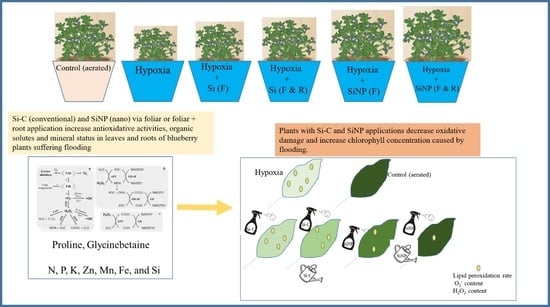
Silicon Nanoparticles Mitigate Hypoxia-Induced Oxidative Damage by Improving Antioxidants Activities and Concentration of Osmolytes in Southern Highbush Blueberry Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Leaf Gas Exchange Parameters and Chlorophylls
2.3. Antioxidant Capacity Determination
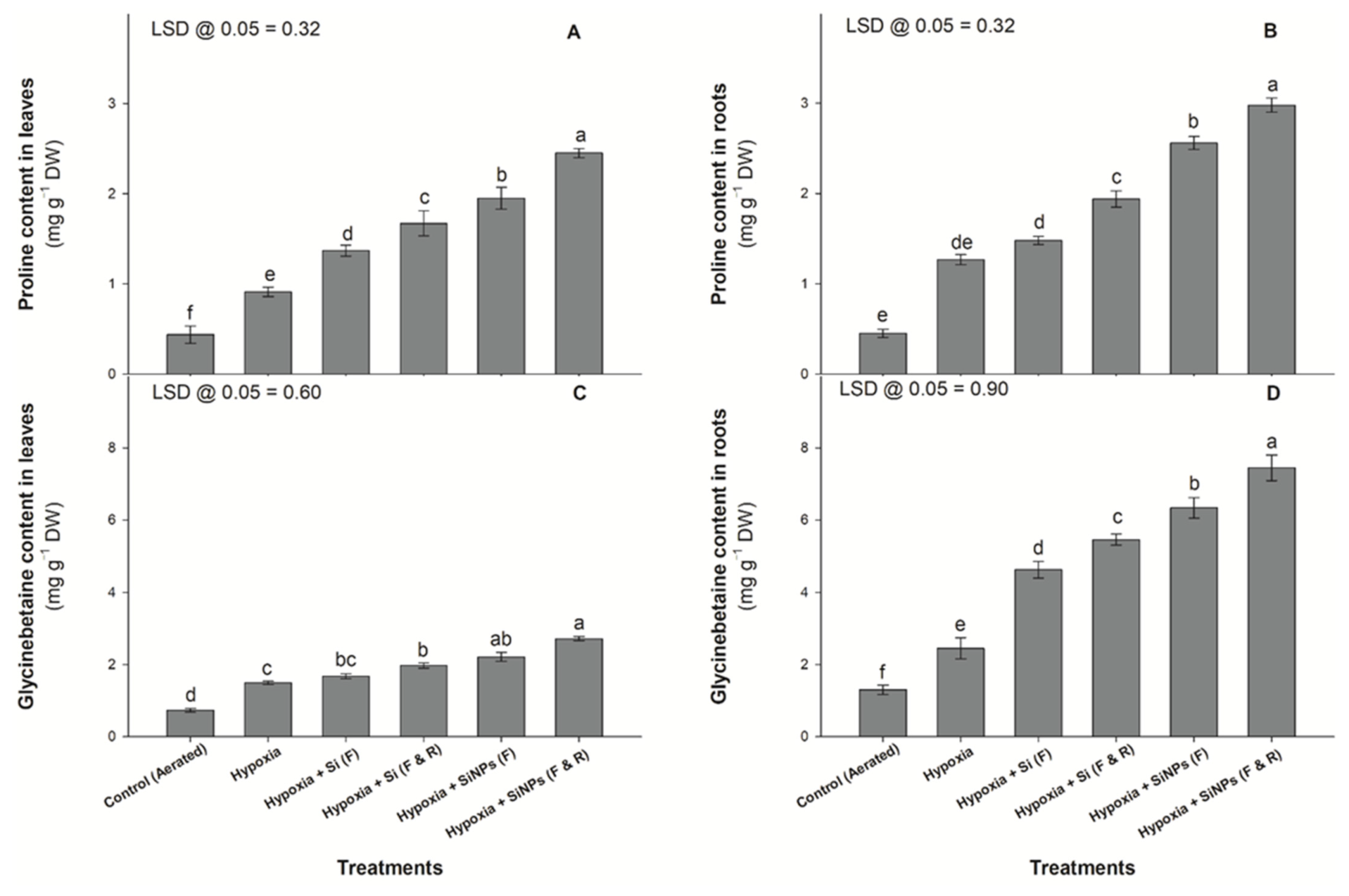
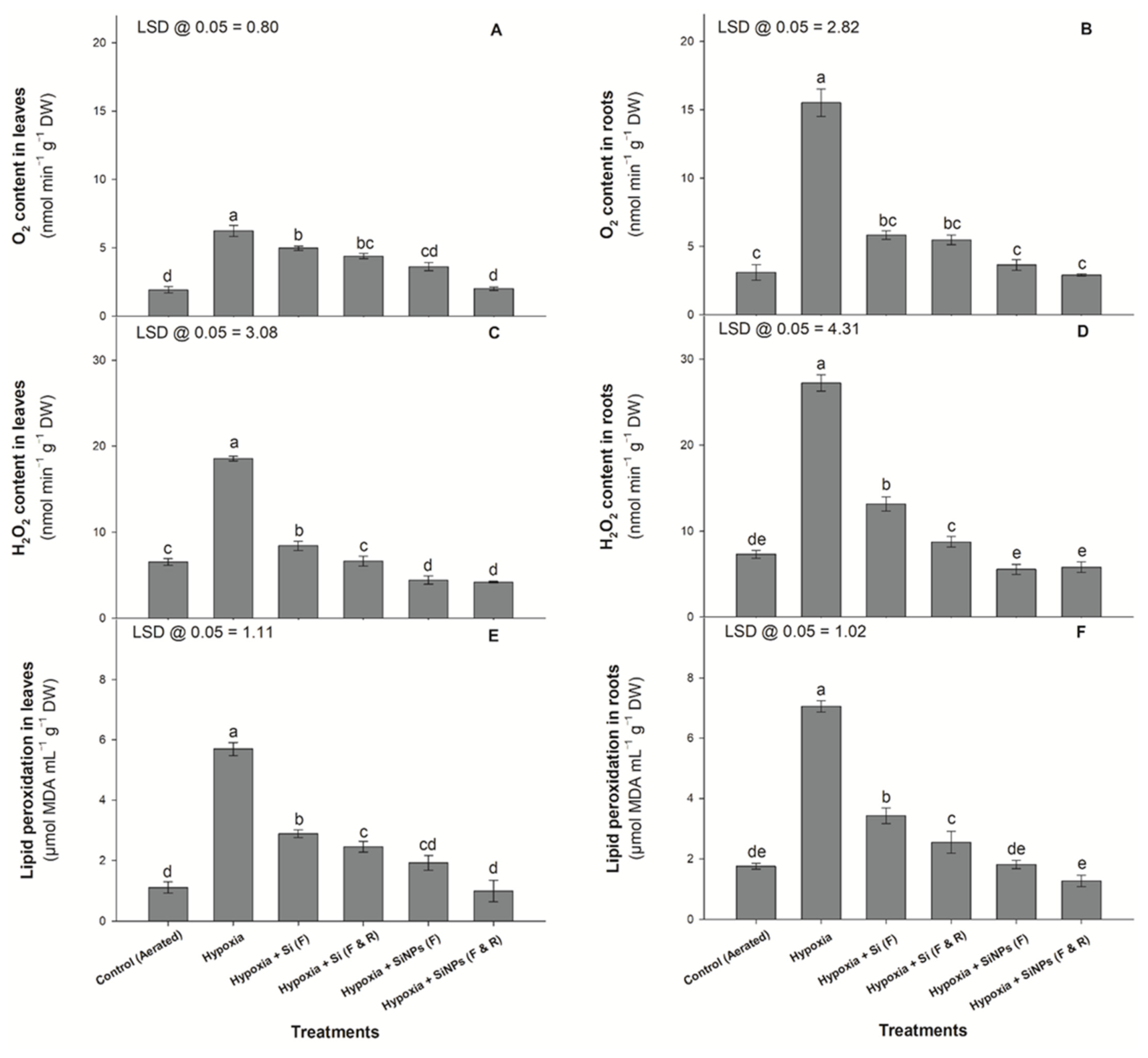
2.4. Glycinebetaine, Lipid Peroxidation, Proline, and ROS Concentrations
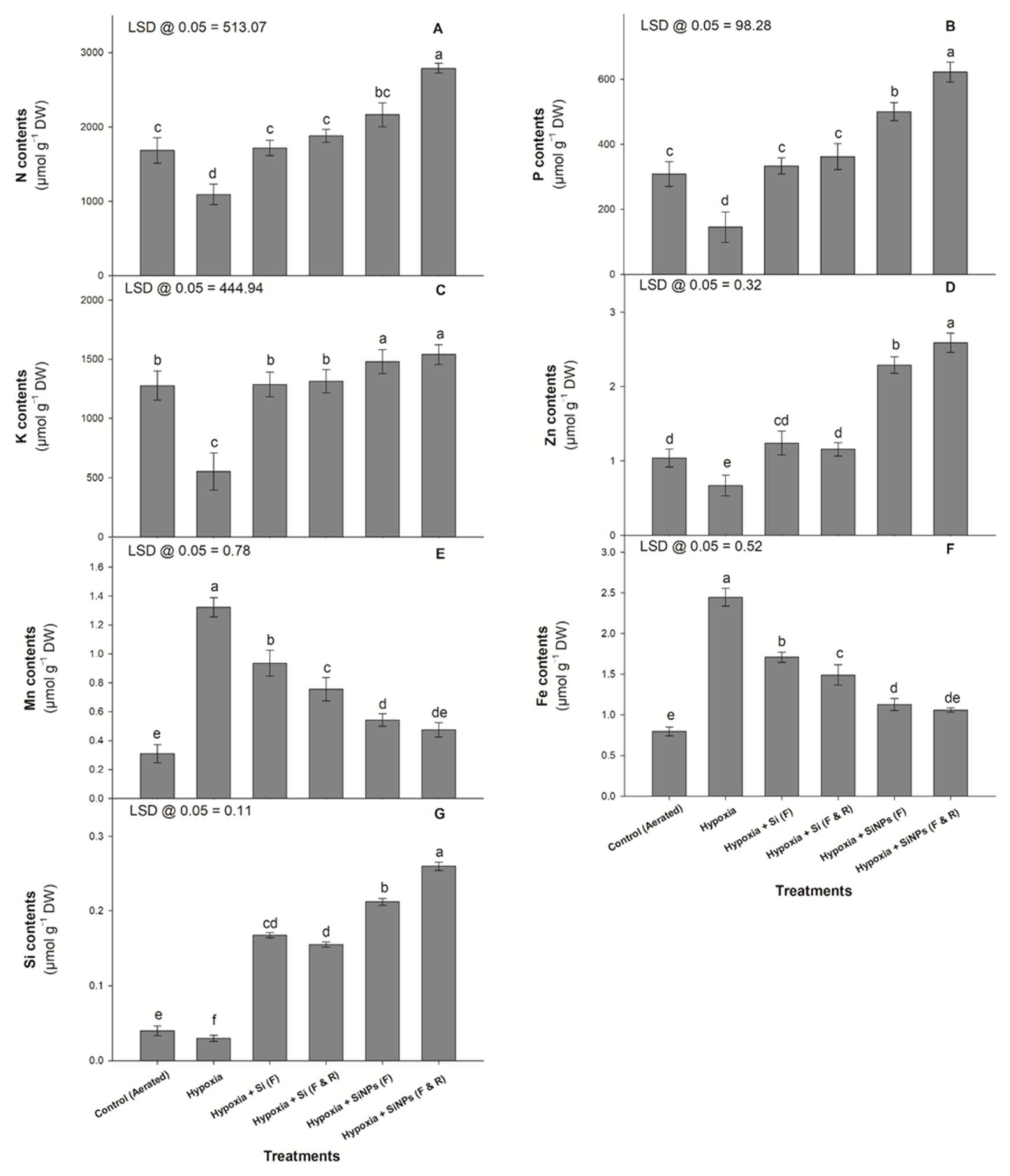
2.5. Mineral Nutrient Concentration in Plant Tissue
2.6. Statistical Analysis
3. Results
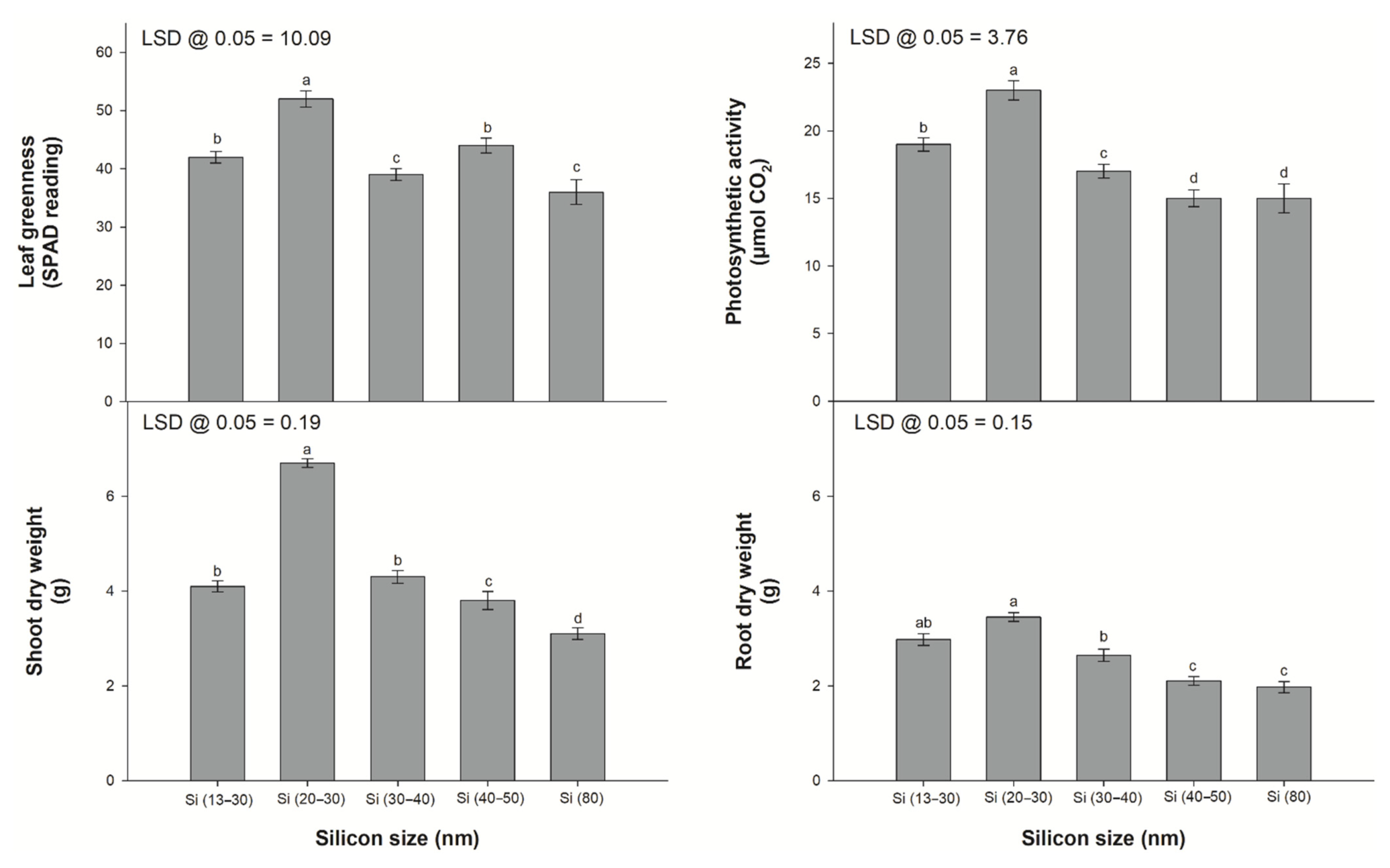
3.1. Leaf Gas Exchange and Leaf Greenness Parameters
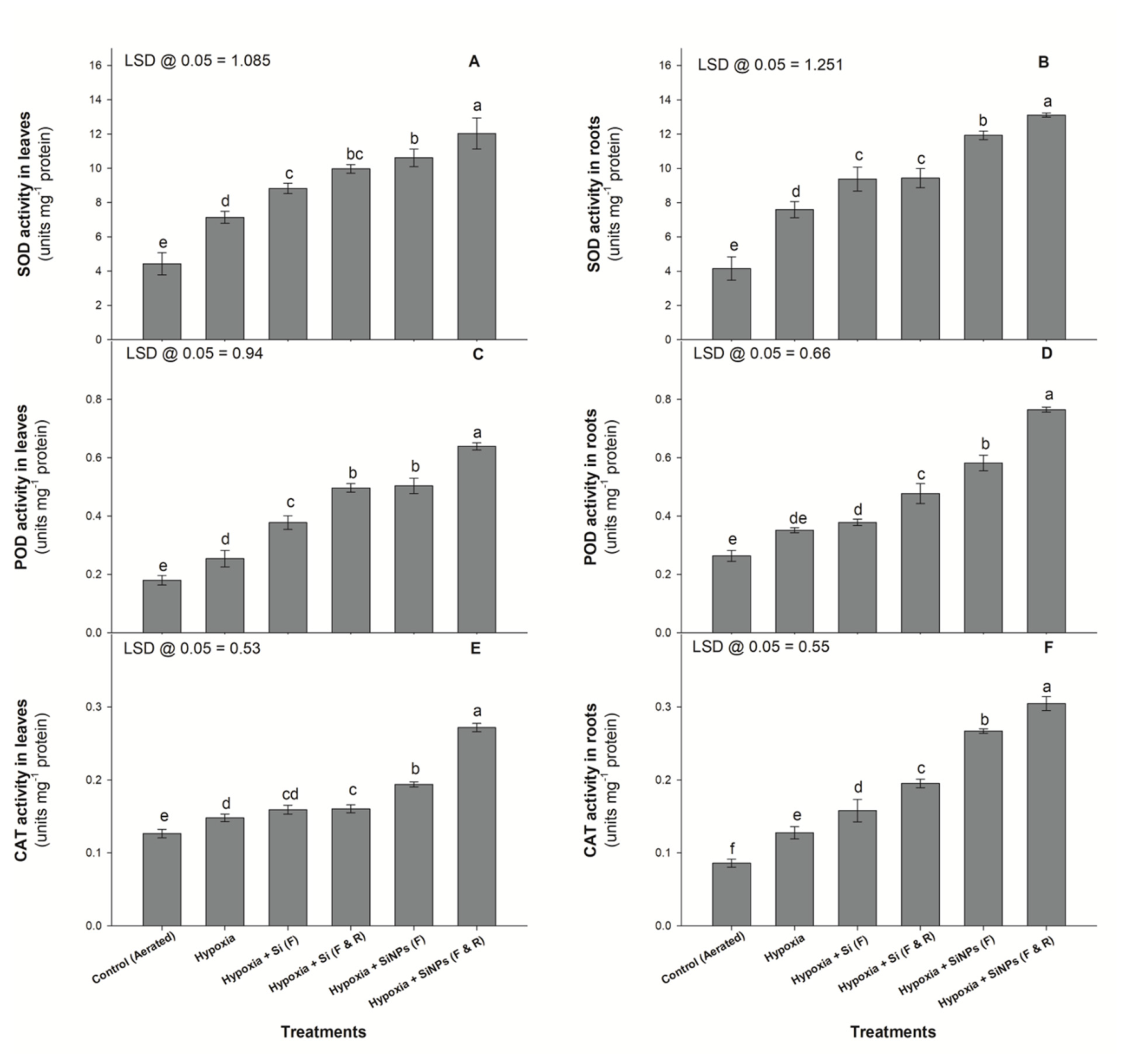
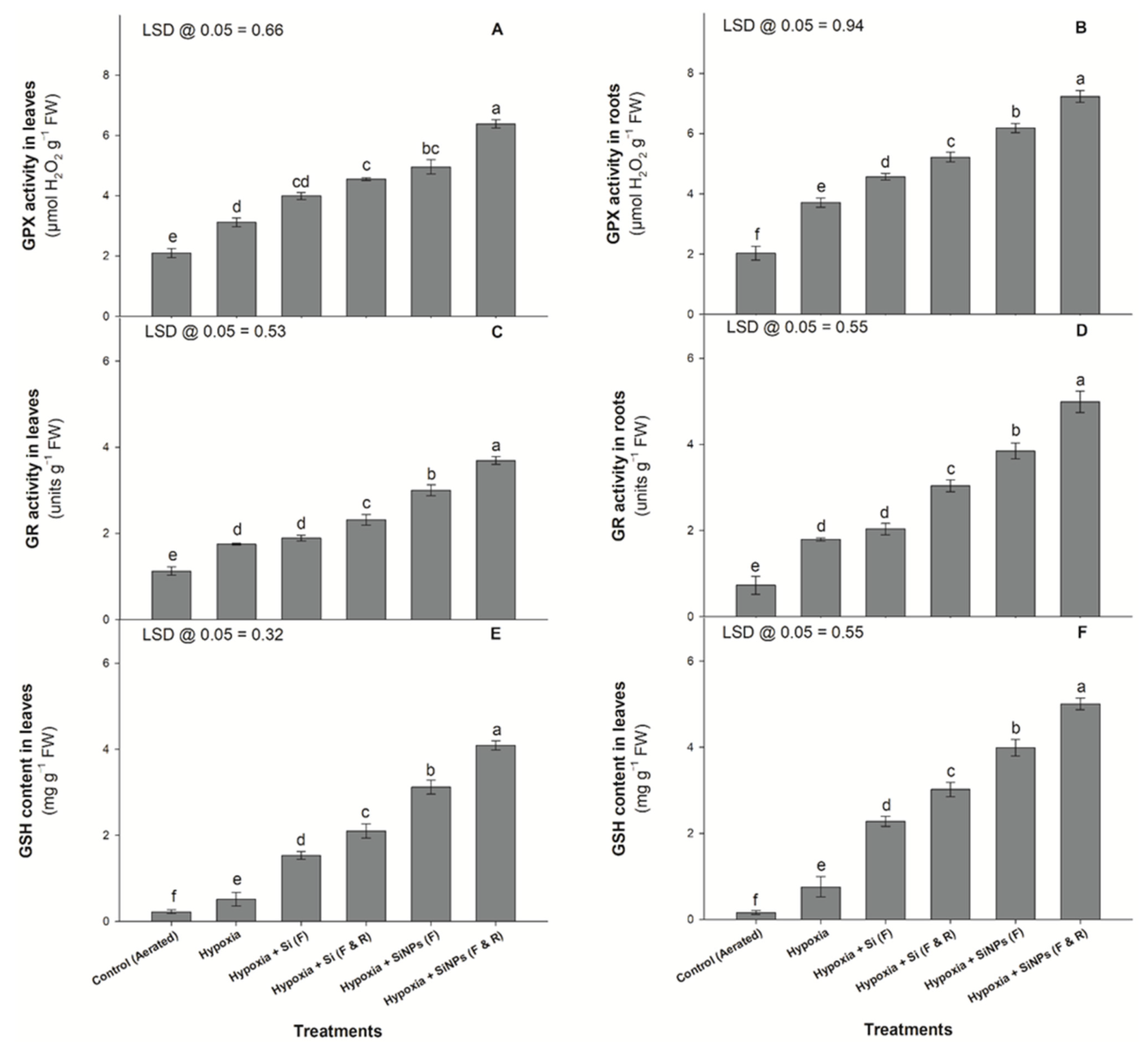
3.2. Enzymatic Activities
3.3. Osmolyte Concentration
3.4. Oxygen, H2O2, and MDA Concentration in Plant Tissue
3.5. Mineral Nutrients Concentration in Plant Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basu, A.; Rhone, M.; Lyons, T.J. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Neto, C.C. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Mol. Nutr. Food Res. 2007, 51, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Retamales, J.B.; Hancock, J.F. Blueberries (Crop Production Science in Horticulture), 2nd ed.; Cabi Publishing: Oxfordshire, UK, 2018; Volume 27. [Google Scholar]
- FAOSTAT Food and Agricultural Organziation of the United Nations Statistics Division. Available online: http://www.fao.org/faostat/en/ (accessed on 28 August 2021).
- Ahsan, N.; Lee, D.-G.; Lee, S.-H.; Lee, K.-W.; Bahk, J.D.; Lee, B.-H. A proteomic screen and identification of waterlogging-regulated proteins in tomato roots. Plant Soil 2007, 295, 37–51. [Google Scholar] [CrossRef]
- Mondal, S.; Khan, M.I.R.; Dixit, S.; Cruz, P.C.; Septiningsih, E.M.; Ismail, A.M. Growth, productivity and grain quality of AG1 and AG2 QTLs introgression lines under flooding in direct-seeded rice system. Field Crops Res. 2020, 248, 107713. [Google Scholar] [CrossRef]
- Christianson, J.A.; Wilson, I.W.; Llewellyn, D.J.; Dennis, E.S. The low-oxygen-induced NAC domain transcription factor ANAC102 affects viability of Arabidopsis seeds following low-oxygen treatment. Plant Physiol. 2009, 149, 1724–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finch-Savage, W.E.; Côme, D.; Lynn, J.R.; Corbineau, F. Sensitivity of Brassica oleracea seed germination to hypoxia: A QTL analysis. Plant Sci. 2005, 169, 753–759. [Google Scholar] [CrossRef]
- Anee, T.I.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Bhuiyan, T.F.; Alam, M.U.; Fujita, M.; Hasanuzzaman, M. Oxidative damage and antioxidant defense in Sesamum indicum after different waterlogging durations. Plants 2019, 8, 196. [Google Scholar] [CrossRef] [Green Version]
- Bailey-Serres, J.; Colmer, T.D. Plant tolerance of flooding stress–recent advances. Plant Cell Environ. 2014, 37, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Davies, F.S.; Flore, J.A. Short-term flooding effects on gas exchange and quantum yield of rabbiteye blueberry (Vaccinium ashei Reade) 1. Plant Physiol. 1986, 81, 289–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eck, P. Blueberry Science; Rutgers University Press: New Brunswick, NJ, USA, 1988; p. 284. [Google Scholar]
- Yu, K.; Zhu, K.; Ye, M.; Zhao, Y.; Chen, W.; Guo, W. Heat tolerance of highbush blueberry is related to the antioxidative enzymes and oxidative protein-repairing enzymes. Sci. Hortic. 2016, 198, 36–43. [Google Scholar] [CrossRef]
- Bhusal, N.; Bhusal, S.J.; Yoon, T.-M. Comparisons of physiological and anatomical characteristics between two cultivars in bi-leader apple trees (Malus × domestica Borkh.) 11Abbreviations: Chl, chlorophyll; DABB, days after bud break; Gs, stomatal conductance; Ψleaf, leaf water potential; ΨMD, midday leaf water potential; ΨPD, pre-dawn leaf water potential; LEC, lower epidermis cell; PPFD, photosynthetic photon flux density; Pn, net photosynthesis rate; Sd, stomatal density; SLW, specific leaf weight; UEC, upper epidermis cell; Ve, vessel; VPD, vapor pressure deficit; Xr, xylem rays. Sci. Hortic. 2018, 231, 73–81. [Google Scholar]
- Miao, B.-H.; Han, X.-G.; Zhang, W.-H. The ameliorative effect of silicon on soybean seedlings grown in potassium-deficient medium. Ann. Bot. 2010, 105, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.A.; Ali, S.; Hameed, A.; Ishaque, W.; Mahmood, K.; Iqbal, Z. Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol. Environ. Saf. 2013, 96, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Khan, A.L.; Kim, D.-H.; Lee, S.-Y.; Kim, K.-M.; Waqas, M.; Jung, H.-Y.; Shin, J.-H.; Kim, J.-G.; Lee, I.-J. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativalow silicon genes, and endogenous phytohormones. BMC Plant Biol. 2014, 14, 13. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.J.; Chen, K.M.; Zhao, Z.G.; Chen, G.C.; Zhou, W.J. Effects of silicon on defense of wheat against oxidative stress under drought at different developmental stages. Biol. Plant. 2008, 52, 592–596. [Google Scholar] [CrossRef]
- Balakhnina, T.I.; Matichenkov, V.V.; Wlodarczyk, T.; Borkowska, A.; Nosalewicz, M.; Fomina, I.R. Effects of silicon on growth processes and adaptive potential of barley plants under optimal soil watering and flooding. Plant Grow. Regul. 2012, 67, 35–43. [Google Scholar] [CrossRef]
- Matichenkov, V.V.; Fomina, I.R.; Biel, K.Y. Protective role of silicon in living organisms. In Complex Biological Systems; Wiley: Hoboken, NJ, USA, 2018; pp. 175–208. [Google Scholar]
- Fraceto, L.F.; Grillo, R.; de Medeiros, G.A.; Scognamiglio, V.; Rea, G.; Bartolucci, C. Nanotechnology in agriculture: Which innovation potential does it have? Front. Environ. Sci. 2016, 4, 20. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles more efficiently alleviate arsenate toxicity than silicon in maize cultiver and hybrid differing in arsenate tolerance. Front. Environ. Sci. 2016, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Haliem, M.E.F.; Hegazy, H.S.; Hassan, N.S.; Naguib, D.M. Effect of silica ions and nano silica on rice plants under salinity stress. Ecol. Eng. 2017, 99, 282–289. [Google Scholar] [CrossRef]
- Rizwan, M.; Meunier, J.-D.; Miche, H.; Keller, C. Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio, W.) grown in a soil with aged contamination. J. Hazard. Mater. 2012, 209, 326–334. [Google Scholar] [CrossRef]
- Hussain, S.; Shuxian, L.; Mumtaz, M.; Shafiq, I.; Iqbal, N.; Brestic, M.; Shoaib, M.; Sisi, Q.; Li, W.; Mei, X.; et al. Foliar application of silicon improves stem strength under low light stress by regulating lignin biosynthesis genes in soybean (Glycine max (L.) Merr.). J. Hazard. Mater. 2021, 401, 123256. [Google Scholar] [CrossRef]
- Iqbal, Z.; Sarkhosh, A.; Balal, R.M.; Gómez, C.; Zubair, M.; Ilyas, N.; Khan, N.; Shahid, M.A. Silicon alleviate hypoxia stress by improving enzymatic and non-enzymatic antioxidants and regulating nutrient uptake in muscadine grape (Muscadinia rotundifolia Michx.). Front. Plant Sci. 2021, 11, 2288. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Maehly, A.C. The assay of catalases and peroxidases. In Methods of Biochemical Analysis; Interscience Publisher Inc.: New York, NY, USA, 1954; pp. 357–424. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9780470110171.ch14 (accessed on 28 August 2021).
- Hossain, M.A.; Nakano, Y.; Asada, K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984, 25, 385–395. [Google Scholar]
- Arakawa, N.; Tsutsumi, K.; Sanceda, N.G.; Kurata, T.; Inagaki, C. A rapid and sensitive method for the determination of ascorbic acid using 4,7-Diphenyl-l,10-phenanthroline. Agric. Biol. Chem. 1981, 45, 1289–1290. [Google Scholar] [CrossRef] [Green Version]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Analyt. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Cueto, Y.R.; Silva, R. Identification of areas exposed to storm surge flooding: Topographic factors and ecosystem changes. J. Cost. Res. 2019, 92 (Suppl. S1), 68–74. [Google Scholar] [CrossRef]
- Loreti, E.; van Veen, H.; Perata, P. Plant responses to flooding stress. Curr. Opin. Plant Biol. 2016, 33, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattioli, R.; Costantino, P.; Trovato, M. Proline accumulation in plants. Plant Signal. Behav. 2009, 4, 1016–1018. [Google Scholar] [CrossRef]
- Jackson, M.B.; Ishizawa, K.; Ito, O. Evolution and mechanisms of plant tolerance to flooding stress. Ann. Bot. 2009, 103, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Du, K.; Xu, L.; Wu, H.; Tu, B.; Zheng, B. Ecophysiological and morphological adaption to soil flooding of two poplar clones differing in flood-tolerance. Flora-Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 96–106. [Google Scholar] [CrossRef]
- Bai, T.; Li, C.; Li, C.; Liang, D.; Ma, F. Contrasting hypoxia tolerance and adaptation in Malus species is linked to differences in stomatal behavior and photosynthesis. Physiol. Plant. 2013, 147, 514–523. [Google Scholar] [CrossRef]
- Bhusal, N.; Kim, H.S.; Han, S.-G.; Yoon, T.-M. Photosynthetic traits and plant–water relations of two apple cultivars grown as bi-leader trees under long-term waterlogging conditions. Environ. Exp. Bot. 2020, 176, 104111. [Google Scholar] [CrossRef]
- Rangwala, T.; Bafna, A.; Vyas, N.; Gupta, R. Role of soluble silica in alleviating oxidative stress in soybean crop. Indian J. Agri. Res. 2018, 52, 9–15. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Intl. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Faisal, M.; Al Sahli, A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014, 33, 2429–2437. [Google Scholar] [CrossRef]
- Mustafa, G.; Sakata, K.; Komatsu, S. Proteomic analysis of flooded soybean root exposed to aluminum oxide nanoparticles. J. Proteom. 2015, 128, 280–297. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol. Biochem. 2017, 110, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Singh, A.; Sudarshan, M.; Roychoudhury, A. Silicon nanoparticle-pulsing mitigates fluoride stress in rice by fine-tuning the ionomic and metabolomic balance and refining agronomic traits. Chemosphere 2021, 262, 127826. [Google Scholar] [CrossRef]
- León, J.; Castillo, M.C.; Gayubas, B. The hypoxia–reoxygenation stress in plants. J. Exp. Bot. 2020, 72, 5841–5856. [Google Scholar] [CrossRef]
- Vives-Peris, V.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Root involvement in plant responses to adverse environmental conditions. Agronomy 2020, 10, 942. [Google Scholar] [CrossRef]
- Ali, S.; Mehmood, A.; Khan, N. Uptake, translocation, and consequences of nanomaterials on plant growth and stress adaptation. J. Nanomater. 2021, 2021, 6677616. [Google Scholar] [CrossRef]
- Ramachandran, S.; Ma, T.S.; Griffin, J.; Ng, N.; Foskolou, I.P.; Hwang, M.-S.; Victori, P.; Cheng, W.-C.; Buffa, F.M.; Leszczynska, K.B.; et al. Hypoxia-induced SETX links replication stress with the unfolded protein response. Nat. Commun. 2021, 12, 3686. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, Z.; Sarkhosh, A.; Balal, R.M.; Rauf, S.; Khan, N.; Altaf, M.A.; Camara-Zapata, J.M.; Garcia-Sanchez, F.; Shahid, M.A. Silicon Nanoparticles Mitigate Hypoxia-Induced Oxidative Damage by Improving Antioxidants Activities and Concentration of Osmolytes in Southern Highbush Blueberry Plants. Agronomy 2021, 11, 2143. https://doi.org/10.3390/agronomy11112143
Iqbal Z, Sarkhosh A, Balal RM, Rauf S, Khan N, Altaf MA, Camara-Zapata JM, Garcia-Sanchez F, Shahid MA. Silicon Nanoparticles Mitigate Hypoxia-Induced Oxidative Damage by Improving Antioxidants Activities and Concentration of Osmolytes in Southern Highbush Blueberry Plants. Agronomy. 2021; 11(11):2143. https://doi.org/10.3390/agronomy11112143
Chicago/Turabian StyleIqbal, Zafar, Ali Sarkhosh, Rashad Mukhtar Balal, Saeed Rauf, Naeem Khan, Muhammad Ahsan Altaf, Jose M. Camara-Zapata, Francisco Garcia-Sanchez, and Muhammad Adnan Shahid. 2021. "Silicon Nanoparticles Mitigate Hypoxia-Induced Oxidative Damage by Improving Antioxidants Activities and Concentration of Osmolytes in Southern Highbush Blueberry Plants" Agronomy 11, no. 11: 2143. https://doi.org/10.3390/agronomy11112143
APA StyleIqbal, Z., Sarkhosh, A., Balal, R. M., Rauf, S., Khan, N., Altaf, M. A., Camara-Zapata, J. M., Garcia-Sanchez, F., & Shahid, M. A. (2021). Silicon Nanoparticles Mitigate Hypoxia-Induced Oxidative Damage by Improving Antioxidants Activities and Concentration of Osmolytes in Southern Highbush Blueberry Plants. Agronomy, 11(11), 2143. https://doi.org/10.3390/agronomy11112143