Organic Amendments Alter Long-Term Turnover and Stability of Soil Carbon: Perspectives from a Data-Model Integration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Datasets
2.2. The RothC Model and Sensitivity Analysis
2.3. Model Modification and Complexity Reduction
2.4. Model Optimization
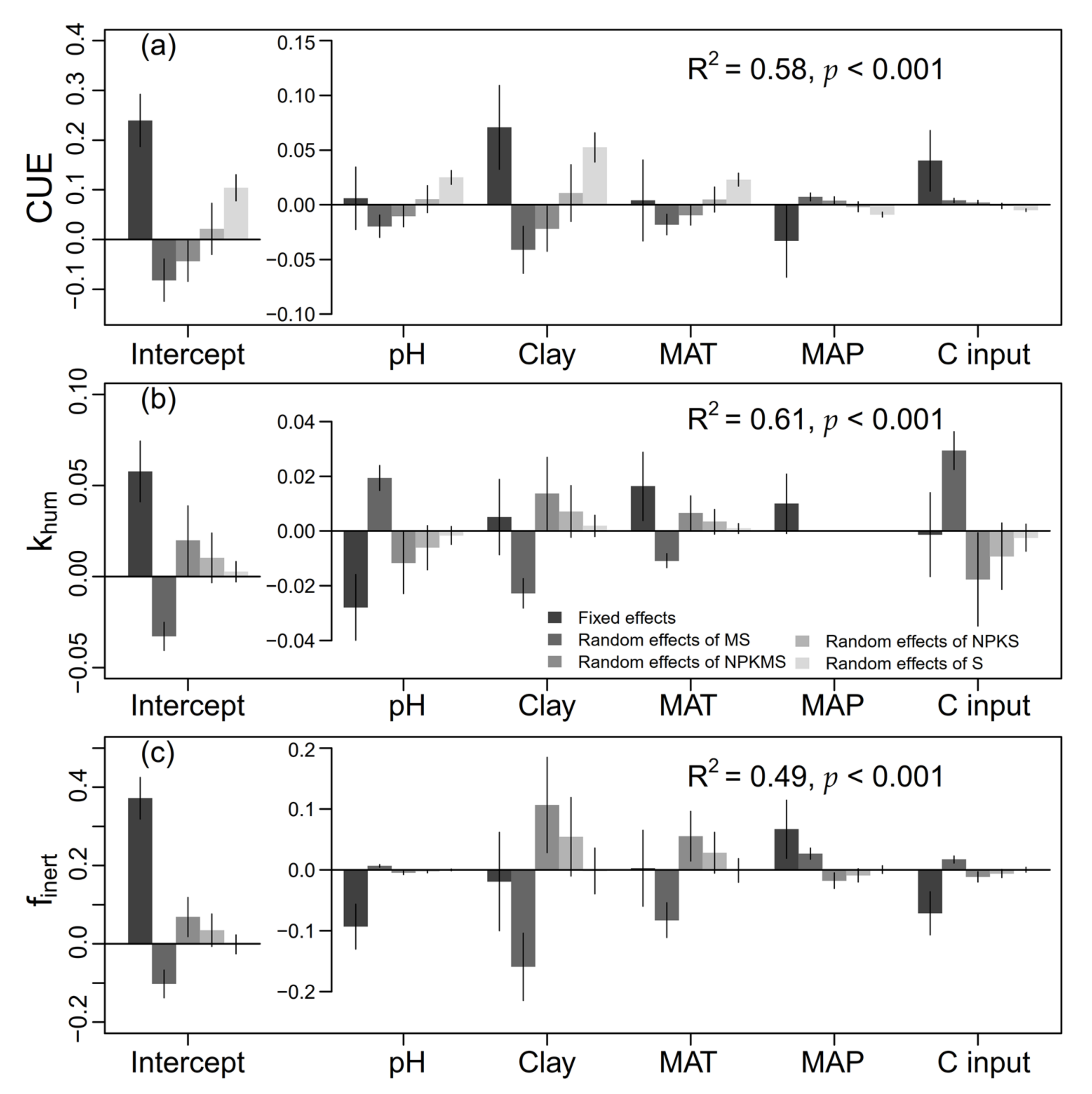
2.5. Drivers of CUE, khum, and finert Changes
2.6. Consequences of OA Addition on Long-Term SOC Dynamics
3. Results
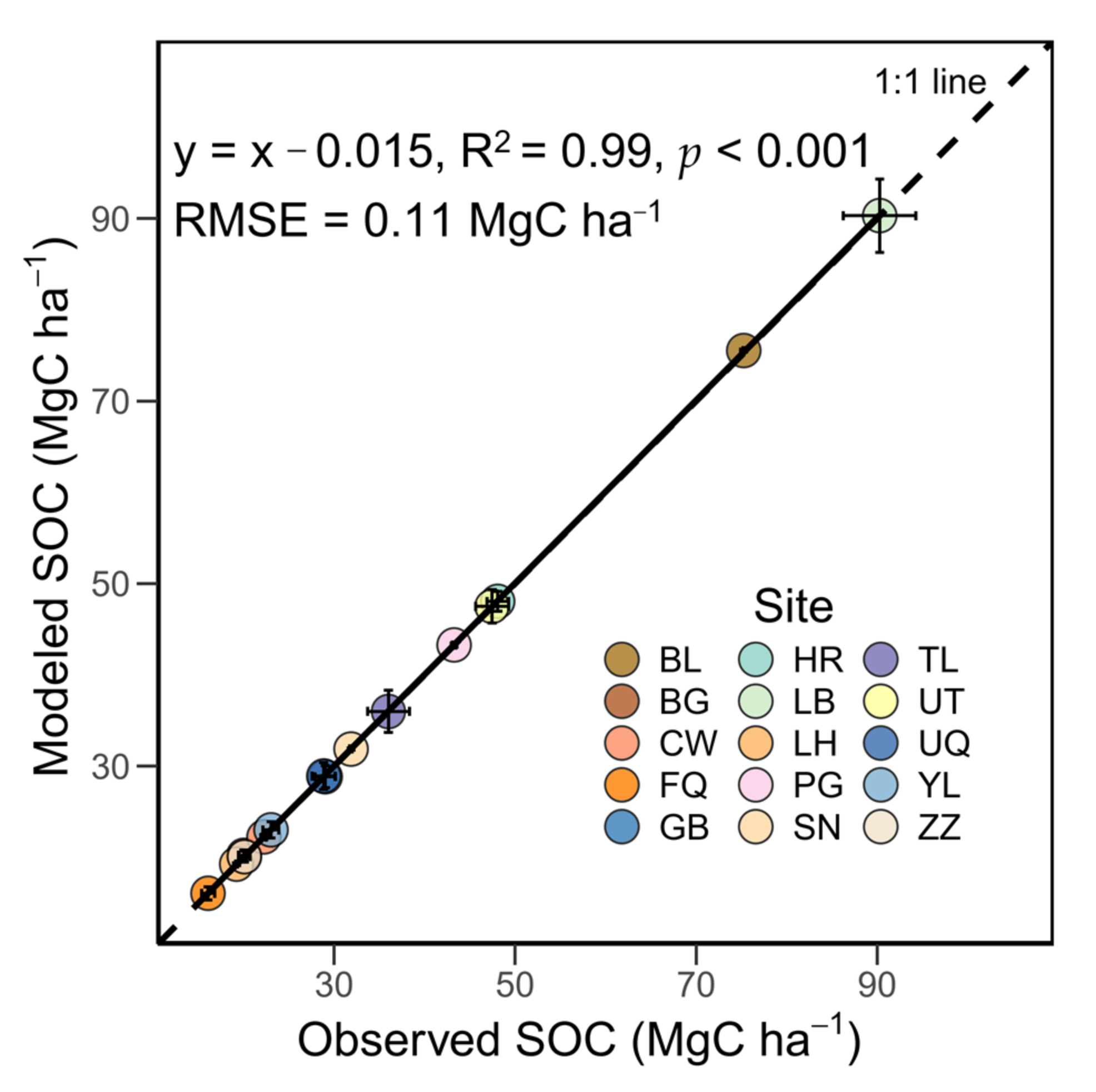
3.1. Performance of the Modified RothC Model
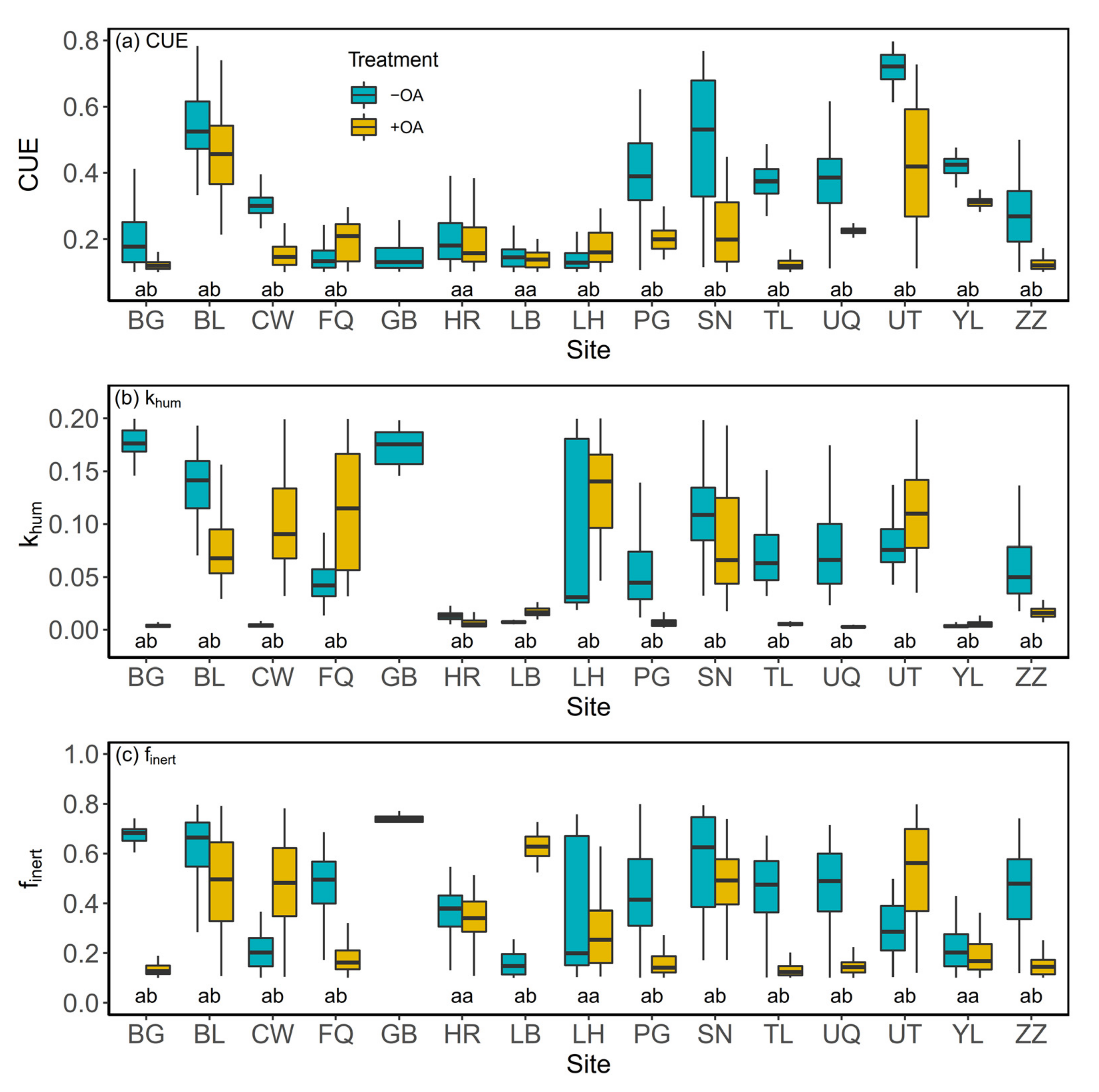
3.2. Microbial Carbon Use efficiency (CUE)
3.3. Decomposition Rate of Humic Organic Matter (khum)
3.4. The Fraction of Inert Organic Matter (finert)
3.5. Long-Term SOC Dynamics under + OA
4. Discussion
4.1. Effects of OA on CUE
4.2. Effects of OA on khum
4.3. Effects of OA on finert
4.4. Implications for Management Practices
4.5. Limitations and Uncertainties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.-S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Rumpel, C.; Amiraslani, F.; Chenu, C.; Garcia Cardenas, M.; Kaonga, M.; Koutika, L.S.; Ladha, J.; Madari, B.; Shirato, Y.; Smith, P.; et al. The 4p1000 initiative: Opportunities, limitations and challenges for implementing soil organic carbon sequestration as a sustainable development strategy. Ambio 2020, 49, 350–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Chang. Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef]
- Lu, F.; Wang, X.K.; Han, B.; Ouyang, Z.Y.; Duan, X.N.; Zheng, H.; Miao, H. Soil carbon sequestrations by nitrogen fertilizer application, straw return and no-tillage in China’s cropland. Glob. Chang. Biol. 2009, 15, 281–305. [Google Scholar] [CrossRef]
- Wang, G.; Luo, Z.; Wang, E.; Zhang, W. Reducing greenhouse gas emissions while maintaining yield in the croplands of Huang-Huai-Hai Plain, China. Agric. For. Meteorol. 2018, 260–261, 80–94. [Google Scholar] [CrossRef]
- Manzoni, S.; Čapek, P.; Porada, P.; Thurner, M.; Winterdahl, M.; Beer, C.; Brüchert, V.; Frouz, J.; Herrmann, A.M.; Lindahl, B.D. Reviews and syntheses: Carbon use efficiency from organisms to ecosystems–definitions, theories, and empirical evidence. Biogeosciences 2018, 15, 5929–5949. [Google Scholar] [CrossRef] [Green Version]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Ågren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Devêvre, O.C.; Horwáth, W.R. Decomposition of rice straw and microbial carbon use efficiency under different soil temperatures and moistures. Soil Biol. Biochem. 2000, 32, 1773–1785. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, J.; Liang, G.; Du, Z.; Zhou, J.; Zhu, C.; Huang, K.; Zhou, X.; Luo, Y.; Yan, L.; et al. Global variation of soil microbial carbon-use efficiency in relation to growth temperature and substrate supply. Sci. Rep. 2019, 9, 5621. [Google Scholar] [CrossRef]
- Frey, S.D.; Lee, J.; Melillo, J.M.; Six, J. The temperature response of soil microbial efficiency and its feedback to climate. Nat. Clim. Chang. 2013, 3, 395. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, E.; Smith, C. Fresh carbon input differentially impacts soil carbon decomposition across natural and managed systems. Ecology 2015, 96, 2806–2813. [Google Scholar] [CrossRef]
- Luo, Z.; Baldock, J.; Wang, E. Modelling the dynamic physical protection of soil organic carbon: Insights into carbon predictions and explanation of the priming effect. Glob. Chang. Biol. 2017, 23, 5273–5283. [Google Scholar] [CrossRef] [PubMed]
- Rovira, P.; Vallejo, V.R. Labile, recalcitrant, and inert organic matter in Mediterranean forest soils. Soil Biol. Biochem. 2007, 39, 202–215. [Google Scholar] [CrossRef]
- Smith, P.; Soussana, J.-F.; Angers, D.; Schipper, L.; Chenu, C.; Rasse, D.P.; Batjes, N.H.; van Egmond, F.; McNeill, S.; Kuhnert, M.; et al. How to measure, report and verify soil carbon change to realize the potential of soil carbon sequestration for atmospheric greenhouse gas removal. Glob. Chang. Biol. 2020, 26, 219–241. [Google Scholar] [CrossRef] [Green Version]
- Jenkinson, D.S.; Andrew, S.P.S.; Lynch, J.M.; Goss, M.J.; Tinker, P.B. The turnover of organic carbon and nitrogen in soil. Philos. Trans. R. Soc. Lond. Ser. B-Biol. 1990, 329, 361–367. [Google Scholar]
- Keating, B.; Carberry, P.; Hammer, G.; Probert, M.; Robertson, M.; Holzworth, D.; Huth, N.; Hargreaves, J.; Meinke, H.; Hochman, Z. An overview of APSIM, a model designed for farming systems simulation. Eur. J. Agron. 2003, 18, 267–288. [Google Scholar] [CrossRef] [Green Version]
- Parton, W.J.; Scurlock, J.M.O.; Ojima, D.S.; Gilmanov, T.G.; Scholes, R.J.; Schimel, D.S.; Kirchner, T.; Menaut, J.C.; Seastedt, T.; Moya, E.G. Observations and modeling of biomass and soil organic matter dynamics for the grassland biome worldwide. Glob. Biogeochem. Cycles 1993, 7, 785–809. [Google Scholar] [CrossRef] [Green Version]
- Conant, R.T.; Ryan, M.G.; Ågren, G.I.; Birge, H.E.; Davidson, E.A.; Eliasson, P.E.; Evans, S.E.; Frey, S.D.; Giardina, C.P.; Hopkins, F.M. Temperature and soil organic matter decomposition rates–synthesis of current knowledge and a way forward. Glob. Chang. Biol. 2011, 17, 3392–3404. [Google Scholar] [CrossRef]
- Dungait, J.A.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Chang. Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- Coleman, K.; Jenkinson, D.; Crocker, G.; Grace, P.; Klir, J.; Korschens, M.; Poulton, P.; Richter, D. Simulating trends in soil organic carbon in long-term experiments using RothC-26.3. Geoderma 1997, 81, 29–44. [Google Scholar] [CrossRef]
- Plaza, C.; Gollany, H.T.; Baldoni, G.; Polo, A.; Ciavatta, C. Predicting long-term organic carbon dynamics in organically amended soils using the CQESTR model. J. Soils Sediments 2012, 12, 486–493. [Google Scholar] [CrossRef]
- Guo, S.; Wu, j.; Dang, T. Effects of crop rotation and fertilization on aboveground biomass and soil organic C in semi-arid region (in Chinese with English abstract). Sci. Agric. Sin. 2008, 41, 744–751. [Google Scholar]
- Xin, X.L.; Zhang, J.B.; Yin, S.W.; Zhu, A.N. Fertility evolution and fertilization technology of alluvial fluvo-aquic soils under long-term fertilization (in Chinese). In Evolution of Soil Fertility in China; Xu, M., Zhang, W., Huang, S., Eds.; China Agricultural Science and Technology Press: Beijing, China, 2015. [Google Scholar]
- Wang, G.; Huang, Y.; Wang, E.; Yu, Y.; Zhang, W. Modeling soil organic carbon change across Australian wheat growing areas, 1960–2010. PLoS ONE 2013, 8, e63324. [Google Scholar] [CrossRef]
- Hao, X.Y.; Zhou, B.K.; Ma, X.Z.; Gao, Z.C.; Zhang, X.L. Fertility evolution and fertilization technology of thick balck soils under long-term fertilization (in Chinese). In Evolution of Soil Fertility in China; Xu, M., Zhang, W., Huang, S., Eds.; China Agricultural Science and Technology Press: Beijing, China, 2015; pp. 67–100. [Google Scholar]
- Monreal, C.; Janzen, H. Soil organic-carbon dynamics after 80 years of cropping a Dark Brown Chernozem. Can. J. Soil Sci. 1993, 73, 133–136. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Pal, D.; Easter, M.; Williams, S.; Paustian, K.; Milne, E.; Chandran, P.; Ray, S.; Mandal, C.; Coleman, K. Evaluating the Century C model using long-term fertilizer trials in the Indo-Gangetic Plains, India. Agric. Ecosyst. Environ. 2007, 122, 73–83. [Google Scholar] [CrossRef]
- Whitmore, A.; Klein-Gunnewiek, H.; Crocker, G.; Klír, J.; Körschens, M.; Poulton, P. Simulating trends in soil organic carbon in long-term experiments using the Verbeme/MOTOR model. Geoderma 1997, 81, 137–151. [Google Scholar] [CrossRef]
- Fan, H.Z.; Feng, W.Q.; Qin, Y.S.; Chen, Q.; Tu, S.H.; Sun, X.F. Fertility evolution and fertilization technology of calcium purple soils under long-term fertilization (in Chinese). In Evolution of Soil Fertility in China; Xu, M., Zhang, W., Huang, S., Eds.; China Agricultural Science and Technology Press: Beijing, China, 2015. [Google Scholar]
- Karhu, K.; Gärdenäs, A.I.; Heikkinen, J.; Vanhala, P.; Tuomi, M.; Liski, J. Impacts of organic amendments on carbon stocks of an agricultural soil—comparison of model-simulations to measurements. Geoderma 2012, 189, 606–616. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.H.; Wang, B.; Lin, Y.H.; Zhang, H.M.; Xie, L.J. Fertility evolution and fertilization technical model of grey desert soils under long-term fertilization (in Chinese). In Evolution of Soil Fertility in China; Xu, M., Zhang, W., Huang, S., Eds.; China Agricultural Science and Technology Press: Beijing, China, 2015; pp. 563–614. [Google Scholar]
- Sun, B.H.; Yang, X.Y.; Zhang, S.L. Fertility evolution and fertilization technology of cinnamon soils under long-term fertilization (in Chinese). In Evolution of Soil Fertility in China; Xu, M., Zhang, W., Huang, S., Eds.; China Agricultural Science and Technology Press: Beijing, China, 2015; pp. 434–473. [Google Scholar]
- Zhang, W.J.; Wang, X.J.; Xu, M.G.; Huang, S.M.; Liu, H.; Peng, C. Soil organic carbon dynamics under long-term fertilizations in arable land of northern China. Biogeosciences 2010, 7, 409–425. [Google Scholar] [CrossRef] [Green Version]
- Jobbagy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, W.; Sun, W.J.; Zheng, X.H. Net primary production of Chinese croplands from 1950 to 1999. Ecol. Appl. 2007, 17, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Skjemstad, J.; Spouncer, L.; Cowie, B.; Swift, R. Calibration of the Rothamsted organic carbon turnover model (RothC ver. 26.3), using measurable soil organic carbon pools. Soil Res. 2004, 42, 79–88. [Google Scholar] [CrossRef]
- Tittonell, P.; Rufino, M.C.; Janssen, B.H.; Giller, K.E. Carbon and nutrient losses during manure storage under traditional and improved practices in smallholder crop-livestock systems—evidence from Kenya. Plant Soil 2010, 328, 253–269. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, T.; Pal, D.K.; Deshmukh, A.S.; Deshmukh, R.R.; Ray, S.K.; Chandran, P.; Mandal, C.; Telpande, B.; Nimje, A.M.; Tiwary, P. Evaluation of RothC model using four Long Term Fertilizer Experiments in black soils, India. Agric. Ecosyst. Environ. 2011, 144, 222–234. [Google Scholar] [CrossRef]
- Falloon, P.; Smith, P. Simulating SOC changes in long-term experiments with RothC and CENTURY: Model evaluation for a regional scale application. Soil Use Manag. 2002, 18, 101–111. [Google Scholar] [CrossRef]
- Smith, J.; Smith, P.; Wattenbach, M.; Zaehle, S.; Hiederer, R.; Jones, R.J.; Montanarella, L.; Rounsevell, M.D.; Reginster, I.; Ewert, F. Projected changes in mineral soil carbon of European croplands and grasslands, 1990–2080. Glob. Chang. Biol. 2005, 11, 2141–2152. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, E.; Zheng, H.; Baldock, J.A.; Sun, O.J.; Shao, Q. Convergent modelling of past soil organic carbon stocks but divergent projections. Biogeosciences 2015, 12, 4373–4383. [Google Scholar] [CrossRef] [Green Version]
- Todd-Brown, K.E.O.; Randerson, J.T.; Hopkins, F.; Arora, V.; Hajima, T.; Jones, C.; Shevliakova, E.; Tjiputra, J.; Volodin, E.; Wu, T.; et al. Changes in soil organic carbon storage predicted by Earth system models during the 21st century. Biogeosciences 2014, 11, 2341–2356. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, Z.R.J.; Meinshausen, M.; Lewis, J.; Gieseke, R.; Dommenget, D.; Dorheim, K.; Fan, C.S.; Fuglestvedt, J.S.; Gasser, T.; Golüke, U.; et al. Reduced Complexity Model Intercomparison Project Phase 1: Introduction and evaluation of global-mean temperature response. Geosci. Model Dev. 2020, 13, 5175–5190. [Google Scholar] [CrossRef]
- Canarini, A.; Wanek, W.; Watzka, M.; Sandén, T.; Spiegel, H.; Šantrůček, J.; Schnecker, J. Quantifying microbial growth and carbon use efficiency in dry soil environments via 18O water vapor equilibration. Glob. Chang. Biol. 2020, 26, 5333–5341. [Google Scholar] [CrossRef]
- Keiluweit, M.; Bougoure, J.J.; Nico, P.S.; Pett-Ridge, J.; Weber, P.K.; Kleber, M. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Chang. 2015, 5, 588–595. [Google Scholar] [CrossRef]
- Heikkinen, J.; Kurganova, I.; de Gerenyu, V.L.; Palosuo, T.; Regina, K. Changes in soil carbon stock after cropland conversion to grassland in Russian temperate zone: Measurements versus model simulation. Nutr. Cycl. Agroecosyst. 2014, 98, 97–106. [Google Scholar] [CrossRef]
- Sierra, C.A.; Müller, M.; Trumbore, S.E. Models of soil organic matter decomposition: The SoilR package, version 1.0. Geosci. Model Dev. 2012, 5, 1045–1060. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021.
- Luo, Z.; Wang, E.; Sun, O. Uncertain future soil carbon dynamics under global change predicted by models constrained by total carbon measurements. Ecol. Appl. 2017, 27, 1001–1009. [Google Scholar] [CrossRef]
- Blagodatskaya, Е.; Kuzyakov, Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: Critical review. Biol. Fertil. Soils 2008, 45, 115–131. [Google Scholar] [CrossRef]
- Cagnarini, C.; Renella, G.; Mayer, J.; Hirte, J.; Schulin, R.; Costerousse, B.; Della Marta, A.; Orlandini, S.; Menichetti, L. Multi-objective calibration of RothC using measured carbon stocks and auxiliary data of a long-term experiment in Switzerland. Eur. J. Soil Sci. 2019, 70, 819–832. [Google Scholar] [CrossRef]
- Spohn, M.; Pötsch, E.M.; Eichorst, S.A.; Woebken, D.; Wanek, W.; Richter, A. Soil microbial carbon use efficiency and biomass turnover in a long-term fertilization experiment in a temperate grassland. Soil Biol. Biochem. 2016, 97, 168–175. [Google Scholar] [CrossRef]
- Sanderman, J.; Baisden, W.T.; Fallon, S. Redefining the inert organic carbon pool. Soil Biol. Biochem. 2016, 92, 149–152. [Google Scholar] [CrossRef]
- Keel, S.G.; Anken, T.; Büchi, L.; Chervet, A.; Fliessbach, A.; Flisch, R.; Huguenin-Elie, O.; Mäder, P.; Mayer, J.; Sinaj, S.; et al. Loss of soil organic carbon in Swiss long-term agricultural experiments over a wide range of management practices. Agric. Ecosyst. Environ. 2019, 286, 106654. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Huang, Y.; Zhang, W.; Yu, Y.; Sun, W. Quantifying carbon input for targeted soil organic carbon sequestration in China’s croplands. Plant Soil 2015, 394, 57–71. [Google Scholar] [CrossRef]
- Wang, G.; Luo, Z.; Han, P.; Chen, H.; Xu, J. Critical carbon input to maintain current soil organic carbon stocks in global wheat systems. Sci. Rep. 2016, 6, 19327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyano, F.E.; Vasilyeva, N.; Menichetti, L. Diffusion limitations and Michaelis–Menten kinetics as drivers of combined temperature and moisture effects on carbon fluxes of mineral soils. Biogeosciences 2018, 15, 5031–5045. [Google Scholar] [CrossRef] [Green Version]
- Manzoni, S.; Moyano, F.; Kätterer, T.; Schimel, J. Modeling coupled enzymatic and solute transport controls on decomposition in drying soils. Soil Biol. Biochem. 2016, 95, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Marschmann, G.L.; Pagel, H.; Kügler, P.; Streck, T. Equifinality, sloppiness, and emergent structures of mechanistic soil biogeochemical models. Environ. Model. Softw. 2019, 122, 104518. [Google Scholar] [CrossRef]
- Sulman, B.N.; Moore, J.A.M.; Abramoff, R.; Averill, C.; Kivlin, S.; Georgiou, K.; Sridhar, B.; Hartman, M.D.; Wang, G.; Wieder, W.R.; et al. Multiple models and experiments underscore large uncertainty in soil carbon dynamics. Biogeochemistry 2018, 141, 109–123. [Google Scholar] [CrossRef] [Green Version]
- Sierra, C.A.; Malghani, S.; Müller, M. Model structure and parameter identification of soil organic matter models. Soil Biol. Biochem. 2015, 90, 197–203. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
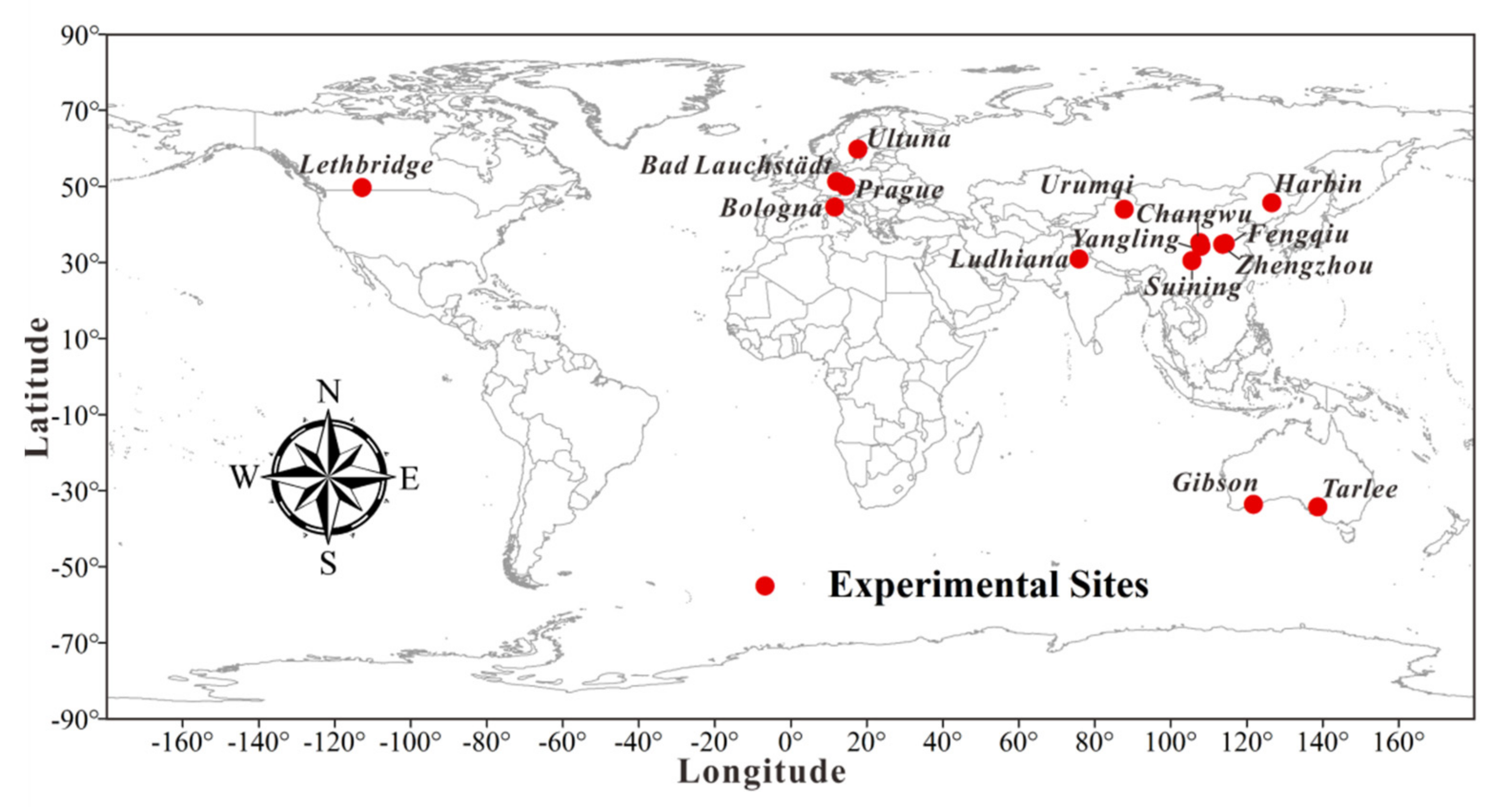



| Site | Initial Soil Properties | Climatic Attributes | Time Span | Carbon Input | Source | ||||
|---|---|---|---|---|---|---|---|---|---|
| SOC0 | pH | Clay | MAT | MAP | (Mg C ha−1 year−1) | ||||
| (Mg ha−1) | (%) | (°C) | (mm) | −OA | +OA | ||||
| Bad Lauchstädt (BL) | 65.5 | 7.0 | 21 | 9.0 | 458 | 1906–1990 | 2.9 | 4.0 | [21] |
| Bologna (BG) | 23.7 | 6.9 | 28 | 13.7 | 752 | 1966–2001 | 0.8 | 1.7 | [22] |
| Changwu (CW) | 22.0 | 8.4 | 24 | 11.4 | 589 | 1984–2002 | 0.6 | 5.8 | [23] |
| Fengqiu (FQ) | 17.7 | 8.7 | 9 | 13.5 | 650 | 1990–2003 | 0.2 | 6.5 | [24] |
| Gibson (GB) | 33.1 | 5.6 | 3 | 16.6 | 467 | 1977–1994 | 0.5 | - | [25] |
| Harbin (HR) | 51.3 | 7.2 | 25 | 3.5 | 533 | 1979–2002 | 0.8 | 1.5 | [26] |
| Lethbridge (LB) | 111.0 | 7.0 | 10 | 5.3 | 362 | 1910–1990 | 0.9 | 1.0 | [27] |
| Ludhiana (LH) | 18.7 | 7.6 | 13 | 24.5 | 695 | 1988–1999 | 1.6 | 6.4 | [28] |
| Prague (PG) | 43.2 | 6.2 | 27 | 8.7 | 477 | 1972–1992 | 1.4 | 2.4 | [29] |
| Suining (SN) | 31.6 | 8.6 | 24 | 17.4 | 1014 | 1981–1998 | 1.0 | 4.0 | [30] |
| Tarlee (TL) | 39.9 | 8.5 | 14 | 16.9 | 464 | 1979–1996 | 0.6 | 0.7 | [25] |
| Ultuna (UT) | 55.8 | 6.6 | 37 | 5.6 | 519 | 1956–1991 | 0.5 | 2.5 | [31] |
| Urumqi (UQ) | 31.2 | 8.1 | 21 | 7.7 | 310 | 1990–2005 | 0.4 | 4.4 | [32] |
| Yangling (YL) | 21.4 | 8.6 | 17 | 13.0 | 575 | 1989–2003 | 0.8 | 2.7 | [33] |
| Zhengzhou (ZZ) | 21.3 | 8.3 | 13 | 14.3 | 632 | 1990–2005 | 0.5 | 3.6 | [34] |
| Parameters | Source of Variation | SS | df | MS | F |
|---|---|---|---|---|---|
| CUE | Sites (S) | 46.90(56%) | 14 | 3.350 | 518.0 *** |
| Treatments (T) | 14.41(17%) | 7 | 2.059 | 319.0 *** | |
| S × T | 3.82(5%) | 7 | 0.546 | 84.7 *** | |
| Residuals | 18.53 | 2871 | 0.006 | ||
| khum | Sites (S) | 4.466(41%) | 14 | 0.3190 | 312.4 *** |
| Treatments (T) | 1.319(12%) | 7 | 0.1885 | 184.6 *** | |
| S × T | 2.102(27%) | 7 | 0.3003 | 294.2 *** | |
| Residuals | 2.931 | 2871 | 0.0010 | ||
| finert | Sites (S) | 28.03(22%) | 14 | 2.002 | 118.9 *** |
| Treatments (T) | 28.27(22%) | 7 | 4.038 | 239.8 *** | |
| S × T | 25.90(20%) | 7 | 3.700 | 219.7 *** | |
| Residuals | 48.34 | 2871 | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Luo, Z. Organic Amendments Alter Long-Term Turnover and Stability of Soil Carbon: Perspectives from a Data-Model Integration. Agronomy 2021, 11, 2134. https://doi.org/10.3390/agronomy11112134
Wang G, Luo Z. Organic Amendments Alter Long-Term Turnover and Stability of Soil Carbon: Perspectives from a Data-Model Integration. Agronomy. 2021; 11(11):2134. https://doi.org/10.3390/agronomy11112134
Chicago/Turabian StyleWang, Guocheng, and Zhongkui Luo. 2021. "Organic Amendments Alter Long-Term Turnover and Stability of Soil Carbon: Perspectives from a Data-Model Integration" Agronomy 11, no. 11: 2134. https://doi.org/10.3390/agronomy11112134
APA StyleWang, G., & Luo, Z. (2021). Organic Amendments Alter Long-Term Turnover and Stability of Soil Carbon: Perspectives from a Data-Model Integration. Agronomy, 11(11), 2134. https://doi.org/10.3390/agronomy11112134







