Indicative Value of the Dominant Plant Species for a Rapid Evaluation of the Nutritional Value of Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Bayesian Statistics and Decision Trees
3. Results
3.1. Bayesian Analysis
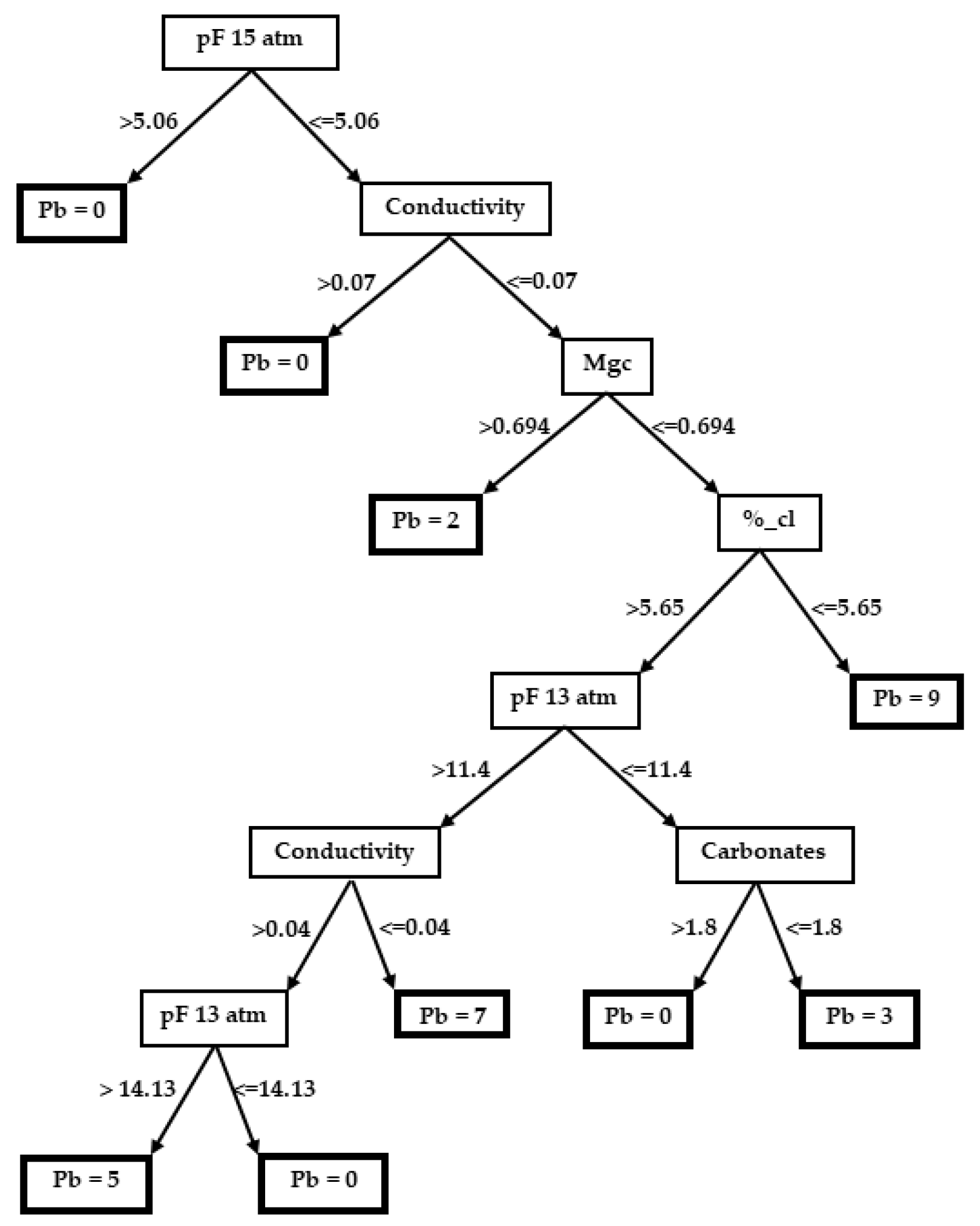
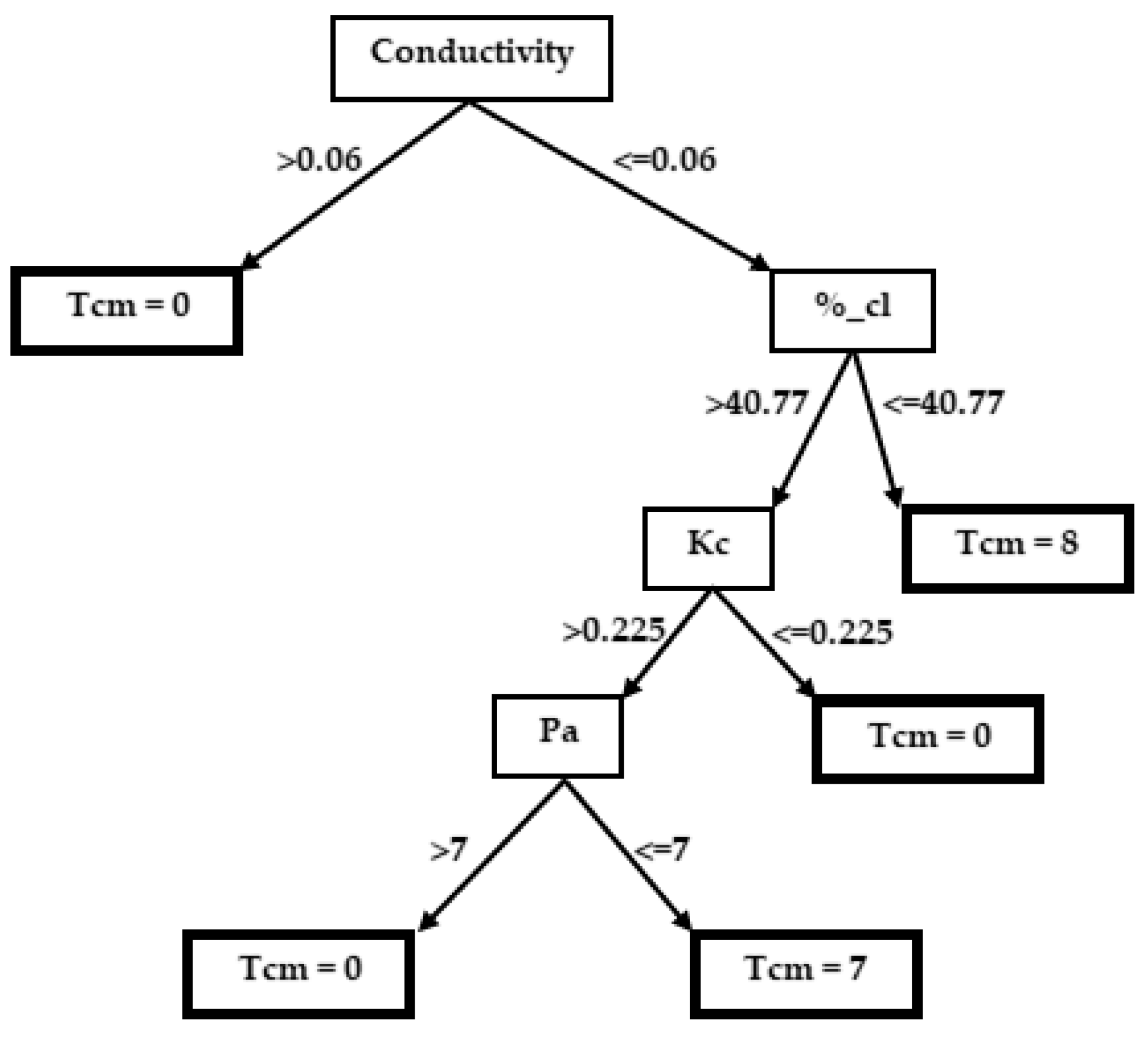
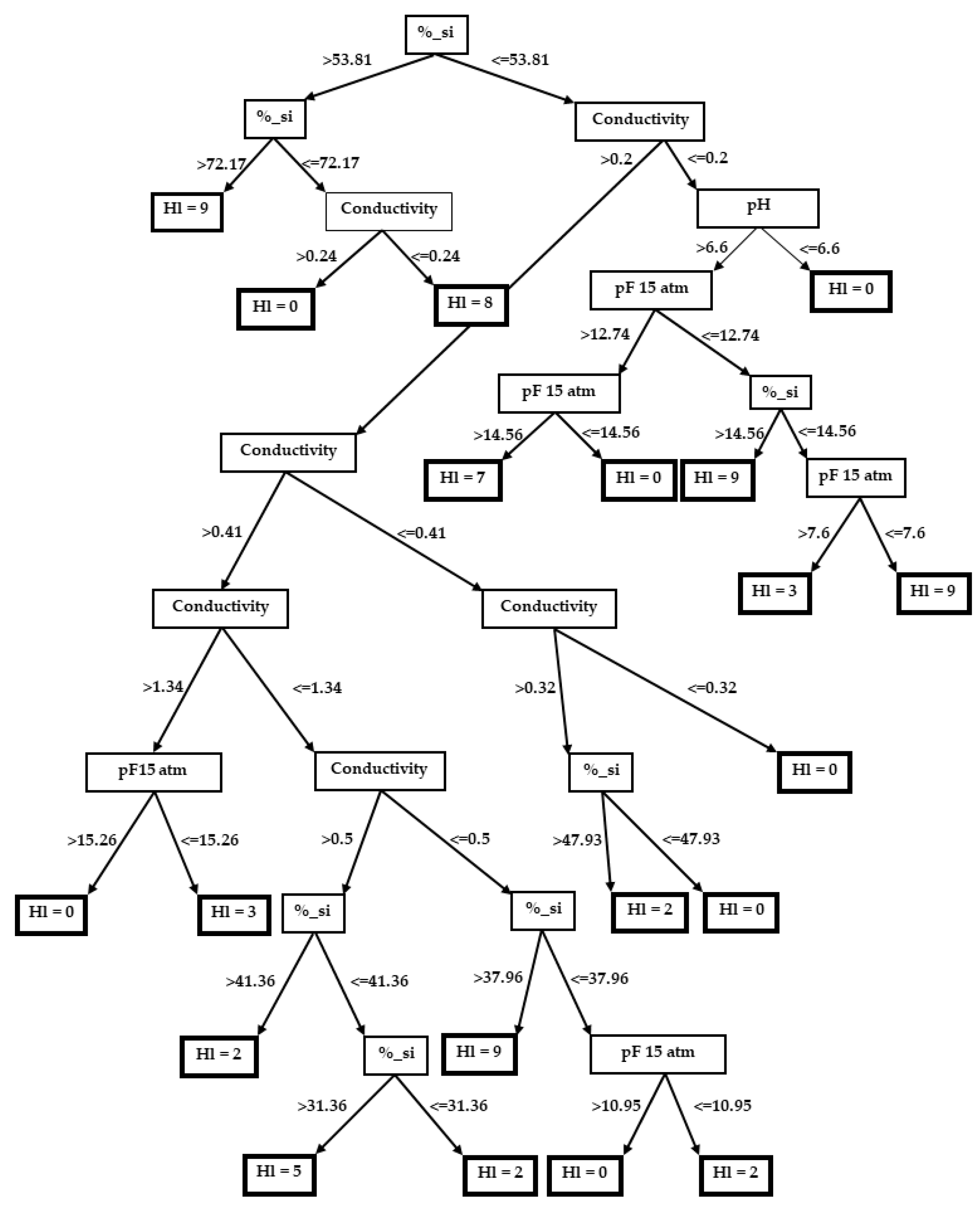
3.2. Decision Trees
3.2.1. Plantago bellardii
3.2.2. Taeniatherum caput-medusae
3.2.3. Hordeum leporinum
3.2.4. Glebionis discolor (=Chrysanthemum coronarium var. discolor)
3.3. Soil Attributes of the Other Species Studied
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cano-Ortiz, A.; Pinto Gomes, C.J.; Cano, E. Current situation of the Gaudinio fragilis—Hordeion bulbosi alliance in the Iberian Peninsula. Acta Bot. Gall. 2009, 156, 19–31. [Google Scholar] [CrossRef][Green Version]
- Perrino, E.V.; Ladisa, G.; Calabrese, G. Flora and plant genetic resources of ancient olive groves of Apulia (southern Italy). Genet. Resour. Crop Evol. 2014, 61, 23–53. [Google Scholar] [CrossRef]
- Perrino, E.V.; Magazzini, P.; Musarella, C.M. Management of grazing “buffalo” to preserve habitats by Directive 92/43 EEC in a wetland protected area of the Mediterranean coast: Palude Frattarolo, Apulia, Italy. Euro-Mediterr. J. Environ. Integr. 2020, in press. [Google Scholar]
- García Fuentes, A.; Cano, E. Malas Hierbas del Olivar Giennense; Instituto de Estudios Giennenses: Jaén, Spain, 1996; 213p. [Google Scholar]
- Pastor Muñoz-Cobo, M. Estudio de Diversos Métodos de Manejo del Suelo Alternativos al Laboreo en el Cultivo del Olivo; Instituto de Estudios Giennenses: Jaén, Spain, 1991; 302p. [Google Scholar]
- Saavedra Saavedra, M.M.; Pastor Muñoz-Cobo, M. Sistemas de Cultivo en Olivar; Agrícola Española, S.A., Ed.; Manejo de mals hierbas y herbicidas: Madrid, Spain, 2002; 429p. [Google Scholar]
- Giusso del Galdo, G.; Marcenò, C.; Musarella, C.M.; Sciandrello, S. La vegetazione costiera della R.N.O. “Torre Salsa” (Siculiana—AG). Inf. Bot. Ital. 2008, 40, 73–89. [Google Scholar]
- Mendes, P.; Meireles, C.; Vila-Viçosa, C.; Musarella, C.M.; Pinto-Gomes, C. Best management practices to face degraded territories occupied by Cistus ladanifer shrublands—Portugal case study. Plant Biosyst. 2015, 149, 494–502. [Google Scholar] [CrossRef]
- Signorino, G.; Cannavò, S.; Crisafulli, A.; Musarella, C.M.; Spampinato, G. Fagonia cretica L. In Schede per una Lista Rossa della Flora vascolare e crittogamica Italiana. Inf. Bot. Ital. 2011, 43, 381–458. [Google Scholar]
- Puglisi, M.; Sciandrello, S.; Musarella, C.M.; Spampinato, G.; Privitera, M.; Tomaselli, V. Bryosociological remarks on garrigue environments in Apulia Region (Southern Italy). Plant Sociol. 2019, 56, 43–52. [Google Scholar] [CrossRef]
- Quinto-Canas, R.; Mendes, P.; Cano-Ortiz, A.; Musarella, C.M.; Pinto-Gomes, C. Forest fringe communities of the southwestern Iberian Peninsula. Rev. Chapingo Ser. Cienc. 2018, 24, 415–434. [Google Scholar] [CrossRef]
- Sciandrello, S.; Musarella, C.M.; Puglisi, M.; Spampinato, G.; Tomaselli, V.; Minissale, P. Updated and new insights on the coastal halophilous vegetation of southeastern Sicily (Italy). Plant Sociol. 2019, 56, 81–98. [Google Scholar] [CrossRef]
- Spampinato, G.; Musarella, C.M.; Cano-Ortiz, A.; Signorino, G. Habitat, occurrence and conservation status of the Saharo-Macaronesian and Southern-Mediterranean element Fagonia cretica L. (Zygophyllaceae) in Italy. J. Arid Land 2018, 10, 140–151. [Google Scholar] [CrossRef]
- Spampinato, G.; Sciandrello, S.; del Galdo, G.; Puglisi, M.; Tomaselli, V.; Cannavò, S.; Musarella, C.M. Contribution to the knowledge of Mediterranean wetland biodiversity: Plant communities of the Aquila Lake (Calabria, Southern Italy). Plant Sociol. 2019, 56, 53–68. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Latini, M.; Iberite, M.; Bonari, G.; Nicolella, G.; Rosati, L.; Salerno, G.; Abbate, G. The segetal flora of winter cereals and allied crops in Italy: Species inventory with chorological, structural and ecological features. Plant Biosyst. 2020, 154, 935–946. [Google Scholar] [CrossRef]
- Cano-Ortiz, A.; Biondi, E.; Pinto-Gomes, C.J.; Del Río González, S.; Cano, E. Soil and phytosociological characterization of grasslands in the western Mediterranean. Am. J. Plant Sci. 2014, 5, 3213–3240. [Google Scholar] [CrossRef][Green Version]
- Cano-Ortiz, A. Bioindicadores ecológicos y manejo de cubiertas vegetales como herramienta para la implantación de una agricultura sostenible. Ph.D. Thesis, University of Jaén, Jaén, Spain, 2007. [Google Scholar]
- Taffetani, F.; Rismondo, M. Bioindicator system for the evaluation of the environmental quality of agro-ecosystems. Fitosociologia 2009, 46, 3–22. [Google Scholar]
- Baldoni, M.; Biondi, E.; Loiotile, A. La vegetazione infestante i vigneti nelle Marche. Fitosociologia 2001, 38, 63–68. [Google Scholar]
- Biondi, E.; Baldoni, M. La vegetazione di margine stradale dell’ordine Brometalia rubenti-tectori nell’Italia Centrale. Ann. Bot. 1991, 49 (Suppl. 8), 214–217. [Google Scholar]
- Biondi, E.; Bagella, S.; Casavecchia, S.; Pinzi, M.; Vagge, I. La vegetazione a Dasypyrum villosum (L.) P. Candargy lungo le coste dell’Italia Settentrionale. Doc. Phytosociol. 1999, 19, 439–446. [Google Scholar]
- Cano-Ortiz, A.; Pinto-Gomes, C.J.; Esteban Ruiz, F.J.; Rodríguez Torres, A.; Goñi, J.; De la Haza, I.; Cano, E. Biodiversity of Hordeion leporini in Portugal: A phytosociological and edaphic análisis. Acta Bot. Gall. 2009, 156, 33–48. [Google Scholar] [CrossRef]
- Cano-Ortiz, A.; Pinto-Gomes, C.J.; Esteban Ruiz, F.J.; Cano, E. Determination of the nutritional state of soils by means of the phytosociological method and different statistical techniques (Bayesian statistics and decision trees) in Spain. Acta Bot. Gall. 2009, 156, 607–624. [Google Scholar] [CrossRef][Green Version]
- Cano-Ortiz, A.; Pinto-Gomes, C.J.; Cano, E. Contribution to the study of the Taeniathero-Aegilopion geniculatae alliance in Portugal. Acta Bot. Gall. 2010, 157, 599–610. [Google Scholar] [CrossRef][Green Version]
- Quinto-Canas, R.; Mendes, P.; Cano-Ortiz, A.; Musarella, C.M.; Pinto-Gomes, C. The Agrostion castellanae Rivas Goday 1957 corr. Rivas Goday & Rivas-Martínez 1963 alliance in the southwestern Iberian Peninsula. Plant Sociol. 2018, 55, 21–29. [Google Scholar] [CrossRef]
- Rivas-Martínez, S. La vegetación de Hordeion leporini en España. Doc. Phytosoc. 1978, 9, 377–392. [Google Scholar]
- Di Pietro, R.; Theurillat, J.P.; Capelo, J.; Fernández-González, F.; Terzi, M.; Carni, A.; Mucina, L. Nomenclature and syntaxonomic notes on some high-rank syntaxa of the European grassland vegetation. Lazaroa 2015, 36, 79–106. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Moretti, M.; Abbate, G. The anthropogenic grasslands of the Securigero securidacae-Dasypyrion villosi in central mediterranean areas: Synecology, distribution and syntaxonomy. Ann. Bot. 2019, 9, 1–38. [Google Scholar] [CrossRef]
- Perrino, E.V.; Tomaselli, V.; Signorile, G.; Angiulli, F.; Silletti, G. Vegetation to Crambe hispanica L. in Apulia Region (Vegetazione a Crambe hispanica L. in Puglia). Fitosociologia 2011, 48, 99–107. [Google Scholar]
- Cano, E.; Musarella, C.M.; Cano-Ortiz, A.; Piñar Fuentes, J.C.; Spampinato, G.; Pinto Gomes, C.J. Morphometric analysis bioclimatic distribution of Glebionis coronaria s.l. (Asteraceae) in the Mediterranean area. PhytoKeys 2017, 81, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Perrino, E.V.; Tomaselli, V.; Costa, R.; Pavone, P. Conservation status of habitats (Directive 92/43 EEC) of coastal and low hill belts in a mediterranean biodiversity hot spot (Gargano—Italy). Plant Biosyst. 2013, 147, 1006–1028. [Google Scholar] [CrossRef]
- Perrino, E.V.; Calabrese, G. Endangered segetal species in southern Italy: Distribution, conservation status, trends, actions and ethnobotanical notes. Genet. Resour. Crop. Evol. 2018, 65, 2107–2134. [Google Scholar] [CrossRef]
- Pesaresi, S.; Galdenzi, D.; Biondi, E.; Casavecchia, S. Bioclimate of Italy: Application of the worldwide bioclimatic classification system. J. Maps 2014, 10, 538–553. [Google Scholar] [CrossRef]
- Pesaresi, S.; Biondi, E.; Casavecchia, S. Bioclimates of Italy. J. Maps 2017, 13, 955–960. [Google Scholar] [CrossRef]
- Sirsat, M.S.; Cernadas, E.; Fernández-Delgado, M.; Khan, R. Classification of agricultural soil parameters in India. Comput. Electron. Agric. 2017, 135, 269–279. [Google Scholar] [CrossRef]
- De Freitas, W.K.; Portz, A.; de Carvalho Peres, A.A.; Martinez Tarré, R.; de Melo Campos, M. Soil nutrient content and plant phytosociology in agroforestry systems of the Rio de Janeiro State highlands, Brazil. Acta Sci. Biol. Sci. 2018, 40, e35368. [Google Scholar] [CrossRef]
- Mansouri, M.; Dumont, B.; Leemans, V.; Destain, M.-F. Bayesian methods for predicting LAI and soil water Content. Precision. Agric. 2014, 15, 184–201. [Google Scholar] [CrossRef]
- Suchithra, M.S.; Pai, M.L. Improving the prediction accuracy of soil nutrient classification by optimizing extreme learning machine parameters. Inf. Process. Agric. 2019, 7, 72–82. [Google Scholar] [CrossRef]
- Braun-Blanquet, J.; Fitosociología, J. Bases para el Estudio de las Comunidades Vegetales; Ediciones Blume: Madrid, Spain, 1979. [Google Scholar]
- Van der Maarel, E. Transformation of cover-abundance values in phytosociology and its effects on community similarity. Vegetatio 1979, 39, 97–114. [Google Scholar]
- Cano, E.; Cano-Ortiz, A.; Musarella, C.M.; Piñar Fuentes, J.C.; Ighbareyeh, J.M.H.; Leiva Gea, F.; del Río, S. Mitigating Climate Change Through Bioclimatic Applications and Cultivation Techniques in Agriculture (Andalusia, Spain). In Sustainable Agriculture, Forest and Environmental; Jhariya, M.K., Banerjee, A., Meena, R.S., Yadav, D.K., Eds.; Springer Nature: Singapore, 2019; pp. 31–69. [Google Scholar]
- Rivas-Martínez, S.; Díez, T.E.; Fernandez-González, F.; Izco, J.; Loidi, J.; Lousã, M.; Penas, A. Vascular Plant Communities of Spain and Portugal. Part II. Itinera Geobotánica 2002, 15, 433–922. [Google Scholar]
- Perrino, E.V.; Brunetti, G.; Farrag, K. Plant communities of multi-metal contaminated soils: A case study in National Park of Alta Murgia (Apulia Region—Southern Italy). Int. J. Phytoremed. 2014, 16, 871–888. [Google Scholar] [CrossRef]
- Bank, R.R. Intraspecific and interspecific pair-wise seedling competition between exotic annual grasses and native perennials: Plant-soil relationship. Plant Soil 2010, 326, 331–343. [Google Scholar] [CrossRef]
- Shiwakoti, S.; Zheljazkov, V.D.; Gollany, H.T.; Kleber, M.; Xing, B. Macronutrients in Soil and Wheat as Affected by a Long-Term Tillage and Nitrogen Fertilization in Winter Wheat–Fallow Rotation. Agronomy 2019, 9, 178. [Google Scholar] [CrossRef]
- Ordoñez, J.C.; Van Bodegom, P.M.; Witte, J.P.H.; Wright, I.J.; Reich, P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- López-Felices, B.; Aznar-Sánchez, J.A.; Velasco-Muñoz, J.F.; Piquer-Rodríguez Mª. Contribution of irrigation ponds to the sustainability of agriculture. A review of worldwide research. Sustainability 2020, 12, 5425. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Wang, L.; Liu, Y.; Feng, Q.; Fang, X.; Liu, Y. Distribution of Shrubland and Grassland Soil Erodibility on the Loess Plateau. Int. J. Environ. Res. Public Health 2018, 15, 1193. [Google Scholar] [CrossRef]
- Pastor, J.; Lacasta, C.; Hernández, A.J. Evaluación de las cubiertas vegetales en el olivar de una zona semiárida del centro de España. Edafología 2000, 7, 165–175. [Google Scholar]
- Jeong, J.; Zhang, X. Model Application for Sustainable Agricultural Water Use. Agronomy 2020, 10, 396. [Google Scholar] [CrossRef]
- Maffia, A.; Pergola, M.; Palese, A.M.; Celano, G. Environmental Impact Assessment of Organic vs. Integrated Olive-Oil Systems in Mediterranean Context. Agronomy 2020, 10, 416. [Google Scholar] [CrossRef]
- Peter, B.G.; Messina, J.P.; Lin, Z.; Snapp, S.S. Crop climate suitability mapping on the cloud: A geovisualization application for sustainable agriculture. Sci. Rep. 2020, 10, 15487. [Google Scholar] [CrossRef]
- Bhattacharyya, C.; Banerjee, S.; Acharya, U.; Mitra, A.; Mallick, I.; Haldar, A.; Haldar, S.; Ghosh, A.; Ghosh, A. Evaluation of plant growth promotion properties and inductionof antioxidative defense mechanism by tea rhizobacteria of Darjeeling, India. Sci. Rep. 2020, 10, 15536. [Google Scholar] [CrossRef]
- Sahib, M.R.; Pervaiz, Z.H.; Williams, M.A.; Saleem, M.; DeBolt, S. Rhizobacterial species richness improves sorghum growth and soil nutrient synergism in a nutrient-poor greenhouse soil. Sci. Rep. 2020, 10, 15454. [Google Scholar] [CrossRef]
- Igor, B.; Leon Josip, T.; Paulo, P. Agriculture Management Impacts on Soil Properties and Hydrological Response in Istria (Croatia). Agronomy 2020, 10, 282. [Google Scholar] [CrossRef]
- He, J.; Jin, Y.; Turner, N.C.; Li, F.-M. Irrigation during Flowering Improves Subsoil Water Uptake and Grain Yield in Rainfed Soybean. Agronomy 2020, 10, 120. [Google Scholar] [CrossRef]
- Caser, M.; Demasi, S.; Victorino, Í.M.M.; Donno, D.; Faccio, A.; Lumini, E.; Bianciotto, V.; Scariot, V. Arbuscular Mycorrhizal Fungi Modulate the Crop Performance and Metabolic Profile of Saffron in Soilless Cultivation. Agronomy 2019, 9, 232. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, C.; Teng, W.; Wang, J.; Lyu, X.; Dong, S.; Kang, S.; Gong, Z.; Ma, C. Accumulation and Distribution of Fertilizer Nitrogen and Nodule-Fixed Nitrogen in Soybeans with Dual Root Systems. Agronomy 2020, 10, 397. [Google Scholar] [CrossRef]
- Dhalaria, R.; Kumar, D.; Kumar, H.; Nepovimova, E.; Kuča, K.; Torequl Islam, M.; Verma, R. Arbuscular Mycorrhizal Fungi as Potential Agents in Ameliorating Heavy Metal Stress in Plants. Agronomy 2020, 10, 815. [Google Scholar] [CrossRef]
- Allito, B.B.; Ewusi-Mensah, N.; Logah, V. Legume-Rhizobium Strain Specificity Enhances Nutrition and Nitrogen Fixation in Faba Bean (Vicia faba L.). Agronomy 2020, 10, 826. [Google Scholar] [CrossRef]
- Kalembasa, S.; Szukała, J.; Faligowska, A.; Kalembasa, D.; Symanowicz, B.; Becher, M.; Gebus-Czupyt, B. Quantification of Biologically Fixed Nitrogen by White Lupin (Lupins albus L.) and Its Subsequent Uptake by Winter Wheat Using the 15N Isotope Dilution Method. Agronomy 2020, 10, 1392. [Google Scholar] [CrossRef]
- Nagaraj, K.; Vanishree, S.; Muthukumar, T. Genotypic variation in response and dependency of Cajanus cajan (L.) Millsp., on arbuscular mycorrhizal fungi in a tropical Alfisol. Plant Biosyst. 2020, 1–13. [Google Scholar] [CrossRef]
- Püschel, D.; Janoušková, M.; Voříšková, A.; Gryndlerová, H.; Vosátka, M.; Jansa, J. Arbuscular mycorrhiza stimulates biological nitrogen fixation in two Medicago spp. through improved phosphorus acquisition. Front Plant Sci. 2017, 8, 390. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; de Bruin, S.; Luckerhoff, L.; van Logtestijn, R.S.P.; Schlaeppi, K. A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME J. 2016, 10, 389–399. [Google Scholar] [CrossRef]
- Jakubus, M.; Graczyk, M. Microelement Variability in Plants as an Effect of Sewage Sludge Compost Application Assessed by Different Statistical Methods. Agronomy 2020, 10, 642. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Zhu, S.; Xu, Y.; Li, H.; Zheng, X.; Shi, R. Combined Application of Organic and Inorganic Nitrogen Fertilizers Affects Soil Prokaryotic Communities Compositions. Agronomy 2020, 10, 132. [Google Scholar] [CrossRef]
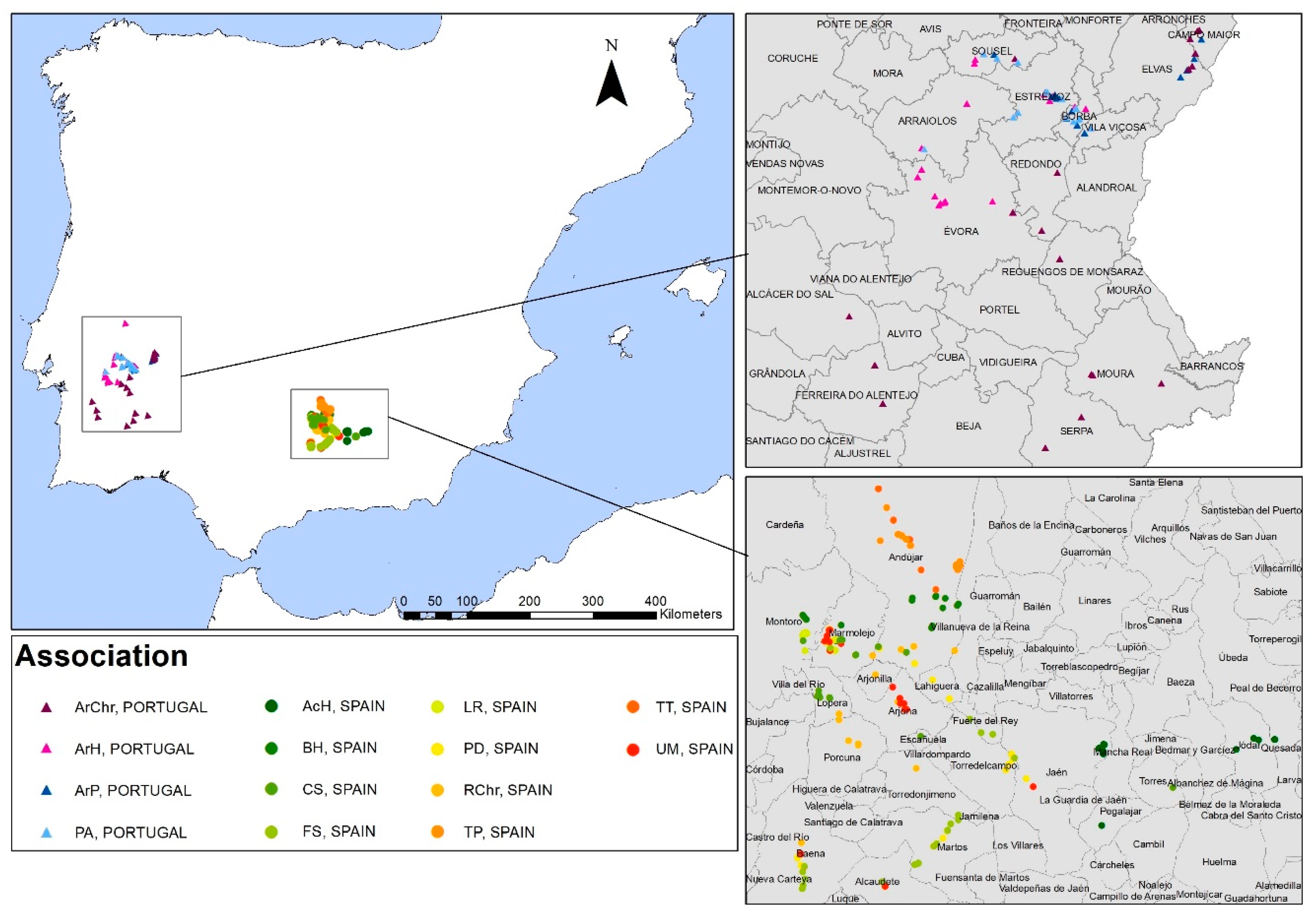
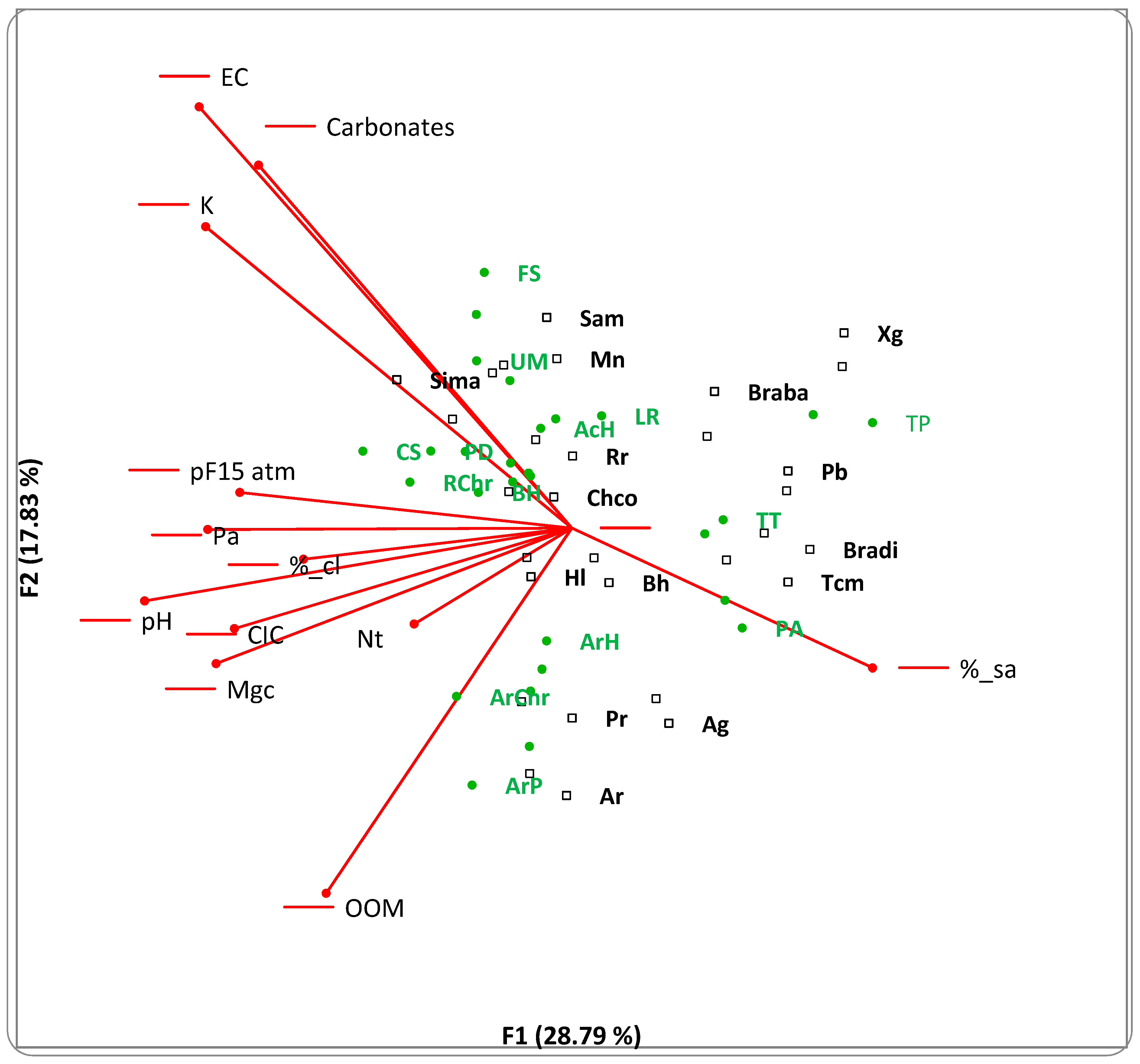

| Plant Association | Dominant Species | Country | Locality | Date | Soil Type and pH | Pasture or Grassland Management | Reference |
|---|---|---|---|---|---|---|---|
| AcH | Hordeum leporinum | Spain | Jaén | Spring 2005 | Cambisols, basic | Pasturage | [22] |
| ArH | Hordeum leporinum | Portugal | Evora | Spring 2005 | Cambisols, acids and neutral | Tillage and herbicides | [17] |
| BH | Hordeum leporinum | Spain | Jaén | Spring 2005 | Regosols, acids | Pasturage | [22] |
| LR | Raphanus raphanistrum | Spain | Jaén | Spring 2005 | Cambisols, neutral | Tillage and herbicides | [17] |
| PD | Diplotaxis virgata | Spain | Jaén | Spring 2005 | Cambisols, basic and neutral | Tillage and herbicides | [17,26] |
| ArP | Papaver rhoeas | Portugal | Evora | Spring 2005 | Cambisols, neutral | Tillage and herbicides | [17] |
| ArChr | Glebionis coronaria | Portugal | Evora | Spring 2005 | Cambisols, neutral | Tillage and herbicides | [16] |
| RChr | Glebionis coronaria | Spain | Jaén | Spring 2005 | Cambisols, basic | Tillage and herbicides | [17,26] |
| UM | Malva neglecta | Spain | Jaén | Spring 2005 | Cambisols, indifferent pH | Tillage and herbicides | [17] |
| PA | Triticum vagans | Portugal | Evora | Spring 2005 | Regosols, neutral | Pasturage | [24] |
| TT | Taeniatherum caput-medusae | Spain | Jaén | Spring 2005 | Regosols, acids | Pasturage | [24] |
| TP | Plantago bellardii | Spain | Jaén | Spring 2005 | Regosols, acids | Pasturage | [17] |
| FS | Sinapis alba subsp. mairei | Spain | Jaén | Spring 2005 | Cambisols, basic | Tillage and herbicides | [17] |
| CS | Sylibum marianum | Spain | Jaén | Spring 2005 | Cambisols, basic and neutral | Tillage and herbicides | [17] |
| Plant Association | CEC | Nt | Pa | Mgc | Kc | %_Carb | %_Cl | %_ Sa | %_Si | pF 15 Atm | EC | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcH | 0.577 | 0.007 | 1.595 | 0.217 | 0.386 | 2.641 | 3.078 | 2.190 | 3.829 | 2.435 | 0.090 | 0.048 |
| ArH | 0.664 | 0.012 | 2.242 | 0.277 | 0.035 | 2.716 | 1.843 | 2.935 | 1.780 | 0.659 | 0.079 | 0.145 |
| BH | 0.964 | 0.020 | 8.420 | 0.176 | 0.101 | 1.630 | 1.164 | 3.474 | 2.653 | 0.819 | 0.012 | 0.146 |
| LR | 0.688 | 0.004 | 0.713 | 0.123 | 0.021 | 0.572 | 1.941 | 2.633 | 1.549 | 0.664 | 0.148 | 0.218 |
| PD | 0.921 | 0.005 | 4.104 | 0.269 | 0.066 | 3.432 | 1.890 | 1.573 | 1.191 | 0.896 | 0.013 | 0.025 |
| ArP | 0.742 | 0.216 | 2.607 | 0.280 | 0.056 | 4.263 | 2.525 | 2.840 | 2.145 | 0.842 | 0.009 | 0.134 |
| ArChr | 0.989 | 0.034 | 3.295 | 0.201 | 0.149 | 2.223 | 1.954 | 3.381 | 1.733 | 1.104 | 0.012 | 0.054 |
| RChr | 1.023 | 0.031 | 8.558 | 0.306 | 0.445 | 2.869 | 2.967 | 3.303 | 3.048 | 1.325 | 0.102 | 0.036 |
| UM | 1.460 | 0.064 | 11.450 | 0.261 | 0.290 | 4.348 | 2.434 | 4.189 | 2.944 | 1.586 | 0.129 | 0.124 |
| PA | 0.678 | 0.009 | 1.842 | 0.240 | 0.029 | 2.034 | 2.245 | 2.511 | 2.124 | 0.819 | 0.007 | 0.091 |
| TT | 1.127 | 0.007 | 1.989 | 0.199 | 0.015 | 0.156 | 1.409 | 4.170 | 3.326 | 0.749 | 0.001 | 0.074 |
| TP | 0.343 | 0.005 | 2.160 | 0.058 | 0.010 | 0.091 | 1.087 | 1.678 | 1.735 | 0.355 | 0.001 | 0.056 |
| FS | 0.858 | 0.007 | 5.076 | 0.731 | 0.118 | 2.641 | 2.614 | 2.265 | 1.840 | 1.002 | 0.180 | 0.042 |
| CS | 1.007 | 0.024 | 6.531 | 0.202 | 0.224 | 2.303 | 2.050 | 2.605 | 2.158 | 0.724 | 0.063 | 0.074 |
| Plant Association | CEC | Nt | Pa | Mgc | Kc | %_Carb | %_Cl | %_Sa | %_Si | pF 15 Atm | EC | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcH | 15.3 | 0.1 | 9.7 | 1.6 | 0.7 | 47.53 | 17.7 | 20.4 | 61.7 | 15.2 | 0.35 | 8.2 |
| ArH | 9.1 | 0.1 | 13.9 | 1.8 | 0.2 | 8.10 | 19.7 | 62.4 | 17.8 | 8.6 | 0.20 | 7.4 |
| BH | 10.5 | 0.1 | 15.4 | 1.0 | 0.3 | 4.49 | 14.5 | 54.2 | 31.2 | 8.2 | 0.12 | 7.4 |
| LR | 6.6 | 0.0 | 4.8 | 0.8 | 0.2 | 2.59 | 17.2 | 64.2 | 18.5 | 7.3 | 0.21 | 6.6 |
| PD | 14.3 | 0.0 | 15.3 | 2.3 | 1.0 | 49.28 | 40.0 | 19.9 | 40.0 | 19.1 | 0.28 | 8.0 |
| ArP | 10.8 | 0.1 | 14.7 | 1.8 | 0.4 | 12.38 | 25.3 | 45.6 | 28.9 | 13.0 | 0.16 | 7.6 |
| ArChr | 12.3 | 0.1 | 26.9 | 2.1 | 0.6 | 7.53 | 19.7 | 55.8 | 24.4 | 11.9 | 0.19 | 7.7 |
| RChr | 11.6 | 0.1 | 20.9 | 2.7 | 1.4 | 35.57 | 24.2 | 37.8 | 37.9 | 14.2 | 0.49 | 7.9 |
| UM | 10.8 | 0.1 | 36.1 | 1.6 | 1.2 | 31.96 | 21.2 | 46.0 | 32.7 | 13.1 | 0.56 | 7.7 |
| PA | 10.5 | 0.1 | 5.6 | 1.7 | 0.2 | 3.59 | 25.8 | 44.9 | 29.2 | 11.8 | 0.10 | 7.5 |
| TT | 9.6 | 0.0 | 5.1 | 1.0 | 0.1 | 1.64 | 13.5 | 64.9 | 21.4 | 6.6 | 0.04 | 6.1 |
| TP | 5.2 | 0.0 | 5.2 | 0.5 | 0.1 | 1.44 | 9.6 | 75.4 | 14.8 | 4.7 | 0.04 | 6.0 |
| FS | 12.2 | 0.0 | 13.1 | 2.8 | 0.8 | 52.18 | 35.1 | 24.2 | 40.6 | 18.7 | 0.68 | 8.0 |
| CS | 11.8 | 0.1 | 27.1 | 2.2 | 1.3 | 26.61 | 25.5 | 45.6 | 28.4 | 12.0 | 0.44 | 8.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Ortiz, A.; Musarella, C.M.; Piñar Fuentes, J.C.; Pinto Gomes, C.J.; Quinto-Canas, R.; del Río, S.; Cano, E. Indicative Value of the Dominant Plant Species for a Rapid Evaluation of the Nutritional Value of Soils. Agronomy 2021, 11, 1. https://doi.org/10.3390/agronomy11010001
Cano-Ortiz A, Musarella CM, Piñar Fuentes JC, Pinto Gomes CJ, Quinto-Canas R, del Río S, Cano E. Indicative Value of the Dominant Plant Species for a Rapid Evaluation of the Nutritional Value of Soils. Agronomy. 2021; 11(1):1. https://doi.org/10.3390/agronomy11010001
Chicago/Turabian StyleCano-Ortiz, Ana, Carmelo M. Musarella, José C. Piñar Fuentes, Carlos J. Pinto Gomes, Ricardo Quinto-Canas, Sara del Río, and Eusebio Cano. 2021. "Indicative Value of the Dominant Plant Species for a Rapid Evaluation of the Nutritional Value of Soils" Agronomy 11, no. 1: 1. https://doi.org/10.3390/agronomy11010001
APA StyleCano-Ortiz, A., Musarella, C. M., Piñar Fuentes, J. C., Pinto Gomes, C. J., Quinto-Canas, R., del Río, S., & Cano, E. (2021). Indicative Value of the Dominant Plant Species for a Rapid Evaluation of the Nutritional Value of Soils. Agronomy, 11(1), 1. https://doi.org/10.3390/agronomy11010001









