Genetic Diversity and Primary Core Collection Construction of Turnip (Brassica rapa L. ssp. rapifera Matzg) Landraces in Tibet Revealed via Morphological and SSR Markers
Abstract
1. Introduction
2. Materials and Methods
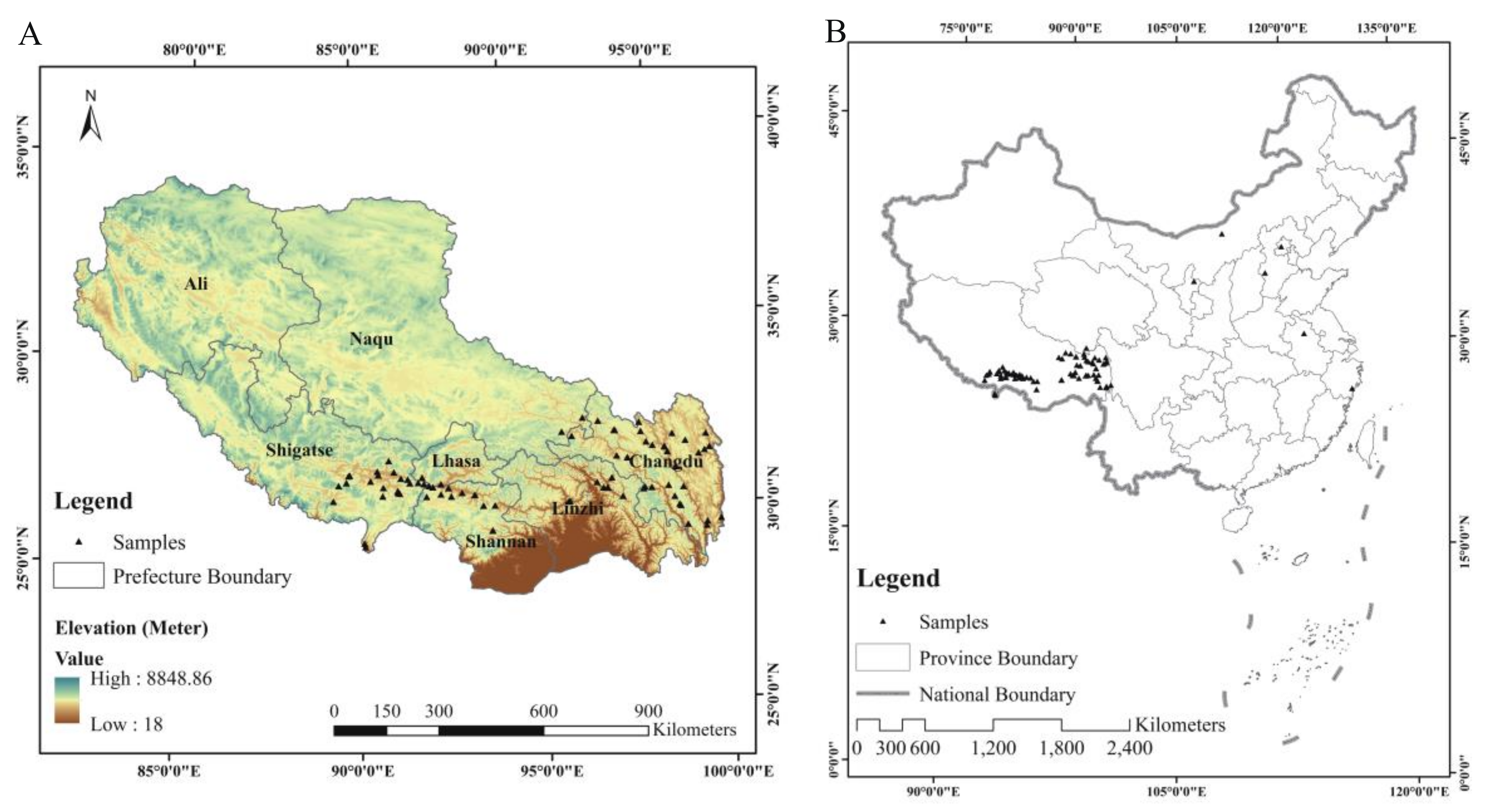
2.1. Plant Materials and Experimental Design
2.2. Investigation and Statistics of Morphological Characteristics
2.3. DNA Extraction and SSR Analysis
2.4. Data Analysis
2.5. Construction of Primary Core Collection in Tibetan Turnip
3. Results
3.1. Analysis of Morphological Characteristics
3.2. Genetic Variation of SSR Markers
3.3. Genetic Similarity Coefficient Analysis and UPGMA Cluster Analysis Based on SSR Markers
3.4. Relationship between Morphological Traits and SSR Markers
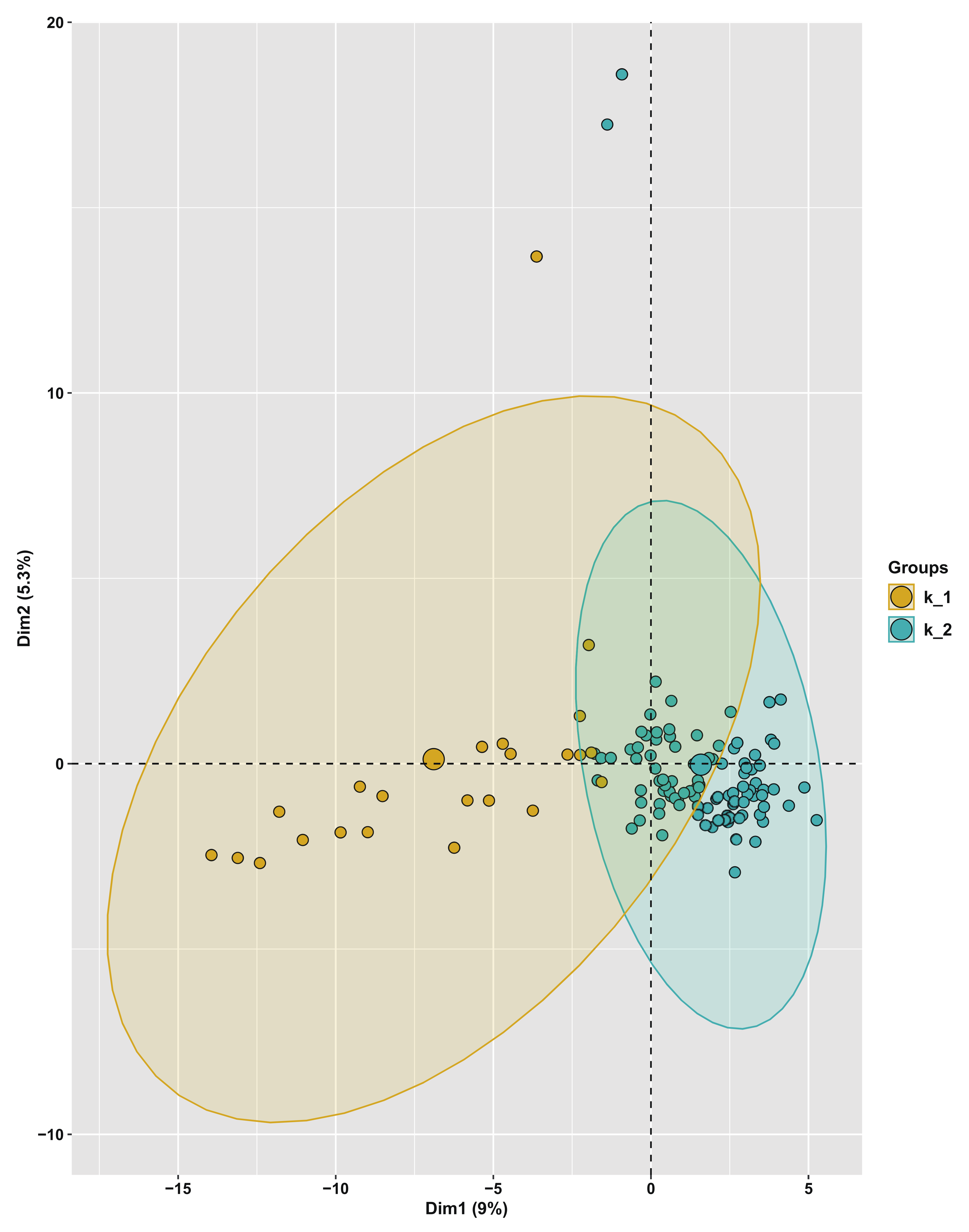
3.5. Population Structure and Principal Component Analysis of the Turnip Germplasm
3.6. Construction and Evaluation of the Primary Core Collection in Tibetan Turnip
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Li, N.; Xuan, Z.; Yang, W. Genetic diversity among "Qamgur" varieties in China revealed by SSR markers. Euphytica 2017, 213, 204. [Google Scholar] [CrossRef]
- Zhao, J.J.; Wang, X.W.; Deng, B.; Lou, P.; Wu, J.; Sun, R.F.; Xu, Z.Y.; Vromans, J.; Koornneef, M.; Bonnema, G. Genetic relationships within Brassica rapa as inferred from AFLP fingerprints. Theor. Appl. Genet. 2005, 110, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Committee of Chinese Herbal Medicine. Chinese Herbal Medicine-Tibetan Medicine; Science&Technology Press of Shanghai: Shanghai, China, 2002; pp. 349–350. [Google Scholar]
- Paul, S.; Geng, C.A.; Yang, T.H.; Yang, Y.P.; Chen, J.J. Phytochemical and health-beneficial progress of turnip (Brassica rapa). J. Food Sci. 2019, 84, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gong, W.; Li, R.; Zhang, L.; Zhang, Y.; Gao, Y.; Lang, J.; Zhao, K.; Liu, K.; Yu, X. Genetic diversity analysis of Tibetan turnip(Brassica rapa L. ssp. rapifera Matzg) revealed by morphological, physiological, and molecular marker. Genet. Resour. Crop. Evol. 2020, 67, 209–223. [Google Scholar] [CrossRef]
- The Investigation Team of the Crop Variety Resources in Tibet. A Collection of Studies on Crop Variety Resources in Tibet; China Agricultural Science and Technology Press: Beijing, China, 1987; p. 146. [Google Scholar]
- Wang, J.; Dan, B.; Hu, S.; Tu, J.; Luan, Y.; Xia, M.; Ga, Z.; Nima, Z.; Tang, L. RAPD analysis for the genetic diversity of Brassica rapa in Tibet. Acta Genet. Sin. 2002, 29, 1021–1027. [Google Scholar] [CrossRef]
- Choudhary, M.; Ghasal, P.C.; Kumar, S.; Yadav, R.P.; Singh, S.; Meena, V.S.; Bisht, J.K. Conservation Agriculture and Climate Change: An Overview. In Conservation Agriculture: An Approach to Combat Climate Change in Indian Himalaya; Bisht, J.K., Meena, V.S., Mishra, P.K., Pattanayak, A., Eds.; Springer: Singapore, 2016; pp. 1–37. [Google Scholar]
- Cao, J.; Qin, L. Germplasm of Horticultural Plants; China Agriculture Press: Beijing, China, 2005; pp. 118–125. [Google Scholar]
- Wang, J. Genetic Resources of Rapeseed in Tibet; Science Press of China: Beijing, China, 2009; pp. 1–215. [Google Scholar]
- Wang, J.; Luan, Y.; Daci, Z.; Chang, T. Geographical distribution and biological characters of wild rapeseed in Tibet. Chin. J. Oil Crop. Sci. 2006, 28, 134–137. [Google Scholar]
- Yao, T.D.; Thompson, L.; Yang, W.; Yu, W.S.; Gao, Y.; Guo, X.J.; Yang, X.X.; Duan, K.Q.; Zhao, H.B.; Xu, B.Q.; et al. Different glacier status with atmospheric circulations in Tibetan Plateau and surroundings. Nat. Clim. Chang. 2012, 2, 663–667. [Google Scholar] [CrossRef]
- Wang, X.F.; Li, Y.H.; Chu, B.Y.; Liu, S.R.; Yang, D.; Luan, J.W. Spatiotemporal dynamics and drivingforces of ecosystem changes: A case study of the National Barrier Zone, China. Sustainability 2020, 12, 6680. [Google Scholar] [CrossRef]
- Shang, Z.; Gibb, M.J.; Leiber, F.; Ismail, M.; Ding, L.; Guo, X.; Long, R. The sustainable development of grassland-livestock systems on the Tibetan plateau: Problems, strategies and prospects. Rangel. J. 2014, 36, 267–296. [Google Scholar] [CrossRef]
- Ramanatha Rao, V.; Hodgkin, T. Genetic diversity and conservation and utilization of plant genetic resources. Plant. Cell Tissue Organ. Cult. 2002, 68, 1–19. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, S.; Li, Z.; Cowling, W.A. Center of origin and centers of diversity in an ancient crop, Brassica rapa (turnip rape). J. Hered. 2014, 105, 555–565. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Mezghani, N.; Mnasri, S.; Mezghani, N.; Garcés-Claver, A. Assessing the genetic diversity and population structure of a tunisian melon (Cucumis melo L.) collection using phenotypic traits and SSR molecular markers. Agronomy 2021, 11, 1121. [Google Scholar] [CrossRef]
- Barabaschi, D.; Tondelli, A.; Desiderio, F.; Volante, A.; Vaccino, P.; Valè, G.; Cattivelli, L. Next generation breeding. Plant Sci. 2016, 242, 3–13. [Google Scholar] [CrossRef]
- McGrath, J.M.; Quiros, C.F. Genetic diversity at isozyme and RFLP loci in Brassica campestris as related to crop type and geographical origin. Theor. Appl. Genet. 1992, 83, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, L.D. Genetics and Morphological Evolution in Plants. Am. Nat. 1984, 123, 681–709. [Google Scholar] [CrossRef]
- Soengas, P.; Cartea, M.E.; Francisco, M.; Lema, M.; Velasco, P. Genetic structure and diversity of a collection of Brassica rapa subsp. rapa L. revealed by simple sequence repeat markers. J. Agric. Sci. 2011, 149, 617–624. [Google Scholar] [CrossRef]
- Zhang, N.W.; Zhao, J.J.; Lens, F.; de Visser, J.; Menamo, T.; Fang, W.; Xiao, D.; Bucher, J.; Basnet, R.K.; Lin, K.; et al. Morphology, carbohydrate composition and vernalization response in a genetically diverse collection of Asian and European turnips (Brassica rapa subsp rapa). PLoS ONE 2014, 9, e114241. [Google Scholar] [CrossRef]
- Cheng, F.; Wu, J.; Cai, C.C.; Fu, L.X.; Liang, J.L.; Borm, T.; Zhuang, M.; Zhang, Y.Y.; Zhang, F.L.; Bonnema, G.; et al. Genome resequencing and comparative variome analysis in a Brassica rapa and Brassica oleracea collection. Sci. Data 2016, 3, 160119. [Google Scholar] [CrossRef] [PubMed]
- Ammar, I.B.; Sonnante, G.; Dridi, B.A. Genetic variability in wild cardoon (Cynara cardunculus L. var. sylvestris) revealed by SSR markers and morphological traits. Sci. Hortic. 2015, 185, 76–81. [Google Scholar] [CrossRef]
- Meng, F.J.; Liu, L.; Peng, M.; Wang, Z.K.; Wang, C.; Zhao, Y.Y. Genetic diversity and population structure analysis in wild strawberry (Fragaria nubicola L.) from Motuo in Tibet Plateau based on simple sequence repeats (SSRs). Biochem. Syst. Ecol. 2015, 63, 113–118. [Google Scholar] [CrossRef]
- Li, X.; Shen, D. Descriptors and Data Standard for Root and Stem Mustard (Brassica Juncea Coss.); Chinese Agricultural Press: Beijing, China, 2008; pp. 1–82. [Google Scholar]
- Wang, S.; Cao, Y.; Redden, R.; Hu, J.; Usher, T. The morphological diversity and classification of adzuki bean [Vigna angularis (Willd.) Ohwi & Ohashi] germplasm resources in China. Acta Agron. Sin. 2002, 28, 727–733. [Google Scholar]
- Kabelka, E.; Franchino, B.; Francis, D.M. Two loci from Lycopersicon hirsutum LA407 confer resistance to strains of Clavibacter michiganensis subsp. michiganensis. Phytopathology 2002, 92, 504–510. [Google Scholar] [CrossRef]
- Wang, F. Study of resistant inheritance and SSR molecular marker for clubroot in turnip. Master’s Thesis, Zhejiang University, Hangzhou, China, 2012. [Google Scholar]
- Wang, X.; Wang, H.; Wang, J.; Sun, R.; Wu, J.; Liu, S.; Bai, Y.; Mun, J.-H.; Bancroft, I.; Cheng, F.; et al. The Brassica rapa Genome Sequencing Project, C. The genome of the mesopolyploid crop species Brassica rapa. Nat. Genet. 2011, 43, 1035–1039. [Google Scholar] [CrossRef]
- Byun, S.O.; Fang, Q.; Zhou, H.; Hickford, J.G. An effective method for silver-staining DNA in large numbers of polyacrylamide gels. Anal. Biochem. 2009, 385, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Muse, S. PowerMarker: New genetic data analysis software. Version 3.23. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chu, Y.G.; Ding, C.J.; Su, X.H.; Huang, Q.J. Genetic diversity and population structure of black cottonwood (Populus deltoides) revealed using simple sequence repeat markers. BMC Genet. 2020, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Brbaklić, L.; Trkulja, D.; Mikić, S.; Mirosavljević, M.; Momčilović, V.; Dudić, B.; Procházková, L.; Aćin, V. Genetic diversity and population structure of serbian barley (Hordeum vulgare L.) collection during a 40-year long breeding period. Agronomy 2021, 11, 118. [Google Scholar] [CrossRef]
- Earl, D.A.; Vonholdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Jakobsson, M.; Rosenberg, N.A. CLUMPP: A cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 2007, 23, 1801–1806. [Google Scholar] [CrossRef]
- Xu, C.; Gao, J.; Du, Z.; Li, D.; Wang, Z.; Li, Y.; Pang, X. Identifying the genetic diversity, genetic structure and a core collection of Ziziphus jujuba Mill. var. jujuba accessions using microsatellite markers. Sci. Rep. 2016, 6, 31503. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Hu, J.; Zhu, J.; Xu, H.M. Methods of constructing core collections by stepwise clustering with three sampling strategies based on the genotypic values of crops. Theor. Appl. Genet. 2000, 101, 264–268. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, Z.; Cao, X.; Jiang, F.L. Genetic diversity of cultivated and wild tomato revealed by morphological traits and SSR markers. Genet. Mol. Res. 2015, 14, 13868–13879. [Google Scholar] [CrossRef] [PubMed]
- Keneni, G.; Bekele, E.; Imtiaz, M.; Dagne, K.; Getu, E.; Assefa, F. Genetic diversity and population structure of ethiopian chickpea (Cicer arietinum L.) germplasm accessions from different geographical origins as revealed by microsatellite markers. Plant. Mol. Biol. Rep. 2012, 30, 654–665. [Google Scholar] [CrossRef]
- Nkhoma, N.; Shimelis, H.; Laing, M.D.; Shayanowako, A.; Mathew, I. Assessing the genetic diversity of cowpea (Vignaun guiculata L. Walp.) germplasm collections using phenotypic traits and SNP markers. BMC Genet. 2020, 21, 110. [Google Scholar] [CrossRef]
- Song, K.; Osborn, T.C.; Williams, P.H. Brassica taxonomy based on nuclear restriction fragment length polymorphisms (RFLPs) 3. Genome relationships in Brassica and related genera and the origin of B. oleracea and B. rapa (syn. Campestris). Theor. Appl. Genet. 1990, 79, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Persson, K.; Fält, A.S.; Bothmer, R. Genetic diversity of allozymes in Turnip (Brassica rapa L. var. rapa) from the Nordic Area. Hereditas 2001, 134, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Wang, G.; Sun, X.; Yang, Y. Variations in genome size of turnip landraces from two high-altitude environments. J. Am. Soc. Hort. Sci 2018, 143, 136–143. [Google Scholar] [CrossRef]
- Cheng, F.; Sun, R.F.; Hou, X.L.; Zheng, H.K.; Zhang, F.L.; Zhang, Y.Y.; Liu, B.; Liang, J.L.; Zhuang, M.; Liu, Y.X.; et al. Subgenome parallel selection is associated with morphotype diversification and convergent crop domestication in Brassica rapa and Brassica oleracea. Nat. Genet. 2016, 48, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- McAlvay, A.C.; Ragsdale, A.P.; Mabry, M.E.; Qi, X.; Bird, K.A.; Velasco, P.; An, H.; Pires, J.C.; Emshwiller, E. Brassica rapa domestication: Untangling wild and feral forms and convergence of crop morphotypes. Mol. Biol. Evol. 2021, 38, 3358–3372. [Google Scholar] [CrossRef]
- Bird, K.A.; An, H.; Gazave, E.; Gore, M.A.; Pires, J.C.; Robertson, L.D.; Labate, J.A. Population structure and phylogenetic relationships in a diverse panel of Brassica rapa L. Front. Plant. Sci. 2017, 8, 321. [Google Scholar] [CrossRef]
- Takahashi, H.; Kimura, M.; Suge, H.; Saito, T. Interactions between vernalization and photoperiod on the flowering and bolting of different turnip varieties. J. Jpn. Soc. Hortic. Sci. 1994, 63, 99–108. [Google Scholar] [CrossRef][Green Version]
- Vogl-Lukasser, B.; Vogl, C.R.; Reiner, H. The turnip (Brassica rapa L. subsp. rapa ) in Eastern Tyrol (Lienz district; Austria). Ethnobot. Res. Appl. 2007, 5, 305–317. [Google Scholar] [CrossRef][Green Version]
- Reiner, H.; Holzner, W.; Ebermann, R. The development of turnip-type and oilseed-type Brassica rapa crops from the wild type in Europe. An overview of botanical, historical and linguistic facts. In Proceedings of the Rapeseed today and tomorrow, 9th International Rapeseed Congress, Cambridge, UK, 4–7 July 1995; pp. 1066–1069. [Google Scholar]
- Zhao, J. Genetic dissection of important agronomic traits in Brassica rapa. Ph.D. Thesis, Chinese Academy of Agricultural Science, Beijing, China, 2005. [Google Scholar]
- Tan, C. The origin, distribution and evolution of the cultivars of Chinese head cabbage (B. compestris ssp. pekinensis). Sci. Agric. Sin. 1979, 12, 68–75. [Google Scholar]
- Takahashi, Y.; Yokoi, S.; Takahata, Y. Genetic divergence of turnip (Brassica rapa L. em. Metzg. subsp. rapa) inferred from simple sequence repeats in chloroplast and nuclear genomes and morphology. Genet. Resour. Crop. Evol. 2016, 63, 869–879. [Google Scholar] [CrossRef]
- Yang, X.; Su, W.; Wang, L.; Lei, J.; Chai, S.; Liu, Q. Molecular diversity and genetic structure of 380 sweetpotato accessions as revealed by SSR markers. J. Integr. Agric. 2015, 14, 633–641. [Google Scholar] [CrossRef]
- Pradhan, A.; Nelson, M.N.; Plummer, J.A.; Cowling, W.A.; Yan, G.J. Characterization of Brassica nigra collections using simple sequence repeat markers reveals distinct groups associated with geographical location, and frequent mislabelling of species identity. Genome 2011, 54, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Yelamo, M.D.; Zumel, L.I. Correlation between the diversity of Brassica montana (Brassicaceae) populations and its geographic distribution in the NE Iberian Peninsula evaluated from enzymatic profiles of samples as a whole. J. Biol. Res. Thessalon 2013, 20, 243–258. [Google Scholar]
- Zamani-Nour, S.; Clemens, R.; Mollers, C. Cytoplasmic diversity of Brassica napus L., Brassica oleracea L. and Brassica rapa L. as determined by chloroplast microsatellite markers. Genet. Resour. Crop. Evol. 2013, 60, 953–965. [Google Scholar] [CrossRef][Green Version]
- Del Carpio, D.P.; Basnet, R.K.; De Vos, R.C.H.; Maliepaard, C.; Visser, R.; Bonnema, G. The patterns of population differentiation in a Brassica rapa core collection. Theor. Appl. Genet. 2011, 122, 1105–1118. [Google Scholar] [CrossRef]
- Zhao, J.; Paulo, M.J.; Jamar, D.; Lou, P.; van Eeuwijk, F.; Bonnema, G.; Vreugdenhil, D.; Koornneef, M. Association mapping of leaf traits, flowering time, and phytate content in Brassica rapa. Genome 2007, 50, 963–973. [Google Scholar] [CrossRef]
- Zhao, J.J.; Artemyeva, A.; Del Carpio, D.P.; Basnet, R.K.; Zhang, N.W.; Gao, J.; Li, F.; Bucher, J.; Wang, X.W.; Visser, R.G.F.; et al. Design of a Brassica rapa core collection for association mapping studies. Genome 2010, 53, 884–898. [Google Scholar] [CrossRef]
- Tanhuanpää, P.; Erkkila, M.; Tenhola-Roininen, T.; Tanskanen, J.; Manninen, O. SNP diversity within and among Brassica rapa accessions reveals no geographic differentiation. Genome 2016, 59, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Zeuli, P.L.S.; Qualset, C.O. Geographical diversity for quantitative spike characters in a world collection of durum wheat. Crop. Sci. 1987, 27, 235–241. [Google Scholar] [CrossRef]
- Miao, L.; Wang, S.; Zou, M.; Li, J.; Kong, L.; Yu, X. Review of the studies on core collection for horticultuala crops. J. Plant. Genet. Resour. 2016, 17, 791–800. [Google Scholar] [CrossRef]
- Brown, A.H.D. Core colection: A practical approach to genetic resources management. Genome 1989, 31, 818–824. [Google Scholar] [CrossRef]
- Li, G.; Li, X.; Shen, D.; Wang, H.; Song, J.; Qiu, Y. Studies on the methods of constructing Chinese cabbage core germplasm based on the morphological data. Acta Hortic. Sin. 2008, 35, 1759–1766. [Google Scholar] [CrossRef]

| The Whole Collection | Collection from Tibet | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Marker | Sample Size | Major. Allele. Frquency | No. Allele (Na) | Gene Diversity | PIC | Band Freq. | Ne | I | He | uHe | Sample Size | Major. Allele. Frquency | No. Allele (Na) | Gene Diversity | PIC | Band Freq. | Ne | I | He | uHe |
| 1 | SSR-00001 | 171 | 0.211 | 9 | 0.854 | 0.837 | 0.472 | 1.561 | 0.521 | 0.344 | 0.345 | 118 | 0.102 | 33 | 0.949 | 0.947 | 0.515 | 1.709 | 0.580 | 0.398 | 0.401 |
| 2 | SSR-00005 | 171 | 0.620 | 5 | 0.573 | 0.539 | 0.642 | 1.763 | 0.567 | 0.398 | 0.399 | 118 | 0.678 | 5 | 0.505 | 0.472 | 0.641 | 1.414 | 0.440 | 0.277 | 0.280 |
| 3 | SSR-00033 | 171 | 0.357 | 6 | 0.735 | 0.689 | 0.338 | 1.344 | 0.364 | 0.225 | 0.226 | 118 | 0.297 | 11 | 0.806 | 0.780 | 0.345 | 1.445 | 0.428 | 0.273 | 0.276 |
| 4 | SSR-00160 | 171 | 0.538 | 4 | 0.632 | 0.583 | 0.350 | 1.408 | 0.425 | 0.265 | 0.266 | 118 | 0.339 | 9 | 0.798 | 0.772 | 0.364 | 1.529 | 0.513 | 0.334 | 0.337 |
| 5 | SSR-00201 | 171 | 0.491 | 6 | 0.645 | 0.586 | 0.338 | 1.466 | 0.406 | 0.271 | 0.272 | 118 | 0.339 | 12 | 0.759 | 0.724 | 0.360 | 1.569 | 0.458 | 0.312 | 0.315 |
| 6 | SSR-00207 | 171 | 0.409 | 6 | 0.725 | 0.682 | 0.401 | 1.496 | 0.467 | 0.300 | 0.301 | 118 | 0.407 | 10 | 0.776 | 0.754 | 0.422 | 1.512 | 0.514 | 0.333 | 0.336 |
| 7 | SSR-06840 | 171 | 0.637 | 3 | 0.508 | 0.438 | 0.599 | 1.519 | 0.512 | 0.333 | 0.334 | 118 | 0.636 | 3 | 0.503 | 0.428 | 0.614 | 1.430 | 0.428 | 0.272 | 0.274 |
| 8 | SSR-00074 | 171 | 0.801 | 5 | 0.338 | 0.312 | 0.443 | 1.411 | 0.341 | 0.229 | 0.230 | 118 | 0.822 | 4 | 0.305 | 0.278 | 0.447 | 1.120 | 0.158 | 0.091 | 0.091 |
| 9 | SSR-00089 | 171 | 0.298 | 7 | 0.776 | 0.741 | 0.373 | 1.509 | 0.467 | 0.306 | 0.307 | 118 | 0.195 | 23 | 0.895 | 0.887 | 0.397 | 1.714 | 0.570 | 0.392 | 0.395 |
| 10 | SSR-23195 | 171 | 0.901 | 3 | 0.182 | 0.171 | 0.312 | 1.236 | 0.218 | 0.142 | 0.142 | 118 | 0.958 | 2 | 0.081 | 0.078 | 0.319 | 1.029 | 0.058 | 0.027 | 0.027 |
| 11 | SSR-00106 | 171 | 0.731 | 5 | 0.410 | 0.351 | 0.167 | 1.217 | 0.183 | 0.116 | 0.117 | 118 | 0.771 | 5 | 0.367 | 0.321 | 0.175 | 1.119 | 0.162 | 0.089 | 0.090 |
| 12 | SSR-19222 | 171 | 0.462 | 5 | 0.628 | 0.555 | 0.286 | 1.367 | 0.361 | 0.227 | 0.228 | 118 | 0.390 | 8 | 0.687 | 0.634 | 0.329 | 1.497 | 0.450 | 0.293 | 0.296 |
| 13 | SSR-25246 | 171 | 0.503 | 6 | 0.671 | 0.630 | 0.317 | 1.437 | 0.381 | 0.248 | 0.248 | 118 | 0.551 | 15 | 0.659 | 0.636 | 0.345 | 1.417 | 0.422 | 0.266 | 0.268 |
| 14 | SSR-24347 | 171 | 0.398 | 4 | 0.697 | 0.641 | 0.198 | 1.261 | 0.285 | 0.173 | 0.174 | 118 | 0.280 | 8 | 0.793 | 0.763 | 0.242 | 1.469 | 0.409 | 0.265 | 0.267 |
| 15 | SSR-28237 | 171 | 0.509 | 4 | 0.633 | 0.571 | 0.173 | 1.199 | 0.229 | 0.131 | 0.131 | 118 | 0.492 | 7 | 0.685 | 0.647 | 0.199 | 1.241 | 0.309 | 0.180 | 0.181 |
| 16 | SSR-39105 | 171 | 0.515 | 4 | 0.559 | 0.464 | 0.112 | 1.147 | 0.170 | 0.099 | 0.099 | 118 | 0.449 | 5 | 0.607 | 0.528 | 0.127 | 1.250 | 0.228 | 0.144 | 0.146 |
| 17 | SSR-39694 | 171 | 0.333 | 7 | 0.780 | 0.748 | 0.281 | 1.374 | 0.388 | 0.242 | 0.243 | 118 | 0.271 | 20 | 0.856 | 0.842 | 0.312 | 1.593 | 0.513 | 0.341 | 0.344 |
| 18 | SSR-16982 | 171 | 0.667 | 4 | 0.500 | 0.450 | 0.336 | 1.447 | 0.374 | 0.244 | 0.245 | 118 | 0.746 | 4 | 0.406 | 0.364 | 0.353 | 1.263 | 0.332 | 0.194 | 0.195 |
| 19 | SSR-00122 | 171 | 0.327 | 7 | 0.755 | 0.717 | 0.313 | 1.335 | 0.350 | 0.216 | 0.217 | 118 | 0.220 | 17 | 0.893 | 0.884 | 0.339 | 1.422 | 0.416 | 0.265 | 0.267 |
| 20 | SSR-00152 | 171 | 0.895 | 3 | 0.191 | 0.176 | 0.310 | 1.254 | 0.229 | 0.149 | 0.150 | 118 | 0.898 | 3 | 0.186 | 0.174 | 0.316 | 1.084 | 0.154 | 0.074 | 0.075 |
| 21 | SSR-00208 | 171 | 0.789 | 4 | 0.345 | 0.306 | 0.218 | 1.249 | 0.202 | 0.133 | 0.134 | 118 | 0.780 | 5 | 0.363 | 0.327 | 0.225 | 1.133 | 0.188 | 0.103 | 0.104 |
| 22 | SSR-36802 | 171 | 0.737 | 4 | 0.419 | 0.378 | 0.326 | 1.266 | 0.311 | 0.186 | 0.187 | 118 | 0.737 | 4 | 0.418 | 0.376 | 0.333 | 1.258 | 0.318 | 0.188 | 0.190 |
| 23 | SSR-43814 | 171 | 0.497 | 4 | 0.633 | 0.570 | 0.208 | 1.196 | 0.247 | 0.141 | 0.142 | 118 | 0.432 | 9 | 0.718 | 0.679 | 0.225 | 1.276 | 0.309 | 0.189 | 0.190 |
| 24 | SSR-00278 | 171 | 0.474 | 8 | 0.693 | 0.653 | 0.402 | 1.517 | 0.447 | 0.298 | 0.299 | 118 | 0.415 | 16 | 0.774 | 0.753 | 0.418 | 1.401 | 0.385 | 0.250 | 0.252 |
| 25 | SSR-29061 | 171 | 0.351 | 5 | 0.694 | 0.632 | 0.507 | 1.446 | 0.415 | 0.270 | 0.271 | 118 | 0.390 | 5 | 0.690 | 0.629 | 0.487 | 1.394 | 0.366 | 0.236 | 0.238 |
| 26 | SSR-16626 | 171 | 0.491 | 5 | 0.607 | 0.530 | 0.367 | 1.394 | 0.347 | 0.229 | 0.230 | 118 | 0.508 | 4 | 0.570 | 0.480 | 0.362 | 1.295 | 0.253 | 0.164 | 0.166 |
| 27 | SSR-19752 | 171 | 0.532 | 5 | 0.625 | 0.568 | 0.254 | 1.298 | 0.321 | 0.197 | 0.198 | 118 | 0.339 | 13 | 0.797 | 0.772 | 0.268 | 1.426 | 0.395 | 0.257 | 0.259 |
| 28 | SSR-37406 | 171 | 0.713 | 8 | 0.475 | 0.459 | 0.210 | 1.175 | 0.245 | 0.135 | 0.135 | 118 | 0.619 | 13 | 0.595 | 0.578 | 0.248 | 1.326 | 0.371 | 0.226 | 0.228 |
| 29 | SSR-36642 | 171 | 0.596 | 6 | 0.557 | 0.493 | 0.181 | 1.130 | 0.178 | 0.098 | 0.098 | 118 | 0.585 | 13 | 0.605 | 0.568 | 0.185 | 1.152 | 0.200 | 0.110 | 0.111 |
| 30 | SSR-31687 | 171 | 0.906 | 4 | 0.174 | 0.166 | 0.200 | 1.148 | 0.161 | 0.095 | 0.095 | 118 | 0.941 | 2 | 0.112 | 0.105 | 0.188 | 1.025 | 0.045 | 0.022 | 0.023 |
| 31 | SSR-06409 | 171 | 0.784 | 5 | 0.373 | 0.357 | 0.542 | 1.522 | 0.437 | 0.293 | 0.293 | 118 | 0.746 | 12 | 0.433 | 0.422 | 0.534 | 1.276 | 0.339 | 0.201 | 0.202 |
| Min | − | − | 0.211 | 3 | 0.174 | 0.166 | 0.112 | 1.130 | 0.161 | 0.095 | 0.095 | − | 0.102 | 2 | 0.081 | 0.078 | 0.127 | 1.025 | 0.045 | 0.022 | 0.023 |
| Max | − | − | 0.906 | 9 | 0.854 | 0.837 | 0.642 | 1.763 | 0.567 | 0.398 | 0.399 | − | 0.958 | 33 | 0.949 | 0.947 | 0.641 | 1.714 | 0.580 | 0.398 | 0.401 |
| Mean | − | − | 0.564 | 5.194 | 0.561 | 0.516 | 0.328 | 1.358 | 0.340 | 0.217 | 0.218 | − | 0.527 | 9.677 | 0.600 | 0.568 | 0.343 | 1.348 | 0.345 | 0.218 | 0.220 |
| Total | − | − | 17.474 | 161 | 17.385 | 15.993 | 10.176 | 42.092 | 10.549 | 6.735 | 6.755 | − | 16.331 | 300 | 18.591 | 17.601 | 10.634 | 41.788 | 10.710 | 6.767 | 6.825 |
| Sampling Scale | Initial Population | 20% Pre-Core Collection | 15% Pre-Core Collection | 10% Pre-Core Collection | |
|---|---|---|---|---|---|
| Evaluation Parameters | |||||
| N | 171 | 34 | 26 | 19 | |
| Morphological traits | MD (%) | - | 4 | 8 | 6 |
| VD (%) | - | 6 | 8 | 8 | |
| CR (%) | - | 81 | 80 | 73 | |
| VR (%) | - | 102.01 | 103.71 | 102.52 | |
| SSR markers | Major allele frequency | 0.564 | 0.505 | 0.504 | 0.504 |
| No. allele (Na) | 5.194 | 7.194 | 6.419 | 5.677 | |
| Gene diversity | 0.561 | 0.630 | 0.634 | 0.634 | |
| PIC | 0.516 | 0.598 | 0.598 | 0.593 | |
| Ne | 1.357 | 1.374 | 1.378 | 1.368 | |
| I | 0.343 | 0.360 | 0.358 | 0.350 | |
| He | 0.218 | 0.229 | 0.229 | 0.224 | |
| uHe | 0.219 | 0.233 | 0.234 | 0.230 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Zhou, F.; Gao, Y.; Liu, C.; Yu, S.; Zhao, K.; Gong, W.; Lang, J.; Zhang, H.; Yu, X. Genetic Diversity and Primary Core Collection Construction of Turnip (Brassica rapa L. ssp. rapifera Matzg) Landraces in Tibet Revealed via Morphological and SSR Markers. Agronomy 2021, 11, 1901. https://doi.org/10.3390/agronomy11101901
Li R, Zhou F, Gao Y, Liu C, Yu S, Zhao K, Gong W, Lang J, Zhang H, Yu X. Genetic Diversity and Primary Core Collection Construction of Turnip (Brassica rapa L. ssp. rapifera Matzg) Landraces in Tibet Revealed via Morphological and SSR Markers. Agronomy. 2021; 11(10):1901. https://doi.org/10.3390/agronomy11101901
Chicago/Turabian StyleLi, Rongrong, Fangyuan Zhou, Yingying Gao, Chenlu Liu, Shubo Yu, Kun Zhao, Wenfeng Gong, Jie Lang, Haijuan Zhang, and Xiaolin Yu. 2021. "Genetic Diversity and Primary Core Collection Construction of Turnip (Brassica rapa L. ssp. rapifera Matzg) Landraces in Tibet Revealed via Morphological and SSR Markers" Agronomy 11, no. 10: 1901. https://doi.org/10.3390/agronomy11101901
APA StyleLi, R., Zhou, F., Gao, Y., Liu, C., Yu, S., Zhao, K., Gong, W., Lang, J., Zhang, H., & Yu, X. (2021). Genetic Diversity and Primary Core Collection Construction of Turnip (Brassica rapa L. ssp. rapifera Matzg) Landraces in Tibet Revealed via Morphological and SSR Markers. Agronomy, 11(10), 1901. https://doi.org/10.3390/agronomy11101901







