Process Optimization for Ultrasound-Assisted Starch Production from Cassava (Manihot esculenta Crantz) Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Sample Preparation
2.2. Extraction of Cassava Starch
2.3. Experimental Design
2.4. Model Validation and Verification
2.5. Kinetics Study
2.6. Starch Characterization
2.7. Data Analysis
3. Results and Discussion
3.1. Evaluation of Extraction Factors on Starch Yield
 ) and had the most significant influence on the amount of the extracted starch. A former study on ultrasound-assisted alkali extractions of polysaccharides from wheat straw reported that the ratio of solvent to sample affected the extraction recovery [29]. This finding was also confirmed by another report revealing the increase of solvent to sample ratio from 10 to 40 could recover 1.5× polysaccharides from BaChu mushroom [30]. Increasing the ratio of solvent to sample will increase the amount of extracted starch. This result was in accordance with the mass transfer principle, where a greater ratio between the solid material and the solvent causes a higher concentration gradient, which is the driving force in solvent extraction [31]. A higher concentration gradient leads to a greater amount of starch extracted from cassava flour that diffuses into the solvent more effectively, thus promoting a higher starch yield. In contrast, the quadratic effect of solvent to sample ratio negatively influenced (
) and had the most significant influence on the amount of the extracted starch. A former study on ultrasound-assisted alkali extractions of polysaccharides from wheat straw reported that the ratio of solvent to sample affected the extraction recovery [29]. This finding was also confirmed by another report revealing the increase of solvent to sample ratio from 10 to 40 could recover 1.5× polysaccharides from BaChu mushroom [30]. Increasing the ratio of solvent to sample will increase the amount of extracted starch. This result was in accordance with the mass transfer principle, where a greater ratio between the solid material and the solvent causes a higher concentration gradient, which is the driving force in solvent extraction [31]. A higher concentration gradient leads to a greater amount of starch extracted from cassava flour that diffuses into the solvent more effectively, thus promoting a higher starch yield. In contrast, the quadratic effect of solvent to sample ratio negatively influenced ( ) the starch yield. This occurrence indicated that some restriction was applied when increasing the solvent to sample ratio to a certain point to reach a higher starch yield. A higher ratio than the optimum solvent to sample ratio will not significantly enhance the extraction yield because the starch has been completely recovered from cassava flour. Additionally, the use of excessive solvent also caused the waste of solvent.
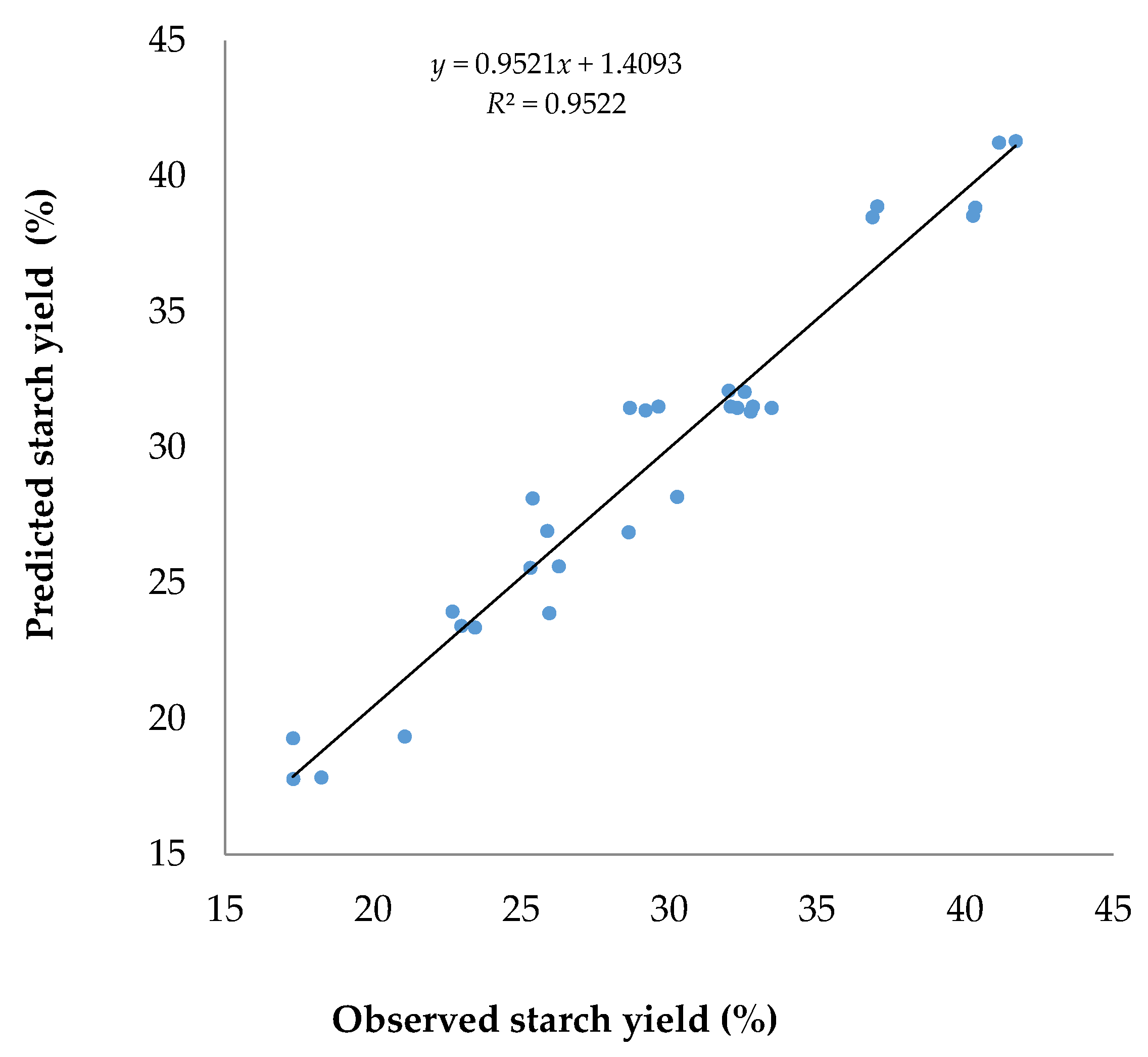
) the starch yield. This occurrence indicated that some restriction was applied when increasing the solvent to sample ratio to a certain point to reach a higher starch yield. A higher ratio than the optimum solvent to sample ratio will not significantly enhance the extraction yield because the starch has been completely recovered from cassava flour. Additionally, the use of excessive solvent also caused the waste of solvent.3.2. The Prediction Capability of the Predicted Model
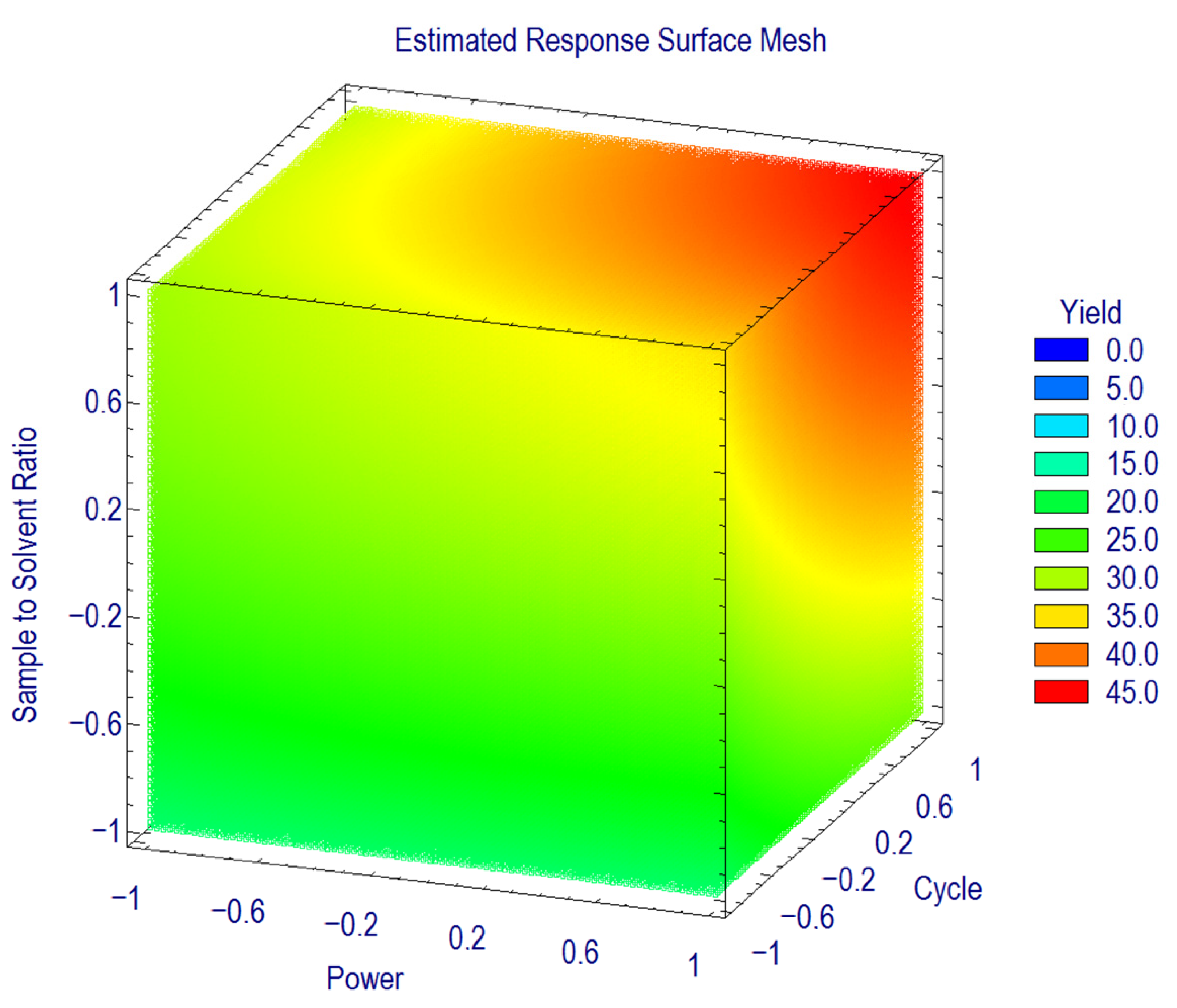
3.3. Optimization of Extraction Conditions
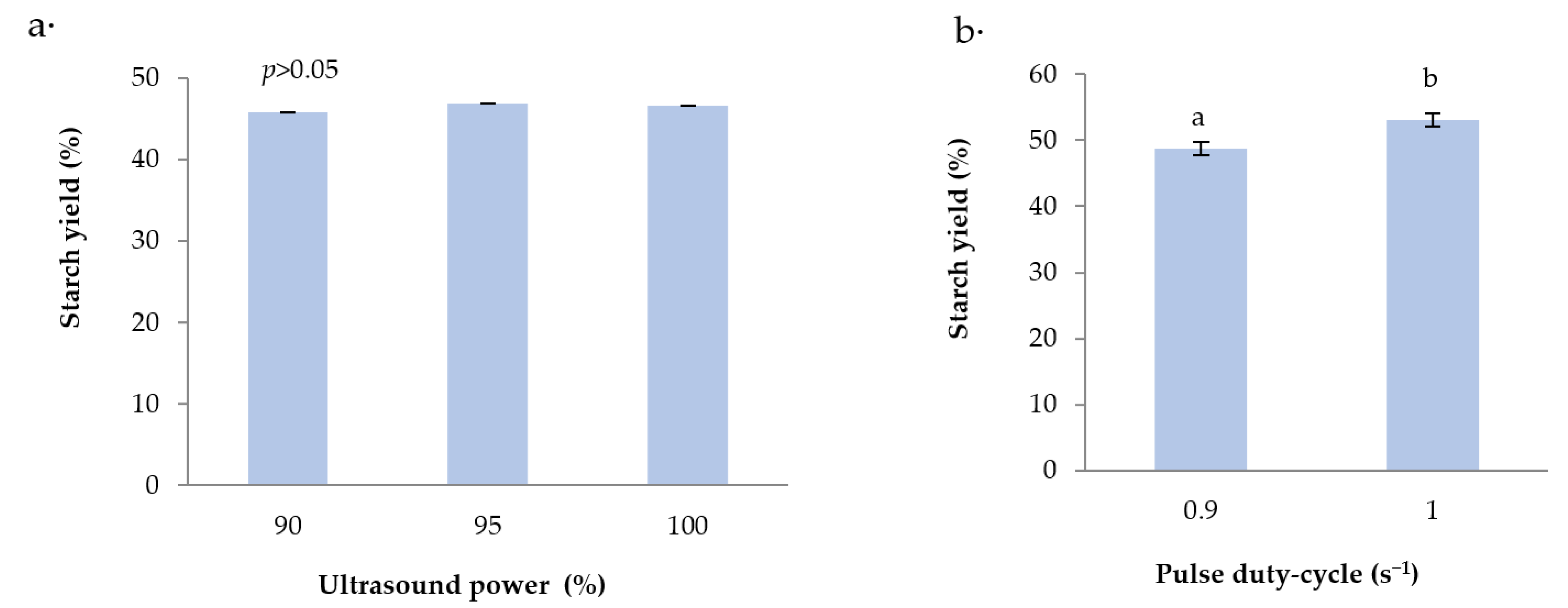
3.4. Further Optimization
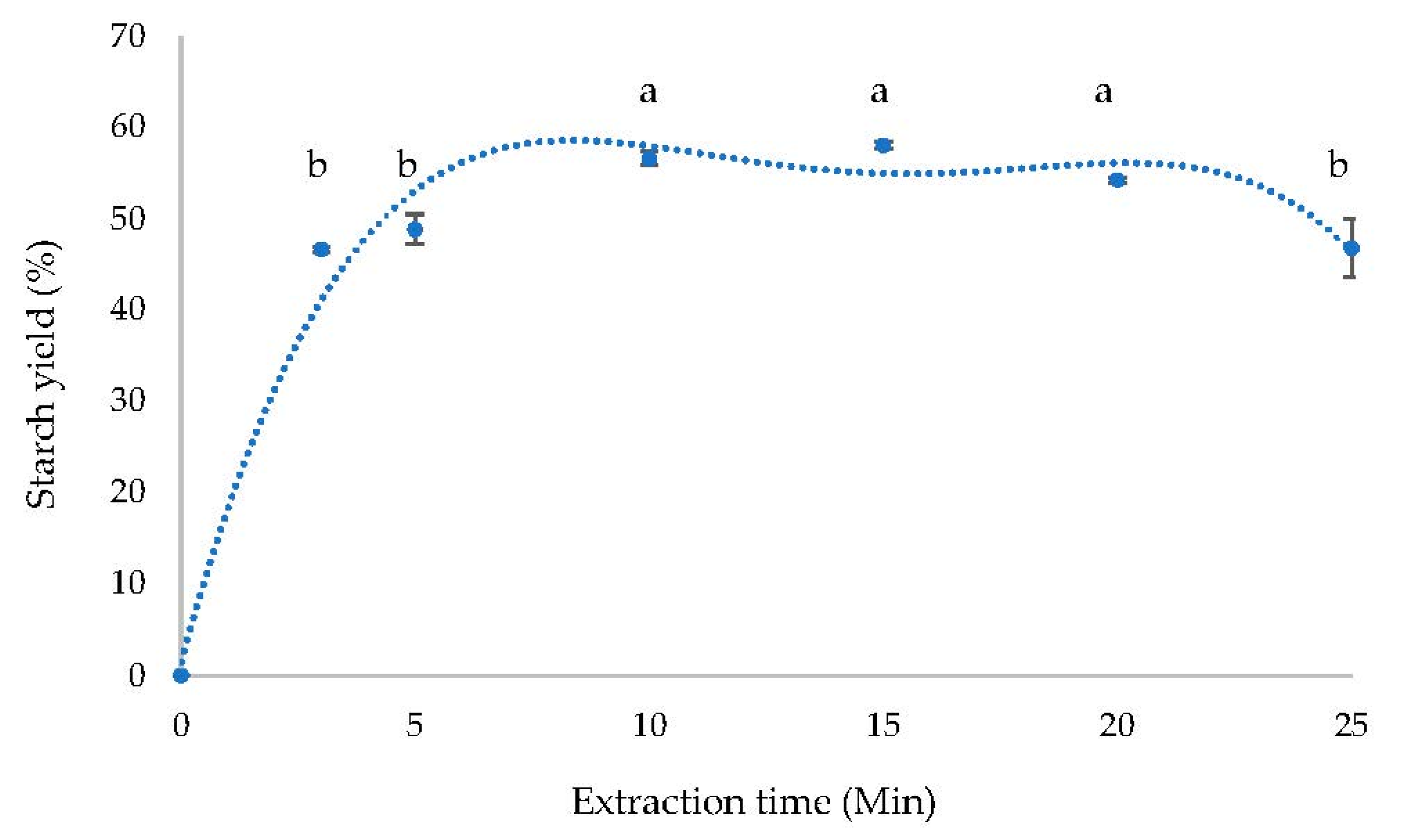
3.5. Kinetics Study
3.6. Ultrasound-Produced Starch Characterization
3.6.1. pH
3.6.2. Starch Content
3.6.3. Viscosity
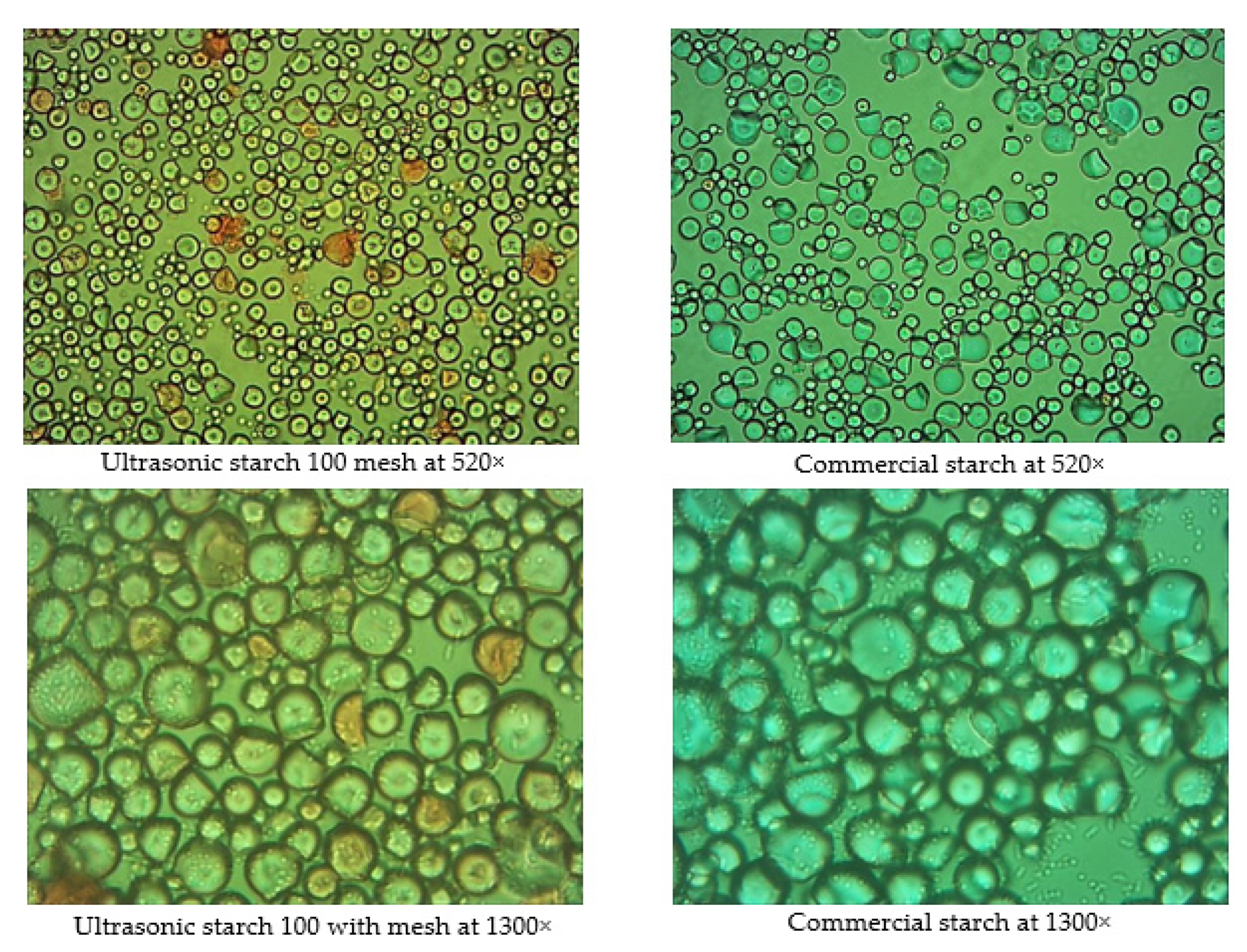
3.6.4. Optical Characteristics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wheatley, C.C.; Chuzel, G.; Zakhia, N. CASSAVA | Uses as a Raw Material. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 969–974. [Google Scholar]
- Chisenga, S.M.; Workneh, T.S.; Bultosa, G.; Alimi, B.A. Progress in research and applications of cassava flour and starch: A review. J. Food Sci. Technol. 2019, 56, 2799–2813. [Google Scholar] [CrossRef] [PubMed]
- Mtunguja, M.K.; Laswai, H.S.; Kanju, E.; Ndunguru, J.; Muzanila, Y.C. Effect of genotype and genotype by environment interaction on total cyanide content, fresh root, and starch yield in farmer-preferred cassava landraces in Tanzania. Food Sci. Nutr. 2016, 4, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Breuninger, W.F.; Piyachomkwan, K.; Sriroth, K. Tapioca/Cassava Starch. In Starch; Elsevier: Amsterdam, The Netherlands, 2009; pp. 541–568. [Google Scholar]
- Eckhoff, S.R.; Watson, S.A. Corn and Sorghum Starches: Production, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; ISBN 9780127462752. [Google Scholar]
- Johnson, R.; Moorthy, S.N.; Padmaja, G. Production of high fructose syrup from cassava and sweet potato flours and their blends with cereal flours. Food Sci. Technol. Int. 2010, 16, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Nand, A.V.; Charan, R.P.; Rohindra, D.; Khurma, J.R. Isolation and properties of starch from some local cultivars of cassava and taro in Fiji. South Pacific J. Nat. Appl. Sci. 2008, 26, 45. [Google Scholar] [CrossRef][Green Version]
- Mweta, D.E.; Labuschagne, M.T.; Koen, E.; Benesi, I.R.M.; John, D.; Saka, K. Some properties of starches from cocoyam (Colocasia esculenta) and cassava (Manihot esculenta Crantz.) grown in Malawi. African J. Food Sci. 2008, 2, 102–111. [Google Scholar]
- Vanier, N.L.; El Halal, S.L.M.; Dias, A.R.G.; Da Rosa Zavareze, E. Molecular structure, functionality and applications of oxidized starches: A review. Food Chem. 2017, 221, 1546–1559. [Google Scholar] [CrossRef]
- Chemat, F.; Huma, Z.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Rahman, S.M.M.; Rakshit, S.K. Effect of Endogenous and Commercial Enzyme on Improving Extraction of Sweet Potato Starch. In Proceedings of the 2004 ASAE Annual Meeting, Ottawa, Canada, 1–4 August 2004. [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Bernardo, C.O.; Ascheri, J.L.R.; Chávez, D.W.H.; Carvalho, C.W.P. Ultrasound Assisted Extraction of Yam (Dioscorea bulbífera) Starch: Effect on Morphology and Functional Properties. Starch Stärke 2018, 70, 1700185. [Google Scholar] [CrossRef]
- González-Lemus, L.B.; Calderón-Domínguez, G.; Salgado-Cruz, M.d.l.P.; Díaz-Ramírez, M.; Ramírez-Miranda, M.; Chanona-Pérez, J.J.; Gϋemes-Vera, N.; Farrera-Rebollo, R.R. Ultrasound-assisted extraction of starch from frozen jicama (P. erosus) roots: Effect on yield, structural characteristics and thermal properties. CyTA J. Food 2018, 16, 738–746. [Google Scholar] [CrossRef]
- Sit, N.; Misra, S.; Deka, S.C. Yield and Functional Properties of Taro Starch as Affected by Ultrasound. Food Bioprocess Technol. 2014, 7, 1950–1958. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Herceg, Z.; Šubarić, D.; Babić, J.; Brnčić, M.; Brnčić, S.R.; Bosiljkov, T.; Čvek, D.; Tripalo, B.; Gelo, J. Ultrasound effect on physical properties of corn starch. Carbohydr. Polym. 2010, 79, 91–100. [Google Scholar] [CrossRef]
- Bendicho, C.; Lavilla, I. Ultrasound-Assisted Metal Extractions; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9780124095472. [Google Scholar]
- Setyaningsih, W.; Saputro, I.E.; Palma, M.; Barroso, C.G. Optimization of the ultrasound-assisted extraction of tryptophan and its derivatives from rice (Oryza sativa) grains through a response surface methodology. J. Cereal Sci. 2017, 75, 192–197. [Google Scholar] [CrossRef]
- Krishnakumar, T.; Rawson, A. Optimization of Ultrasound-Assisted Extraction (UAE) of Starch from Cassava (Manihot esculenta Grantz) Using Response Surface Methodology (RSM); ICAT-CTCRI: Tamil Nadu, India, 2016. [Google Scholar]
- Mandal, S.C.; Mandal, V.; Das, A.K. Classification of Extraction Methods, in: Essentials of Botanical Extraction; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 83–136. [Google Scholar]
- Suhaimi, S.H.; Hasham, R.; Hafiz Idris, M.K.; Ismail, H.F.; Mohd Ariffin, N.H.; Abdul Majid, F.A. Optimization of Ultrasound-Assisted Extraction Conditions Followed by Solid Phase Extraction Fractionation from Orthosiphon stamineus Benth (Lamiace) Leaves for Antiproliferative Effect on Prostate Cancer Cells. Molecules 2019, 24, 4183. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Li, J.; Zhu, C. Effect of ultrasound pretreatment on enzymolysis and physicochemical properties of corn starch. Adv. Eng. Res. 2018, 164, 572–576. [Google Scholar]
- Jia, X.; Zhang, C.; Hu, J.; He, M.; Bao, J.; Wang, K.; Li, P.; Chen, M.; Wan, J.; Su, H.; et al. Ultrasound-assisted extraction, antioxidant and anticancer activities of the polysaccharides from Rhynchosia minima root. Molecules 2015, 20, 20901–20911. [Google Scholar] [CrossRef]
- Charpe, T.W.; Rathod, V.K. Kinetics of ultrasound assited extrction of wedelolactone from Eclipta alba. Braz. J. Chem. Eng. 2016, 33, 1003–1010. [Google Scholar] [CrossRef]
- Standar Nasional Indonesia. BSN SNI 3451:2011 Tapioka; Standar Nasional Indonesia: Jakarta, Indonesia, 2011; pp. 1–38. [Google Scholar]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Ebringerová, A.; Hromádková, Z. An overview on the application of ultrasound in extraction, separation and purification of plant polysaccharides. Cent. Eur. J. Chem. 2010, 8, 243–257. [Google Scholar] [CrossRef]
- XuJie, H.; Na, Z.; SuYing, X.; ShuGang, L.; BaoQiu, Y. Extraction of BaChu mushroom polysaccharides and preparation of a compound beverage. Carbohydr. Polym. 2008, 73, 289–294. [Google Scholar] [CrossRef]
- Elboughdiri, N. Effect of time, solvent-solid ratio, ethanol concentration and temperature on extraction yield of phenolic compounds from olive leaves. Eng. Technol. Appl. Sci. Res. 2018, 8, 2805–2808. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.M. Mempelajari Karakteristik Kimia Dan Fisik Tepung Tapioka Dan Mocal (Modified Cassava Flour) Sebagai Penyalut Kacang Pada Produk Kacang Salut. Ph.D. Thesis, IPB University, Dramaga, Indonesia, 2007; pp. 34–81. [Google Scholar]
- Stevenson, D.L.; Kennedy, J.F. Starch conversion technology: Edited by C. M. A. Beynum and J. A. Roels, Marcel Dekker Inc., New York, 1985. pp. 362. ISBN 0-8247-7194-X. Br. Polym. J. 1987, 19, 417. [Google Scholar] [CrossRef]
- Iida, Y.; Tuziuti, T.; Yasui, K.; Towata, A.; Kozuka, T. Control of viscosity in starch and polysaccharide solutions with ultrasound after gelatinization. Innov. Food Sci. Emerg. Technol. 2008, 9, 140–146. [Google Scholar] [CrossRef]
- Ashokkumar, M. Applications of ultrasound in food and bioprocessing. Ultrason. Sonochem. 2015, 25, 17–23. [Google Scholar] [CrossRef]
- Zisu, B.; Schleyer, M.; Chandrapala, J. Application of ultrasound to reduce viscosity and control the rate of age thickening of concentrated skim milk. Int. Dairy J. 2013, 31, 41–43. [Google Scholar] [CrossRef]
- Manchun, S.; Nunthanid, J.; Limmatvapirat, S.; Sriamornsak, P. Effect of ultrasonic treatment on physical properties of tapioca starch. Adv. Mater. Res. 2012, 506, 294–297. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Fang, G.; Man, Y.; Liu, Y. Effect of Ultrasound-Assisted Isolation on Yield and Properties of High-Amylose Starch from Amylomaize. Starch Staerke 2019, 71, 1800292. [Google Scholar] [CrossRef]
- Herceg, I.L.; Jambrak, A.R.; Šubarić, D.; Brnčić, M.; Brnčić, S.R.; Badanjak, M.; Tripalo, B.; Ježek, D.; Novotni, D.; Herceg, Z. Texture and pasting properties of ultrasonically treated corn starch. Czech J. Food Sci. 2010, 28, 83–93. [Google Scholar] [CrossRef]
- Zhu, F. Composition, structure, physicochemical properties, and modifications of cassava starch. Carbohydr. Polym. 2015, 122, 456–480. [Google Scholar] [CrossRef]
- Wu, Y.; Du, X.; Ge, H.; Lv, Z. Preparation of microporous starch by glucoamylase and ultrasound. Starch Stärke 2011, 63, 217–225. [Google Scholar] [CrossRef]
 indicates a positive effect while
indicates a positive effect while  indicates a negative effect.
indicates a negative effect.
 indicates a positive effect while
indicates a positive effect while  indicates a negative effect.
indicates a negative effect.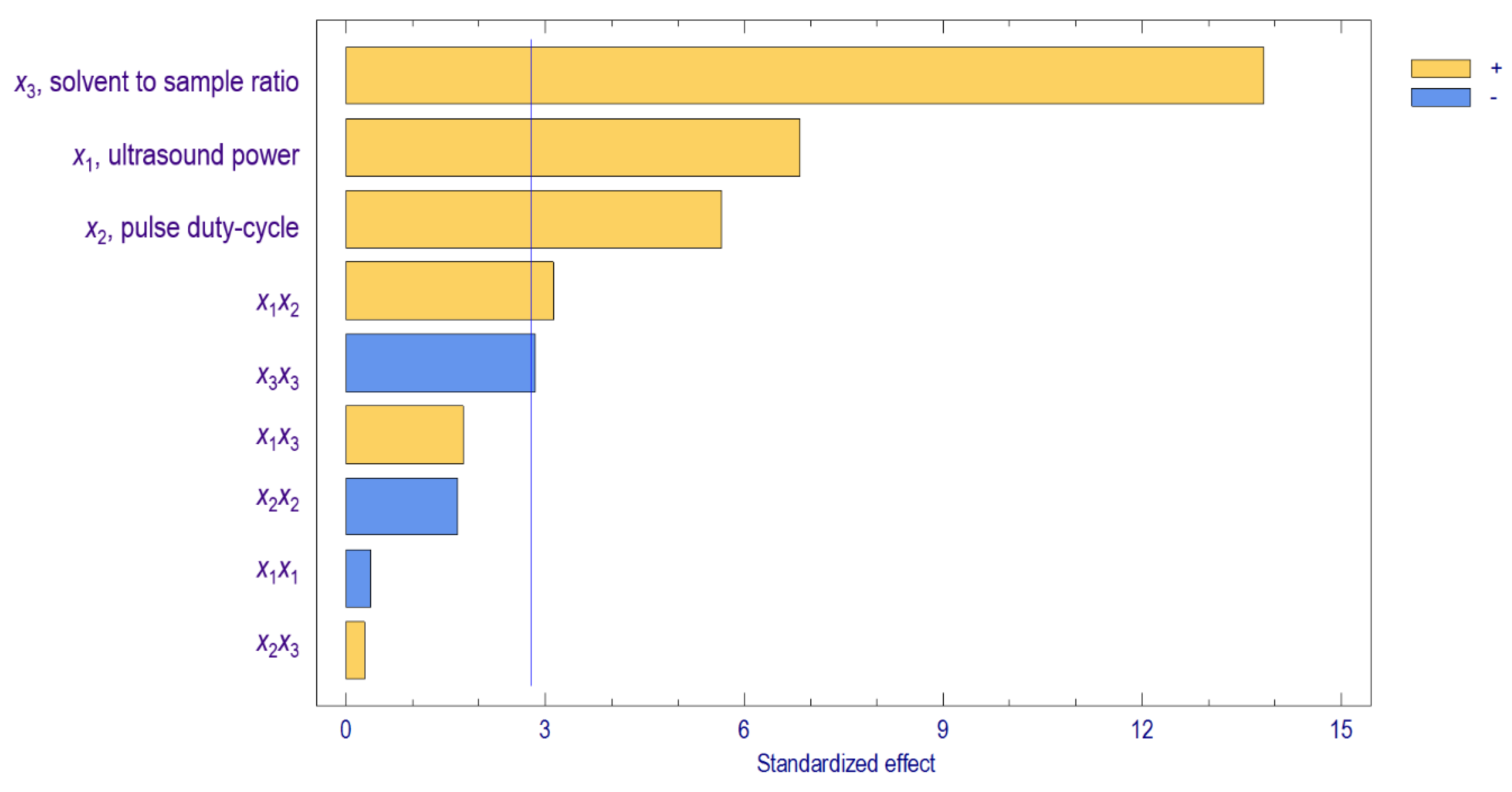
| Variable | Code | −1 | 0 | +1 | Unit |
|---|---|---|---|---|---|
| Ultrasound power | x1 | 30 | 60 | 90 | % |
| Pulse duty-cycle | x2 | 0.3 | 0.6 | 0.9 | s−1 |
| Solvent to sample ratio | x3 | 10:1 | 20:1 | 30:1 | (v/w) |
| Run | x1 | x2 | x3 | Starch Yield (%) |
|---|---|---|---|---|
| 1 | 1 | −1 | 0 | 30.26 |
| 2 | −1 | 1 | 0 | 25.87 |
| 3 | 1 | 1 | 0 | 37.02 |
| 4 | 1 | 0 | 1 | 41.70 |
| 5 | −1 | 0 | −1 | 21.06 |
| 6 | 0 | 0 | 0 | 32.82 |
| 7 | 0 | 1 | 1 | 40.25 |
| 8 | 0 | −1 | 1 | 32.00 |
| 9 | 0 | 0 | 0 | 29.63 |
| 10 | −1 | −1 | 0 | 26.27 |
| 11 | 0 | 1 | −1 | 22.97 |
| 12 | 0 | −1 | −1 | 18.24 |
| 13 | −1 | 0 | 1 | 29.19 |
| 14 | 0 | 0 | 0 | 32.07 |
| 15 | 1 | 0 | −1 | 22.67 |
| 16 | 1 | 0 | −1 | 25.94 |
| 17 | 1 | 0 | 1 | 41.13 |
| 18 | −1 | −1 | 0 | 25.30 |
| 19 | 0 | 0 | 0 | 32.29 |
| 20 | 1 | −1 | 0 | 25.38 |
| 21 | −1 | 1 | 0 | 28.62 |
| 22 | −1 | 0 | 1 | 32.74 |
| 23 | 0 | 0 | 0 | 28.66 |
| 24 | 0 | −1 | 1 | 32.54 |
| 25 | 0 | 1 | −1 | 23.43 |
| 26 | 0 | 0 | 0 | 33.45 |
| 27 | 1 | 1 | 0 | 40.33 |
| 28 | 0 | 1 | 1 | 36.86 |
| 29 | 0 | −1 | −1 | 17.30 |
| 30 | −1 | 0 | −1 | 17.29 |
| Temperature (°C) | Viscosity (cP) | |
|---|---|---|
| Ultrasonic Results | Commercial | |
| 60 | 2.4 ± 0.06 | 2.0 ± 0.10 |
| 65 | 1868.0 ± 2.88 | 1972.0 ± 2.51 |
| 70 | 1920.0 ± 0.57 | 1996.0 ± 5.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setyaningsih, W.; Karmila; Fathimah, R.N.; Cahyanto, M.N. Process Optimization for Ultrasound-Assisted Starch Production from Cassava (Manihot esculenta Crantz) Using Response Surface Methodology. Agronomy 2021, 11, 117. https://doi.org/10.3390/agronomy11010117
Setyaningsih W, Karmila, Fathimah RN, Cahyanto MN. Process Optimization for Ultrasound-Assisted Starch Production from Cassava (Manihot esculenta Crantz) Using Response Surface Methodology. Agronomy. 2021; 11(1):117. https://doi.org/10.3390/agronomy11010117
Chicago/Turabian StyleSetyaningsih, Widiastuti, Karmila, Rohmah Nur Fathimah, and Muhammad Nur Cahyanto. 2021. "Process Optimization for Ultrasound-Assisted Starch Production from Cassava (Manihot esculenta Crantz) Using Response Surface Methodology" Agronomy 11, no. 1: 117. https://doi.org/10.3390/agronomy11010117
APA StyleSetyaningsih, W., Karmila, Fathimah, R. N., & Cahyanto, M. N. (2021). Process Optimization for Ultrasound-Assisted Starch Production from Cassava (Manihot esculenta Crantz) Using Response Surface Methodology. Agronomy, 11(1), 117. https://doi.org/10.3390/agronomy11010117