Seed Germination and Seedling Growth of Four Bedding Plants in Substrate Containing Coal Bottom Ash Mixed with Coir Dust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates Characterization
2.2. Physical Properties of the Substrates
2.3. Hydraulic Property of Substrates
2.4. EC and pH of Substrates
2.5. Seed Germination and Seedling Growth of Ornamental Plants
2.6. Heavy Metal Analysis of Plants
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Mixed Coir Dust and BA Substrates
3.2. Seed Germination
3.3. Seedling Growth
3.3.1. Periwinkles
3.3.2. Globe Amaranth
3.3.3. Impatiens
3.3.4. Petunia
3.3.5. Seedling Growth Comparisons among the Four Bedding Plants
3.4. Heavy Metals in Plants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Energy Outlook. 2020. Available online: https://www.iea.org/reports/world-energy-outlook-2020 (accessed on 12 March 2021).
- USEPA (United States Environmental Protection Agency). Coal Ash Basics. Available online: https://www.epa.gov/coalash/coal-ash-basics (accessed on 13 March 2020).
- Cary, E.E.; Gilbert, M.; Bache, C.A.; Gutenmann, W.H.; Lisk, D.J. Elemental composition of potted vegetables and millet grown on hard coal bottom ash-amended soil. Bull. Environ. Contam. Toxicol. 1983, 31, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Bruder-Hubscher, V.; Lagarde, F.; Leroy, M.; Coughanowr, C.; Enguehard, F. Utilisation of bottom ash in road construction: Evaluation of the environmental impact. Waste Manag. Res. 2001, 19, 545–556. [Google Scholar] [CrossRef]
- Schlossberg, M.J.; Miller, W.P. Coal combustion by-product (CCB) utilization in turfgrass sod production. HortScience 2004, 39, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.R.; Buck, J.S.; Sambo, P. The pH, electrical conductivity, and primary macronutrient concentration of sphagnum peat and ground parboiled fresh rice hull substrates over time in a greenhouse environment. HortTechnology 2011, 21, 103–108. [Google Scholar] [CrossRef]
- Jackson, B.E.; Wright, R.D.; Browder, J.F.; Harris, J.R.; Niemiera, A.X. Effect of fertilizer rate on growth of azalea and holly in pine bark and pine tree substrates. HortScience 2008, 43, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Jackson, B.E.; Wright, R.D.; Seiler, J.R. Changes in chemical and physical properties of pine tree substrate and pine bark during long-term nursery crop production. HortScience 2009, 44, 791–799. [Google Scholar] [CrossRef] [Green Version]
- Abad, M.; Fornes, F.; Carrión, C.; Noguera, V.; Noguera, P.; Maquieira, Á.; Puchades, R. Physical properties of various coconut coir dusts compared to peat. HortScience 2005, 40, 2138–2144. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.R.; Stamps, R.H. Growth of bedding plants in sphagnum peat and coir dust-based substrates. J. Environ. Hortic. 1996, 14, 187–190. [Google Scholar] [CrossRef]
- Evans, M.R.; Konduru, S.; Stamps, R.H. Source variation in physical and chemical properties of coconut coir dust. HortScience 1996, 31, 965–967. [Google Scholar] [CrossRef] [Green Version]
- Rhie, Y.H.; Kang, S.; Choi, J.M.; Kim, J. Physical and chemical properties of bottom ash and coir dust mix used as horticultural substrates. Hortic. Sci. Technol. 2018, 36, 161–171. [Google Scholar]
- Woodard, M.; Bearce, B.; Cluskey, S.; Townsend, E. Coal bottom ash and pine wood peelings as root substrates in a circulating nutriculture system. HortScience 1993, 28, 636–638. [Google Scholar] [CrossRef] [Green Version]
- Engstrom, K.B.; Myers, S.G.; Bearce, B.C. Growth and flowering responses of ‘Dark Red Hegg’ poinsettias in coal ash-amended root media to 3 nutrient solutions in a closed-loop nutriculture system. HortScience 1994, 29, 452. [Google Scholar] [CrossRef] [Green Version]
- Myers, S.S.; Bearce, B. Growth and flowering response of ‘Brilliant Diamond’ poinsettias in a peat vermiculite mix amended with coal bottom ash. HortScience 1993, 28, 256. [Google Scholar] [CrossRef] [Green Version]
- Pitchay, D.S.; Bearce, B. Chemical and physical properties of coal bottom ash-based root media and their effect on poinsettia nutritive status, growth, and flowering. HortScience 1996, 31, 912. [Google Scholar] [CrossRef] [Green Version]
- Pitchay, D.S.; Myers, S.; Bearce, B.C. Dry weight partitioning among roots, shoot bottoms and tops, and leaves of Hydrangea macrophylla cuttings rooted in coal bottom ash or peat: Perlite media containing four levels of dolomitic limestone. HortScience 1998, 33, 465. [Google Scholar] [CrossRef]
- Pitchay, D.S.; Sherratt, M.D.; Bearce, B.C. Inflorescence color manipulation in hydrangea forced in media containing coal bottom ash and mine soil. HortScience 1996, 31, 658. [Google Scholar] [CrossRef] [Green Version]
- Kafkafi, U. Functions of the root system. In Soilless Culture; Elsevier BV: Alpharetta, GA, USA, 2008; pp. 13–40. [Google Scholar]
- Liang, J.; Zhang, J.; Wong, M.H. Effects of air-filled soil porosity and aeration on the initiation and growth of secondary roots of maize (Zea mays). Plant Soil 1996, 186, 245–254. [Google Scholar] [CrossRef]
- Ball Seed Company. Available online: https://www.ballseed.com/quickculture/ProductionGuides (accessed on 18 March 2021).
- USDA (United States Department of Agriculture). Available online: https://www.nass.usda.gov/Publications/AgCensus/2017 (accessed on 18 March 2021).
- Fonteno, W.; Hardin, C.; Brewster, J. Procedures for Determining Physical Properties of Horticultural Substrates Using the NCSU Porometer; Horticultural Substrates Laboratory, North Carolina State University: Raleigh, NC, USA, 1995. [Google Scholar]
- Bilderback, T.E.; Fonteno, W.C.; Johnson, D.R. Physical properties of media composed of peanut hulls, pine bark, and peatmoss and their effects on azalea growth. J. Am. Soc. Hortic. Sci. 1982, 107, 522–525. [Google Scholar]
- Milks, R.R.; Fonteno, W.C.; Larson, R.A. Hydrology of horticultural substrates. II. Predicting physical properties of media in containers. J. Am. Soc. Hortic. Sci. 1989, 114, 53–56. [Google Scholar]
- Wallach, R.; Da Silva, F.; Chen, Y. Unsaturated hydraulic characteristics of composted agricultural wastes, tuff, and their mixtures. Soil Sci. 1992, 153, 434–441. [Google Scholar] [CrossRef]
- Warncke, D. Analyzing greenhouse growth media by the saturation extraction method. HortScience 1986, 21, 223–225. [Google Scholar]
- Novozamsky, I.; Lexmond, T.M.; Houba, V. A single extraction procedure of soil for evaluation of uptake of some heavy metals by plants. Int. J. Environ. Anal. Chem. 1993, 51, 47–58. [Google Scholar] [CrossRef]
- RDA (Rural Development Administration). Standard of Research Methods and Analysis for Agricultural Science; Rural Development Administration: Jeonju, Korea, 2012. [Google Scholar]
- Li, Q.; Chen, J.; Caldwell, R.D.; Deng, M. Cowpeat as a substitute for peat in container substrates for foliage plant propagation. HortTechnology 2009, 19, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Atiyeh, R.M.; Edwards, C.A.; Subler, S.; Metzger, J.D. Pig manure vermicompost as a component of a horticultural bedding plant medium: Effects on physicochemical properties and plant growth. Bioresour. Technol. 2001, 78, 11–20. [Google Scholar] [CrossRef]
- Terman, G.L.; Kilmer, V.J.; Hunt, C.M.; Buchanan, W. Fluidized bed boiler waste as a source of nutrients and lime. J. Environ. Qual. 1978, 7, 147–150. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, P.; Puchades, R.; Maquieira, A.; Noguera, V. Physico-chemical and chemical properties of some coconut coir dusts for use as a peat substitute for containerised ornamental plants. Bioresour. Technol. 2002, 82, 241–245. [Google Scholar] [CrossRef]
- Warncke, D.D.; Krauskopf, D. Ball Redbook: Greenhouse Growth Media: Testing & Nutrition Guidelines, 15th ed.; Ball, G.J., Ed.; Ball Publishing: West Chicago, IL, USA, 1991; Volume 1, pp. 245–255. [Google Scholar]
- Noguera, P.; Abad, M.; Noguera, V.; Puchades, R.; Maquieira, A. Coconut coir waste, a new and viable ecologically-friendly peat substitute. Acta Hortic. 2000, 517, 279–286. [Google Scholar] [CrossRef]
- Tilt, K.M.; Bilderback, T.E.; Fonteno, W.C. Particle size and container size effects on growth of three ornamental species. J. Amer. Soc. Hortic. Sci. 1987, 112, 981–984. [Google Scholar]
- Ben-Noah, I.; Friedman, S.P. Review and evaluation of root respiration and of natural and agricultural processes of soil aeration. Vadose Zone J. 2018, 17, 1–47. [Google Scholar] [CrossRef] [Green Version]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Ann. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [Green Version]
- EUR-Lex. Commission regulation (EC) No 1881/2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2006.364.01.0005.01.ENG (accessed on 15 November 2020).
- Zhang, J.H.; Tian, G.M.; Zhou, G.D.; He, M.M.; Wang, F.; Yao, J.H. Evaluation of organic solid wastes composts as peat substitutes for seedling production. J. Plant Nutr. 2013, 36, 1780–1794. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Kiyani, A.; Mirza, C.R.; Butt, T.A.; Barros, R.; Ali, B.; Iqbal, M.; Yousaf, S. Ornamental plants for the phytoremediation of heavy metals: Present knowledge and future perspectives. Environ. Res. 2021, 195, 110780. [Google Scholar] [CrossRef] [PubMed]
- Gianoncelli, A.; Zacco, A.; Struis, R.P.W.J.; Borgese, L.; Depero, L.E.; Bontempi, E. Fly ash pollutants, treatment and recycling. In Pollutant Diseases, Remediation and Recycling; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer International Publishing: Cham, Switzerland, 2013; Volume 4, pp. 103–213. [Google Scholar]
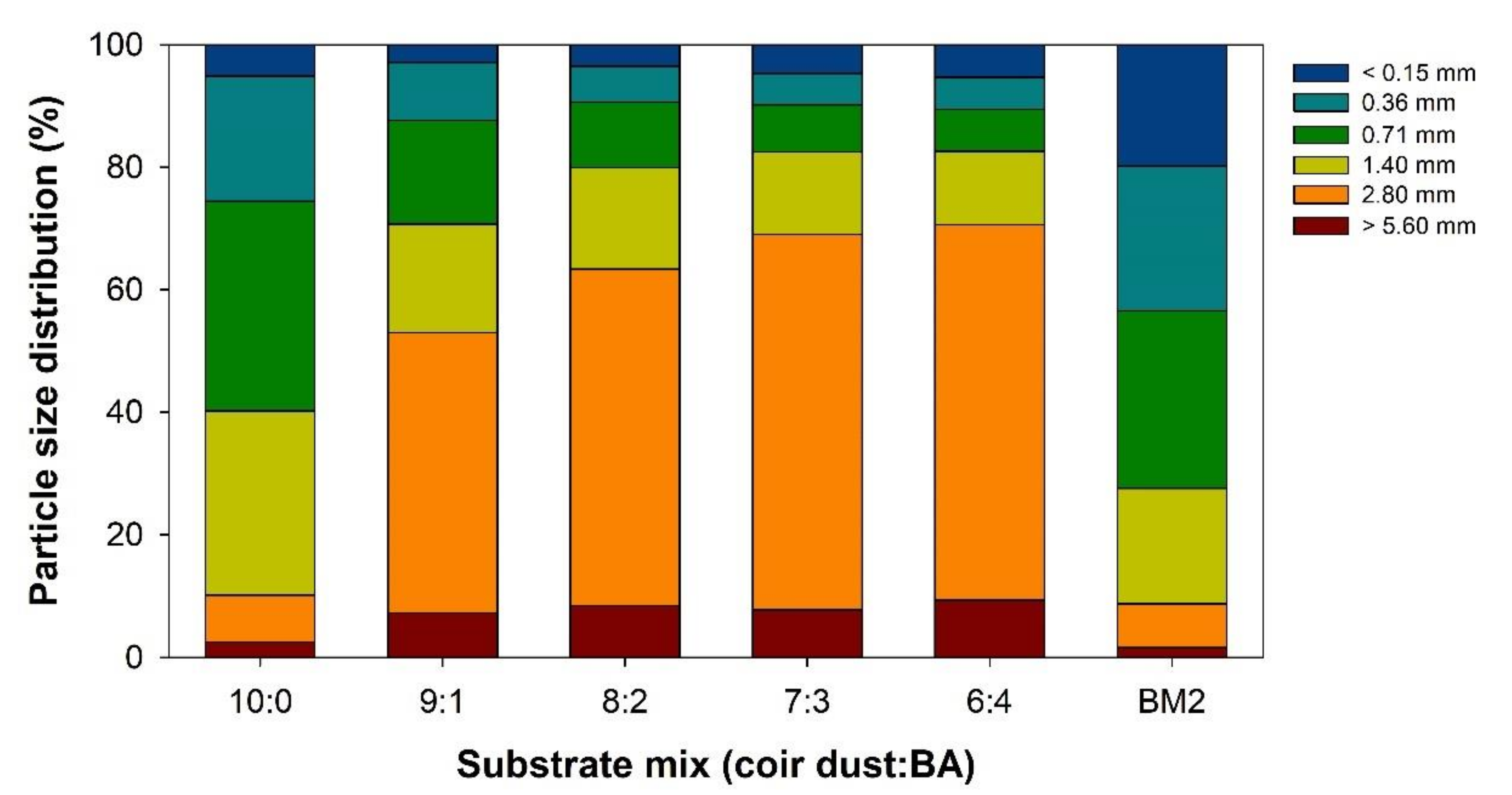
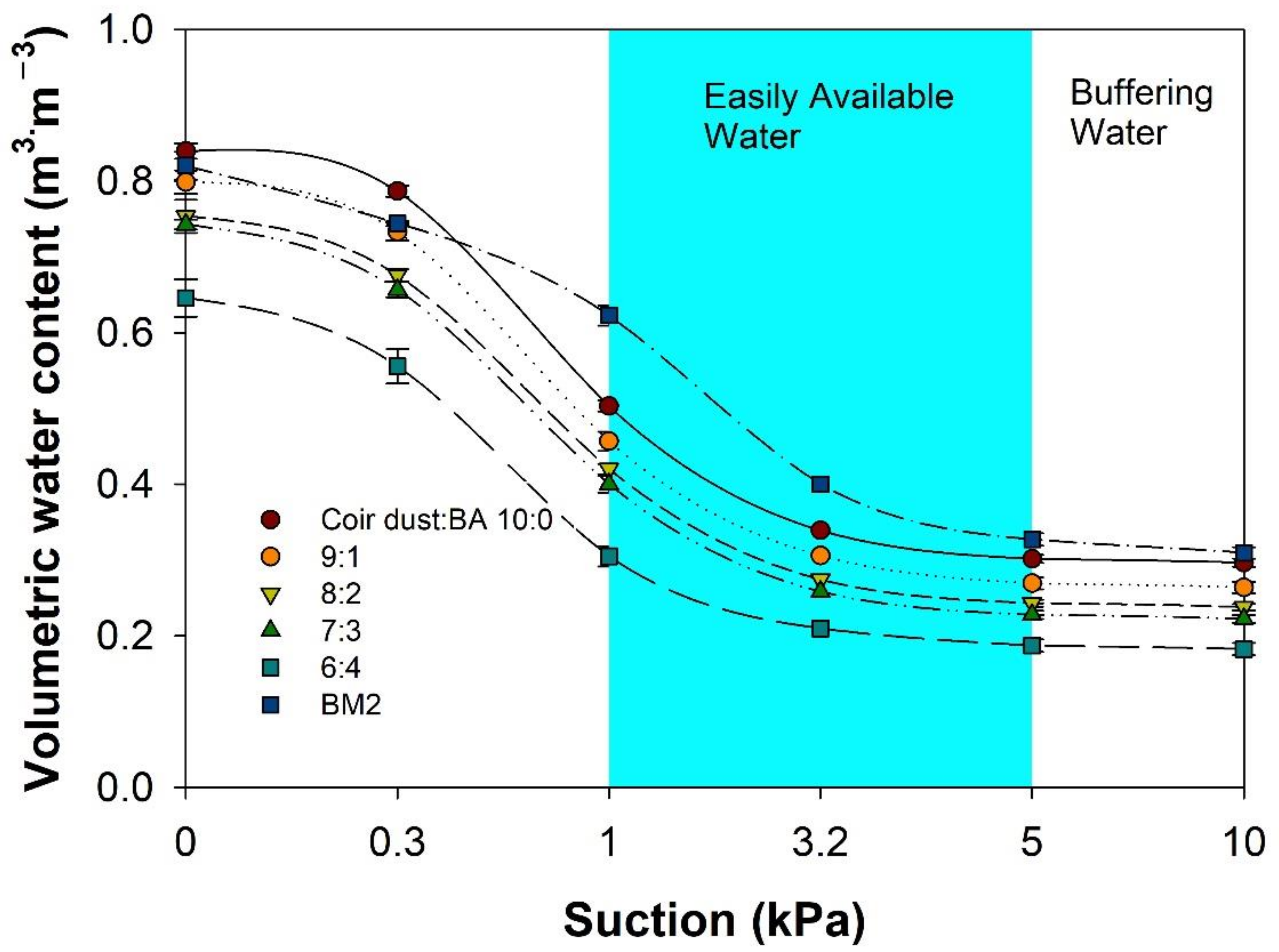
| Substrates (Coir Dust:BA) | Air Space (%) | Container Capacity (%) | Solid (%) | Bulk Density (g·cm−3) | ||||
|---|---|---|---|---|---|---|---|---|
| 10:0 | 25.5 ± 2.21 | b | 63.2 ± 1.82 | b | 11.4 ± 0.84 | c | 0.06 ± 0.001 | f |
| 9:1 | 26.5 ± 0.78 | ab | 61.1 ± 1.07 | b | 12.4 ± 0.54 | c | 0.12 ± 0.001 | d |
| 8:2 | 26.2 ± 1.64 | ab | 58.6 ± 1.36 | c | 15.3 ± 0.78 | b | 0.16 ± 0.003 | c |
| 7:3 | 28.0 ± 0.74 | a | 54.9 ± 1.84 | d | 17.0 ± 1.18 | ab | 0.21 ± 0.004 | b |
| 6:4 | 27.7 ± 0.95 | ab | 53.7 ± 0.18 | d | 18.5 ± 0.99 | a | 0.25 ± 0.003 | a |
| BM2 | 11.7 ± 1.33 | c | 76.6 ± 0.94 | a | 11.7 ± 2.25 | c | 0.10 ± 0.001 | e |
| Significance | *** | *** | *** | *** | ||||
| Substrates (Coir Dust:BA) | EC (dS·m−1) | pH |
|---|---|---|
| 10:0 | 0.85 ± 0.168 | 4.59 ± 0.420 |
| 9:1 | 0.69 ± 0.068 | 4.82 ± 0.287 |
| 8:2 | 0.63 ± 0.110 | 4.88 ± 0.167 |
| 7:3 | 0.60 ± 0.095 | 4.92 ± 0.099 |
| 6:4 | 0.57 ± 0.061 | 4.86 ± 0.159 |
| BM2 | 0.87 ± 0.199 | 5.36 ± 0.236 |
| Significance | NS | NS |
| Substrates (Coir Dust:BA) | Germination Rate (%) | Days to Germination | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C. roseus | G. globosa | I. walleriana | P. multiflora | C. roseus | G.globosa | I. walleriana | P. multiflora | ||
| 10:0 | 77 ± 4.7 | 75 ± 10.2 | 97 ± 1.8 | 77 ± 4.7 | 15 ± 0.3 | 7 ± 0.3 | 12 ± 0.2 | 9 ± 0.3 | |
| 9:1 | 61 ± 9.0 | 95 ± 3.0 | 100 ± 0.0 | 91 ± 9.0 | 14 ± 0.3 | 7 ± 0.3 | 13 ± 0.2 | 9 ± 0.2 | |
| 8:2 | 66 ± 5.4 | 88 ± 7.7 | 95 ± 1.6 | 92 ± 5.4 | 15 ± 0.4 | 7 ± 0.3 | 12 ± 0.2 | 9 ± 0.2 | |
| 7:3 | 75 ± 2.6 | 89 ± 3.9 | 98 ± 1.6 | 84 ± 2.6 | 15 ± 0.3 | 7 ± 0.3 | 12 ± 0.2 | 9 ± 0.2 | |
| 6:4 | 80 ± 8.2 | 86 ± 6.4 | 97 ± 1.8 | 88 ± 8.2 | 15 ± 0.3 | 7 ± 0.3 | 12 ± 0.2 | 9 ± 0.2 | |
| BM2 | 77 ± 4.7 | 92 ± 3.9 | 100 ± 0.0 | 88 ± 4.7 | 15 ± 0.3 | 8 ± 0.3 | 11 ± 0.2 | 10 ± 0.5 | |
| Significance | NS | NS | NS | NS | NS | NS | NS | NS | |
| Substrates (Coir Dust:BA) | Plant Height (cm) | Total Chlorophyll Content (SPAD Units) | No. of Leaves | Leaf Area (cm2) | Shoot Dry Weight (mg) | Root Dry Weight (mg) | Total Dry Weight (mg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catharanthus roseus ‘Pacifica XP Polka Dot’ | ||||||||||||||
| 10:0 | 4 ± 0.2 | c | 39 ± 1.3 | b | 12 ± 0.3 | 19 ± 0.9 | c | 44 ± 4.6 | c | 7 ± 3.1 | 51 ± 5.9 | c | ||
| 9:1 | 4 ± 0.1 | c | 39 ± 1.0 | b | 12 ± 0.2 | 23 ± 0.9 | b | 59 ± 5.4 | b | 3 ± 0.3 | 62 ± 5.5 | bc | ||
| 8:2 | 4 ± 0.1 | c | 41 ± 0.8 | b | 12 ± 0.3 | 22 ± 0.8 | b | 61 ± 2.5 | b | 4 ± 0.5 | 65 ± 3.0 | bc | ||
| 7:3 | 4 ± 0.1 | bc | 40.6 ± 1.0 | b | 12 ± 0.2 | 24 ± 1.0 | ab | 63 ± 5.1 | b | 5 ± 0.3 | 68 ± 5.5 | b | ||
| 6:4 | 4 ± 0.1 | a | 41 ± 0.6 | b | 12 ± 0.3 | 24 ± 1.0 | ab | 64 ± 5.6 | ab | 4 ± 1.2 | 68 ± 0.6 | ab | ||
| BM2 | 4 ± 0.1 | ab | 44 ± 1.2 | a | 11 ± 0.3 | 26 ± 1.0 | a | 77 ± 4.2 | a | 6 ± 0.9 | 83 ± 0.5 | a | ||
| Significance | * | ** | NS | *** | ** | NS | * | |||||||
| Gomphrena globosa ‘Buddy Purple’ | ||||||||||||||
| 10:0 | 7 ± 0.4 | abc | 30 ± 0.6 | b | 9 ± 0.4 | bc | 28 ± 1.3 | 72 ± 5.1 | 14 ± 0.9 | 86 ± 5.8 | ab | |||
| 9:1 | 8 ± 0.2 | ab | 30 ± 0.7 | b | 8 ± 0.3 | c | 26 ± 1.2 | 62 ± 5.4 | 13 ± 1.7 | 75 ± 7.0 | b | |||
| 8:2 | 7 ± 0.2 | abc | 30 ± 0.9 | ab | 9 ± 0.5 | bc | 26 ± 1.0 | 66 ± 6.0 | 14 ± 1.1 | 79 ± 6.9 | b | |||
| 7:3 | 7 ± 0.1 | bc | 32 ± 0.6 | a | 10 ± 0.5 | a | 29 ± 0.7 | 87 ± 4.4 | 16 ± 1.5 | 103 ± 5.3 | a | |||
| 6:4 | 7 ± 0.2 | c | 33 ± 0.5 | a | 10 ± 0.5 | ab | 28 ± 1.3 | 76 ± 2.6 | 16 ± 1.2 | 93 ± 3.7 | ab | |||
| BM2 | 8 ± 0.3 | a | 31 ± 0.9 | ab | 9 ± 0.3 | c | 30 ± 1.7 | 87 ± 12.9 | 18 ± 1.7 | 105 ± 12.2 | a | |||
| Significance | * | * | * | NS | NS | NS | * | |||||||
| Impatiens walleriana ‘Super Elfin XP Deep Pink’ | ||||||||||||||
| 10:0 | 4 ± 0.1 | b | 29 ± 1.4 | c | 14 ± 0.2 | b | 27 ± 0.7 | b | 46 ± 1.6 | b | 7 ± 1.5 | ab | 53 ± 1.8 | ab |
| 9:1 | 4 ± 0.0 | b | 30 ± 0.5 | bc | 14 ± 0.2 | b | 29 ± 0.7 | b | 51 ± 3.2 | b | 4 ± 0.4 | bc | 55 ± 3.6 | ab |
| 8:2 | 4 ± 0.1 | b | 32 ± 0.6 | b | 14 ± 0.2 | b | 29 ± 0.7 | b | 47 ± 1.7 | b | 4 ± 0.5 | bc | 50 ± 1.6 | ab |
| 7:3 | 4 ± 0.1 | b | 3 ± 0.5 | b | 13 ± 0.2 | c | 28 ± 0.9 | b | 43 ± 2.0 | b | 3 ± 0.4 | bc | 46 ± 2.3 | b |
| 6:4 | 4 ± 0.1 | b | 32 ± 0.7 | b | 14 ± 0.2 | b | 29 ± 1.5 | b | 50 ± 2.0 | b | 3 ± 0.3 | c | 53 ± 1.7 | ab |
| BM2 | 7 ± 0.2 | a | 37 ± 0.6 | a | 17 ± 0.4 | a | 41 ± 1.6 | a | 89 ± 9.3 | a | 11 ± 1.9 | a | 101 ± 11.0 | a |
| Significance | *** | *** | *** | *** | *** | * | * | |||||||
| Petunia multiflora ‘Damask Blue’ | ||||||||||||||
| 10:0 | 4 ± 0.2 | c | 32 ± 1.5 | 10 ± 0.5 | c | 26 ± 2.1 | c | 60 ± 4.8 | b | 5 ± 0.8 | b | 65 ± 4.7 | c | |
| 9:1 | 4 ± 0.1 | b | 33 ± 1.5 | 11 ± 0.2 | ab | 31 ± 1.4 | bc | 73 ± 5.6 | b | 5 ± 1.2 | b | 78 ± 6.2 | bc | |
| 8:2 | 4 ± 0.2 | ab | 34 ± 2.0 | 11 ± 0.4 | bc | 34 ± 1.9 | b | 80 ± 9.9 | b | 6 ± 0.8 | ab | 86 ± 10.1 | b | |
| 7:3 | 4 ± 0.1 | b | 37 ± 1.0 | 12 ± 0.4 | a | 34 ± 1.0 | b | 80 ± 5.2 | b | 6 ± 0.5 | ab | 86 ± 5.3 | b | |
| 6:4 | 4 ± 0.1 | b | 34 ± 1.4 | 11 ± 0.4 | bc | 32 ± 1.2 | b | 76 ± 5.3 | b | 6 ± 0.9 | ab | 83 ± 5.4 | bc | |
| BM2 | 5 ± 0.2 | a | 34 ± 1.0 | 12 ± 0.4 | a | 50 ± 2.5 | a | 135 ± 9.1 | a | 20 ± 3.0 | a | 155 ± 8.9 | a | |
| Significance | ** | NS | ** | *** | *** | * | *** | |||||||
| Substrates (Coir Dust:BA) | Cadmium (mg·kg−1) | Lead (mg·kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C. roseus | G. globosa | I. walleriana | P. multiflora | C. roseus | G. globosa | I. walleriana | P. multiflora | ||
| 10:0 | 0.2 ± 0.01 a | 0.4 ± 0.07 | 1.3 ± 0.40 a | 0.6 ± 0.02 a | - | - | - | - | |
| 9:1 | 0.1 ± 0.02 b | 0.1 ± 0.01 | 1.1 ± 0.06 ab | 0.2 ± 0.02 c | - | - | - | - | |
| 8:2 | 0.1 ± 0.02 b | 0.3 ± 0.10 | 1.3 ± 0.04 a | 0.4 ± 0.05 b | - | - | - | 0.2 ± 0.10 | |
| 7:3 | 0.0 ± 0.01 c | 0.3 ± 0.03 | 0.9 ± 0.04 ab | 0.1 ± 0.05 d | - | - | - | - | |
| 6:4 | - | 0.2 ± 0.07 | 0.7 ± 0.01 ab | 0.0 ± 0.03 d | - | - | 0.0 ± 0.01 | - | |
| BM2 | - | - | 0.1 ± 0.02 b | 0.1 ± 0.02 d | - | - | 0.1 ± 0.04 | - | |
| Significance | *** | NS | * | *** | NS | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhie, Y.-H.; Nam, S.; Kim, J. Seed Germination and Seedling Growth of Four Bedding Plants in Substrate Containing Coal Bottom Ash Mixed with Coir Dust. Agronomy 2021, 11, 1902. https://doi.org/10.3390/agronomy11101902
Rhie Y-H, Nam S, Kim J. Seed Germination and Seedling Growth of Four Bedding Plants in Substrate Containing Coal Bottom Ash Mixed with Coir Dust. Agronomy. 2021; 11(10):1902. https://doi.org/10.3390/agronomy11101902
Chicago/Turabian StyleRhie, Yong-Ha, Suyun Nam, and Jongyun Kim. 2021. "Seed Germination and Seedling Growth of Four Bedding Plants in Substrate Containing Coal Bottom Ash Mixed with Coir Dust" Agronomy 11, no. 10: 1902. https://doi.org/10.3390/agronomy11101902
APA StyleRhie, Y.-H., Nam, S., & Kim, J. (2021). Seed Germination and Seedling Growth of Four Bedding Plants in Substrate Containing Coal Bottom Ash Mixed with Coir Dust. Agronomy, 11(10), 1902. https://doi.org/10.3390/agronomy11101902








