Modification of Yield and Fiber Fractions Biosynthesis in Phaseolus vulgaris L. by Treatment with Biostimulants Containing Amino Acids and Seaweed Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Plant Yielding
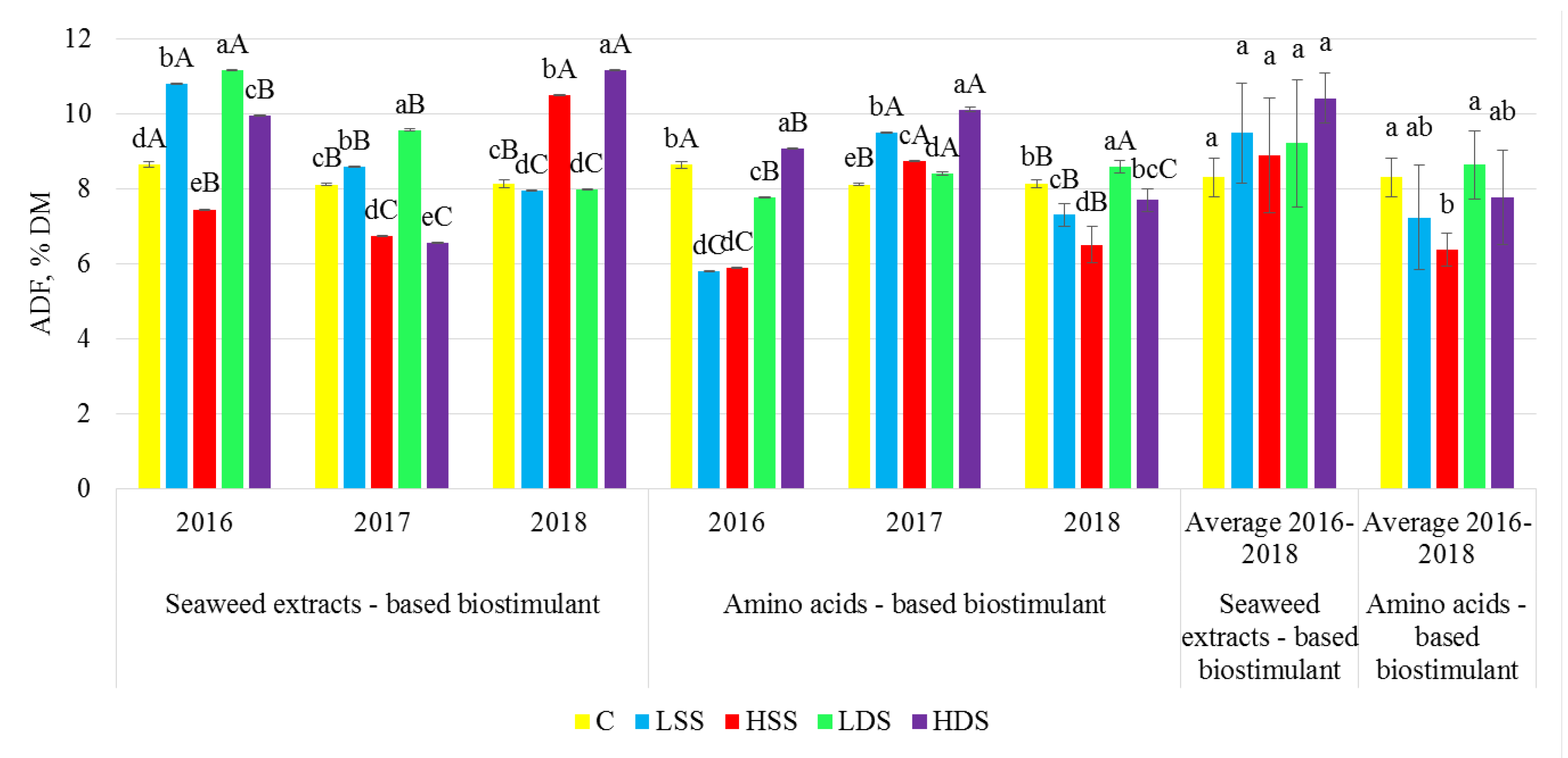
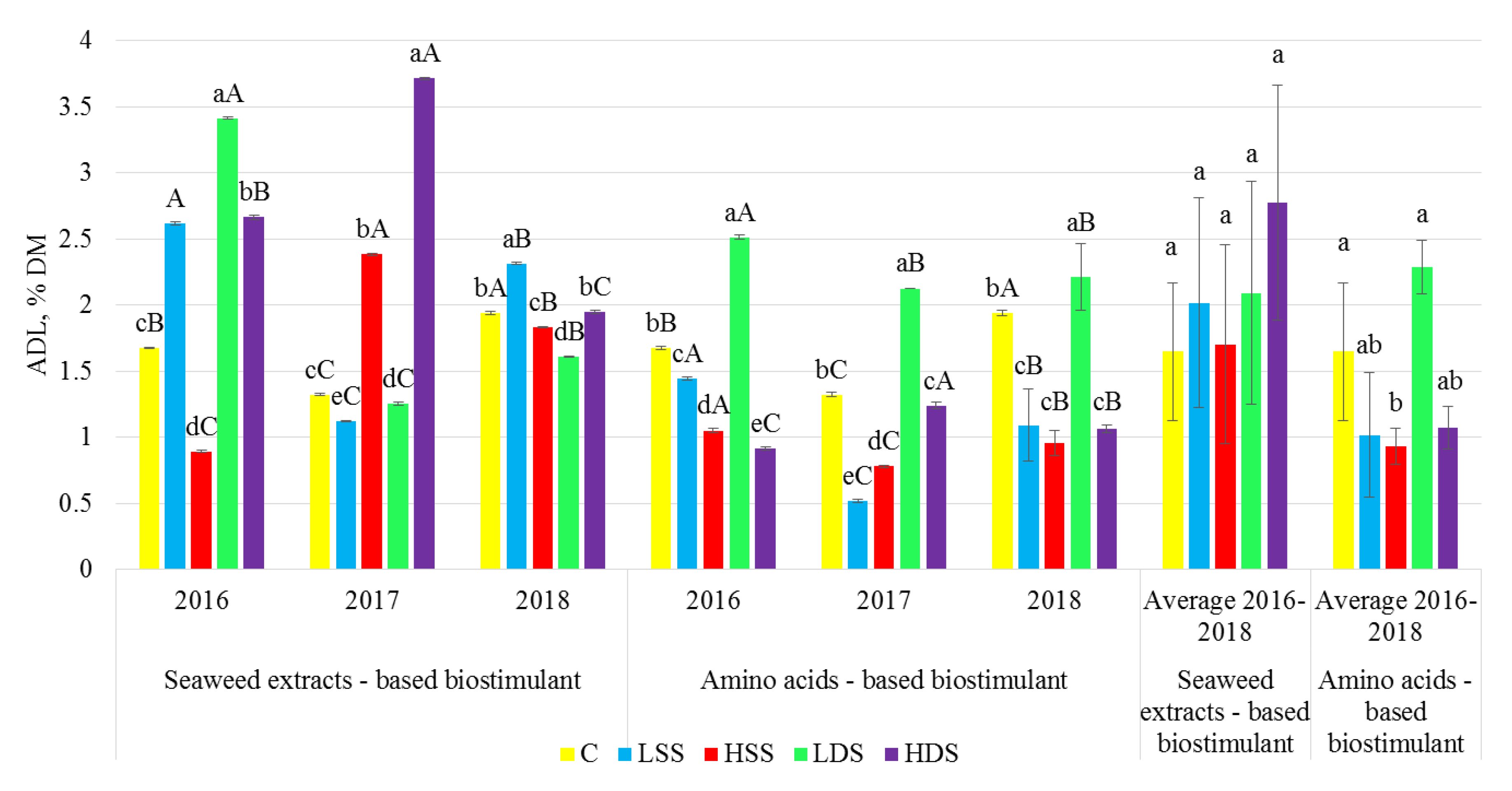
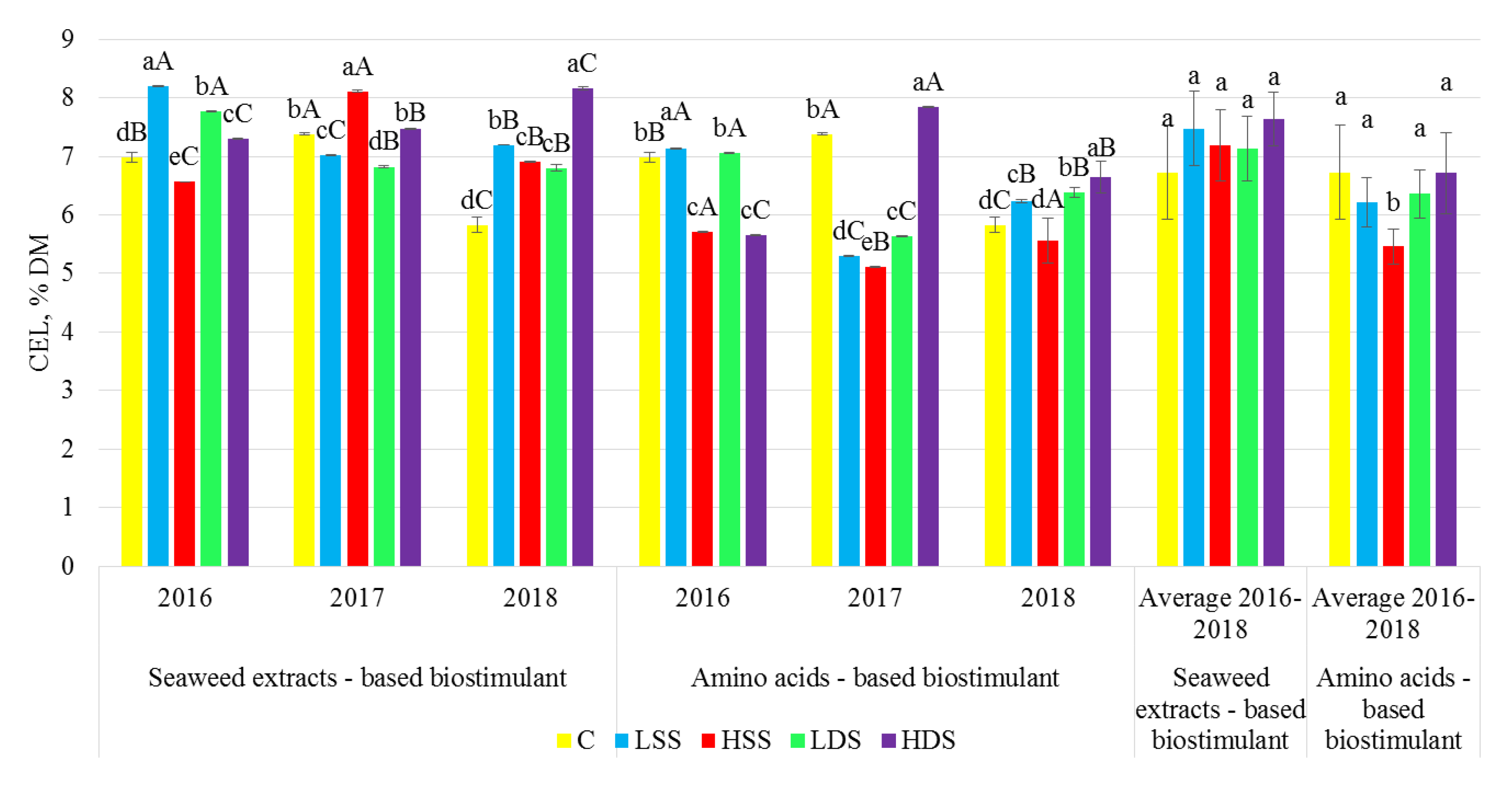
2.3. Dietary Fiber Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carvalho, S.; Vasconcelos, M.W. Producing more with less: Strategies and novel technologies for plant-based food biofortification. Food Res. Int. 2013, 54, 961–971. [Google Scholar] [CrossRef]
- Szparaga, A. Wybrane Właściwości Fizyczne, Mechaniczne, Chemiczne i Plon Nasion Fasoli Zwykłej (Phaseolus vulgaris L.) w Zależności od Metody Aplikacji Biostymulatorów; Polskie Towarzystwo Inżynierii Rolniczej: Kraków, Poland, 2019. [Google Scholar]
- Szparaga, A.; Kocira, S. Generalized logistic functions in modelling emergence of Brassica napus L. PLoS ONE 2018, 13, e0201980. [Google Scholar] [CrossRef] [PubMed]
- Kocira, S.; Hara, P.; Szparaga, A.; Czerwińska, E.; Beloev, H.; Findura, P.; Bajus, P. Evaluation of the Effectiveness of the Use of Biopreparations as Seed Dressings. Agriculture 2020, 10, 90. [Google Scholar] [CrossRef]
- Dymkowska–Malesa, M.; Szparaga, A.; Czerwińska, E. Evaluation of polychlorinated biphenyls content in chosen vegetables from Warmia and Mazury region. Rocz. Ochr. Srodowiska 2014, 16, 290–299. [Google Scholar]
- Eckardt, N.A.; Cominelli, E.; Galbiati, M.; Tonelli, C. The future of science: Food and water for life. Plant Cell 2009, 21, 368–372. [Google Scholar] [CrossRef]
- Rajabi Hamedani, S.; Rouphael, Y.; Colla, G.; Colantoni, A.; Cardarelli, M. Biostimulants as a Tool for Improving Environmental Sustainability of Greenhouse Vegetable Crops. Sustainability 2020, 12, 5101. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019R1009 (accessed on 24 June 2020).
- Godlewska, A.; Ciepiela, G.A. Yield Performance and Content of Selected Organic Compounds in Trifolium pratense Treated with Various Biostimulants against the Background of Nitrogen Fertilisation. Legume Res. 2020, LR-522. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Kumar, H.D.; Aloke, P. Role of Biostimulant Formulations in Crop Production: An Overview. Int. J. Appl. Res. Vet. M 2020, 8, 38–46. [Google Scholar]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable Sources of Plant Biostimulation: Microalgae as a Sustainable Means to Improve Crop Performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y. Microalgae: New Source of Plant Biostimulants. Agronomy 2020, 10, 1240. [Google Scholar] [CrossRef]
- Norrie, J.; Keathley, J.P. Benefits of Ascophyllum nodosum marine-plant extract applications to ‘Thompson seedless’ grape production. Acta Hortic. 2006, 727, 243–247. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kuboń, M.; Czerwińska, E.; Piskier, T. Morphological and Biochemical Responses of Glycine max (L.) Merr. to the Use of Seaweed Extract. Agronomy 2019, 9, 93. [Google Scholar] [CrossRef]
- Kocira, S.; Kocira, A.; Kornas, R.; Koszel, M.; Szmigielski, M.; Krajewska, M.; Szparaga, A.; Krzysiak, Z. Effects of seaweed extract on yield and protein content of two common bean (Phaseolus vulgaris L.) cultivars. Legume Res. 2018, 41, 589–593. [Google Scholar] [CrossRef]
- Michałek, W.; Kocira, A.; Findura, P.; Szparaga, A.; Kocira, S. The Influence of Biostimulant Asahi SL on the Photosynthetic Activity of Selected Cultivars of Phaseolus vulgaris L. Rocz. Ochr. Sr. 2018, 20, 1286–1301. [Google Scholar]
- Hamza, B.; Suggars, A. Biostimulants: Myths and realities. TurfGrass Trends 2001, 10, 6–10. [Google Scholar]
- Ertani, A.; Pizzeghelio, D.; Altissimo, A.; Nardi, S. Use of meat hydrolyzate derived from tanning residues as plant biostimulant for hydroponically grown maize. J. Plant Nutr. Soil Sci. 2013, 176, 287–295. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Kauffman, G.L., III; Kneival, D.P.; Watschke, T.L. Effects of biostimulant on the heat tolerance associated with photosynthetic capacity, membrane thermostability, and polphenol production of perennial ryegrass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Apone, F.; Tito, A.; Carola, A.; Arciello, S.; Tortora, A.; Filippini, L. A mixture of peptides and sugars derived from plant cell walls increases plant defense responses to stress and attenuates ageing-associated molecular changes in cultured skin cells. J. Biotechnol. 2010, 145, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2013, 364, 145–158. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kocira, A.; Czerwińska, E.; Wójtowicz, A.; Bronowicka-Mielniczuk, U.; Koszel, M.; Findura, P. Modeling biometric traits, yield and nutritional and antioxidant properties of seeds of three soybean cultivars through the application of biostimulant containing seaweed and amino acids. Front. Plant Sci. 2018, 9, 388. [Google Scholar] [CrossRef]
- Kocira, S.; Kocira, A.; Szmigielski, M.; Piecak, A.; Sagan, A.; Malaga-Toboła, U. Effect of an amino acids-containing biostimulator on common bean crop. Przem. Chem. 2015, 94, 1732–1736. [Google Scholar] [CrossRef]
- Kapusta, F. Legumes as protein source for humans and animals. Int. J. Eng. Sci. Technol. 2019, 1, 16–32. [Google Scholar]
- Ulloa, J.A.; Rosas, U.P.; Ramírez, R.J.C.; Rangel, U.B.E. El frijol (Phaseolus vulgaris): Su importancia nutricional y como fuente de fitoquímicos. Rev. Fuente 2011, 3, 5–9. [Google Scholar]
- Suárez-Martínez, S.E.; Ferriz-Martínez, R.A.; Campos-Vega, R.; Elton-Puente, J.E.; de la Torre Carbot, K.; García-Gasca, T. Bean seeds: Leading nutraceutical source for human health. CyTA-J. Food. 2016, 14, 131–137. [Google Scholar] [CrossRef]
- Los, F.G.B.; Zielinski, A.A.F.; Wojeicchowski, J.P.; Nogueira, A.; Demiate, I.M. Beans (Phaseolus vulgaris L.): Whole seeds with complex chemical composition. Curr. Opin. Food Sci. 2018, 19, 63–71. [Google Scholar] [CrossRef]
- Chávez-Mendoza, C.; Sánchez, E. Bioactive Compounds from Mexican Varieties of the Common Bean (Phaseolus vulgaris): Implications for Health. Molecules 2017, 22, 1360. [Google Scholar] [CrossRef] [PubMed]
- Tosh, S.M.; Yada, S. Dietary fibers in pulse seeds and fractions: Characterization, functional attributes, and applications. Food Res. Int. 2010, 43, 450–460. [Google Scholar] [CrossRef]
- Dueñas, M.; Fernández, D.; Hernández, T.; Estrella, I.; Muñoz, R. Bioactive phenolic compounds of cowpeas (Vigna sinensis L). Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J. Sci. Food Agric. 2005, 85, 297–304. [Google Scholar] [CrossRef]
- Oomah, B.D.; Cardador-Martínez, A.; Loarca-Piña, G. Phenolics and antioxidative activities in common beans (Phaseolus vulgaris L). J. Sci. Food Agric. 2005, 85, 935–942. [Google Scholar] [CrossRef]
- Rocha-Guzman, N.E.; Gallegos-Infante, J.A.; Gonzalez-Laredo, R.F.; Cardoza-Cervantes, V.; Reynoso-Camacho, R.; Ramos-Gomez, M.; Garcia-Gasca, T.; De Anda Salazar, A. Evaluation of culinary quality and antioxidant capacity for Mexican common beans (Phaseolus vulgaris L.) canned in pilot plant. Int. Food Res. J. 2013, 20, 1087–1093. [Google Scholar]
- Moreno-Franco, B.; León-Latre, M.; Andrés-Esteban, E.M.; Ordovás, J.M.; Casasnovas, J.A.; Peñalvo, J.L. Soluble and insoluble dietary fibre intake and risk factors for cvd and metabolic syndrome in middle-aged adults: The AWHS cohort. Atherosclerosis 2014, 235, e279–e280. [Google Scholar] [CrossRef]
- Guzmán, M.S.H.; Gallegos, J.A.A.; Muñoz, M.D.L.Á.Á.; Delgado, S.G.; Piña, G.L. Calidad alimentariay potencial nutracéutico del frijol (Phaseolus vulgaris L.) [Food quality and nutraceutical potential of common bean (Phaseolus vulgaris L.)]. Agric. Téc. México 2002, 28, 159–173. [Google Scholar]
- Karr-Lilienthala, L.K.; Kadzereb, C.T.; Grieshopc, C.M.; Fahey, G.C., Jr. Chemical and nutritional properties of soybean carbohydrates as related to nonruminants: A review. Livest. Prod. Sci. 2005, 97, 1–12. [Google Scholar] [CrossRef]
- Joubert, J.M.; Lefranc, G. Seaweed biostimulants in agriculture: Recent studies on mode of action two types of products from alga: Growth and nutrition stimulants and stimulants of plant Demence reactions. In Proceedings of the Biostimulators in Modern Agriculture, Warsaw, Poland, 7–8 February 2008. [Google Scholar]
- Szparaga, A.; Kuboń, M.; Kocira, S.; Czerwińska, E.; Pawłowska, A.; Hara, P. Towards Sustainable Agriculture—Agronomic and Economic Effects of Biostimulant Use in Common Bean Cultivation. Sustainability 2019, 11, 4575. [Google Scholar] [CrossRef]
- Kocira, S. Effect of amino acid biostimulant on the yield and nutraceutical potential of soybean. Chil. J. Agric. Res. 2019, 79, 17–25. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Bettoni, M.M.; Mogor, A.F.; Pauletti, V.; Goicoechea, N.; Aranjuelo, I.; Garmendia, I. Nutritional quality and yield of onion as affected by different application methods and doses of humic substances. J. Food Comp. Anal. 2016, 51, 37–44. [Google Scholar] [CrossRef]
- Shahabivand, S.; Padash, A.; Aghaee, A.; Nasiri, Y.; Rezaei, P.F. Plant biostimulants (Funneliformis mosseae and humic substances) rather than chemical fertilizer improved biochemical responses in peppermint. Iran. J. Plant Physiol. 2018, 8, 2333–2344. [Google Scholar]
- Lötze, E.; Hoffman, E.W. Nutrient composition and content of various biological active compounds of three South African-based commercial seaweed biostimulants. J. Appl. Phycol. 2016, 28, 1379–1386. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Siłtys, R. Evaluation of the content of crude fibre and its fractionsin cereal products. Bromat. Chem. Toksykol. 2009, 4, 1083–1088. [Google Scholar]
- Jenkins, D.J.A.; Kendall, C.W.C.; Ransom, T.P.P. Dietary fiber, the evolution of the human diet and coronary heart disease. Nutr. Res. 1998, 18, 633–652. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Sánchez-Muniz, F.J. Dietary fibre from Edible seaweeds: Chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr. Res. 2000, 20, 585–598. [Google Scholar] [CrossRef]
- Nawirska-Olszanska, A.; Kucharska, A.Z.; Sokol-Letowska, A. Dietary fibre fractions in Cornelian cherry fruit [Cornus mas L.]. Zywn. Nauka Technol. Jakosc 2010, 2, 95–103. [Google Scholar] [CrossRef]
- Baer, D.J.; Rumpler, W.V.; Miles, C.W.; Fahey, G.C., Jr. Dietary Fiber Decreases the Metabolizable Energy Content and Nutrient Digestibility of Mixed Diets Fed to Humans. J. Nutr. 1997, 4, 579–586. [Google Scholar] [CrossRef]
- Segura, C.M.; Barbosa, M.E.; Matus, B.Á.; Cabrera, A.D.; Murguía, O.M.; Moguel, O.Y.; Betancur, A.D. Comparison of Chemical and Functional Properties of Stevia rebaudiana (Bertoni) Varieties Cultivated in Mexican Southeast. Am. J. Plant Sci. 2014, 5, 286–293. [Google Scholar] [CrossRef]
- Hillman, L.C.; Peters, S.G.; Fisher, A. The Effects of the Fiber Components Pectin, Cellulose and Lignin on Serum Cholesterol Levels. Am. J. Clin. Nutr. 1985, 42, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, A.; Ciepiela, G.A. The effect of growth regulator on dry matter yield and some chemical components in selected grass species and cultivars. J. Soil Sci. Plant Nutr. 2016, 62, 297–302. [Google Scholar] [CrossRef]
- Botta, A. Enhancing plant tolerance to temperature stress with amino acids: An approach to their mode of action. Acta Hortic. 2013, 1009, 29–35. [Google Scholar] [CrossRef]
- Colla, G.; Svecova, E.; Rouphael, Y.; Cardarelli, M.; Reynaud, H.; Canaguier, R.; Rouphael, Y. Effectiveness of a plant-derived protein hydrolysate to improve crop performances under different growing conditions. Acta Hortic. 2013, 1009, 175–179. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived protein hydrolysate on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Xu, C.; Leskovar, D. Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition valued under drought stress. Sci. Hortic. 2015, 183, 39–47. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Rouphael, Y.; De Micco, V.; Arena, C.; Raimondi, G.; Colla, G.; De Pascale, S. Effect of Ecklonia maxima seaweed extract on yield, mineral composition, gas exchange and leaf anatomy of zucchini squash grown under saline conditions. J. Appl. Phycol. 2017, 29, 459–470. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef]
- Di Stasio, E.; Rouphael, Y.; Colla, G.; Raimondi, G.; Giordano, M.; Pannico, A.; El-Nakhel, C.; De Pascale, S. The influence of Ecklonia maxima seaweed extract on growth, photosynthetic activity and mineral compositionof Brassica rapa L. subsp. sylvestris under nutrient stress conditions. Eur. J. Hortic. Sci. 2017, 82, 286–293. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. Hortic. Sci. 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguiere, R.; Rouphael, J. Protein hydrolysates as biostimulants in horticulture. Hortic. Sci. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Hortic. Sci. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Salwa, A.R.; Hammada Osama, A.M. Ali Physiological and biochemical studies on drought tolerance of wheat plants by application of amino acids and yeast extract. Ann. Agric. Sci. 2014, 59, 133–145. [Google Scholar] [CrossRef]
- Barnett, J.A.; Payne, R.W.; Yarrow, D. The Yeasts: Characteristics and Identification, 2nd ed.; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- El-Nabarawy, M.A. Mitigation of dark induced senescence. 1—By some amino acids. Ann. Agric. Sci. Moshtohor Univ. 2001, 39, 225–232. [Google Scholar]
- Abbas, S.M. The influence of biostimulants on the growth and on the biochemical composition of vicia faba CV. Giza 3 beans. Rom. Biotech. Lett. 2013, 18, 8061–8068. [Google Scholar]
- Aloni, R.; Langhans, M.; Aloni, E.; Ullrich, C.I. Role of cytokinin in the regulation of root gravitropism. Planta 2004, 220, 177–182. [Google Scholar] [CrossRef]
- Aloni, R.; Aloni, E.; Langhans, M.; Ullrich, C.I. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 2006, 97, 883–893. [Google Scholar] [CrossRef]
- Aloni, R.; Tollier, M.T.; Monties, B. The role of auxin and gibberellin in controlling lignin formation in primary phloem fibers and in xylem of Coleus-blumei stems. Plant Physiol. 1990, 94, 1743–1747. [Google Scholar] [CrossRef]
- Mauriat, M.; Moritz, T. Analyses of GA20ox- and GID1-over-expressing aspen suggest that gibberellins play two distinct roles in wood formation. Plant J. 2009, 58, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Dayan, J.; Schwarzkopf, M.; Avni, A.; Aloni, R. Enhancing plant growth and fiber production by silencing GA 2-oxidase. Plant Biotechnol. J. 2010, 8, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Hooley, R. Gibberellins: Perception, transduction and responses. Plant Mol. Biol. 1994, 26, 1529–1555. [Google Scholar] [CrossRef] [PubMed]
- Kende, H.; Zeevaart, J. The five “classical” plant hormones. Plant Cell 1997, 9, 1197–1210. [Google Scholar] [CrossRef]
- Bai, W.Q.; Xiao, Y.H.; Zhao, J.; Song, S.Q.; Hu, L.; Zeng, J.Y.; Li, X.B.; Hou, L.; Luo, M.; Li, D.M.; et al. Gibberellin overproduction promotes sucrose synthase expression and secondary cell wall deposition in cotton fibers. PLoS ONE 2014, 9, e96537. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Ward, D.A.; Hedden, P.; Phillips, A.L.; Power, J.B.; Davey, M.R. Engineering gibberellin metabolism in Solanum nigrum L. by ectopic expression of gibberellin oxidase genes. Plant Cell 2012, 31, 945–953. [Google Scholar] [CrossRef]
- Gou, J.; Ma, C.; Kadmiel, M.; Gai, Y.; Strauss, S.; Jiang, X.; Busov, V. Tissue-specific expression of Populus C19 GA 2-oxidases differentially regulate above- and below-ground biomass growth through control of bioactive GA concentrations. New Phytol. 2011, 192, 626–639. [Google Scholar] [CrossRef]
- Xiao, Y.-H.; Li, D.-M.; Yin, M.-H.; Li, X.-B.; Zhang, M.; Wang, Y.-J.; Dong, J.; Zhao, J.; Luo, M.; Luo, X.-Y.; et al. Gibberellin 20- oxidase promotes initiation and elongation of cotton fibers by regulating gibberellin synthesis. J. Plant Physiol. 2010, 167, 829–837. [Google Scholar] [CrossRef]
- Vidal, A.M.; Gisbert, C.; Talón, M.; Primo-Millo, E.; López-Díaz, I.; García-Martínez, J.L. The ectopic overexpression of a citrus gibberellin 20-oxidase enhances the non-13- hydroxylation pathway of gibberellin biosynthesis and induces an extremely elongated phenotype in tobacco. Physiol. Plant. 2001, 112, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.E.; Israelsson, M.; Olsson, O.; Moritz, T. Increased gibberellins biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat. Biotechnol. 2000, 18, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Grabber, J.H.; Ralph, J.; Lapierre, C.; Barrière, Y. Genetic and molecular basis of grass cell-wall degradability. I. Lignin–cell wall matrix interactions. C. R. Biol. 2004, 327, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Anterola, A.M.; Lewis, N.G. Trends in lignin modification: A comprehensive analysis of the effects of genetic manipulations/mutations on lignification and vascular integrity. Phytochemistry 2002, 61, 221–294. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Goffner, D.; Joffroy, I.; Grima-Pettenati, J.; Halpin, C.; Knight, M.E.; Schuch, W.; Boudet, A.M. Purification and characterization of isoforms of cinnamyl alcohol dehydrogenase from Eucalyptus xylem. Planta 1992, 188, 48–53. [Google Scholar] [CrossRef]
- Fan, L.; Shi, W.J.; Hu, W.R.; Hao, X.Y.; Wang, D.M.; Yuan, H.; Yan, H.Y. Molecular and biochemical evidence for phenylpropanoid synthesis and presence of wall-linked phenolics in cotton fibers. J. Integr. Plant Biol. 2009, 51, 626–637. [Google Scholar] [CrossRef]
- Moghaddam, S.M.; Brick, M.A.; Echeverria, D.; Thompson, A.J.; Brick, L.A.; Lee, R.; Mamidi, S.; McClean, P.E. Genetic Architecture of Dietary Fiber and Oligosaccharide Content in a Middle American Panel of Edible Dry Bean. Plant Genome 2018, 11, 170074. [Google Scholar] [CrossRef]
- Szparaga, A.; Kocira, S.; Kocira, A.; Czerwińska, E.; Świeca, M.; Lorencowicz, E.; Kornas, R.; Koszel, M.; Oniszczuk, T. Modification of growth, yield, and the nutraceutical and antioxidative potential of soybean through the use of synthetic biostimulants. Front. Plant Sci. 2018, 9, 1401. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kocira, A.; Czerwińska, E.; Depo, K.; Erlichowska, B.; Deszcz, E. Effect of applying a biostimulant containing seaweed and amino acids on the content of fiber fractions in three soybean cultivars. Legume Res. 2019, 42, 341–347. [Google Scholar] [CrossRef]
- Kim, H.; Triplet, B.A. Cotton fiber growth in planta and in vitro. Models for plant cell elongation and cell wall biogenesis. Plant Physiol. 2001, 127, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-B.; Fan, X.-P.; Wang, X.-L.; Cai, L.; Yang, W.-C. The cotton ACTIN1 gene is functionally expressed in fibers and participates in fiber elongation. Plant Cell 2005, 17, 859–875. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Li, Q.; Fan, X.; Yang, W.; Xue, Y. The R2R3 MYB transcription factor GhMYB109 is required for cotton fiber development. Genetics 2008, 180, 811–820. [Google Scholar] [CrossRef]
- Qin, Y.-M.; Hu, C.-Y.; Pang, Y.; Kastaniotis, A.J.; Hiltunen, J.K.; Zhu, Y.-X. Saturated very-long- chain fatty acids promote cotton fiber and Arabidopsis cell elongation by activating ethylene biosynthesis. Plant Cell 2007, 19, 3692–3704. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-Q.; Xu, K.-X.; Luo, B.; Wang, J.-W.; Chen, X.-Y. An ATP-binding cassette transporter GhWBC1 from elongating cotton fibers. Plant Physiol. 2003, 133, 580–588. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, Y.-H.; Zhu, S.-W.; Mao, X.-Z.; Feng, J.-X.; Qin, Y.-M.; Zhang, L.; Cheng, J.; Wei, L.-P.; Wang, Z.-Y.; Zhu, Y.-X. Transcriptome profiling, molecular biological, and physiological studies reveal a major role for ethylene in cotton fiber cell elongation. Plant Cell 2006, 18, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.S.; Cheung, F.; Lee, J.J.; Ha, M.; Wei, N.E.; Sze, S.-H.; Stelly, D.M.; Thaxton, P.; Triplett, B.; Town, C.D.; et al. Accumulation of genome- specific transcripts, transcription factors and phytohormonal regulators during early stages of fiber cell development in allotetraploid cotton. Plant J. 2006, 47, 761–775. [Google Scholar] [CrossRef]


| Biostimulant | Biostimulant Composition [17,28] | Number of Sprays and Plant Developmental Stages (BBCH) in which the Biostimulants Were Applied | Concentration | Volume of Working Solution/Working Pressure |
|---|---|---|---|---|
| Seaweed extracts -based product | Auxins (11 mg dm−3), cytokinins (0.031 mg dm−3), alginates (1.5 g L−1), amino acids (total 441.3 mg 100 g−1), mannitol (2261 mg L−1), neutral sugars (1.08 g L−1). Macro-elements (N 0.09%, P 90.7 mg kg−1, K 7163.3 mg kg−1, Ca 190.4 mg kg−1, Mg 337.2 mg kg−1, Na 1623.7 mg kg−1). Microelements Mn 17.3 mg kg−1, Fe 40.7 mg kg−1, Cu 13.5 mg kg−1, Zn 17.0 mg kg−1, B 33.0 mg kg−1) | Single spraying: BBCH 13–15 (LSS) | 0.7% | 300 L·ha−1/ 0.30 MPa |
| Single spraying: BBCH 13–15 (HSS) | 1.0% | |||
| Double spraying: BBCH 13–15, BBCH 61 (LDS) | 0.7% | |||
| Double spraying: BBCH 13–15, BBCH 61 (HDS) | 1.0% | |||
| Amino acids -based product | Aliphatic amino acids (glycine, alanine, valine, leucine, isoleucine, proline). Hydroxy-amino acids (serine, threonine). S-containing amino acids (cysteine, methionine). Aromatic amino acids (phenylalanine, tryptophan, tyrosine). Acidic amino acids (aspartic acid, glutamic acid). Basic amino acids (histidine, arginine, lysine). Organic N (5.0%), B (1.5%), Mg (0.8%), Fe (1%), Zn (0.1%), Mn (0.1%), Mo (0.001%), and many micro-elements | Single spraying: BBCH 13–15 (LSS) | 0.3% | 300 L·ha−1/ 0.30 MPa |
| Single spraying: BBCH 13–15 (HSS) | 0.5% | |||
| Double spraying: BBCH 13–15, BBCH 61 (LDS) | 0.3% | |||
| Double spraying: BBCH 13–15, BBCH 61 (HDS) | 0.5% |
| Month | Year | Average from 2002 to 2015 | ||||||
|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | ||||||
| T (°C) Average (min/max) | Rainfall (mm) | T (°C) Average (min/max) | Rainfall (mm) | T (°C) Average (min/max) | Rainfall (mm) | T (°C) | Rainfall (mm) | |
| IV | 9.2 (−1.2/22.6) | 68.4 | 7.7 (−1.6/23.3) | 37.2 | 11.5 (−1.0/23.1) | 29.6 | 8.6 | 41.9 |
| V | 13.8 (2.6/26.7) | 61.3 | 13.7 (−1.4/26.9) | 100.0 | 14.2 (1.9/25.8) | 54.7 | 12.6 | 64.1 |
| VI | 18.1 (4.2/31.5) | 97.1 | 18.3 (5.7/30.2) | 38.6 | 18.0 (5.2/30.6) | 77.1 | 17.8 | 68.3 |
| VII | 19.5 (8.8/31.2) | 107.6 | 18.5 (5.3/32.9) | 61.1 | 19.1 (7.6/32.4) | 93.7 | 18.8 | 79.4 |
| VIII | 18.2 (7.1/30.7) | 95.3 | 19.5 (4.3/34.4) | 25.5 | 19.8 (6.3/31.9) | 64.5 | 19.5 | 71.5 |
| IX | 15.2 (1.6/28.7) | 41.2 | 13.2 (−0.3/27.3) | 100.4 | 15.1 (1.9/26.9) | 44.3 | 14.0 | 69.6 |
| Average/Total | 17.1 | 470.9 | 15.2 | 362.8 | 16.3 | 363.9 | 15.2 | 394.8 |
| Parameters | Biostimulant Treatment | Biostimulant | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Kelpak SL | Terra Sorb Complex | ||||||||
| Season | Average | Season | Average | ||||||
| 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | ||||
| 1000 seed weight (g 1000−1) | C | 177.1 bA | 152.8 aB | 151.7 abB | 160.5 a | 177.1 aA | 152.8 aB | 151.7 aB | 160.5 a |
| LSS | 167.2 cdA | 148.9 abC | 156.4 aB | 157.5 a | 167.2 cbA | 155.4 aA | 154.9 aA | 159.2 a | |
| HSS | 166.5dA | 138.4 bA | 138.0 bA | 147.6 a | 159.3 cA | 158.5 aA | 147.7 aA | 155.2 a | |
| LDS | 172.2 bA | 147.0 abB | 158.6 aB | 159.3 a | 171.9 abA | 153.6 aB | 152.5 aB | 159.3 a | |
| HDS | 182.8 aA | 155.7 aB | 159.3 aB | 165.9 a | 168.7 bA | 137.0 bB | 137.1 bB | 147.6 a | |
| Seed yield (g m−2) | C | 247.9 cC | 278.4dB | 316.2 cA | 280.8 b | 247.9 c | 278.4 c | 316.2 c | 280.8 a |
| LSS | 320.3 bB | 359.9 abB | 393.9 abAB | 358.0 a | 258.0 bB | 329.8 bA | 340.8 bA | 309.5 a | |
| HSS | 322.5 bA | 319.2 cA | 334.3 bcA | 325.3 a | 266.7 bB | 393.6 aA | 358.2 abA | 339.5 a | |
| LDS | 330.2 abB | 330.7 bcB | 411.9 aA | 357.6 a | 284.1 aB | 381.1 aA | 362.4 aA | 342.5 a | |
| HDS | 338.6 aA | 370.1 aA | 403.9 aA | 370.9 a | 257.8 bB | 334.5 bA | 340.6 bA | 311.0 a | |
| Number of pods (per m−2) | C | 349dB | 421 cA | 427 cA | 399 b | 349 cB | 421 cA | 427 cA | 399 b |
| LSS | 490 cA | 600 aA | 627 aA | 572 a | 494 bA | 483 cA | 505 bA | 494 ab | |
| HSS | 576 bA | 584 aA | 587 abA | 582 a | 517 abB | 663 aA | 655 aA | 612 a | |
| LDS | 654dA | 491 bA | 585 abA | 577 a | 515 abB | 704 aA | 690 aA | 636 a | |
| HDS | 551 bA | 556 aA | 557 bA | 555 a | 520 aA | 578 bA | 567 aA | 555 ab | |
| Number of seeds (per m−2) | C | 1399 cB | 1822dA | 2084 bA | 1768 a | 1399 cB | 1822 cA | 2084 cA | 1768 a |
| LSS | 1915 aB | 2415 aA | 2518 aA | 2283 a | 1543 bB | 1969 cA | 2050 cA | 1854 a | |
| HSS | 1937 aB | 2308 bcA | 2425 aA | 2223 a | 1675 aB | 2799 bA | 2815 bA | 2430 a | |
| LDS | 1917 aC | 2248 cB | 2588 aA | 2251 a | 1652 aB | 2985 aA | 2992 aA | 2543 a | |
| HDS | 1852 bB | 2376 abA | 2535 aA | 2254 a | 1528 bB | 2740 bA | 2830 bA | 2366 a | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocira, S.; Szparaga, A.; Findura, P.; Treder, K. Modification of Yield and Fiber Fractions Biosynthesis in Phaseolus vulgaris L. by Treatment with Biostimulants Containing Amino Acids and Seaweed Extract. Agronomy 2020, 10, 1338. https://doi.org/10.3390/agronomy10091338
Kocira S, Szparaga A, Findura P, Treder K. Modification of Yield and Fiber Fractions Biosynthesis in Phaseolus vulgaris L. by Treatment with Biostimulants Containing Amino Acids and Seaweed Extract. Agronomy. 2020; 10(9):1338. https://doi.org/10.3390/agronomy10091338
Chicago/Turabian StyleKocira, Sławomir, Agnieszka Szparaga, Pavol Findura, and Krzysztof Treder. 2020. "Modification of Yield and Fiber Fractions Biosynthesis in Phaseolus vulgaris L. by Treatment with Biostimulants Containing Amino Acids and Seaweed Extract" Agronomy 10, no. 9: 1338. https://doi.org/10.3390/agronomy10091338
APA StyleKocira, S., Szparaga, A., Findura, P., & Treder, K. (2020). Modification of Yield and Fiber Fractions Biosynthesis in Phaseolus vulgaris L. by Treatment with Biostimulants Containing Amino Acids and Seaweed Extract. Agronomy, 10(9), 1338. https://doi.org/10.3390/agronomy10091338








