Modified Humic Substances as Soil Conditioners: Laboratory and Field Trials
Abstract
1. Introduction
2. Materials and Methods
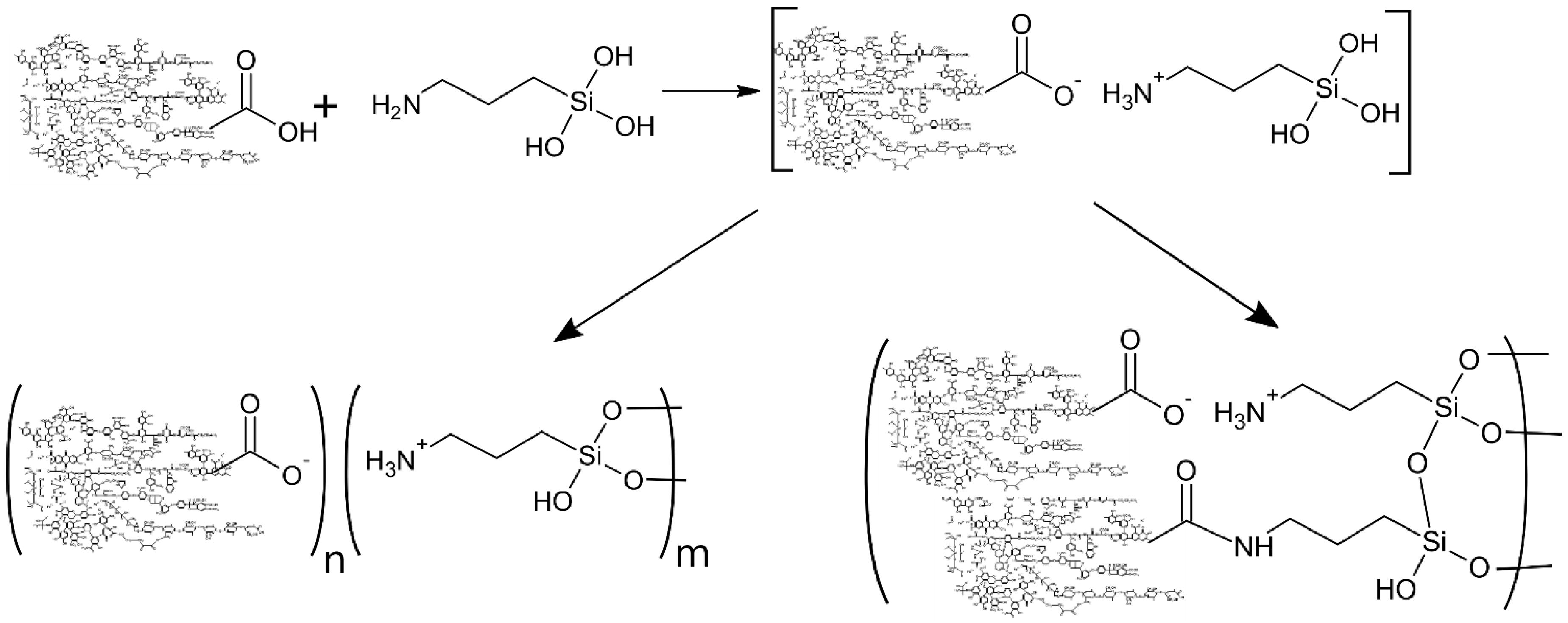
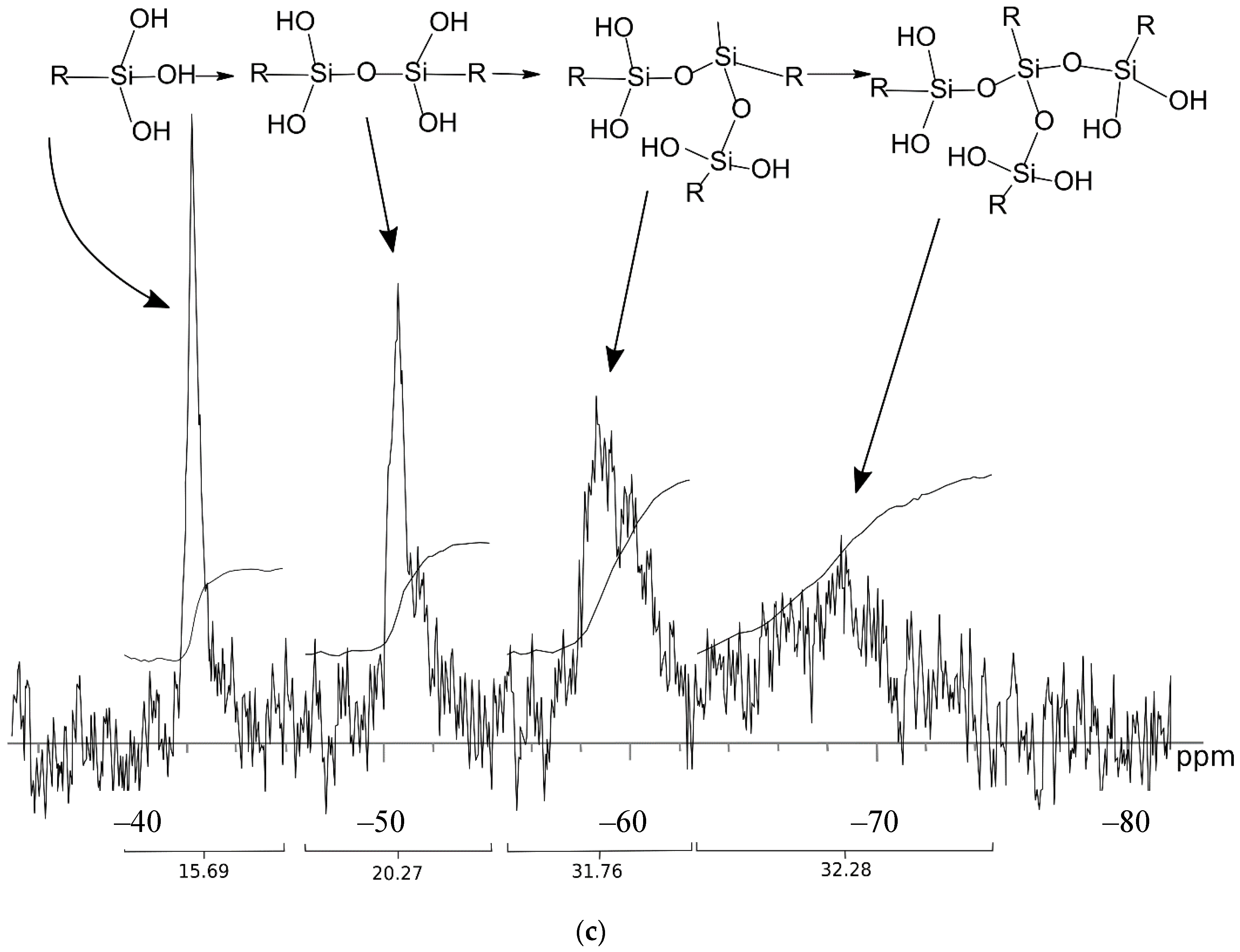
2.1. Synthesis and Characterization of the Silane-Modified HS
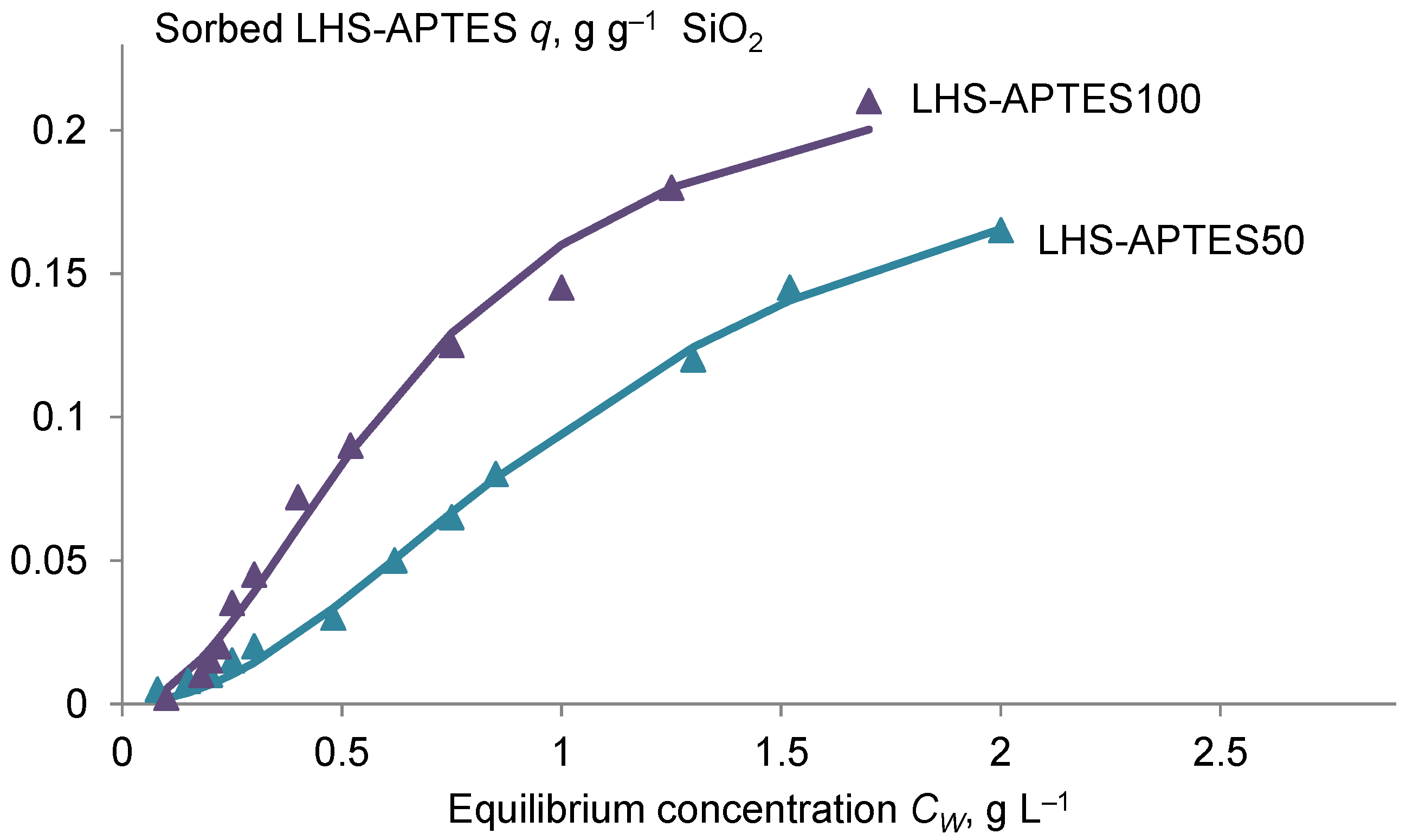
2.2. Sorption Experiments on Silica Gel
2.3. Soil and Its Characteristics
2.4. Laboratory Experiments with Soil Aggregates
2.5. Field Experiments Layout
2.6. Water-Stable Aggregates and Micro Aggregates’ Assessment
2.7. Soil Properties
2.7.1. pH Measurements
2.7.2. DOC and UV-Vis Measurements
2.7.3. Substrate-Induced Respiration
2.7.4. Determination of Labile NH4+ and NO3−
2.8. Bioassay
2.9. Statistical data Treatment
3. Results
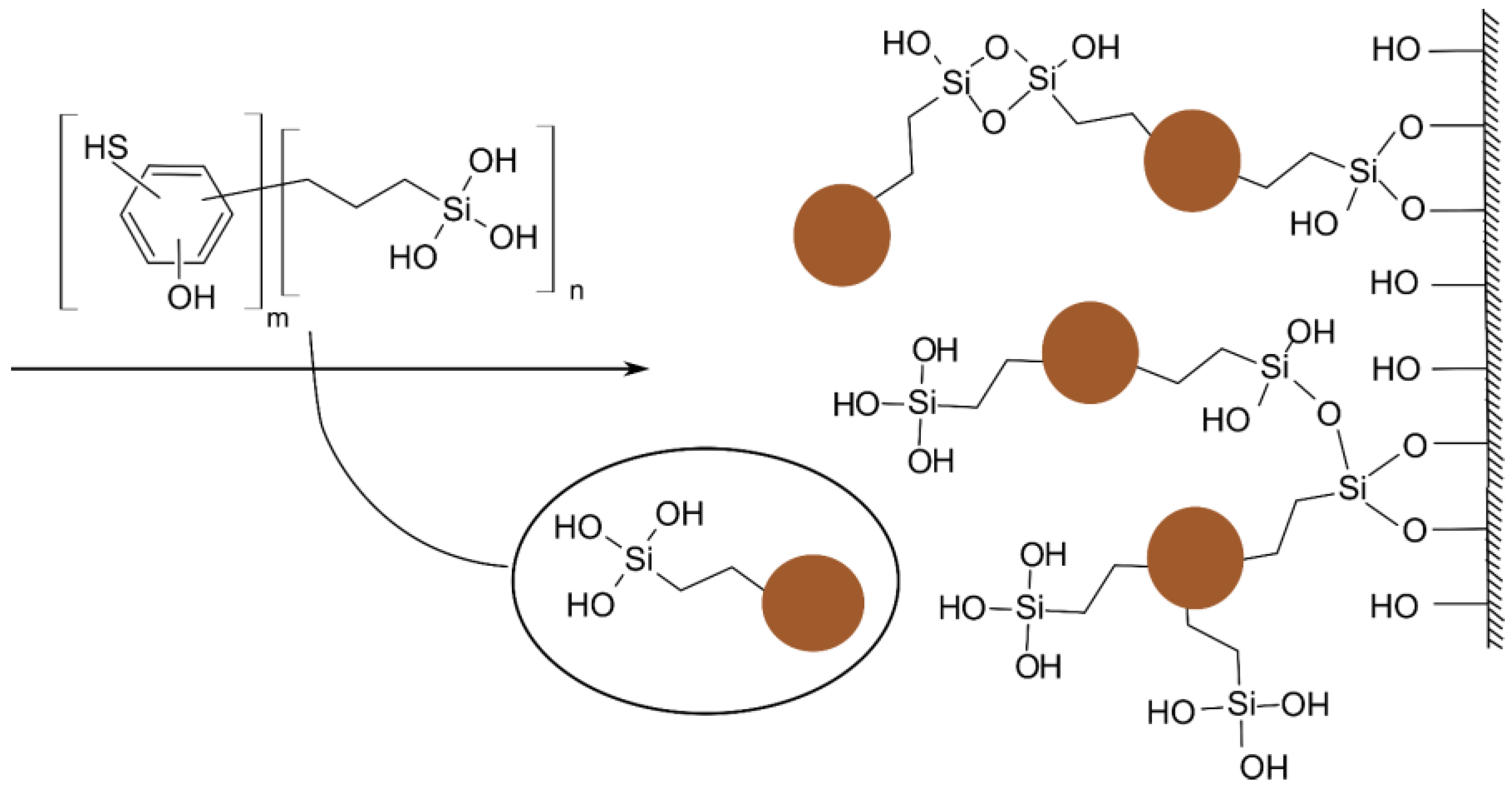
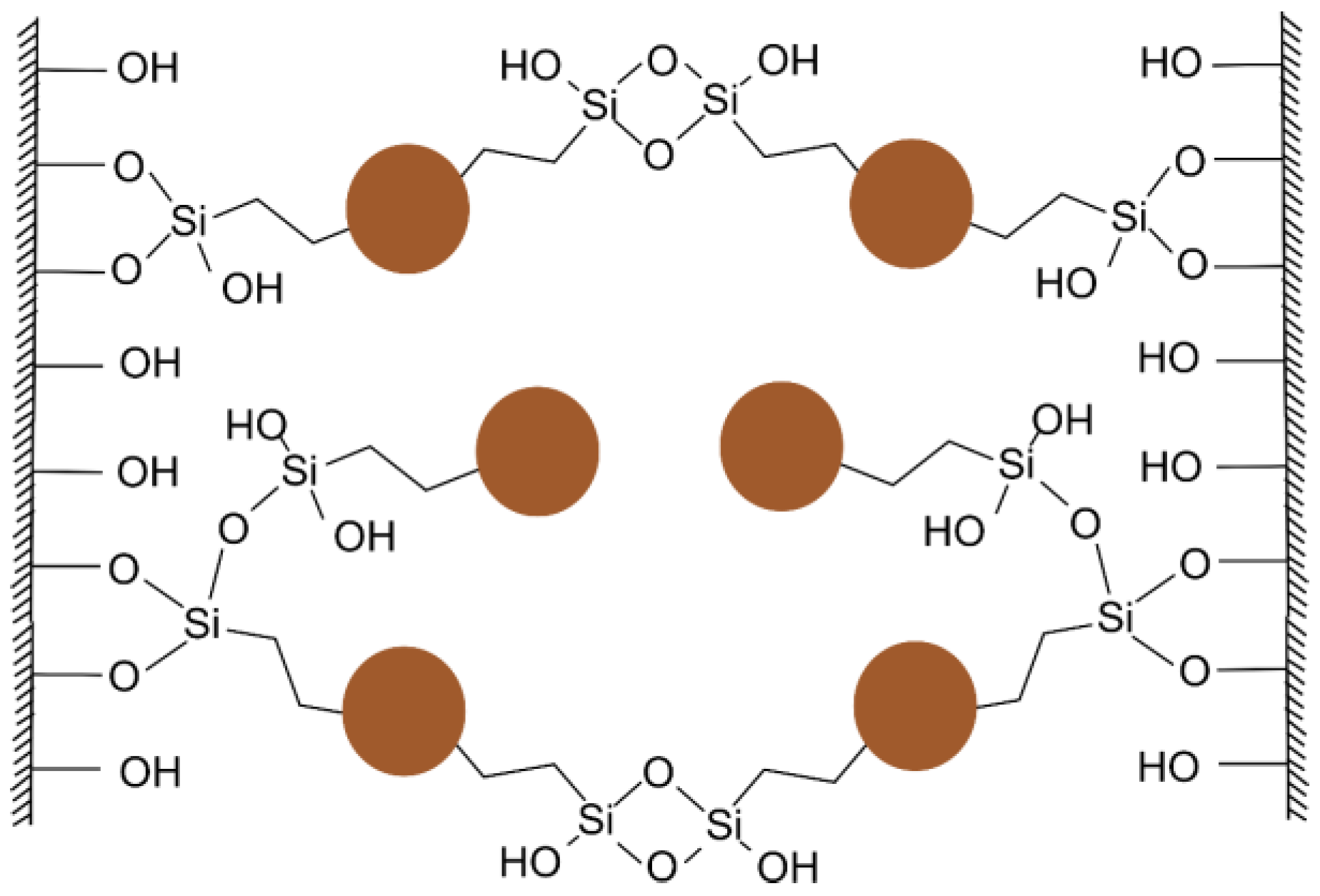
3.1. Properties and Sorption Characteristics of the HS-Silane Polyelectrolyte Complexes
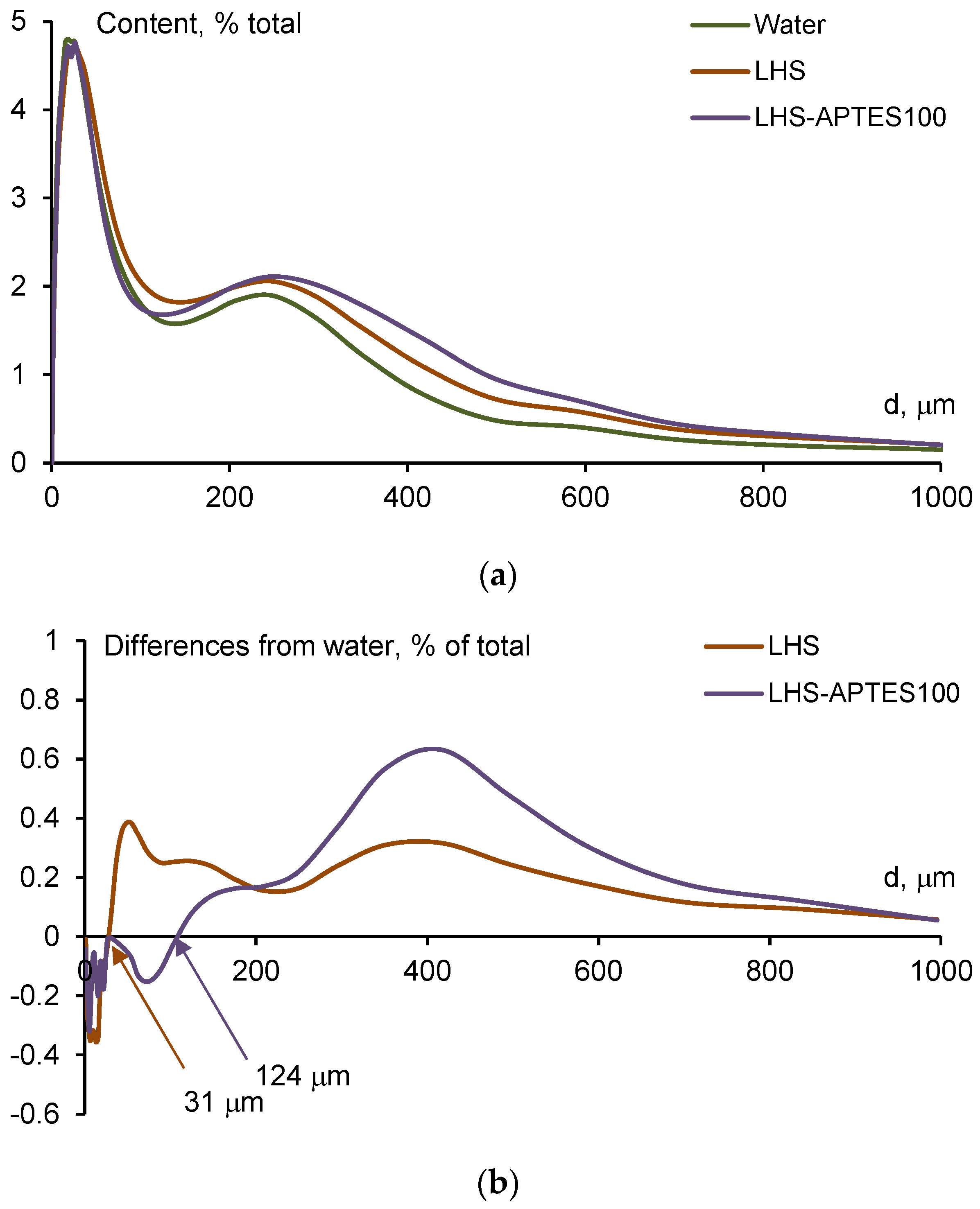
3.2. Effect of LHS-APTES Complexes on Water-Stable Soil Aggregates: Laboratory Studies
3.3. Effect of the HS-Silane Polyelectrolyte Complexes on Soil Properties: Field Studies
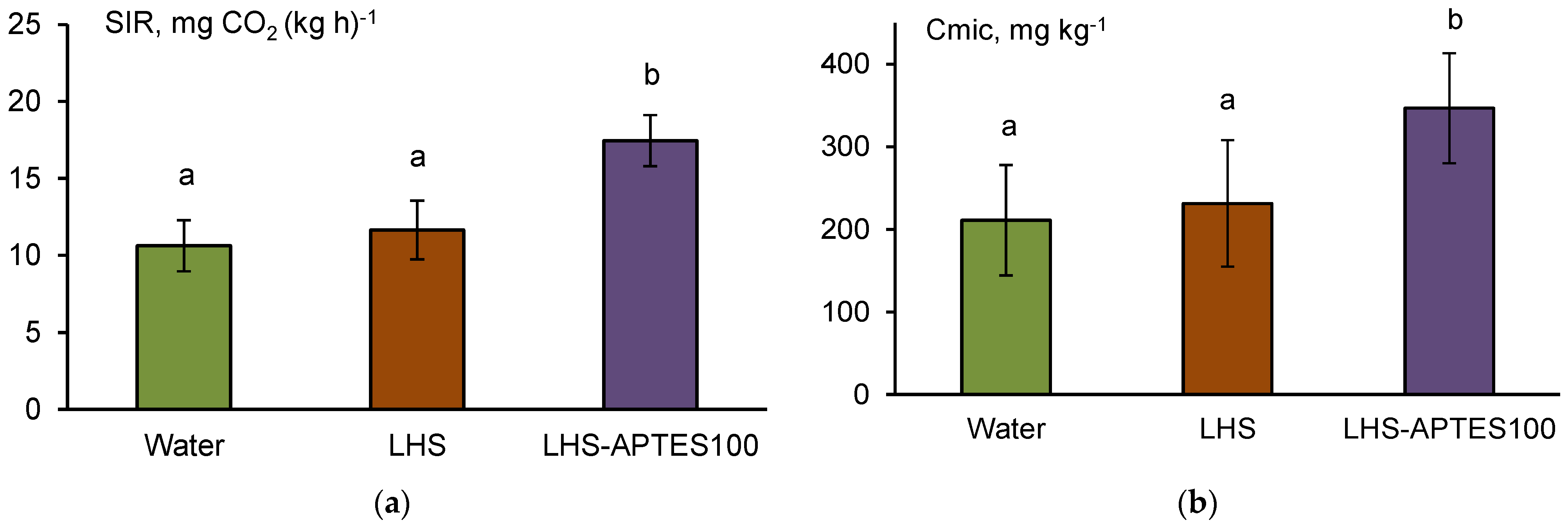
3.4. Biological Effects of the Soil Amendment with HS-Silane Polyelectrolyte Complexes
4. Discussion
4.1. On Mechanism of Soil Structure Improvement due to the Treatment with LHS-APTES Conditioner
4.2. Biological Effects of the Modified HS Used in This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dexter, A.R.; Czyż, E.A. Applications of S-theory in the study of soil physical degradation and its consequences. Land Degrad. Dev. 2007, 18, 369–381. [Google Scholar] [CrossRef]
- Loss, A.; Pereira, M.G.; Costa, E.M.; Beutler, S.J. Soil fertility, physical and chemical organic matter fractions, natural C-13 and N-15 abundance in biogenic and physicogenic aggregates in areas under different land use systems. Soil Res. 2014, 52, 685–697. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K.; Elliott, E.T.; Combrink, C. Soil structure and organic matter: I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci. Soc. Am. J. 2000, 64, 681–689. [Google Scholar] [CrossRef]
- Bieganowski, A.; Zaleski, T.; Kajdas, B.; Sochan, A.; Józefowska, A.; Beczek, M.; Lipiec, J.; Turski, M.; Ryżak, M. An improved method for determination of aggregate stability using laser diffraction. Land Degrad. Dev. 2018, 29, 1376–1384. [Google Scholar] [CrossRef]
- Larney, F.J.; Angers, D.A. The role of organic amendments in soil reclamation: A review. Can. J. Soil Sci. 2012, 92, 19–38. [Google Scholar] [CrossRef]
- Dong, X.; Guan, T.; Li, G.; Lin, Q.; Zhao, X. Long-term effects of biochar amount on the content and composition of organic matter in soil aggregates under field conditions. J. Soils Sediments 2016, 16, 1481–1497. [Google Scholar] [CrossRef]
- Sharifi, Z.; Azadi, N.; Certini, G. Fire and tillage as degrading factors of soil structure in Northern Zagros Oak Forest, West Iran. Land Degrad. Dev. 2016, 28, 1068–1077. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; Li, C.; Zhang, J.; Wang, L. Effects of temperature on soil organic carbon fractions contents, aggregate stability and structural characteristics of humic substances in a Mollisol. J. Soils Sediments 2016, 16, 1849–1857. [Google Scholar] [CrossRef]
- Kraaijvanger, R.; Veldkamp, T. Grain productivity, fertilizer response and nutrient balance of farming systems in Tigray, Ethiopia: A multiperspective view in relation to soil fertility degradation. Land Degrad. Dev. 2014, 26, 701–710. [Google Scholar] [CrossRef]
- Grimmond, S. Urbanization and global environmental change: Local effects of urban warming. Geogr. J. 2007, 173, 83–88. [Google Scholar] [CrossRef]
- Edvardsson, K. Gravel roads and dust suppression. Road Mater. Pavement Des. 2009, 10, 439–469. [Google Scholar] [CrossRef]
- Kononova, M.M. Soil Organic Matter: Its Nature, Its Role in Soil Formation and in Soil Fertility, 2nd ed.; Pergamon Press: Oxford, UK, 1966; pp. 1–544. [Google Scholar]
- Tisdall, J.M.; Oades, J.M. Organic matter and water stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Piccolo, A.; Pietramellara, G.; Mbagwu, J.S.C. Use of humic substances as soil conditioners to increase aggregate stability. Geoderma 1997, 75, 267–277. [Google Scholar] [CrossRef]
- Lugato, E.; Simonetti, G.; Morari, F.; Nardi, S.; Berti, A.; Giardini, L. Distribution of organic and humic carbon in wet-sieved aggregates of different soils under long-term fertilization experiment. Geoderma 2010, 157, 80–85. [Google Scholar] [CrossRef]
- Jin, X.; An, T.; Gall, A.R.; Li, S.; Sun, L.; Pei, J.; Gao, X.; He, X.; Fu, S.; Ding, X.; et al. Long-term plastic film mulching and fertilization treatments changed the annual distribution of residual maize straw C in soil aggregates under field conditions: Characterization by 13C tracing. J. Soils Sediments 2018, 18, 169–178. [Google Scholar] [CrossRef]
- Mitran, T.; Mani, P.K.; Bandyopadhyay, P.K.; Basak, N. Influence of organic amendments on soil physical attributes and aggregate associated phosphorus under long-term rice-wheat cropping. Pedosphere 2018, 28, 823–832. [Google Scholar] [CrossRef]
- Ozgöz, E.; Günal, H.; Acir, N.; Gökmen, F.; Birol, M.; Budak, M. Soil quality and spatil variability assessment of land use effects in a Typic Haplustoll. Land Degrad. Dev. 2013, 24, 277–286. [Google Scholar] [CrossRef]
- Lyons, G.; Genc, Y. Commercial humates in agriculture: Real substance or smoke and mirrors? Agronomy 2016, 6, 50. [Google Scholar] [CrossRef]
- Wang, D.Y.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M.; Liu, H. Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 2017, 303, 110–117. [Google Scholar] [CrossRef]
- Sojka, R.E.; Bjorneberg, D.L.; Entry, J.A.; Lentzl, R.D.; Orts, W.J. Polyacrylamide in agriculture and environmental land management. Adv. Agron. 2007, 92, 75–162. [Google Scholar] [CrossRef]
- Mamedov, A.I.; Huang, C.; Aliev, F.A.; Levy, G.J. Aggregate stability and water retention near saturation characteristics as affected by soil texture, aggregate size and polyacrylamide application. Land Degrad. Dev. 2017, 28, 543–552. [Google Scholar] [CrossRef]
- Sojka, R.E.; Entry, J.A.; Furhmann, J.J. The influence of high application rates of polyacrylamide on microbial metabolic potential in an agricultural soil. Appl. Soil Ecol. 2006, 32, 243–252. [Google Scholar] [CrossRef]
- Ding, X.; Xu, G.; Zhou, W.; Kuruppu, M. Effect of synthetic and natural polymers on reducing bauxite residue dust pollution. Environ. Technol. 2020, 41, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Filippini, G. Environmental health criteria 49—Acrylamide. Ital. J. Neuro. Sci. 1986, 7, 171. [Google Scholar] [CrossRef]
- Mroczek, E.; Kleiber, T.; Konieczny, P.; Waśkiewicz, A. Effect of residual monomer from polyacrylamide on head lettuce grown in peat substrate. Food Addit. Contam. Part A 2015, 32, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Singh, Y.; Iqbal, T.; Knoblauch, C.; Simon, P.; Wichern, F. Short-term effects of polyacrylamide and dicyandiamide on C and N mineralization in a sandy loam soil. Soil Use Manag. 2016, 32, 127–136. [Google Scholar] [CrossRef]
- Du, Z.-L.; Zhao, J.K.; Wang, Y.D.; Zhang, Q.-Z. Biochar addition drives soil aggregation and carbon sequestration in aggregate fractions from an intensive agricultural system. J. Soils Sediments 2017, 17, 581–589. [Google Scholar] [CrossRef]
- Volikov, A.B.; Kholodov, V.A.; Kulikova, N.A.; Philippova, O.I.; Ponomarenko, S.A.; Lasareva, E.V.; Parfyonova, A.M.; Hatfield, K.; Perminova, I.V. Silanized humic substances act as hydrophobic modifiers of soil separates inducing formation of water-stable aggregates in soils. Catena 2016, 137, 229–236. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. In World Soil Resources Reports; FAO: Rome, Italy, 2015; Volume 106, p. 138. [Google Scholar]
- Grant, P.G.; Lemke, S.L.; Dwyer, M.R.; Phillips, T.D. Modified Langmuir equation for S-shaped and multisite isotherm plots. Langmuir 1998, 14, 4292–4299. [Google Scholar] [CrossRef]
- Shein, E.V.; Rusanov, A.M.; Nikolaeva, E.I.; Khaidapova, D.D. Parametric estimation of soil physical functions. Mosc. Univ. Soil Sci. Bull. 2007, 62, 101–106. [Google Scholar] [CrossRef]
- Elliott, E.T. Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci. Soc. Am. J. 1985, 50, 627–633. [Google Scholar] [CrossRef]
- Amelung, W.; Zech, W. Minimization of organic matter disruption during particle-size fractionation of grassland epipedons. Geoderma 1999, 92, 73–85. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Rumpel, C.; Kögel-Knabner, I. Evaluation of an ultrasonic dispersion procedure to isolate primary organomineral complexes from soils. Eur. J. Soil Sci. 1999, 50, 87–94. [Google Scholar] [CrossRef]
- Ryżak, M.; Bieganowski, A. Methodological aspects of determining soil particle-size distribution using the laser diffraction method. J. Plant. Nutr. Soil Sci. 2011, 174, 624–633. [Google Scholar] [CrossRef]
- Amezkeka, E. Soil aggregate stability: A review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Ananyeva, N.D.; Susyan, E.A.; Chernova, O.V.; Stephan, W. Microbial respiration activities of soils from different climatic regions of European Russia. Eur. J. Soil Biol. 2008, 44, 147–157. [Google Scholar] [CrossRef]
- Kulikova, N.A.; Filippova, O.I.; Volikov, A.B.; Perminova, I.V. Slow nitrogen release from humic substances modified with aminoorganosilanes. J. Soils Sediments 2018, 18, 1400–1408. [Google Scholar] [CrossRef]
- Klein, O.I.; Kulikova, N.A.; Stepanova, E.V.; Filippova, O.I.; Fedorova, T.V.; Maloshenok, L.G.; Filimonov, I.S.; Koroleva, O.V. Preparation and characterization of bioactive products obtained via the solubilization of brown coal by white rot fungi. Appl. Biochem. Microbiol. 2014, 50, 730–736. [Google Scholar] [CrossRef]
- Mueller, L.; Shepherd, G.; Schindler, U.; Ball, B.C.; Munkholm, L.; Hennings, V.; Smolentseva, E.; Rukhovic, O.; Lukin, S.; Hui, C. Evaluation of soil structure in the framework of an overall soil quality rating. Soil Tillage Res. 2013, 127, 74–84. [Google Scholar] [CrossRef]
- Beari, F.; Brand, M.; Jenkner, P.; Lehnert, R.; Metternich, H.J.; Monkiewicz, J.; Siesler, H.W. Organofunctional alkoxysilanes in dilute aqueous solution: New accounts on the dynamic structural mutability. J. Organometal. Chem. 2001, 625, 208–216. [Google Scholar] [CrossRef]
- Volikov, A.B.; Ponomarenko, S.A.; Gutsche, A.; Nirschl, H.; Hatfield, K.; Perminova, I.V. Targeted design of water-based humic substances-silsesquioxane soft materials for nature-inspired remedial applications. RSC Adv. 2016, 6, 48222–48230. [Google Scholar] [CrossRef]
- Gümüs, I.; Seker, C. Influence of humic acid applications on modulus of rupture, aggregate stability, electrical conductivity, carbon and nitrogen content of a crusting problem soil. Solid Earth 2015, 6, 1231–1236. [Google Scholar] [CrossRef]
- David, M.B.; McIsaac, G.F.; Darmody, R.G.; Omonode, R.A. Long-term changes in Mollisol organic carbon and nitrogen. J. Environ. Qual. 2009, 38, 200–211. [Google Scholar] [CrossRef]
- Volikov, A.B.; Ponomarenko, S.A.; Konstantinov, A.I.; Hatfield, K.; Perminova, I.V. Nature-like solution for removal of direct brown 1 azo dye from aqueous phase using humics-modified silica gel. Chemosphere 2016, 145, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Goebel, M.O.; Woche, S.K.; Bachmann, J.; Lamparter, A.; Fischer, W.R. Significance of wettability-induced changes in microscopic water distribution for soil organic matter decomposition. Soil Sci. Soc. Am. J. 2007, 71, 1593–1599. [Google Scholar] [CrossRef]
- Plaza, I.; Ontiveros-Ortega, A.; Calero, J.; Aranda, V. Implication of zeta potential and surface free energy in the description of agricultural soil quality: Effect of different cations and humic acids on degraded soils. Soil Tillage Res. 2015, 146, 148–158. [Google Scholar] [CrossRef]
- Piccolo, A.; Mbagwu, J.S.C. Effects of different organic waste amendments on soil microaggregates stability and molecular sizes of humic substances. Plant Soil 1990, 123, 27–37. [Google Scholar] [CrossRef]
- Piccolo, A.; Mbagwu, J.S.C. Humic substances and surfactants effects on the stability of two tropical soils. Soil Sci. Soc. Am. J. 1994, 58, 950–955. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takei, T.; Yazawa, Y.; Wong, M.T.F.; Gilkes, R.J.; Swift, R.S. Effect of humic acids, sodium, and calcium additions on the formation of water-stable aggregates in Western Australian wheat belt soil. Aust. J. Soil Res. 2004, 42, 435–439. [Google Scholar] [CrossRef]
- Colazo, J.C.; Buschiazzo, D. The impact of agriculture on soil texture due to wind erosion. Land Degrad. Dev. 2015, 26, 62–70. [Google Scholar] [CrossRef]
- Aimar, S.B.; Méndez, M.J.; Funk, R.; Buschiazzo, D.E. Soil properties related to potential particulate matter emissions (PM10) of sandy soils. Aeolian Res. 2012, 3, 437–443. [Google Scholar] [CrossRef]
- Wang, X.; Chow, J.C.; Kohl, S.D.; Narasimh, L.; Yatavelli, R.; Percy, K.E.; Legge, A.H.; Watson, J.G. Wind erosion potential for fugitive dust sources in the Athabasca Oil Sands Region. Aeolian Res. 2015, 18, 121–134. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, W.; Wang, H.; Bao, Q.; Jin, H.; Liu, Y. Preparation and experimental dust suppression performance characterization of a novel guar gum-modification-based environmentally-friendly degradable dust suppressant. Powder Technol. 2018, 339, 314–325. [Google Scholar] [CrossRef]
- Amato, F.; Karanasiou, A.; Cordoba, P.; Alastuey, A.; Moreno, T.; Lucarelli, F.; Nava, S.; Calzolai, G.; Querol, X. Effects of road dust suppressants on PM levels in a Mediterranean urban area. Environ. Sci. Technol. 2014, 48, 8069–8077. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nie, W.; Mu, Y.; Zhang, H.; Wang, H.; Jin, H.; Liu, Z. A synthesis and performance evaluation of a highly efficient ecological dust depressor based on the sodium lignosulfonate–acrylic acid graft copolymer. RSC Adv. 2018, 8, 11498–11508. [Google Scholar] [CrossRef]
- Orlov, D.S.; Grishina, L.A. Practical Guide on Humic Chemistry; Lomonosov Moscow State University: Moscow, Russia, 1981; pp. 1–273. (In Russian) [Google Scholar]
- Jiang, X.; Wright, A.L.; Wang, X.; Liang, F. Tillage-induced changes in fungal and bacterial biomass associated with soil aggregates: A long-term field study in a subtropical rice soil in China. Appl. Soil Ecol. 2011, 48, 168–173. [Google Scholar] [CrossRef]
- Milne, R.M.; Haynes, R.J. Comparative effects of annual and permanent dairy pastures on soil physical properties in the Tsitsikamma region of South Africa. Soil Use Manag. 2004, 20, 81–88. [Google Scholar] [CrossRef]
- Gil, S.V.; Meriles, J.; Conforto, C.; Basanta, M.; Radl, V.; Hagn, A.; Schloter, M.; March, G.J. Response of soil microbial communities to different management practices in surface soils of a soybean agroecosystem in Argentina. Eur. J. Soil Biol. 2011, 47, 55–60. [Google Scholar] [CrossRef]



| Sample | Content, % (wt.) | Atomic Ratio | ||||
|---|---|---|---|---|---|---|
| C | H | N | Ash | H/C | C/N | |
| LHS | 31.7 | 2.9 | 1.2 | 39 | 1.08 | 26 |
| LHS-APTES50 | 31.6 | 3.5 | 2.5 | 43 | 1.34 | 13 |
| LHS-APTES100 | 31.0 | 3.1 | 3.4 | 44 | 1.21 | 9.1 |
| Sample | N/C * | Qmax, g g−1 SiO2 | K′, L g−1 | R2 |
|---|---|---|---|---|
| LHS-APTES50 | 0.082 ± 0.007 a | 0.22 ± 0.02 a | 78 ± 6 a | 0.996 |
| LHSAPTES100 | 0.12 ± 0.01 b | 0.23 ± 0.02 a | 230 ± 20 b | 0.990 |
| Treatment | WSA | WSMA | ||
|---|---|---|---|---|
| Content, % | MWD, mm | Content, % | MWD, μm | |
| Water (control) | 86 ± 3 a | 1.6 ± 0.3 a | 8 ± 1 a | 59 ± 5 a |
| LHS | 87 ± 4 a | 1.7 ± 0.5 a | 8 ± 1 a | 70 ± 14 b |
| LHS-APTES100 | 87 ± 3 a | 2.9 ± 0.5 b | 11 ± 2 b | 73 ± 15 b |
| Treatment | pH | Labile Mineral N, mg kg−1 | DOC in Water Extract | ||
|---|---|---|---|---|---|
| NH4+ | NO3− | mg OC kg−1 | E4650.01% | ||
| Water (control) | 7.39 ± 0.08 a | 1.5 ± 0.6 a | 2.0 ± 0.5 a | 123 ± 43 a | 0.007 ± 0.003 a |
| LHS | 7.41 ± 0.07 a | 9 ± 5 b | 3 ± 1 a | 200 ± 80 ab | 0.025 ± 0.009 c |
| LHS-APTES100 | 7.4 ± 0.1 a | 12 ± 5 b | 4 ± 2 a | 249 ± 93 b | 0.016 ± 0.006 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikova, N.A.; Volikov, A.B.; Filippova, O.I.; Kholodov, V.A.; Yaroslavtseva, N.V.; Farkhodov, Y.R.; Yudina, A.V.; Roznyatovsky, V.A.; Grishin, Y.K.; Zhilkibayev, O.T.; et al. Modified Humic Substances as Soil Conditioners: Laboratory and Field Trials. Agronomy 2021, 11, 150. https://doi.org/10.3390/agronomy11010150
Kulikova NA, Volikov AB, Filippova OI, Kholodov VA, Yaroslavtseva NV, Farkhodov YR, Yudina AV, Roznyatovsky VA, Grishin YK, Zhilkibayev OT, et al. Modified Humic Substances as Soil Conditioners: Laboratory and Field Trials. Agronomy. 2021; 11(1):150. https://doi.org/10.3390/agronomy11010150
Chicago/Turabian StyleKulikova, Natalia A., Alexander B. Volikov, Olga I. Filippova, Vladimir A. Kholodov, Nadezhda V. Yaroslavtseva, Yulian R. Farkhodov, Anna V. Yudina, Vitaly A. Roznyatovsky, Yuri K. Grishin, Oral T. Zhilkibayev, and et al. 2021. "Modified Humic Substances as Soil Conditioners: Laboratory and Field Trials" Agronomy 11, no. 1: 150. https://doi.org/10.3390/agronomy11010150
APA StyleKulikova, N. A., Volikov, A. B., Filippova, O. I., Kholodov, V. A., Yaroslavtseva, N. V., Farkhodov, Y. R., Yudina, A. V., Roznyatovsky, V. A., Grishin, Y. K., Zhilkibayev, O. T., & Perminova, I. V. (2021). Modified Humic Substances as Soil Conditioners: Laboratory and Field Trials. Agronomy, 11(1), 150. https://doi.org/10.3390/agronomy11010150





