Hap3 | T | G | G | T | 64 | 3.82 bc | 4.08 b | ||
| Hap4 | T | G | T | T | 18 | 1.68 c | 1.7 b | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.-G.; Park, S.-Y.; Lar, S.M.; Zhang, H.; Lee, A.-R.; Cao, F.-Y.; Seo, J.; Ham, T.-H.; Lee, J.; Kwon, S.-W. Genome-Wide Association Study (GWAS) of Mesocotyl Length for Direct Seeding in Rice. Agronomy 2021, 11, 2527. https://doi.org/10.3390/agronomy11122527
Jang S-G, Park S-Y, Lar SM, Zhang H, Lee A-R, Cao F-Y, Seo J, Ham T-H, Lee J, Kwon S-W. Genome-Wide Association Study (GWAS) of Mesocotyl Length for Direct Seeding in Rice. Agronomy. 2021; 11(12):2527. https://doi.org/10.3390/agronomy11122527
Chicago/Turabian StyleJang, Seong-Gyu, So-Yeon Park, San Mar Lar, Hongjia Zhang, Ah-Rim Lee, Fang-Yuan Cao, Jeonghwan Seo, Tae-Ho Ham, Joohyun Lee, and Soon-Wook Kwon. 2021. "Genome-Wide Association Study (GWAS) of Mesocotyl Length for Direct Seeding in Rice" Agronomy 11, no. 12: 2527. https://doi.org/10.3390/agronomy11122527
APA StyleJang, S.-G., Park, S.-Y., Lar, S. M., Zhang, H., Lee, A.-R., Cao, F.-Y., Seo, J., Ham, T.-H., Lee, J., & Kwon, S.-W. (2021). Genome-Wide Association Study (GWAS) of Mesocotyl Length for Direct Seeding in Rice. Agronomy, 11(12), 2527. https://doi.org/10.3390/agronomy11122527







