Effect of Biostimulants on Apple Quality at Harvest and After Storage
Abstract
1. Introduction
“…stimulate plant nutrition processes independently of the product’s nutrient content with the sole aim of improving one or more of the following characteristics of the plant or the plant rhizosphere: (i) nutrient use efficiency, (ii) tolerance to abiotic stress, (iii) quality traits, or (iv) availability of confined nutrients in the soil or rhizosphere”.[13]
2. Materials and Methods
2.1. Site Description and Experimental Layout
2.2. Productive and Qualitative Parameters
2.3. Biochemical Analysis of Apple Fruits
2.3.1. Phenolic Compounds
2.3.2. Mineral Element Content
2.4. Statistical Analysis
3. Results
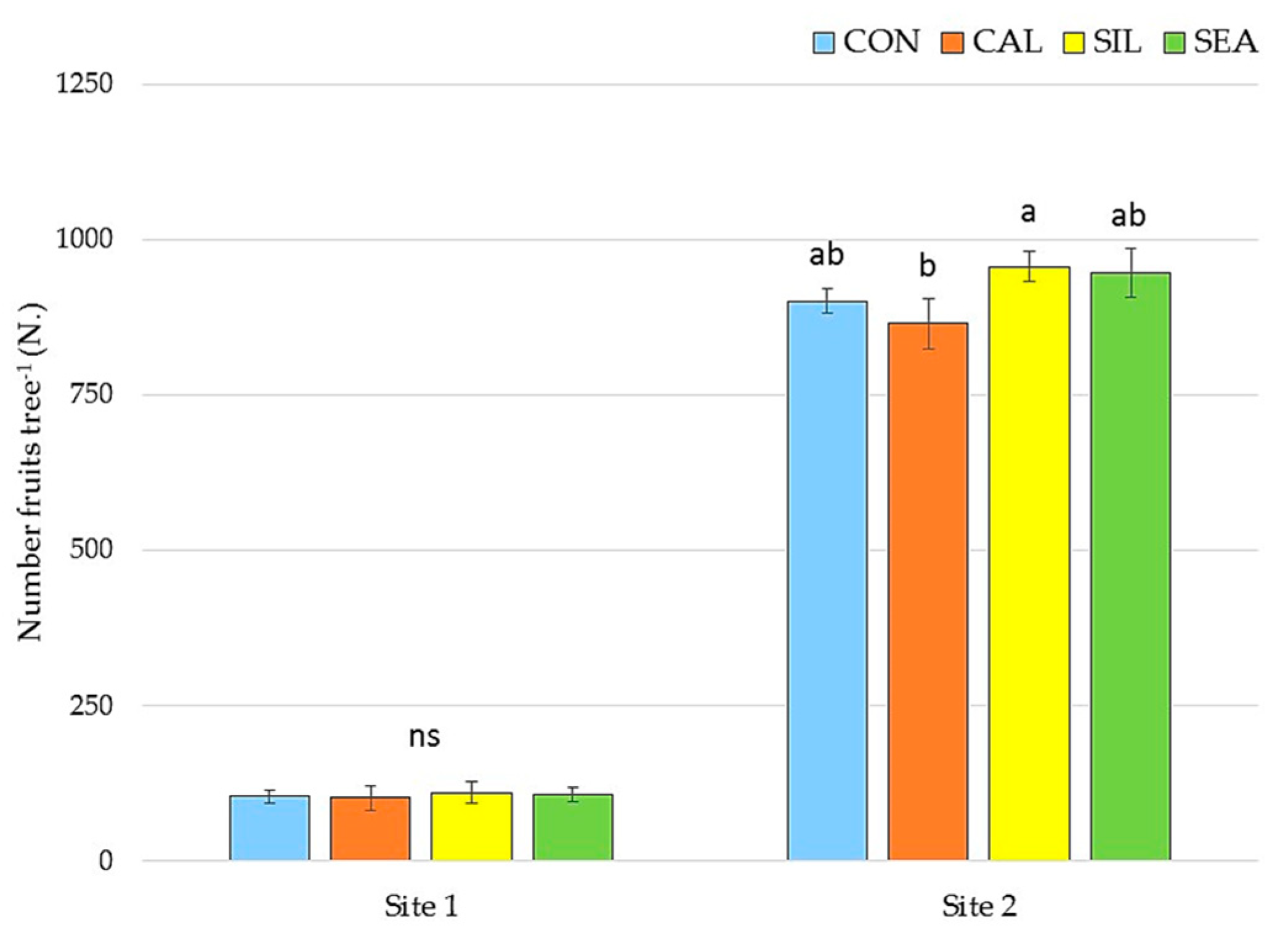
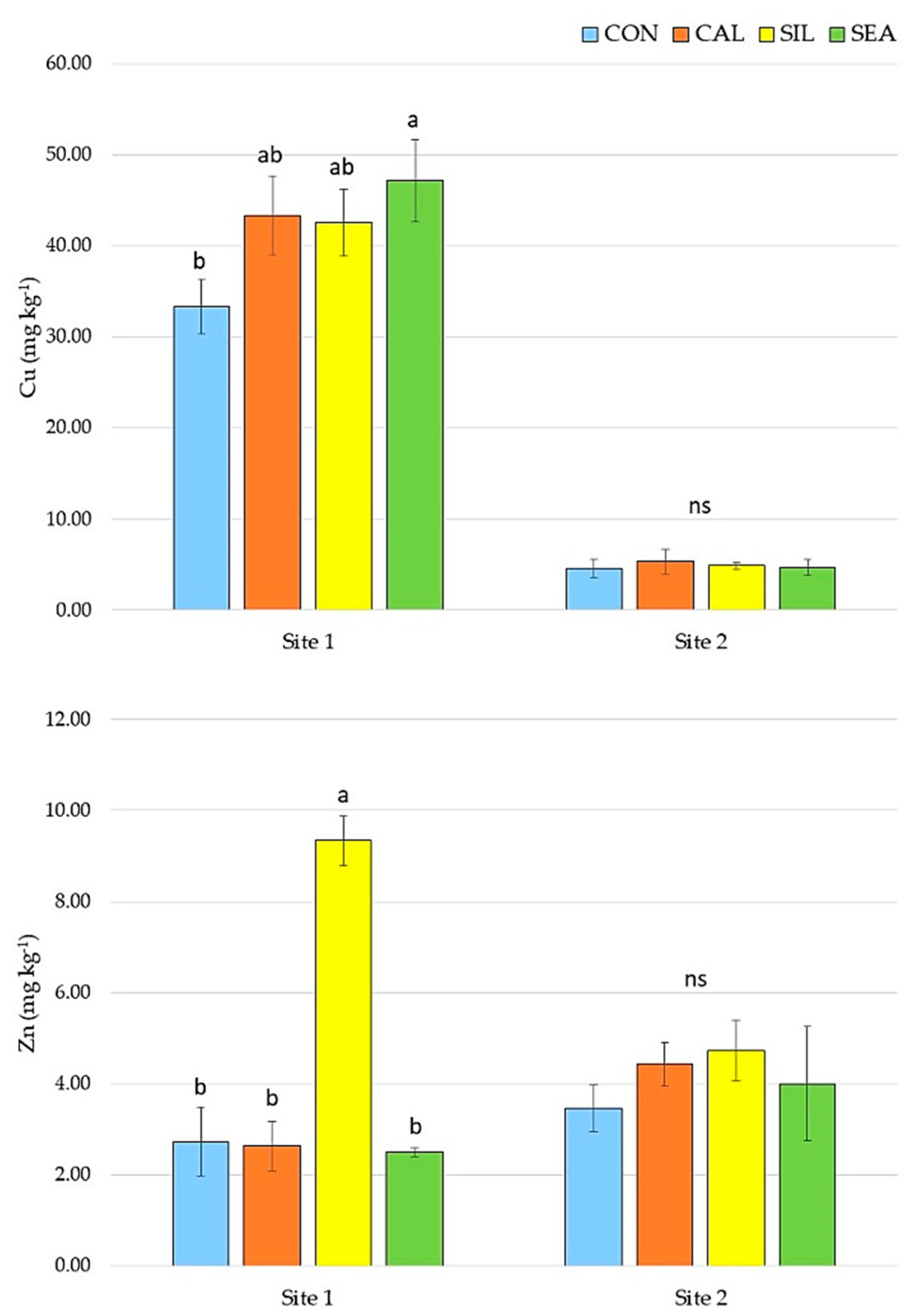
3.1. Yield and Quality Components at Harvest
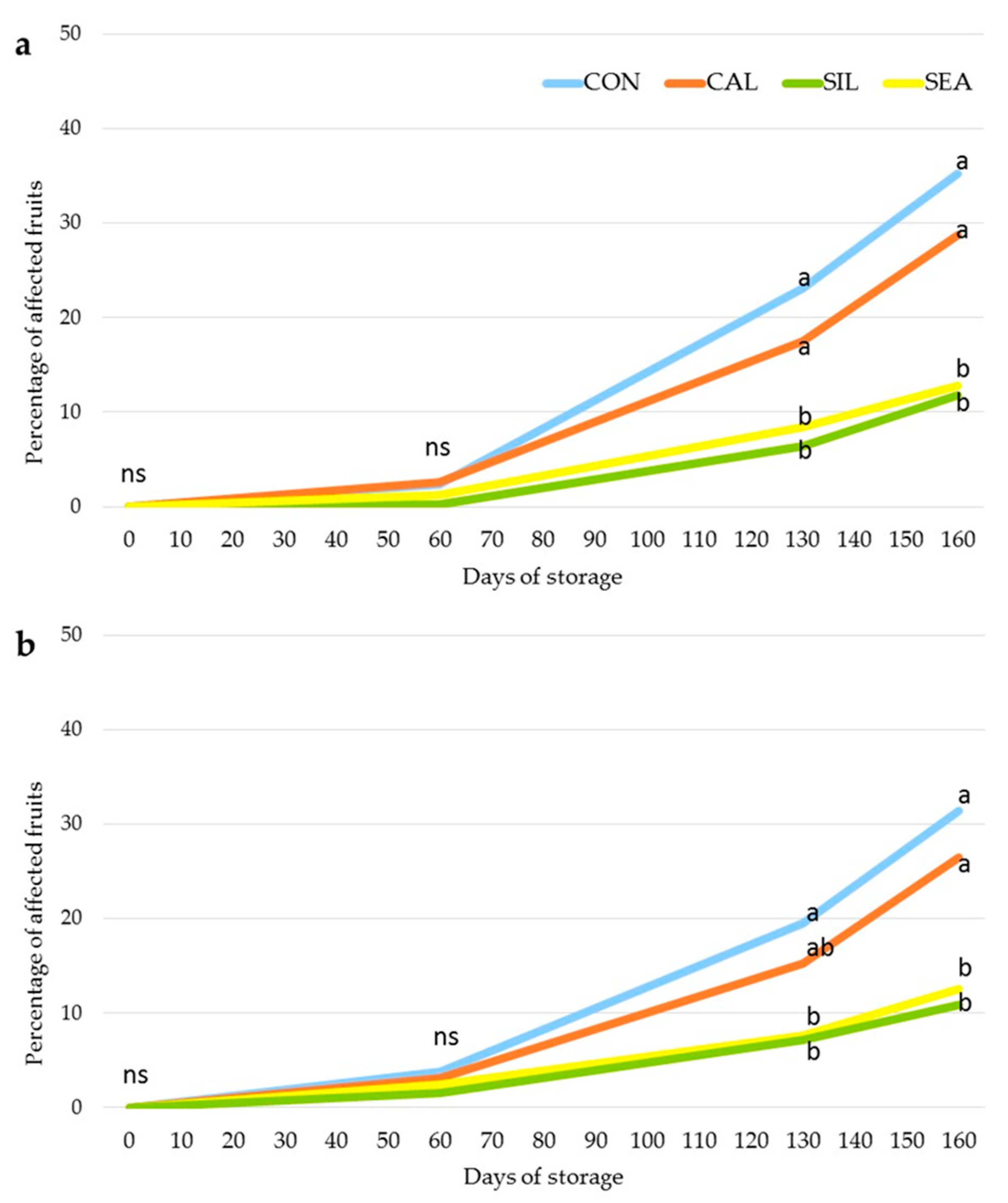
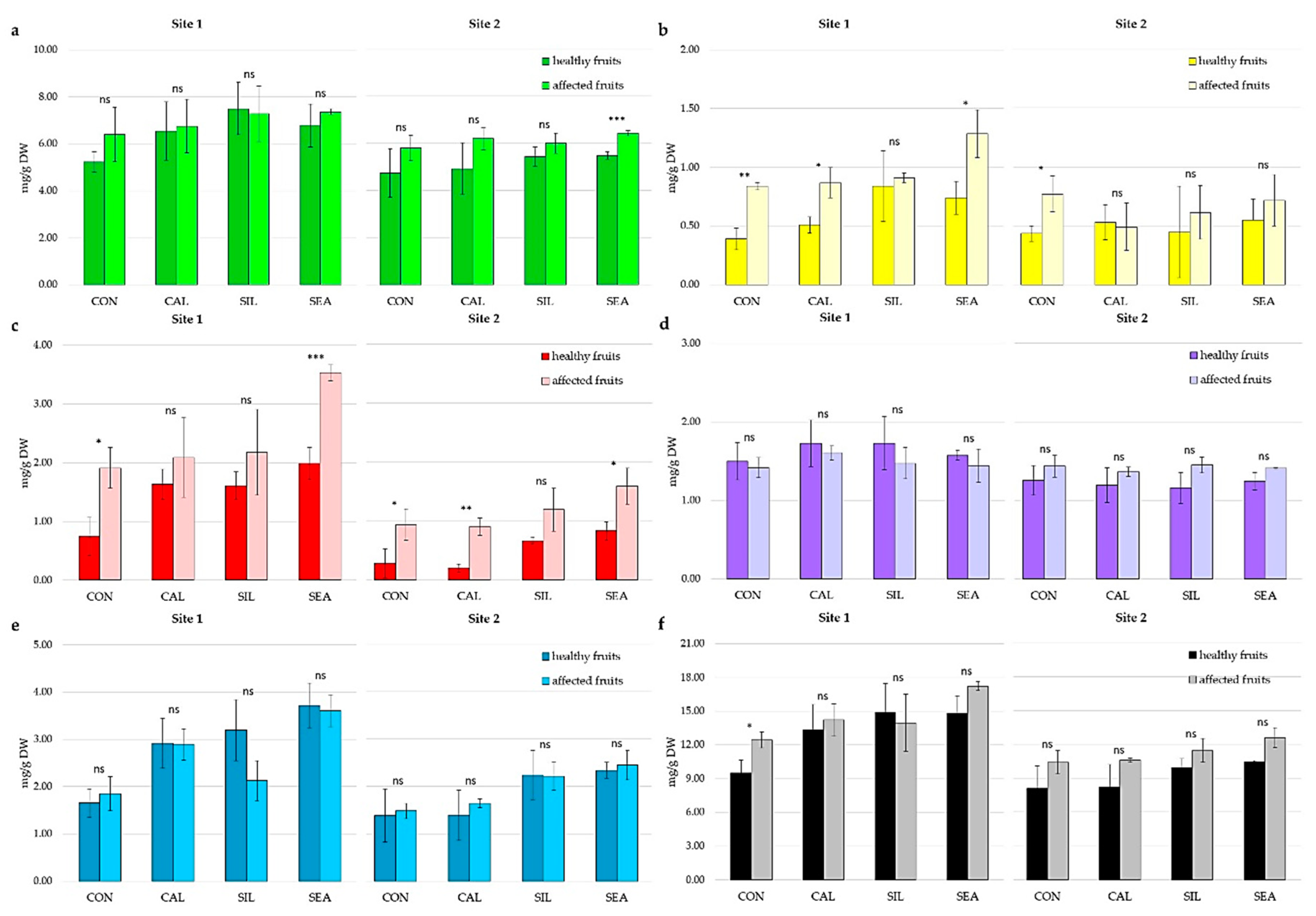
3.2. Fruit Quality and Disorder Incidence during Storage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Gago, C.M.L.; Guerreiro, A.C.; Miguel, G.; Panagopoulos, T.; da Silva, M.M.; Antunes, M.D.C. Effect of calcium chloride and 1-MCP (SmartfreshTM) postharvest treatment on ‘Golden Delicious’ apple cold storage physiological disorders. Sci. Hortic. 2016, 211, 440–448. [Google Scholar] [CrossRef]
- Bangerth, F. Calcium-related physiological disorders of plants. Annu. Rev. Phytopathol. 1979, 17, 97–122. [Google Scholar] [CrossRef]
- Fallahi, E.; Conway, W.S.; Hickey, K.D.; Sams, C.E. The role of calcium and nitrogen in postharvest quality and disease resistance of apples. Hort. Sci. 1997, 32, 831–835. [Google Scholar] [CrossRef]
- Dris, R.; Niskanen, R. Calcium chloride sprays decrease physiological disorders following long-term cold storage of apple. Plant Foods Hum. Nutr. 1999, 54, 159–171. [Google Scholar] [CrossRef]
- de Freitas, S.T.; do Amarante, C.V.T.; Mitcham, E.J. Mechanisms regulating apple cultivar susceptibility to bitter pit. Sci. Hortic. 2015, 186, 54–60. [Google Scholar] [CrossRef]
- Perring, M.A.; Pearson, K. Redistribution of minerals in apple fruit during storage: The effect of storage atmosphere on calcium concentration. J. Sci. Food Agric. 1987, 40, 37–42. [Google Scholar] [CrossRef]
- Saure, M.C. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Sci. Hortic. 2005, 105, 65–89. [Google Scholar] [CrossRef]
- Amarante, C.V.T.; Silveira, J.P.G.; Steffens, C.A.; Paes, F.N.; Argenta, L.C. Tissue sampling method and mineral attributes to predict bitter pit occurrence in apple fruit: A multivariate approach. VII Int. Postharvest Symp. 1012 2013, 1133–1139. [Google Scholar] [CrossRef]
- Tagliavini, M.; Toselli, M. Foliar applications of nutrients. In Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; pp. 53–59. [Google Scholar]
- Yuri, J.A.; Retamales, J.B.; Moggia, C.; Vásquez, J.L. Bitter pit control in apples cv. ′Braeburn′ through foliar sprays of different calcium sources. Acta Hortic. 2002, 594, 453–460. [Google Scholar] [CrossRef]
- Lötze, E.; Joubert, J.; Theron, K.I. Evaluating pre-harvest foliar calcium applications to increase fruit calcium and reduce bitter pit in ′Golden Delicious′ apples. Sci. Hortic. 2008, 116, 299–304. [Google Scholar] [CrossRef]
- EU, European Union Fertilizing Products Regulation. Available online: http://data.europa.eu/eli/reg/2019/1009/oj (accessed on 1 July 2020).
- Basile, B.; Rouphael, Y.; Colla, G.; Soppelsa, S.; Andreotti, C. Appraisal of emerging crop management opportunities in fruit trees, grapevines and berry crops facilitated by the application of biostimulants. Sci. Hortic. 2020, 267, 109330. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Andreotti, C. Use of biostimulants for organic apple production: Effects on tree growth, yield, and fruit quality at harvest and during storage. Front. Plant Sci. 2018, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol oxidases in crops: Biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 2017, 18, 377. [Google Scholar] [CrossRef]
- Ghasemi, S.; Khoshgoftarmanesh, A.H.; Hadadzadeh, H.; Jafari, M. Synthesis of iron-amino acid chelates and evaluation of their efficacy as iron source and growth stimulator for tomato in nutrient solution culture. J. Plant Growth Regul. 2012, 31, 498–508. [Google Scholar] [CrossRef]
- Popko, M.; Michalak, I.; Wilk, R.; Gramza, M.; Chojnacka, K.; Górecki, H. Effect of the new plant growth biostimulants based on amino acids on yield and grain quality of winter wheat. Molecules 2018, 23, 470. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, L.; Yang, C.; Lv, Q. Effects of iron and zinc foliar applications on rice plants and their grain accumulation and grain nutritional quality. J. Sci. Food Agric. 2013, 93, 254–261. [Google Scholar] [CrossRef]
- Aziz, R.A.; Naira, A.; Moieza, A. Effect of plant biostimulants on fruit cracking and quality attributes of pomegranate cv. ‘Kandhari kabuli’. Sci. Res. Essays 2013, 8, 2171–2175. [Google Scholar] [CrossRef]
- Correia, S.; Oliveira, I.; Queirós, F.; Ribeiro, C.; Ferreira, L.; Luzio, A.; Silva, A.P.; Gonçalves, B. Preharvest application of seaweed based biostimulant reduced cherry (Prunus Avium L.) cracking. Procedia Environ. Sci. 2015, 29, 251–252. [Google Scholar] [CrossRef]
- Ma, J.F. Uptake of silicon in different plant species. In Handbook of Biomineralization; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 113–124. [Google Scholar]
- Sorrenti, G.; Quartieri, M.; Caruso, S.; Lugli, S.; Rombolà, A.D. Efficace impiego di silicato di sodio e cloruro di calcio per ridurre lo spacco dei frutti. Riv. Fruttic. E Ortofloric. 2008, 70, 28–32. [Google Scholar]
- Agrios, Direttive per la Produzione Frutticola Integrata. Available online: http://www.agrios.it/en/ (accessed on 1 July 2020).
- Tessmer, M.A.; Appezzato-da-Glória, B.; Antoniolli, L.R. Influence of growing sites and physicochemical features on the incidence of lenticel breakdown in ′Gala′ and ′Galaxy′ apples. Sci. Hortic. 2016, 205, 119–126. [Google Scholar] [CrossRef]
- Andreotti, C.; Ravaglia, D.; Ragaini, A.; Costa, G. Phenolic compounds in peach (Prunus persica) cultivars at harvest and during fruit maturation. Ann. Appl. Biol. 2008, 153, 11–23. [Google Scholar] [CrossRef]
- Guerrero-Chavez, G.; Scampicchio, M.; Andreotti, C. Influence of the site altitude on strawberry phenolic composition and quality. Sci. Hortic. 2015, 192, 21–28. [Google Scholar] [CrossRef]
- ISO 16634-1:2008. Food Products—Determination of the Total Nitrogen Content by Combustion according to the Dumas Principle and Calculation of the Crude Protein Content—Part 1: Oilseeds and Animal Feeding Stuffs; International Organization for Standardization: Geneva, Switzerland, 2008. [Google Scholar]
- EPA 3052. Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrics; USA Environmental Protection Agency: Washington, DC, USA, 1996.
- EPA 6010D. Inductively Coupled Plasma—Optical Emission Spectrometry; USA Environmental Protection Agency: Washington, DC, USA, 2018.
- Bradshaw, T.L.; Berkett, L.P.; Griffith, M.C.; Kingsley-Richards, S.L.; Darby, H.M.; Parsons, R.L.; Moran, R.E.; Garcia, M.E. Assessment of kelp extract biostimulants on tree growth, yield, and fruit quality in a certified organic apple orchard. Acta Hortic. 2013, 1010, 191–198. [Google Scholar] [CrossRef]
- Malaguti, D.; Rombolà, A.D.; Gerin, M.; Simoni, G.; Tagliavini, M.; Marangoni, B. Effect of seaweed extracts-based leaf sprays on the mineral status, yield and fruit quality of apple. Acta Hortic. 2002, 594, 357–359. [Google Scholar] [CrossRef]
- Spinelli, F.; Fiori, G.; Noferini, M.; Sprocatti, M.; Costa, G. Perspectives on the use of a seaweed extract to moderate the negative effects of alternate bearing in apple trees. J. Hortic. Sci. Biotechnol. 2009, 84, 131–137. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar applications of biostimulants promote growth, yield and fruit quality of strawberry plants grown under nutrient limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef]
- Hajiboland, R.; Moradtalab, N.; Eshaghi, Z.; Feizy, J. Effect of silicon supplementation on growth and metabolism of strawberry plants at three developmental stages. N. Z. J. Crop Hortic. Sci. 2018, 46, 144–161. [Google Scholar] [CrossRef]
- Agostinho, F.; Tubana, B.; Martins, M.; Datnoff, L. Effect of different silicon sources on yield and silicon uptake of rice grown under varying phosphorus rates. Plants 2017, 6, 35. [Google Scholar] [CrossRef]
- Artyszak, A. Effect of silicon fertilization on crop yield quantity and quality—A literature review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef]
- Lordan, J.; Francescatto, P.; Dominguez, L.; Robinson, T. Long-term effects of tree density and tree shape on apple orchard performance, a 20 year study—Part 1, agronomic analysis. Sci. Hortic. 2018, 238, 303–317. [Google Scholar] [CrossRef]
- Frioni, T.; Sabbatini, P.; Tombesi, S.; Norrie, J.; Poni, S.; Gatti, M.; Palliotti, A. Effects of a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci. Hortic. 2018, 232, 97–106. [Google Scholar] [CrossRef]
- Weber, N.; Schmitzer, V.; Jakopic, J.; Stampar, F. First fruit in season: Seaweed extract and silicon advance organic strawberry (Fragaria×ananassa Duch.) fruit formation and yield. Sci. Hortic. 2018, 242, 103–109. [Google Scholar] [CrossRef]
- Wally, O.; Critchley, A.; Hiltz, D.; Craigie, J.; Han, X.; Zaharia, I.; Abrams, S.; Prithiviraj, B. Regulation of phytohormone biosynthesis and accumulation in Arabidopsis following treatment with commercial extract from the marine macroalga Ascophyllum nodosum. J. Plant Growth Regul. 2013, 32, 324–339. [Google Scholar] [CrossRef]
- Fageria, N.K. Nutrient management for improving upland rice productivity and sustainability. Commun. Soil Sci. Plant Anal. 2001, 32, 2603–2629. [Google Scholar] [CrossRef]
- de Freitas, S.T.; Jiang, C.-Z.; Mitcham, E.J. Mechanisms involved in calcium deficiency development in tomato fruit in response to gibberellins. J. Plant Growth Regul. 2012, 31, 221–234. [Google Scholar] [CrossRef]
- Saure, M. Reassessment of the role of calcium in development of bitter pit in apple. Funct. Plant Biol. 1996, 23, 237–243. [Google Scholar] [CrossRef]
- Ferguson, I.; Volz, R.; Woolf, A. Preharvest factors affecting physiological disorders of fruit. Postharvest Biol. Technol. 1999, 15, 255–262. [Google Scholar] [CrossRef]
- Henriques, A.R.; Chalfun-Junior, A.; Aarts, M. Strategies to increase zinc deficiency tolerance and homeostasis in plants. Braz. J. Plant Physiol. 2012, 24, 3–8. [Google Scholar] [CrossRef]
- de Freitas, S.T.; do Amarante, C.V.T.; Labavitch, J.M.; Mitcham, E.J. Cellular approach to understand bitter pit development in apple fruit. Postharvest Biol. Technol. 2010, 57, 6–13. [Google Scholar] [CrossRef]
- Guerriero, G.; Hausman, J.-F.; Legay, S. Silicon and the plant extracellular matrix. Front. Plant Sci. 2016, 7, 463. [Google Scholar] [CrossRef] [PubMed]
- Pais, L.; Petho, F. Investigations on causes of ‘Jonathan spot’. I. Szolo Es Gyumtermeszt. 1970, 6, 31–42. [Google Scholar]
- Johnson, D.S.; Dover, C.J. The effect of calcium and zinc sprays on the texture of ′Cox′s Orange Pippin′ apples in controlled atmosphere storage. Acta Hortic. 2002, 594, 427–433. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Treutter, D. Biosynthesis of phenolic compounds and its regulation in apple. Plant Growth Regul. 2001, 34, 71–89. [Google Scholar] [CrossRef]
- Zupan, A.; Mikulic-Petkovsek, M.; Cunja, V.; Stampar, F.; Veberic, R. Comparison of phenolic composition of healthy apple tissues and tissues affected by bitter pit. J. Agric. Food Chem. 2013, 61, 12066–12071. [Google Scholar] [CrossRef]
| Treatment | Active Ingredient | Commercial Name | Dose (mL 100 L−1) | Number of Applications | Dates of Applications | Other Characteristics |
|---|---|---|---|---|---|---|
| CON 1 | H2O | - | - | 0 | - | - |
| CAL | CaCl2 | Alical® (SIPCAM Italia spa, Pero, Italy) | 400 | 6 | 28 May 2014 (45 DAFB 2) 11 June 2014 (59 DAFB) 25 June 2014 (73 DAFB) 9 July 2014 (87 DAFB) 23 July 2014 (101 DAFB) 6 August 2014 (115 DAFB, 3 weeks before harvest) | Calcium chloride solution. Calcium oxide water-soluble: 17% |
| SIL | Si, Zn, Mo + CaCl2 | Siliforce® (ILSA spa, Arzignano, Italy) + Alical® | 200 + 400 | 6 | Same as for CAL treatment | Fluid mixture of micro-elements containing silicon (8 g kg−1), molybdenum (0.2%), and zinc (1.8%) |
| SEA | Seaweed cream + CaCl2 | Algavis® (SERBIOS srl, Polesine, Italy) + Alical® | 200 + 400 | 6 | Same as for CAL treatment | Filtered seaweeds cream solution (Ascophyllum nodosum). Organic carbon of biological origin (4%), mannitol (0.7 g L−1) |
| Yield (kg Tree−1) | Yield (t ha−1) | Fruit Weight (g) | Fruit Diameter (mm) | |
|---|---|---|---|---|
| Treatment (T) | ||||
| CON | 65.54 ± 57.27 ab | 47.53 ± 6.72 | 133.37 ± 7.52 | 73.40 ± 3.05 |
| CAL | 63.87 ± 55.81 b | 46.42 ± 8.35 | 135.93 ± 6.41 | 74.75 ± 1.65 |
| SIL | 69.61 ± 60.81 a | 50.54 ± 7.91 | 134.70 ± 6.44 | 74.48 ± 4.05 |
| SEA | 68.57 ± 59.90 ab | 49.78 ± 6.93 | 131.45 ± 7.82 | 76.38 ± 3.72 |
| Significance | * | ns | ns | ns |
| Site (S) | ||||
| Site 1 | 13.60 ± 1.51 | 42.50 ± 4.70 | 137.89 ± 5.50 | 77.06 ± 2.25 |
| Site 2 | 120.20 ± 5.47 | 54.63 ± 2.49 | 129.83 ± 5.60 | 72.45 ± 2.24 |
| Significance | *** | *** | *** | *** |
| T × S | ns | ns | ns | ns |
| Firmness (kg cm−2) | Total Soluble Solids (°Brix) | Acidity (g L−1) | Color Index | |
|---|---|---|---|---|
| Treatment (T) | ||||
| CON | 7.75 ± 0.24 | 12.42 ± 1.10 | 7.58 ± 1.06 | 32.13 ± 8.87 b |
| CAL | 7.87 ± 0.25 | 12.25 ± 1.20 | 7.60 ± 1.00 | 32.05 ± 7.33 b |
| SIL | 7.63 ± 0.20 | 12.21 ± 1.04 | 7.08 ± 0.96 | 32.33 ± 5.94 b |
| SEA | 7.85 ± 0.28 | 12.28 ± 1.11 | 7.50 ± 0.94 | 40.47 ± 12.39 a |
| Significance | ns | ns | ns | * |
| Site (S) | ||||
| Site 1 | 7.73 ± 0.24 | 11.33 ± 0.19 | 8.20 ± 0.55 | 40.58 ± 8.88 |
| Site 2 | 7.82 ± 0.25 | 13.25 ± 0.48 | 6.68 ± 0.57 | 27.92 ± 2.81 |
| Significance | ns | *** | *** | *** |
| T × S | ns | ns | ns | ns |
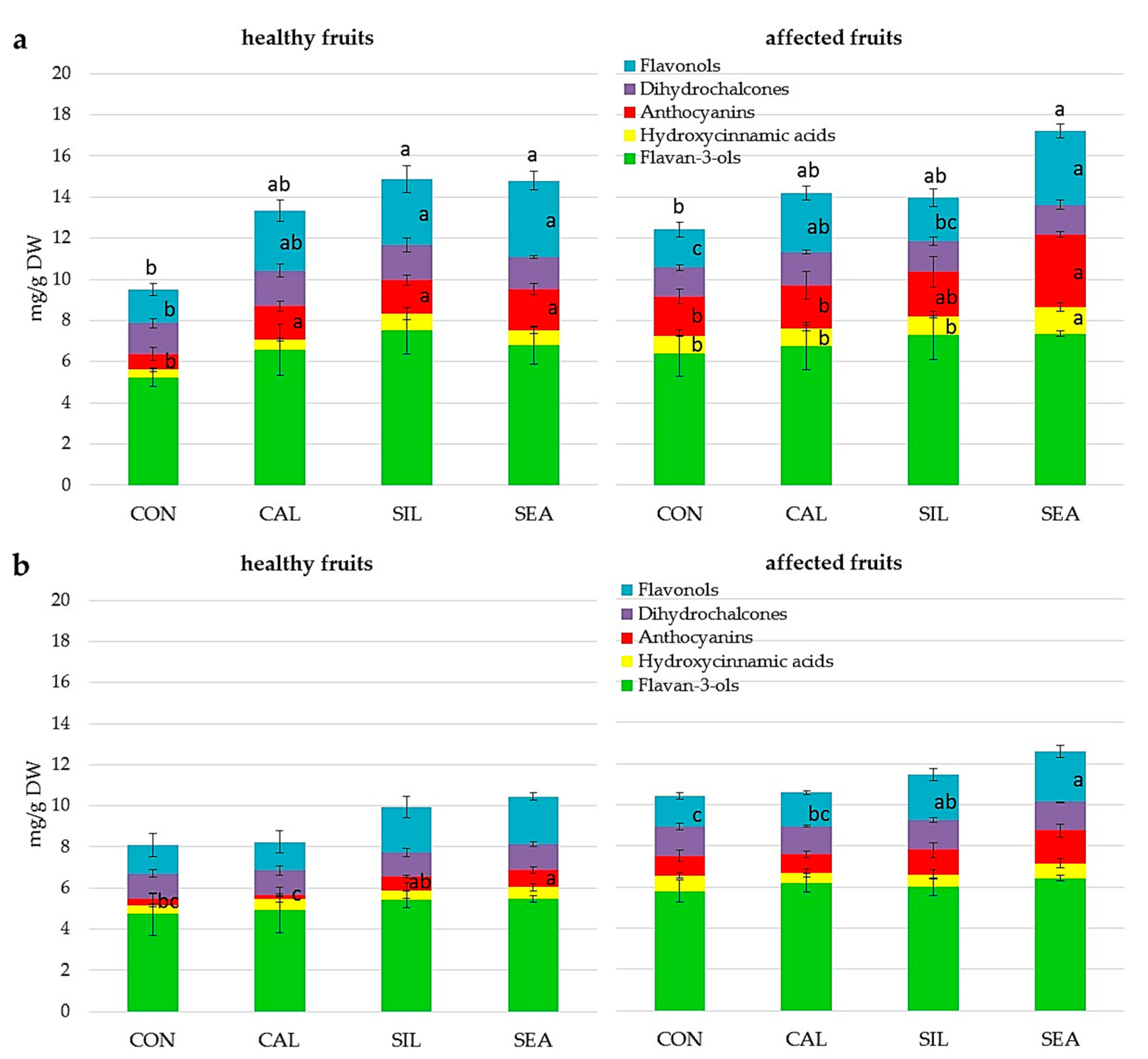
| Flavan-3-ols (mg g−1 DW) | Hydroxycinnamic Acids (mg g−1 DW) | Anthocyanins (mg g−1 DW) | Dihydro-Chalcones (mg g−1 DW) | Flavonols (mg g−1 DW) | Total (mg g−1 DW) | |
|---|---|---|---|---|---|---|
| Treatment (T) | ||||||
| CON | 5.76 ± 1.08 | 0.28 ± 0.59 | 2.02 ± 0.72 b | 1.59 ± 0.15 | 2.78 ± 0.64 | 12.43 ± 2.02 |
| CAL | 5.42 ± 0.75 | 0.44 ± 0.41 | 3.18 ± 0.71 ab | 2.00 ± 0.23 | 4.94 ± 0.99 | 15.98 ± 1.82 |
| SIL | 5.88 ± 2.99 | 0.26 ± 0.23 | 2.60 ± 1.21 ab | 2.02 ± 0.81 | 4.88 ± 3.02 | 15.65 ± 7.72 |
| SEA | 6.92 ± 3.80 | 0.40 ± 0.52 | 3.99 ± 2.18 a | 2.15 ± 0.75 | 5.14 ± 2.39 | 18.62 ± 9.52 |
| Significance | ns | ns | * | ns | ns | ns |
| Site (S) | ||||||
| Site 1 | 7.26 ± 2.88 | 0.53 ± 0.52 | 3.73 ± 1.66 | 2.17 ± 0.72 | 4.98 ± 2.66 | 18.67 ± 7.54 |
| Site 2 | 4.74 ± 0.54 | 0.17 ± 0.23 | 2.16 ± 0.62 | 1.71 ± 0.24 | 3.89 ± 1.29 | 12.67 ± 2.36 |
| Significance | * | * | *** | * | ns | * |
| T × S | ns | ns | ns | ns | ns | ns |
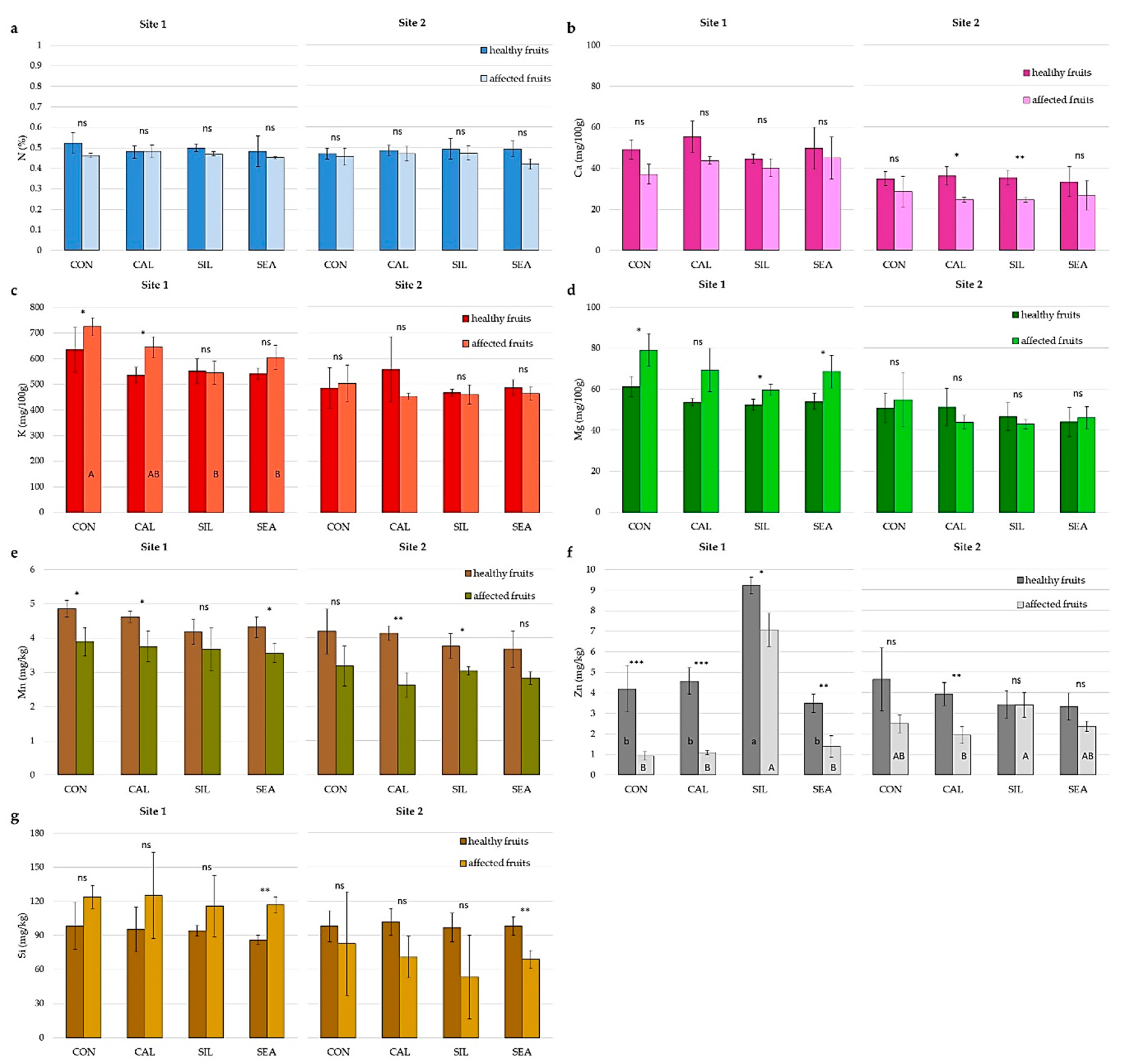
| N (% DW) | P (mg 100 g−1 DW) | K (mg 100 g−1 DW) | Ca (mg 100 g−1 DW) | Mg (mg 100 g−1 DW) | |
|---|---|---|---|---|---|
| Treatment (T) | |||||
| CON | 0.48 ± 0.02 | 61.46 ± 4.91 | 645.56 ± 40.92 a | 46.06 ± 9.60 b | 86.47 ± 5.79 |
| CAL | 0.51 ± 0.02 | 59.30 ± 5.68 | 613.46 ± 109.49 ab | 58.89 ± 13.58 ab | 88.43 ± 10.07 |
| SIL | 0.48 ± 0.02 | 56.71 ± 3.93 | 581.39 ± 67.62 ab | 55.39 ± 16.76 ab | 85.41 ± 5.58 |
| SEA | 0.50 ± 0.03 | 54.73 ± 6.10 | 550.41 ± 49.37 b | 61.84 ± 18.66 a | 85.98 ± 4.78 |
| Significance | ns | ns | * | * | ns |
| Site (S) | |||||
| Site 1 | 0.50 ± 0.03 | 55.82 ± 6.42 | 645.17 ± 62.60 | 66.95 ± 11.72 | 91.13 ± 5.59 |
| Site 2 | 0.48 ± 0.02 | 60.28 ± 3.43 | 550.24 ± 57.55 | 44.14 ± 8.21 | 82.02 ± 3.48 |
| Significance | * | * | *** | *** | *** |
| T × S | ns | ns | ns | ns | ns |
| B (mg kg−1 DW) | Fe (mg kg−1 DW) | Mn (mg kg−1 DW) | Si (mg kg−1 DW) | Se (µg kg−1 DW) | |
|---|---|---|---|---|---|
| Treatment (T) | |||||
| CON | 21.66 ± 7.89 | 40.72 ± 10.12 | 4.08 ± 0.73 | 79.35 ± 21.54 | 1.47 ± 1.38 |
| CAL | 21.39 ± 5.76 | 38.69 ± 11.11 | 4.50 ± 1.10 | 81.53 ± 25.25 | 1.24 ± 1.28 |
| SIL | 23.99 ± 10.63 | 43.88 ± 18.09 | 4.24 ± 0.34 | 115.38 ± 113.48 | 1.88 ± 1.54 |
| SEA | 22.48 ± 9.82 | 35.59 ± 5.62 | 4.34 ± 0.82 | 117.07 ± 92.80 | 1.70 ± 1.34 |
| Significance | ns | ns | ns | ns | ns |
| Site (S) | |||||
| Site 1 | 15.10 ± 1.44 | 35.75 ± 8.65 | 4.69 ± 0.85 | 89.38 ± 69.17 | 2.63 ± 1.03 |
| Site 2 | 29.67 ± 4.79 | 43.69 ± 13.28 | 3.89 ± 0.37 | 107.28 ± 77.53 | 0.52 ± 0.39 |
| Significance | *** | ns | * | ns | *** |
| T × S | ns | ns | ns | ns | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soppelsa, S.; Kelderer, M.; Testolin, R.; Zanotelli, D.; Andreotti, C. Effect of Biostimulants on Apple Quality at Harvest and After Storage. Agronomy 2020, 10, 1214. https://doi.org/10.3390/agronomy10081214
Soppelsa S, Kelderer M, Testolin R, Zanotelli D, Andreotti C. Effect of Biostimulants on Apple Quality at Harvest and After Storage. Agronomy. 2020; 10(8):1214. https://doi.org/10.3390/agronomy10081214
Chicago/Turabian StyleSoppelsa, Sebastian, Markus Kelderer, Raffaele Testolin, Damiano Zanotelli, and Carlo Andreotti. 2020. "Effect of Biostimulants on Apple Quality at Harvest and After Storage" Agronomy 10, no. 8: 1214. https://doi.org/10.3390/agronomy10081214
APA StyleSoppelsa, S., Kelderer, M., Testolin, R., Zanotelli, D., & Andreotti, C. (2020). Effect of Biostimulants on Apple Quality at Harvest and After Storage. Agronomy, 10(8), 1214. https://doi.org/10.3390/agronomy10081214







