Niche Shifts, Hybridization, Polyploidy and Geographic Parthenogenesis in Western North American Hawthorns (Crataegus subg. Sanguineae, Rosaceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Sampling, and Geographic Ranges
Data Analyses
| Crataegus Sample | Sample Sizes Voucher Data (with Links to Online Databases) | ||||
|---|---|---|---|---|---|
| Taxa and Ploidy Level (Entries Refer to Sporophytic Ploidy Levels (2n), in Terms of the Base Chromosome Number in Crataegus, x = 17) | Climate Supplementary Data Table S1 | Morphology Supplementary Data Table S2 | Anatomy Supplementary Data Table S3 1 | Additional X-ray Images 2 (Specimens/Leaves) | Flow Cytometry Supplementary Data Table S4 |
| Crataegus subg. Americanae El-Gazzar | |||||
| C. sect. Coccineae Loudon https://morphobank.org/index.php/Projects/FoliosList/folio_id/936/project_id/3190 | |||||
| C. chrysocarpa Ashe (4x) 3 | 125 | 20 | 3 | 4/7 | 25 4 |
| C. sect. Macracanthae Loudon | |||||
| C. macracantha Lodd. ex Loudon (4x) 5 | 46 | 7 | 3 | - | 21 |
| Crataegus subg. Sanguineae Ufimov | |||||
| C. sect. Salignae T.A. Dickinson and Ufimov https://morphobank.org/index.php/Projects/FoliosList/folio_id/882/project_id/3190 | |||||
| C. erythropoda Ashe (4x) | 43 | 10 | 3 | 4/8 | 18 |
| C. rivularis Nuttall ex Torr. & Gray (4x) | 86 | 11 | 4 | 5/19 | 25 |
| C. saligna Greene (2x) | 35 | 4 | 5 | 5/17 | 9 |
| C. sect. Douglasianae Rehder ex C.K. Schneid. https://morphobank.org/index.php/Projects/FoliosList/folio_id/883/project_id/3190 | |||||
| C. douglasii Lindley (4x) | 51 + 154 6 | 24 | 5 | 8/16 | 133 |
| C. gaylussacia A. Heller (3x) 7 | 12 + 3 6 | - | - | - | 12 |
| C. suksdorfii (Sarg.) Kruschke (2x) | 17 + 59 6 | 20 | 4 | 4/7 1/1 8 | 24 + 25 8 |
| C. suksdorfii (Sarg.) Kruschke (3, 4x) | 25 + 8 + 83 6 | 10 9 | 4 | 4/9 | 42 |
2.2. Morphological Variation
2.2.1. Taxon Differentiation
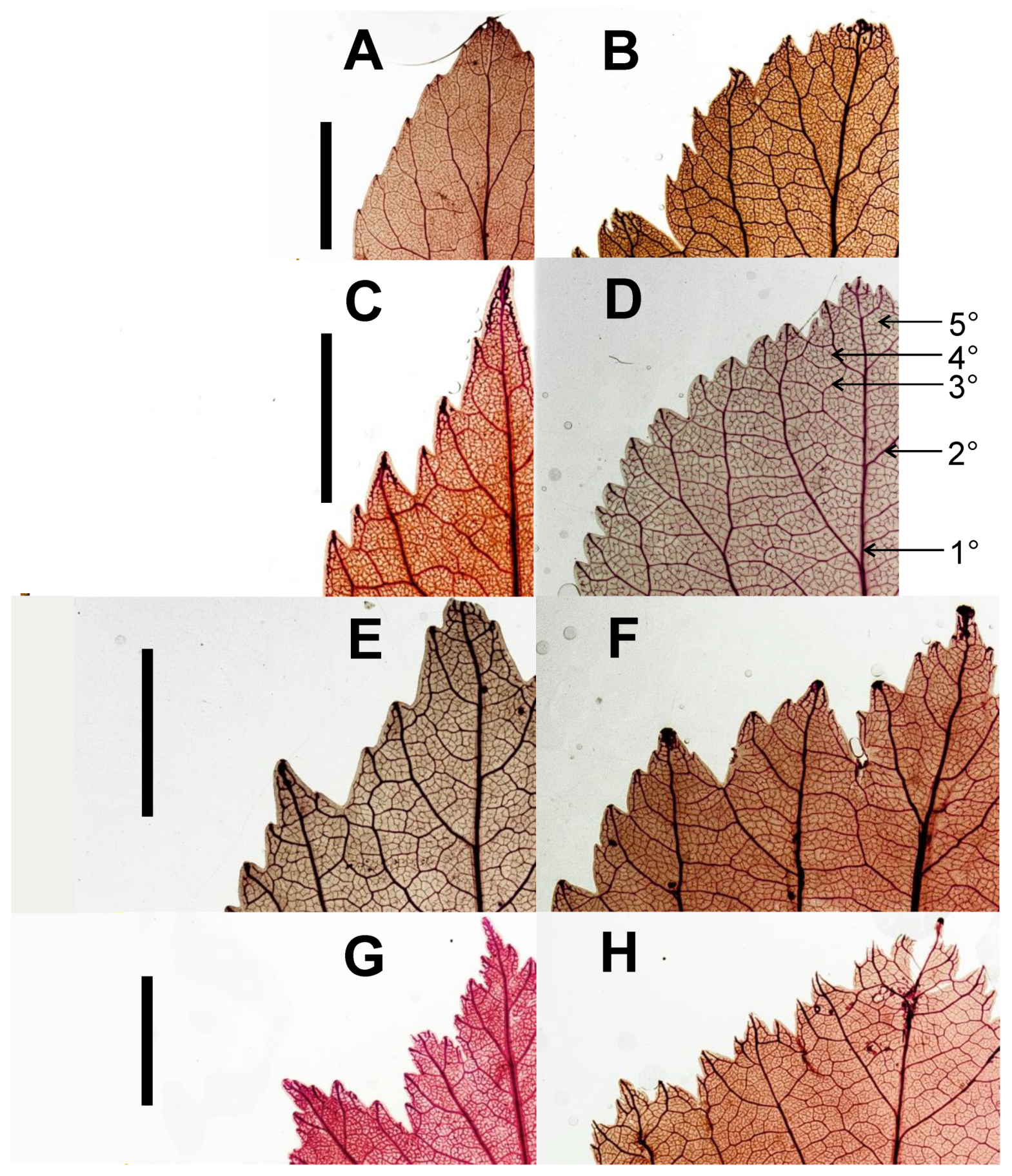
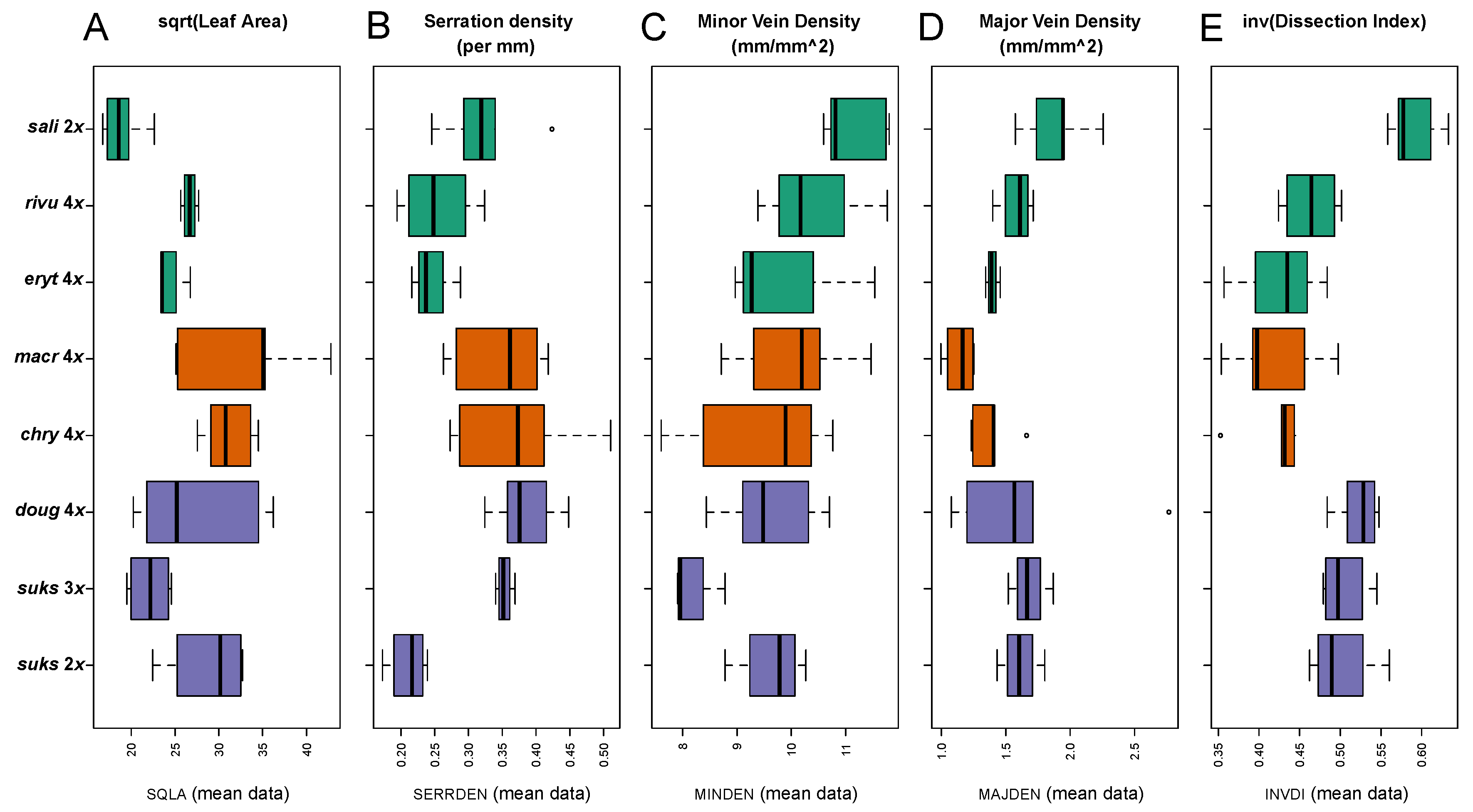
2.2.2. Leaf Architectural Data
2.3. Flow Cytometry
2.4. Climate Niche Comparisons
3. Results
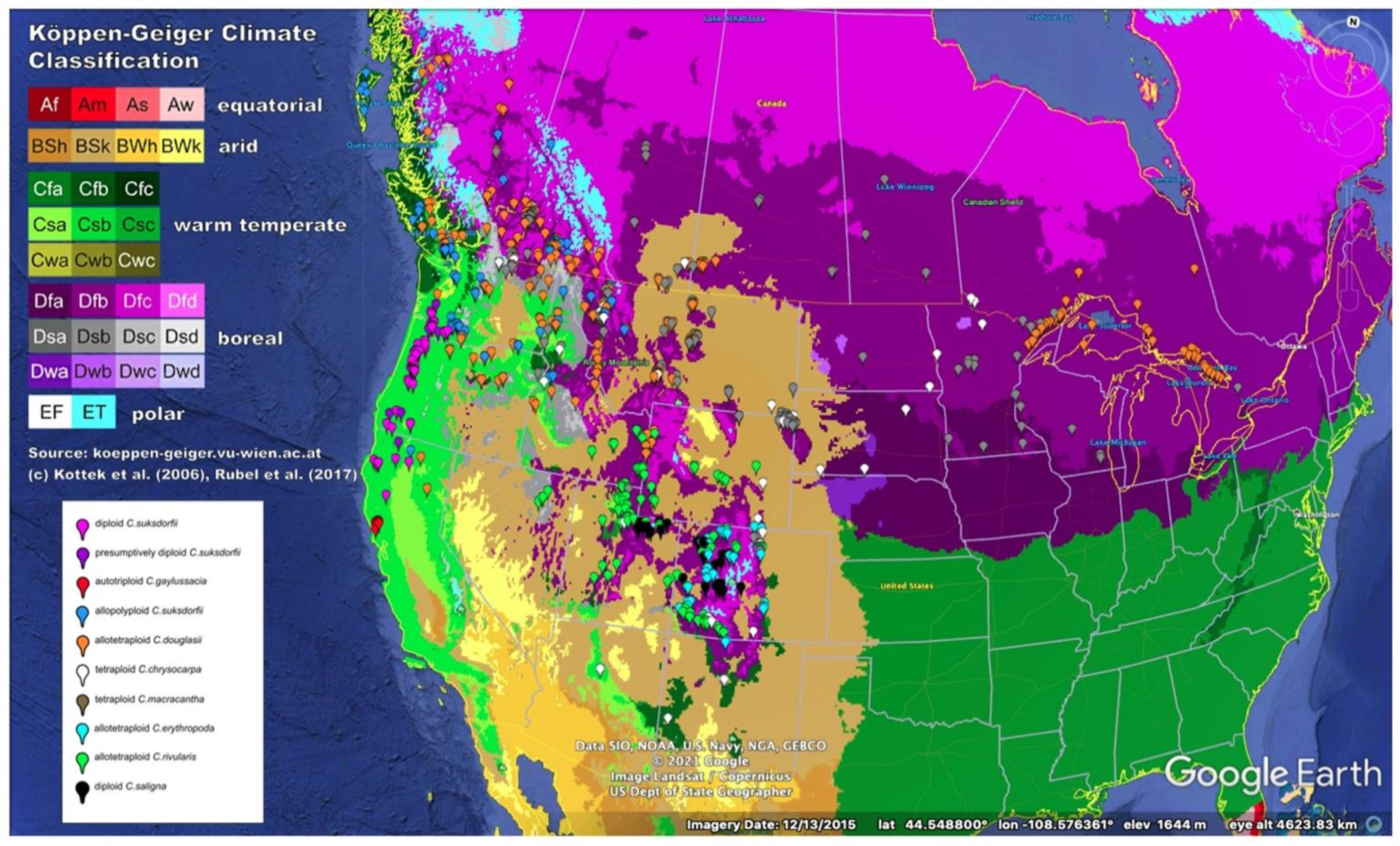
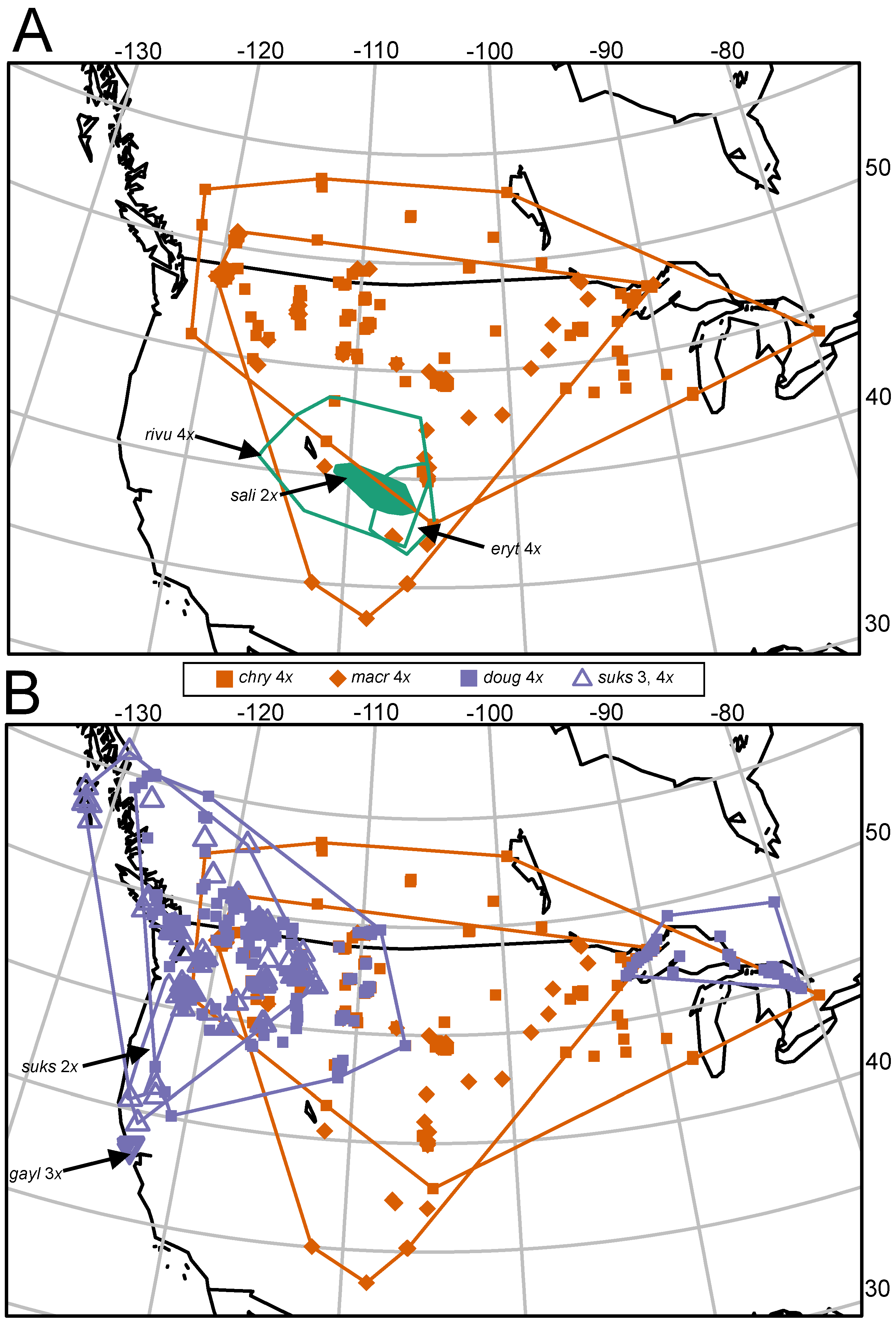
3.1. Differences in Geographic Ranges
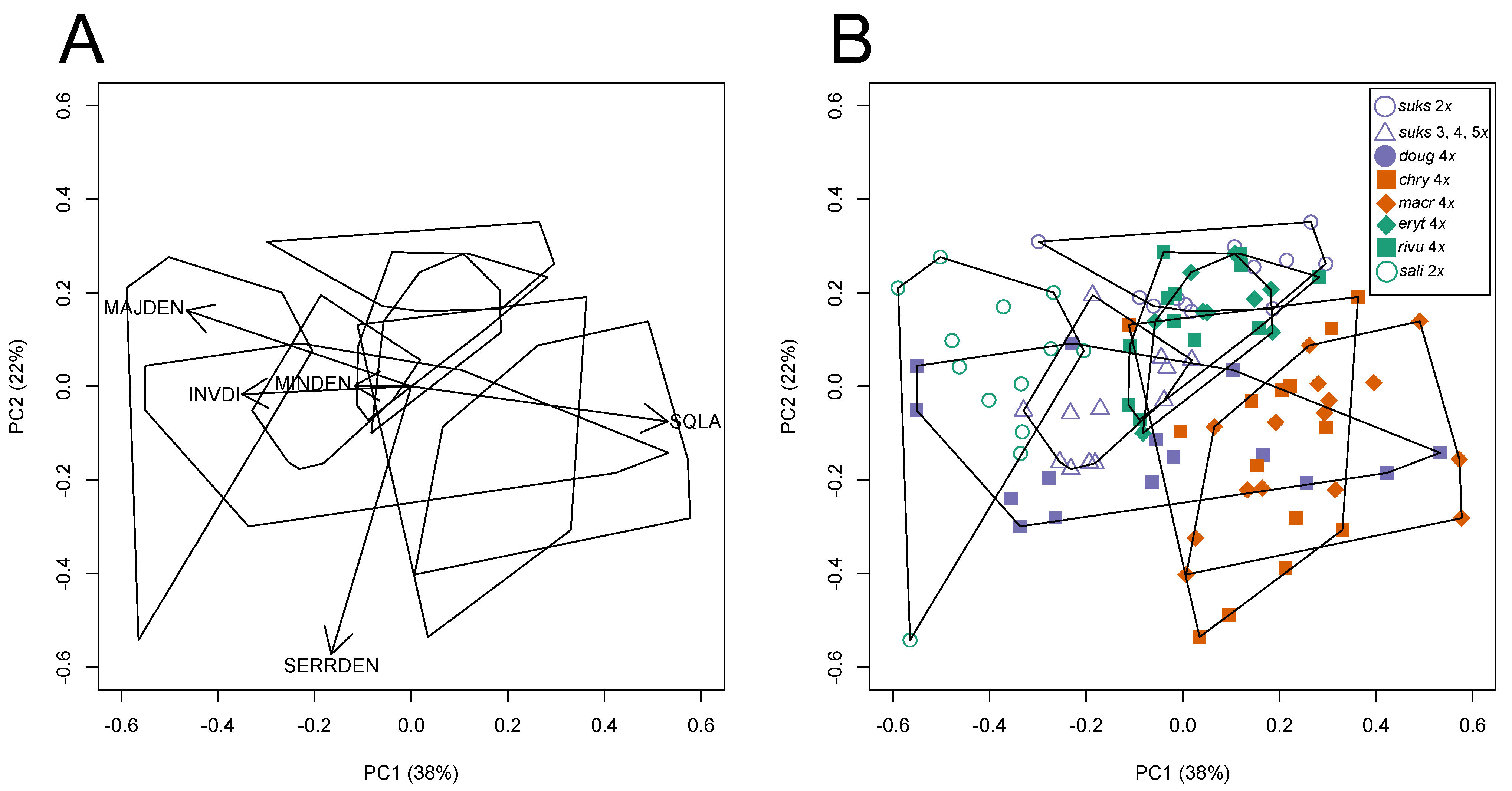
3.2. Morphological Variation
3.2.1. Taxon Differentiation
3.2.2. Leaf Vascular Architecture
3.3. Flow Cytometry
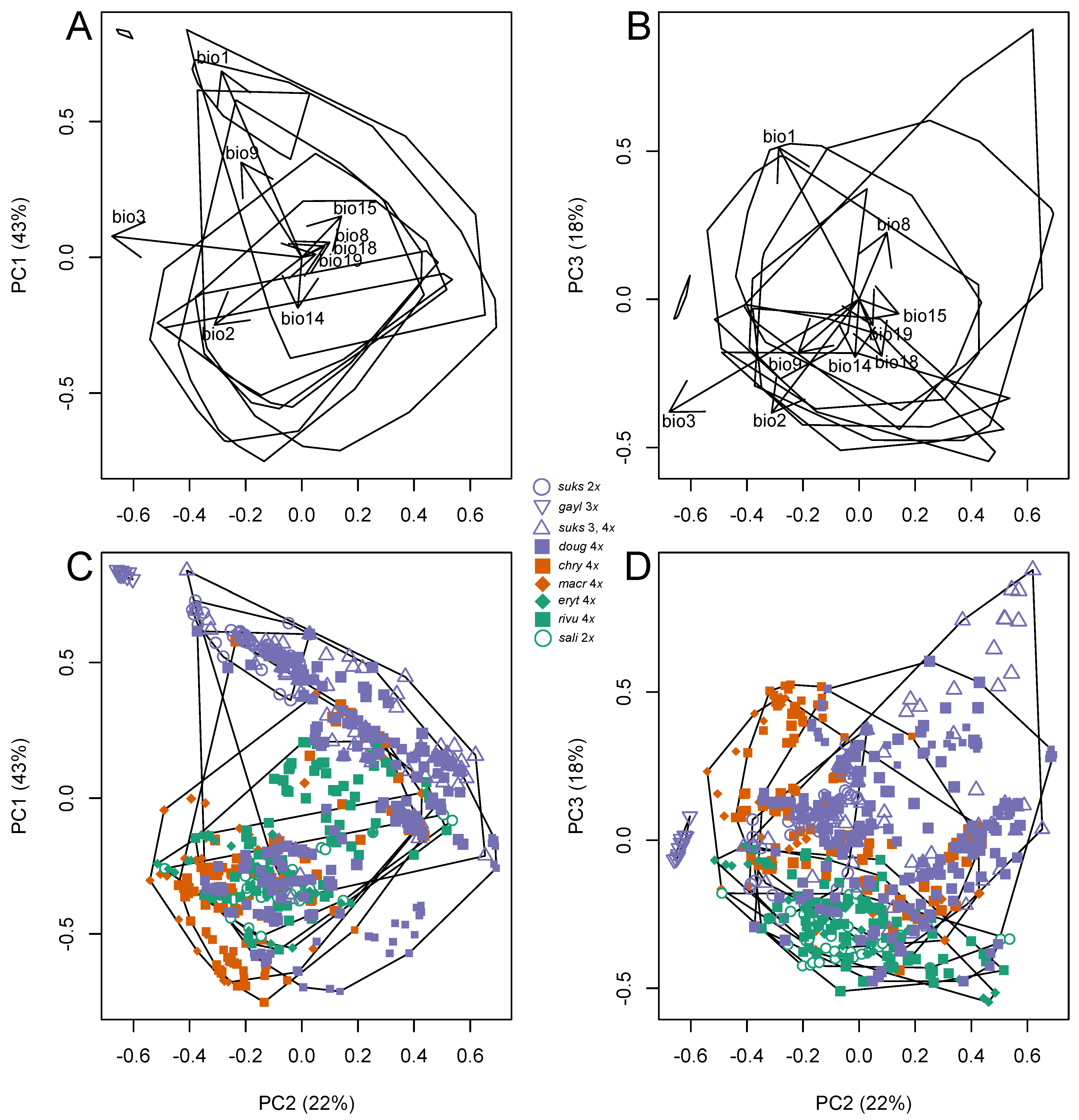
3.4. Climate Correlates of the Differences in Taxon Ranges
3.4.1. Climate Niche Comparisons
3.4.2. Climate Niche Breadth and Overlap
4. Discussion
4.1. Are the Taxa Studied Here Distinct?
4.1.1. Morphological Differentiation
4.1.2. Genetic Differentiation
4.2. Geographic Parthenogenesis?
4.2.1. Biogeographic Differentiation
4.2.2. Ecological Differentiation
4.2.3. Geographic Parthenogenesis?
4.3. If Geographic Parthenogenesis: Adaptation?
4.4. If Geographic Parthenogenesis: Breeding System?
4.5. Hybridization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iezzoni, A.F.; McFerson, J.; Luby, J.; Gasic, K.; Whitaker, V.; Bassil, N.; Yue, C.; Gallardo, K.; McCracken, V.; Coe, M.; et al. RosBREED: Bridging the chasm between discovery and application to enable DNA-informed breeding in rosaceous crops. Hortic. Res. 2020, 7, 177. [Google Scholar] [CrossRef]
- Baird, J.R.; Thieret, J.W. The Medlar (Mespilus germanica, Rosaceae) from antiquity to obscurity. Econ. Bot. 1989, 43, 328–372. [Google Scholar] [CrossRef]
- Phipps, J.B.; O’Kennon, R.J.; Lance, R.W. Hawthorns and Medlars; Timber Press: Portland, OR, USA, 2003; p. 139. [Google Scholar]
- Zarrei, M.; Talent, N.; Kuzmina, M.; Lee, J.; Lund, J.; Shipley, P.R.; Stefanović, S.; Dickinson, T.A. Universal plant DNA barcode loci are poor indicators of species diversity in hawthorns (Crataegus; Rosaceae). AoB Plants 2015, 7, plv045. [Google Scholar] [CrossRef]
- Bourcier, P.G. In Excellent Order—the Gentleman Farmer Views His Fences, 1790–1860. Agric. Hist. 1984, 58, 546–564. [Google Scholar]
- Standing Conference for Local History (Ed.) Hedges and Local History; Bedford Square Press for the Standing Conference for Local History, Botanical Society of the British Isles: London, UK, 1979. [Google Scholar]
- Billington, C. Shrubs of Michigan, 2nd ed.; Cranbrook Institute of Science: Bloomfield Hills, MI, USA, 1968; Volume 20, p. 339. [Google Scholar]
- Dickinson, T.A. The biology of Canadian weeds. 68. Crataegus crus galli sensu lato. Can. J. Plant Sci. 1985, 65, 641–654. [Google Scholar] [CrossRef]
- Martin, A.C.; Zim, H.S.; Nelson, A.L. American Wildlife and Plants—A Guide to Wildlife Food Habits; McGraw-Hill Book Company: New York, NY, USA, 1951; p. 500. [Google Scholar]
- Alonso-Castro, A.J.; Domínguez, F.; Zapata-Morales, J.R.; Carranza-Álvarez, C. Plants used in the traditional medicine of Mesoamerica (Mexico and Central America) and the Caribbean for the treatment of obesity. J. Ethnopharmacol. 2015, 175, 335–345. [Google Scholar] [CrossRef]
- Diane, A.; Borthwick, F.; Wu, S.; Lee, J.; Brown, P.N.; Dickinson, T.A.; Croft, K.D.; Vine, D.F.; Proctor, S.D. Hypolipidemic and cardioprotective benefits of a novel fireberry hawthorn fruit extract in the JCR:LA-cp rodent model of dyslipidemia and cardiac dysfunction. Food Funct. 2016, 7, 3943–3952. [Google Scholar] [CrossRef]
- Edwards, J.E.; Brown, P.N.; Talent, N.; Dickinson, T.A.; Shipley, P.R. A review of the chemistry of the genus Crataegus. Phytochemistry 2012, 79, 5–26. [Google Scholar] [CrossRef]
- Lardos, A.; Heinrich, M. Continuity and change in medicinal plant use: The example of monasteries on Cyprus and historical iatrosophia texts. J. Ethnopharmacol. 2013, 150, 202–214. [Google Scholar] [CrossRef]
- Robles-Botero, M.V.; Ronquillo-de Jesús, E.; Quiroz-Reyes, C.N.; Aguilar-Méndez, M.A. Caracterización e identificación de compuestos bioactivos con actividad antioxidante de la cáscara, pulpa y semilla del fruto de tejocote (Crataegus mexicana). TIP Rev. Espec. En Cienc. Químico-Biológicas 2020, 23, 1–10. [Google Scholar] [CrossRef]
- Soares, S.S.; Bekbolatova, E.; Cotrim, M.D.; Sakipova, Z.; Ibragimova, L.; Kukula-Koch, W.; Giorno, T.B.S.; Fernandes, P.D.; Fonseca, D.A.; Boylan, F. Chemistry and Pharmacology of the Kazakh Crataegus almaatensis Pojark: An Asian Herbal Medicine. Antioxidants 2019, 8, 300. [Google Scholar] [CrossRef]
- Tassell, M.C.; Kingston, R.; Gilroy, D.; Lehane, M.; Furey, A. Hawthorn (Crataegus spp.) in the treatment of cardiovascular disease. Pharmacogn. Rev. 2010, 4, 32–41. [Google Scholar] [CrossRef]
- Hojsgaard, D.; Hörandl, E. Apomixis as a Facilitator of Range Expansion and Diversification in Plants. In Evolutionary Biology: Biodiversification from Genotype to Phenotype; Pontarotti, P., Ed.; Springer International Publishing: Cham, Switzerland; New York, NY, USA, 2015; pp. 305–327. [Google Scholar]
- Hörandl, E. The complex causality of geographical parthenogenesis. New Phytol. 2006, 171, 525–538. [Google Scholar] [CrossRef]
- Hörandl, E. Geographical Parthenogenesis: Opportunities for Asexuality. In Lost Sex: The Evolutionary Biology of Parthenogenesis; Schön, I., Martens, K., van Dijk, P., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 161–186. [Google Scholar]
- Vandel, A. La parthénogenese geographique. Contribution a l’étude biologique et cytologique de la parthénogenese naturelle. Bull. Biol. De La Fr. Et De La Belg. 1928, 62, 164–182. [Google Scholar]
- Bierzychudek, P. Patterns in plant parthenogenesis. Experientia 1985, 41, 1255–1264. [Google Scholar] [CrossRef]
- Hörandl, E.; Cosendai, A.C.; Temsch, E.M. Understanding the geographic distributions of apomictic plants: A case for a pluralistic approach. Plant Ecol. Divers. 2008, 1, 309–320. [Google Scholar] [CrossRef]
- Tilquin, A.; Kokko, H. What does the geography of parthenogenesis teach us about sex? Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150538. [Google Scholar] [CrossRef]
- Brown, J.H.; Stevens, G.C.; Kaufman, D.M. The geographic range: Size, shape, boundaries, and internal structure. Annu. Rev. Ecol. Syst. 1996, 27, 597–623. [Google Scholar] [CrossRef]
- Morueta-Holme, N.; Enquist, B.J.; McGill, B.J.; Boyle, B.; Jørgensen, P.M.; Ott, J.E.; Peet, R.K.; Šímová, I.; Sloat, L.L.; Thiers, B.; et al. Habitat area and climate stability determine geographical variation in plant species range sizes. Ecol. Lett. 2013, 16, 1446–1454. [Google Scholar] [CrossRef]
- Dickinson, T.A.; Lo, E.; Talent, N. Polyploidy, reproductive biology, and Rosaceae: Understanding evolution and making classifications. Plant Syst. Evol. 2007, 266, 59–78. [Google Scholar] [CrossRef]
- Talent, N.; Dickinson, T.A. Endosperm formation in aposporous Crataegus (Rosaceae, Spiraeoideae, tribe Pyreae): Parallels to Ranunculaceae and Poaceae. New Phytol. 2007, 173, 231–249. [Google Scholar] [CrossRef]
- Burgess, M.B.; Cushman, K.R.; Doucette, E.T.; Talent, N.; Frye, C.T.; Campbell, C.S. Effects of apomixis and polyploidy on diversification and geographic distribution in Amelanchier (Rosaceae). Am. J. Bot. 2014, 101, 1375–1387. [Google Scholar] [CrossRef]
- Campbell, C.S.; Greene, C.W.; Dickinson, T.A. Reproductive biology in subfamily Maloideae (Rosaceae). Syst. Bot. 1991, 16, 333–349. [Google Scholar] [CrossRef]
- de Nettancourt, D. Incompatibility in Angiosperms; Springer: Berlin, Germany, 1977. [Google Scholar]
- Dickinson, T.A.; Belaoussoff, S.; Love, R.M.; Muniyamma, M. North American black-fruited hawthorns: I. Variation in floral construction, breeding system correlates, and their possible evolutionary significance in Crataegus sect. Douglasii Loudon. Folia Geobot. Phytotaxon. 1996, 31, 355–371. [Google Scholar] [CrossRef]
- Dickinson, T.A.; Phipps, J.B. Studies in Crataegus (Rosaceae: Maloideae) XIV. The breeding system of Crataegus crus galli sensu lato in Ontario (Canada). Am. J. Bot. 1986, 73, 116–130. [Google Scholar] [CrossRef]
- Lewis, D. Competition and dominance of incompatibility alleles in diploid pollen. Heredity 1947, 1, 85–108. [Google Scholar] [CrossRef]
- Ludwig, S.; Robertson, A.; Rich, T.C.G.; Djordjevic, M.; Cerovic, R.; Houston, L.; Harris, S.A.; Hiscock, S.J. Breeding systems, hybridization and continuing evolution in Avon Gorge Sorbus. Ann. Bot. 2013, 111, 563–575. [Google Scholar] [CrossRef]
- Ufimov, R.A.; Dickinson, T.A. Infrageneric nomenclature adjustments in Crataegus L. (Maleae, Rosaceae). Phytologia 2020, 102, 177–199. [Google Scholar]
- Dickinson, T.A.; Lo, E.Y.Y.; Talent, N.; Love, R.M. Black-fruited Hawthorns of Western North America—One or more Agamic Complexes? Botany 2008, 86, 846–865. [Google Scholar] [CrossRef]
- Zarrei, M.; Stefanović, S.; Dickinson, T.A. Reticulate evolution in North American black-fruited hawthorns (Crataegus section Douglasia; Rosaceae): Evidence from nuclear ITS2 and plastid sequences. Ann. Bot. 2014, 114, 253–269. [Google Scholar] [CrossRef]
- Coughlan, J.M.; Stefanović, S.; Dickinson, T.A. Relative resource allocation to dispersal and competition demonstrates the putative role of hybridity in geographical parthenogenesis. J. Biogeogr. 2014, 41, 1603–1613. [Google Scholar] [CrossRef]
- Lo, E.Y.Y.; Stefanović, S.; Dickinson, T.A. Geographical parthenogenesis in Pacific Northwest hawthorns (Crataegus; Rosaceae). Botany 2013, 91, 107–116. [Google Scholar] [CrossRef]
- McGoey, B.V.; Chau, K.; Dickinson, T.A. Stomata size in relation to ploidy level in North American hawthorns (Crataegus, Rosaceae). Madrono 2014, 61, 177–193. [Google Scholar] [CrossRef]
- Talent, N.; Dickinson, T.A. Polyploidy in Crataegus and Mespilus (Rosaceae, Maloideae): Evolutionary inferences from flow cytometry of nuclear DNA amounts. Can. J. Bot. 2005, 83, 1268–1304. [Google Scholar] [CrossRef]
- Canadensys. Crataegus species. Acadia University, Université de Montréal Biodiversity Centre, University of Toronto Mississauga, University of British Columbia. 2020. Available online: http://data.canadensys.net/explorer (accessed on 21 March 2012).
- Liston, A.; Weitemier, K.A.; Letelier, L.; Podani, J.; Zong, Y.; Lieu, L.; Dickinson, T.A. Phylogeny of Crataegus (Rosaceae) based on 257 nuclear loci and chloroplast genomes: Evaluating the impact of hybridization. PeerJ 2021, 9, e12418. [Google Scholar] [CrossRef]
- Phipps, J.B. Crataegus. In Flora of North America North of Mexico; 22+ vols. 1993+; Flora of North America Editorial Committee, Ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 2015; Volume 9, pp. 491–643. [Google Scholar]
- Coughlan, J.M. Mechanisms for Range Size and Distribution Variation in the Polyploid Complex Black-Fruited Hawthorn (Crataegus Series Douglasianae): Biogeographic Implications for the Maintenance of Cytotype Diversity. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2012. [Google Scholar]
- Talent, N.; Dickinson, T.A. Apomixis and hybridization in Rosaceae subtribe Pyrineae Dumort.: A new tool promises new insights. In Apomixis: Evolution, Mechanisms and Perspectives; Grossniklaus, U., Hörandl, E., Sharbel, T., van Dijk, P., Eds.; Regnum Vegetabile vol. 147; Gantner Verlag: Ruggell, Liechtenstein, 2007; pp. 301–316. [Google Scholar]
- Talent, N.; Dickinson, T.A. The potential for ploidy level increases and decreases in Crataegus (Rosaceae, Spiraeoideae, tribe Pyreae). Can. J. Bot. 2007, 85, 570–584. [Google Scholar] [CrossRef]
- Thiers, B. Index Herbariorum: A global directory of public herbaria and associated staff. Available online: http://sweetgum.nybg.org/science/ih/ (accessed on 7 November 2015).
- Consortium_of_Pacific_Northwest_Herbaria. 2019. Specimen Database (CPNWH). University of Washington Herbarium. 2019. Available online: https://www.pnwherbaria.org (accessed on 9 February 2019).
- Intermountain_Regional_Herbarium_Network. 2019. Specimen Database. Utah State University. 2019. Available online: https://www.intermountainbiota.org/portal/index.php (accessed on 9 February 2019).
- Phipps, J.B. Introduction to the red-fruited hawthorns (Crataegus, Rosaceae) of western North America. Can. J. Bot. 1998, 76, 1863–1899. [Google Scholar]
- Clark, W. Earth Point—Tools for Google Earth. Available online: http://www.earthpoint.us/ (accessed on 19 February 2019).
- GBIF: The Global Biodiversity Information Facility. What is GBIF? Available online: https://www.gbif.org/what-is-gbif (accessed on 30 August 2020).
- Kottek, M.; Grieser, J.r.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Shorthouse, D.P. SimpleMappr, an Online Tool to Produce Publication-Quality Point Maps. Available online: http://www.simplemappr.net (accessed on 7 November 2015).
- Hijmans, R.J. Geosphere: Spherical Trigonometry (R Package Version 1.5-10). 2019. Available online: https://CRAN.R-project.org/package=geosphere (accessed on 1 June 2019).
- Legendre, L.; Legendre, P. Numerical Ecology. In Developments in Environmental Modelling, 2nd ed.; Jorgensen, S.E., Ed.; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1998; Volume 3, p. 419. [Google Scholar]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. cluster: Cluster Analysis Basics and Extensions. R Package Version 2.1.2. Available online: https://cran.r-project.org/web/packages/cluster/cluster.pdf (accessed on 1 May 2021).
- Gower, J.C. A general coefficient of similarity and some of its properties. Biometrics 1971, 27, 857–871. [Google Scholar] [CrossRef]
- Paradis, E.; Blomberg, S.; Bolker, B.; Claude, J.; Cuong, H.S.; Desper, R.; Didier, G.; Durand, B.; Dutheil, J.; Gascuel, O.; et al. Package ‘ape’ (Analyses of Phylogenetics and Evolution). 2015. Available online: https://cran.r-project.org/web/packages/ape/ape.pdf (accessed on 15 January 2016).
- Frontier, S. Étude de la décroissance des valeurs propres dans une analyses en composantes principales: Comparaison avec le modèle du bâton brisé. J. Exp. Mar. Biol. Ecol. 1976, 25, 67–75. [Google Scholar] [CrossRef]
- Oksanen, J. Multivariate Analysis of Ecological Communities in R: Vegan Tutorial. 2015. Available online: https://www.mooreecology.com/uploads/2/4/2/1/24213970/vegantutor.pdf (accessed on 16 October 2021).
- Dickinson, T.A.; Yan, X.Q. Variation in Leaf Venation in Rocky Mountain Hawthorns: Implications for Geographical Parthenogenesis. 2021. Available online: http://morphobank.org/permalink/?P2423.
- Dickinson, T.A.; Yan, X.Q. Variation in Leaf Venation in Pacific Northwest Hawthorns. 2021. Available online: http://morphobank.org/permalink/?P2523.
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Mangiafico, S.S. Nested ANOVA. Available online: https://rcompanion.org/rcompanion/d_07.html (accessed on 21 December 2020).
- McDonald, J.H. Nested ANOVA. Available online: http://www.biostathandbook.com/nestedanova.html (accessed on 21 December 2020).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-151. Available online: https://CRAN.R-project.org/package=nlme (accessed on 15 December 2020).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. R Package Version 3.4-10. Available online: https://CRAN.R-project.org/package=raster (accessed on 15 May 2021).
- Franklin, J. Mapping Species Distributions; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef]
- Therneau, T.; Atkinson, B.; Ripley, B. Rpart: Recursive Partitioning and Regression Trees. R Package Version 4.1-15. Available online: https://CRAN.R-project.org/package=rpart (accessed on 6 November 2016).
- Ripley, B. Tree: Classification and Regression Trees. R Package Version 1.0-40. 2019. Available online: https://cran.r-project.org/web/packages/tree/tree.pdf (accessed on 1 May 2021).
- Roberts, D. Lab 6—Classification Tree Models. Undated. Available online: http://ecology.msu.montana.edu/labdsv/R/labs/lab6/lab6.html (accessed on 1 May 2021).
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling (R Package Version 1.1-1). 2016. Available online: https://CRAN.R-project.org/package=dismo (accessed on 20 July 2015).
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2009, 33, 607–611. [Google Scholar] [CrossRef]
- McKown, A.D.; Cochard, H.; Sack, L. Decoding leaf hydraulics with a spatially explicit model: Principles of venation architecture and implications for its evolution. Am. Nat. 2010, 175, 447–460. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Leigh, A.; Boyce, C.K.; Jones, C.S.; Niklas, K.J.; Royer, D.L.; Tsukaya, H. The evolution and functional significance of leaf shape in the angiosperms. Funct. Plant Biol. 2011, 38, 535–552. [Google Scholar] [CrossRef]
- Scoffoni, C.; Rawls, M.; McKown, A.; Cochard, H.; Sack, L. Decline of Leaf Hydraulic Conductance with Dehydration: Relationship to Leaf Size and Venation Architecture. Plant Physiol. 2011, 156, 832–843. [Google Scholar] [CrossRef]
- Buechler, W.K. Alternative Leaf Clearing and Mounting Procedures (Update of 2004 Original); By the author: Boise, ID, USA, 2010; p. 29. [Google Scholar]
- Yan, B.X. Leaf Venation in Rocky Mountain Hawthorns—Methods (Document). 2016. Available online: http://morphobank.org/permalink/?P2423.
- Ellis, B.; Daly, D.C.; Hickey, L.J.; Johnson, K.R.; Mitchell, J.D.; Wilf, P.; Wing, S.L. Manual of Leaf Architecture; Comstock Publishing Associates (Cornell University Press) in association with The New York Botanical Garden Press: Ithaca, NY, USA, 2009. [Google Scholar]
- Rasband, W.S. ImageJ. 1997–2018. Available online: https://imagej.nih.gov/ij/ (accessed on 30 June 2015).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Scoffoni, C.; Sack, L.; PrometheusWiki_Contributors. Quantifying Leaf Vein Traits. 2013. Available online: https://prometheuswiki.rsb.anu.edu.au/tiki-index.php?page=Quantifying+leaf+vein+traits (accessed on 1 April 2016).
- Kincaid, D.T.; Schneider, R.B. Quantification of leaf shape with a microcomputer and Fourier transform. Can. J. Bot. 1978, 61, 2333–2342. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Yoder, J.B. Species distribution models. In R. The Molecular Ecologist. 2013. Available online: https://www.molecularecologist.com/2013/04/23/species-distribution-models-in-r/ (accessed on 11 December 2016).
- O’Donnell, M.S.; Ignizio, D.A. Bioclimatic Predictors for Supporting Ecological Applications in the Conterminous United States: USGS Data Series 691; United States Geological Survey: Reston, VA, USA, 2012; p. 10. [Google Scholar]
- Rivas-Martinez, S.; Rivas-Saenz, S. Worldwide Bioclimatic Classification System, 1996–2021. Available online: http://www.globalbioclimatics.org (accessed on 5 July 2020).
- Zepner, L.; Karrasch, P.; Wiemann, F.; Bernard, L. ClimateCharts.net—An Interactive Climate Analysis Web Platform. Available online: https://climatecharts.net/ (accessed on 16 December 2020).
- Blonder, B. Frequently Asked Questions (FAQ) for Hypervolume R Package. 2014. Available online: http://www.benjaminblonder.org/hypervolume_faq.html (accessed on 30 April 2021).
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.-J.; Randin, C.; Zimmermann, N.E.; et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Coughlan, J.M.; Han, S.; Stefanović, S.; Dickinson, T.A. Widespread generalist clones are associated with range and niche expansion in allopolyploids of Pacific Northwest Hawthorns (Crataegus L.). Mol. Ecol. 2017, 26, 5484–5499. [Google Scholar] [CrossRef]
- Phipps, J.B.; Muniyamma, M. [Studies in Crataegus (Rosaceae: Maloideae) III.] A taxonomic revision of Crataegus (Rosaceae) in Ontario. Can. J. Bot. 1980, 58, 1621–1699. [Google Scholar] [CrossRef]
- Patrias, K.; Citing Medicine: The NLM Style Guide for Authors, Editors, and Publishers [Internet]. 2nd edition, Appendix E: Two-Letter Abbreviations for Canadian Provinces and Territories and U.S. States and Territories. Available online: https://www.ncbi.nlm.nih.gov/books/NBK7254/ (accessed on 19 December 2020).
- Marquis, R.J.; Voss, E.G. Distributions of some western North American plants disjunct in the Great Lakes region. Mich. Bot. 1981, 20, 53–82. [Google Scholar]
- Lo, E.Y.Y.; Stefanović, S.; Christensen, K.I.; Dickinson, T.A. Evidence for genetic association between East Asian and Western North American Crataegus L. (Rosaceae) and rapid divergence of the Eastern North American lineages based on multiple DNA sequences. Mol. Phylogenetics Evol. 2009, 51, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Matzk, F.; Meister, A.; Schubert, I. An efficient screen for reproductive pathways using mature seeds of monocots and dicots. Plant J. 2000, 21, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Ranney, T.; Eaker, T. Variation in Ploidy Levels and Reproductive Pathways Among Flowering Crabapples. HortScience 2004, 39, 773B-773. [Google Scholar] [CrossRef]
- Dickinson, T.A.; Coughlan, J.M.; Han, S. [North American black-fruited hawthorns IV.] What IS Suksdorf’s hawthorn?—Reexamining the black-fruited hawthorns of the Pacific Northwest (Crataegus section Douglasianae, Rosaceae tribe Maleae). in press.
- Phipps, J.B.; O’Kennon, R.J. Hawthorns (Crataegus: Rosaceae) of the Cypress Hills, Alberta and Saskatchewan. J. Bot. Res. Inst. Tex. 2007, 1, 1031–1090. [Google Scholar]
- Coyne, J.A.; Orr, H.A. Speciation; Sinauer Associates, Inc.: Sunderland, MA, USA, 2004. [Google Scholar]
- Dickinson, T.A. Sex and Rosaceae apomicts. Taxon 2018, 67, 1093–1107. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Haslinger, K.; Auer, I. The Climate of the European Alps: Shift of Very High Resolution Köppen-Geiger Climate Zones 1800–2100. Meteorol. Z. 2017, 26, 115–125. [Google Scholar] [CrossRef]
- Phipps, J.B.; O’Kennon, R.J. Three new species of Crataegus (Rosaceae) from western North America: C. okennonii, C. okanaganensis, and C. phippsii. SIDA 1998, 18, 169–191. [Google Scholar]
- Dickinson, T.A. Book Review—Hawthorns and Medlars. Can. Bot. Assoc. Bull. 2004, 37, 30–31. [Google Scholar]
- Dickinson, T.A.; Love, R.M. [North American black-fruited hawthorns: III.] What is Douglas hawthorn? In Conservation and Management of Oregon’s Native Flora; Kaye, T., Liston, A., Love, R.M., Luoma, D.L., Meinke, R.J., Wilson, M.V., Eds.; Native Plant Society of Oregon: Corvallis, OR, USA, 1997; pp. 162–171. [Google Scholar]
- Phipps, J.B. The relationships of the American black-fruited hawthorns Crataegus erythropoda, C. rivularis, C. saligna, and C. brachyacantha to C. Ser. Douglasianae (Rosaceae). SIDA 1999, 18, 647–660. [Google Scholar]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis, Version 3.10. 2016. Available online: http://www.mesquiteproject.org (accessed on 3 October 2016).
- Lo, E.Y.Y.; Stefanović, S.; Dickinson, T.A. Molecular reappraisal of relationships between Crataegus and Mespilus (Rosaceae, Pyreae)—Two genera or one? Syst. Bot. 2007, 32, 596–616. [Google Scholar]
- Dickinson, T.A.; Campbell, C.S. Population structure and reproductive ecology in the Maloideae (Rosaceae). Syst. Bot. 1991, 16, 350–362. [Google Scholar] [CrossRef]
- Dickinson, T.A.; Phipps, J.B. Studies in Crataegus L. (Rosaceae: Maloideae) IX. Short shoot leaf heteroblasty in Crataegus crus-galli L. sensu lato. Can. J. Bot. 1984, 62, 1775–1780. [Google Scholar] [CrossRef]
- Dickinson, T.A.; Phipps, J.B. Studies in Crataegus L. (Rosaceae: Maloideae) XIII. Degree and pattern of variation in Crataegus section Crus-galli in Ontario. Syst. Bot. 1985, 10, 322–337. [Google Scholar] [CrossRef]
- Vander Kloet, S.P.; Dickinson, T.A. The taxonomy of Vaccinium section Myrtillus (Ericaceae). Brittonia 1999, 51, 231–254. [Google Scholar] [CrossRef]
- Lo, E.Y.Y.; Stefanović, S.; Dickinson, T.A. Reconstructing reticulation history in a phylogenetic framework and the potential of allopatric speciation driven by polyploidy in an agamic complex in Crataegus (Rosaceae). Evolution 2010, 64, 3593–3608. [Google Scholar] [CrossRef] [PubMed]
- Ufimov, R.; Zeisek, V.; Píšová, S.; Baker, W.J.; Fér, T.; van Loo, M.; Dobeš, C.; Schmickl, R. Relative performance of customized and universal probe sets in target enrichment: A case study in subtribe Malinae. Appl. Plant Sci. 2021, 9, e11442. [Google Scholar] [CrossRef]
- Lo, E.Y.Y.; Stefanović, S.; Dickinson, T.A. Population genetic structure of diploid sexual and polyploid apomictic hawthorns (Crataegus; Rosaceae) in the Pacific Northwest. Mol. Ecol. 2009, 18, 1145–1160. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, J.; Shery Han, S.H.; Stefanović, S.; Dickinson, T. Data from: Widespread generalist clones are associated with range and niche expansion in allopolyploids of Pacific Northwest Hawthorns (Crataegus L.), Dryad, Dataset. Available online:. (accessed on 5 July 2020). [CrossRef]
- Fortin, M.-J.; Keitt, T.H.; Maurer, A.B.; Taper, M.L.; Kaufman, D.M.; Blackburn, T.M. Species’ geographic ranges and distributional limits: Pattern analysis and statistical issues. OIKOS 2005, 108, 7–17. [Google Scholar] [CrossRef]
- Ritchie, J.C. Postglacial Vegetation of Canada; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Bailey, R.G. Map. Ecoregions of North America; Revised; USDA Forest Service: Washington, DC, USA, 1997. [Google Scholar]
- Bouchal, J.M.; Güner, T.H.; Velitzelos, D.; Velitzelos, E.; Denk, T. Messinian vegetation and climate of the intermontane Florina–Ptolemais–Servia Basin, NW Greece inferred from palaeobotanical data: How well do plant fossils reflect past environments? R. Soc. Open Sci. 2020, 7, 192067. [Google Scholar] [CrossRef]
- Denk, T.; Grimm, G.W.; Grímsson, F.; Zetter, R. Evidence from “Köppen signatures” of fossil plant assemblages for effective heat transport of Gulf Stream to subarctic North Atlantic during Miocene cooling. Biogeosciences 2013, 10, 7927–7942. [Google Scholar] [CrossRef]
- Beatty, B.L.; Jennings, W.F.; Rawlinson, R.C. Crataegus Saligna Greene (Willow Hawthorn): A Technical Conservation Assessment; USDA Forest Service, Rocky Mountain Region: Lakewood, CO, USA, 2004. Available online: https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5206835.pdf (accessed on 30 September 2007).
- Phipps, J.B. Monograph of northern Mexican Crataegus (Rosaceae, subfam. Maloideae). Sida Bot. Misc. 1997, 15, 1–94. [Google Scholar]
- Brodribb, T.J.; Feild, T.S.; Sack, L. Viewing leaf structure and evolution from a hydraulic perspective. Funct. Plant Biol. 2010, 37, 488–498. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C.; McKown, A.D.; Frole, K.; Rawls, M.; Havran, J.C.; Tran, H.; Tran, T. Developmentally based scaling of leaf venation architecture explains global ecological patterns. Nat. Commun. 2012, 3, 837. Available online: http://www.nature.com/ncomms/journal/v3/n5/suppinfo/ncomms1835_S1.html (accessed on 20 July 2015). [CrossRef]
- Jordano, P. What is long-distance dispersal? And a taxonomy of dispersal events. J. Ecol. 2017, 105, 75–84. [Google Scholar] [CrossRef]
- Nathan, R. Long-Distance Dispersal of Plants. Science 2006, 313, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.I.; Zarrei, M.; Kuzmina, M.; Talent, N.; Lin, C.; Dickinson, T.A. Crataegus ×ninae-celottiae and C. ×cogswellii (Rosaceae, Maleae), two spontaneously formed intersectional nothospecies. Phytokeys 2014, 36, 1–26. [Google Scholar] [CrossRef] [PubMed]
- EDDMapS. Crataegus monogyna. Available online: http://www.eddmaps.org/distribution/uscounty.cfm?sub=5392 (accessed on 23 August 2016).
- Dickson, E.E. Malus. In Flora of North America North of Mexico; 22+ vols. 1993+; Flora of North America Editorial Committee, Ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 2015; Volume 9, pp. 472–479. [Google Scholar]
- Kurtto, A.; Sennikov, A.; Lampinen, R. Atlas Flora Europaeae. Distribution of Vascular Plants in Europe. 16. Rosaceae (Cydonia to Prunus, excl. Sorbus); The Committee for Mapping the Flora of Europe & Societas Biologica Fennica Vanamo: Helsinki, Finland, 2013. [Google Scholar]
- Muniyamma, M.; Phipps, J.B. Studies in Crataegus. XII. Cytological evidence for sexuality in some diploid and tetraploid species of North American hawthorns. Can. J. Bot. 1985, 63, 1319–1324. [Google Scholar] [CrossRef]
- Baker, H.G. Support for Baker’s Law-As a Rule. Evolution 1967, 21, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Key, B.A. Soil Enrichment under Chalk Scrub and re-Establishment of Chalk Grassland after Scrub Clearance. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 1979. [Google Scholar]
- Borys, M.W.; Leszczyñska-Borys, H. Roots Attributes of Mexican Crataegus spp. trees. Rev. Chapingo Ser. Hortic. 2004, 10, 85–95. [Google Scholar] [CrossRef]
- Brinkman, K.A. Crataegus L. In Seeds of Woody Plants in the United States; Agriculture Handbook; United States Forest Service, United States Department of Agriculture: Washington, DC, USA, 1974; Volume 450, pp. 356–360. [Google Scholar]
- Hinckley, T.M.; Duhme, F.; Hinckley, A.R.; Richter, H. Drought relations of shrub species: Assessment of the mechanisms of drought resistance. Oecologia 1983, 59, 344–350. [Google Scholar] [CrossRef]
- Hobbs, J.J. Mount Sinai; University of Texas Press: Austin, TX, USA, 1995. [Google Scholar]
- Alonso-Marcos, H.; Nardi, F.D.; Scheffknecht, S.; Tribsch, A.; Hülber, K.; Dobeš, C. Difference in reproductive mode rather than ploidy explains niche differentiation in sympatric sexual and apomictic populations of Potentilla puberula. Ecol. Evol. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Campbell, C.S.; Evans, R.C.; Morgan, D.R.; Dickinson, T.A.; Arsenault, M.P. Phylogeny of subtribe Pyrinae (formerly the Maloideae, Rosaceae): Limited resolution of a complex evolutionary history. Plant Syst. Evol. 2007, 266, 119–145. [Google Scholar] [CrossRef]
- Evans, R.C.; Campbell, C.S. The origin of the apple subfamily (Maloideae; Rosaceae) is clarified by DNA sequence data from duplicated GBSSI genes. Am. J. Bot. 2002, 89, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus [×] domestica Borkh.). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef] [PubMed]
- De Storme, N.; Mason, A. Plant speciation through chromosome instability and ploidy change: Cellular mechanisms, molecular factors and evolutionary relevance. Curr. Plant Biol. 2014, 1, 10–33. [Google Scholar] [CrossRef]
- Talent, N. Evolution of gametophytic apomixis in flowering plants: An alternative model from Maloid Rosaceae. Theory Biosci. 2009, 128, 121–138. [Google Scholar] [CrossRef]
- Vašková, D.; Kolarčik, V. Breeding Systems in Diploid and Polyploid Hawthorns (Crataegus): Evidence from Experimental Pollinations of C. monogyna, C. subsphaerica, and Natural Hybrids. Forests 2019, 10, 1059. [Google Scholar] [CrossRef]
- Hajrudinović, A.; Siljak-Yakovlev, S.; Brown, S.C.; Pustahija, F.; Bourge, M.; Ballian, D.; Bogunić, F. When sexual meets apomict: Genome size, ploidy level and reproductive mode variation of Sorbus aria s.l. and S. austriaca (Rosaceae) in Bosnia and Herzegovina. Ann. Bot. 2015, 116, 301–312. [Google Scholar] [CrossRef]
- Dyke, A.S. Late Quaternary Vegetation History of Northern North America Based on Pollen, Macrofossil, and Faunal Remains. Géographie Phys. Et Quat. 2005, 59, 211–262. [Google Scholar] [CrossRef]
- Baniaga, A.E.; Marx, H.E.; Arrigo, N.; Barker, M.S. Polyploid plants have faster rates of multivariate niche differentiation than their diploid relatives. Ecol. Lett. 2020, 23, 68–78. [Google Scholar] [CrossRef]
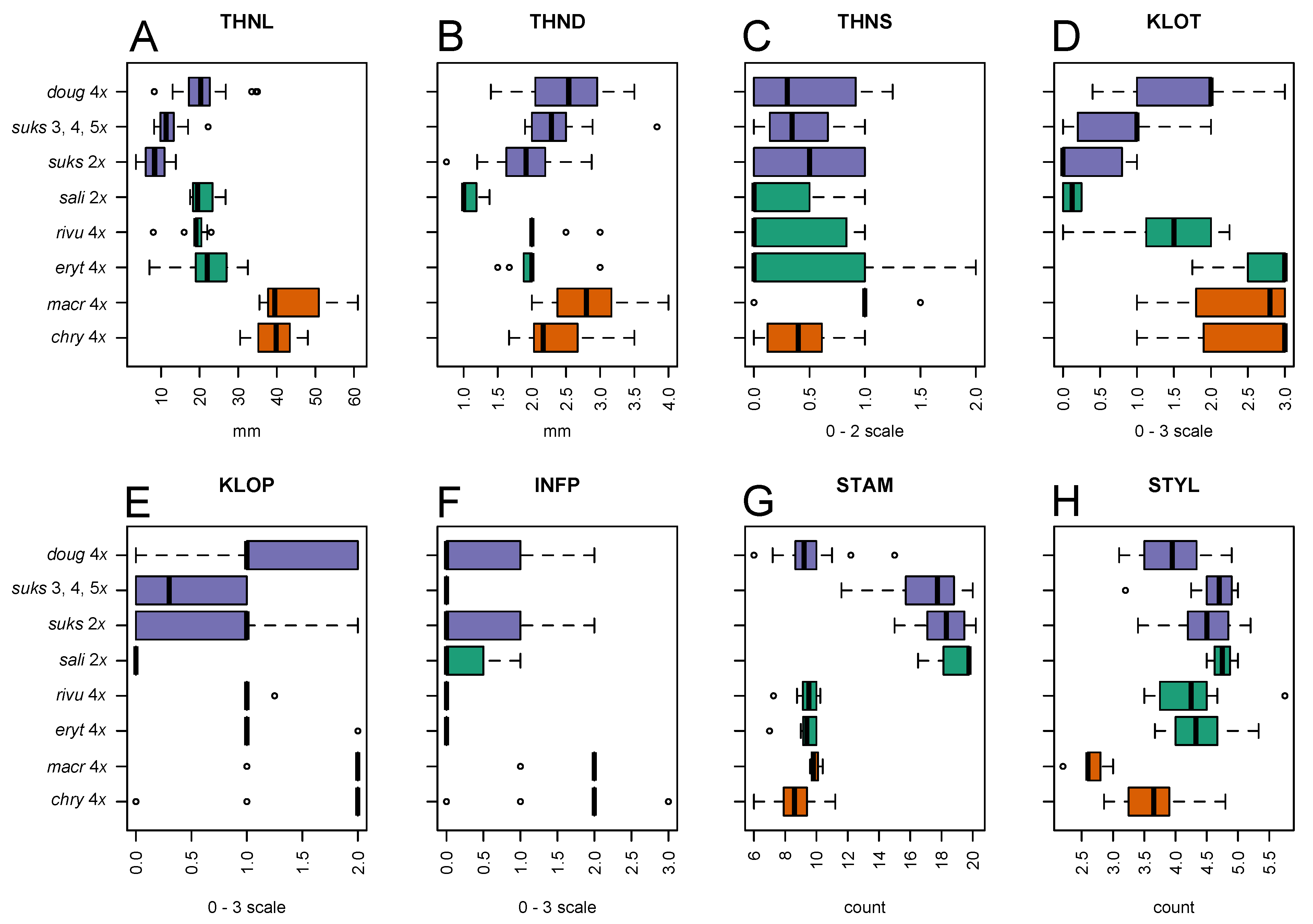

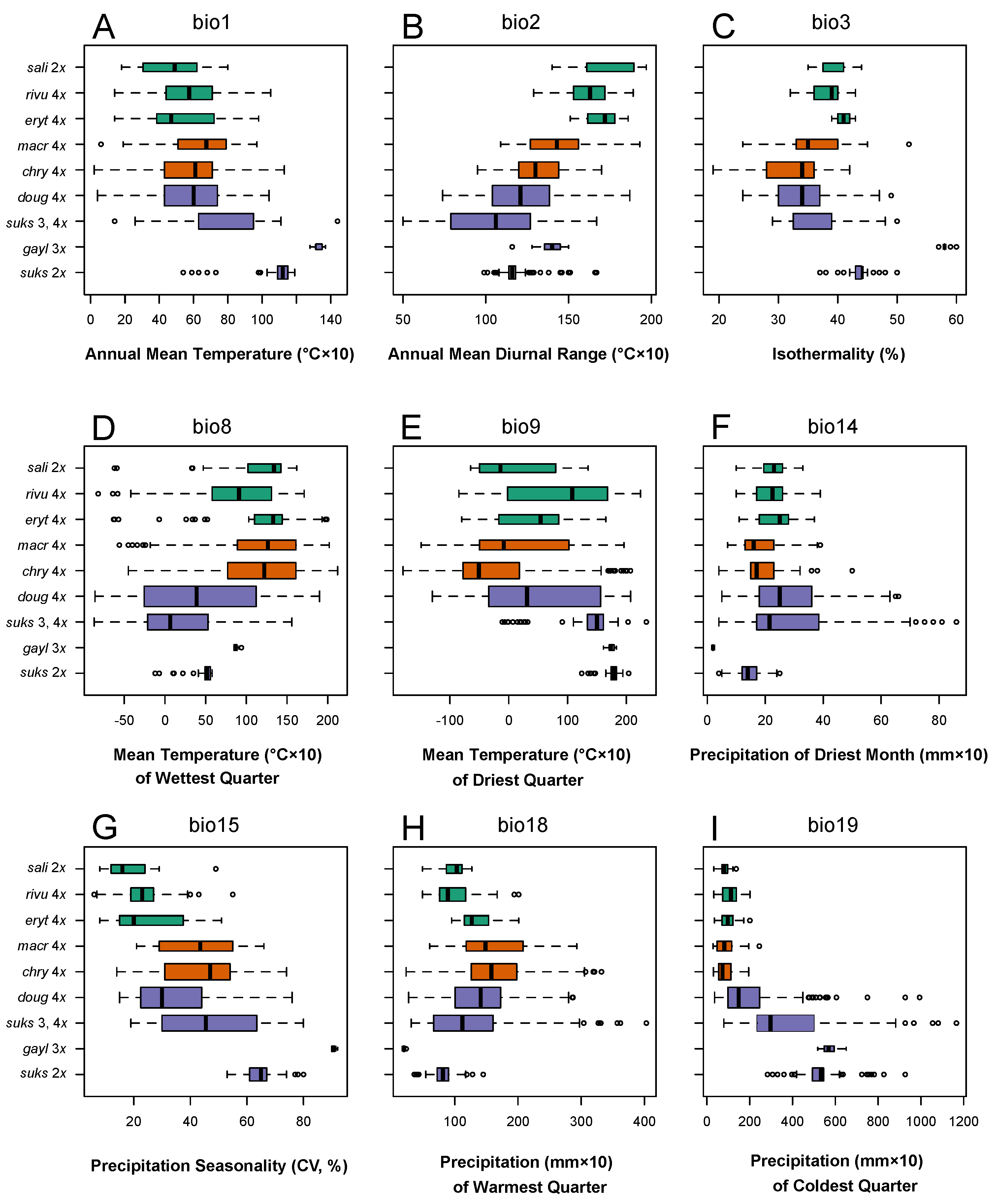
| Acronym Used in Figures and Text | Description |
|---|---|
| THNL | Thorn length (mm) |
| THND | Thorn diameter at base (mm) |
| THNS | Thorn curvature: 0, straight; 1, moderately curved; 2, very curved |
| KLOT | Calyx lobe toothing: 0, no toothing, or only 1–2 teeth on a single lobe; 1, isolated teeth on more than one lobe; 2, several teeth on most lobes; densely toothed on each lobe |
| KLOP | Calyx lobe pubescence: 0, completely glabrous; 1, scattered hairs, or hairs dense only locally; 2, dense enough to overlap; 3, hairs very dense, over the entire region |
| INFP | Inflorescence axis pubescence along the secondary veins: 0, completely glabrous; 1, scattered hairs, or hairs dense only locally; 2, dense enough to overlap; 3, hairs very dense, over the entire region |
| STAM | Number of stamens per flower. |
| STYL | Number of styles per flower. |
| Descriptor and Acronym | Explanation |
|---|---|
| Square root-transformed Leaf Area0.5 (SQLA) | Leaf Area (mm2) 1/2 |
| Serration Density (SERRDEN) | Leaf teeth/mm perimeter (calculated for entire leaf) |
| Minor Vein Density (MINDEN); = Sum of vein lengths for quaternary and higher order veins per unit area (mm/mm2) | Minor vein length per unit Area (mm/mm2) |
| Major Vein Density (MAJDEN); = Sum of vein lengths for primary, secondary, and tertiary veins per unit area (mm/mm2) | Major vein length per unit Area (mm/mm2) |
| Dissection Index−1 (INVDI); = 1 for a perfect circle (minimum P, for any given A) and approaches 0 as a leaf is increasingly lobed [36] | Inverse of the dissection index [89], 2(Aπ)1/2/P, for A = leaf area, and P = leaf perimeter |
| Taxon | Range, Areal Extent (km2) and as Percent of C. chrysocarpa Polygon (Figure 1B) | Elevational Range (m above Sea Level) | States/Provinces (Phipps 2015) |
|---|---|---|---|
| Crataegus chrysocarpa (4x) | 3,417,010 | 143–2546 | All Canadian provinces; northernmost tier of states plus OR, WY, UT, CO. SD, IA, IL, CT, RI, MA |
| Crataegus macracantha (4x) | 2,479,523 (73%) | 231–2303 | All Canadian provinces except NS, NL, PE; northernmost tier of states except ID and ME, plus OR, WY, UT, CO. AZ, NM, SD, NE, KS, IA, MO, IL, IN, OH, WV, PA, VA, MD, CT, RI, MA |
| Crataegus saligna (2x) | 54,000 (2%) | 1551–2652 | UT, CO |
| Crataegus rivularis (4x) | 449,000 (13%) | 1272–3138 | ID, WY, NV, UT, CO, AZ, NM |
| Crataegus erythropoda (4x) | 107,000 (3%) | 1597–3138 | WY, CO, NM |
| Crataegus douglasii (4x) | (western) 1,565,955 (Great Lakes) 274,936 (total) 1,840,891 (54%) | 4–2098 | BC, AB, SK, WA, ID, MT, OR, CA, ON, MN, WI, MI |
| Crataegus gaylussacia (auto 3x) | 295 (<<1%) | 33–191 | Marin and Sonoma counties CA |
| Crataegus suksdorfii (2x) | 39,513 (1%) | 3–1499 | WA, OR, CA |
| Crataegus suksdorfii (allo 3, 4x) | 1,264,913 (37%) | 5–1594 | AK, BC, WA, ID, MT, OR, CA |
| Morphologically Distinct? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Taxon | sali — | rivu apo | eryt apo | chry apo | macr apo | doug apo | 3x suks apo | 2x suks — | gayl apo | |
| Fruit color, stamen number | B20 | B10 | B10 | R10 | R10 | B10 | B20 | B20 | B20 | |
| sali (2x) | B20 | — | Y | Y | Y | Y | Y | (Y 1) | (Y 1) | (Y 1) |
| rivu (4x) | B10 | N | — | (Y 2) | Y | Y | (Y 2) | Y | Y | Y |
| eryt (4x) | B10 | N | N | — | Y | Y | (N) | Y | Y | Y |
| chry (4x) | R10 | N | N | N | — | Y 3 | Y | Y | Y | Y |
| macr (4x) | R10 | N | N | N | N | — | Y | Y | Y | Y |
| doug (4x) | B10 | Y | (Y 4) | (Y 4) | N | N | — | Y | Y | Y |
| suks (3x) | B20 | Y | Y | Y | N | N | N | — | N | N |
| suks (2x) | B20 | Y | Y | Y | Y | Y | Y | Y 5 | — | (N) |
| gayl (3x 6) | B20 | Y | Y | Y | Y | Y | Y | Y | Y | — |
| Allopatric? | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dickinson, T.A.; Yan, B.X.; Han, S.; Zarrei, M. Niche Shifts, Hybridization, Polyploidy and Geographic Parthenogenesis in Western North American Hawthorns (Crataegus subg. Sanguineae, Rosaceae). Agronomy 2021, 11, 2133. https://doi.org/10.3390/agronomy11112133
Dickinson TA, Yan BX, Han S, Zarrei M. Niche Shifts, Hybridization, Polyploidy and Geographic Parthenogenesis in Western North American Hawthorns (Crataegus subg. Sanguineae, Rosaceae). Agronomy. 2021; 11(11):2133. https://doi.org/10.3390/agronomy11112133
Chicago/Turabian StyleDickinson, Timothy A., Brigitte Xueqi Yan, Shery Han, and Mehdi Zarrei. 2021. "Niche Shifts, Hybridization, Polyploidy and Geographic Parthenogenesis in Western North American Hawthorns (Crataegus subg. Sanguineae, Rosaceae)" Agronomy 11, no. 11: 2133. https://doi.org/10.3390/agronomy11112133
APA StyleDickinson, T. A., Yan, B. X., Han, S., & Zarrei, M. (2021). Niche Shifts, Hybridization, Polyploidy and Geographic Parthenogenesis in Western North American Hawthorns (Crataegus subg. Sanguineae, Rosaceae). Agronomy, 11(11), 2133. https://doi.org/10.3390/agronomy11112133






