Evaluation of the Antifungal Activity of Gold–Chitosan and Carbon Nanoparticles on Fusarium oxysporum
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Chitosan–Stabilized Gold Nanoparticles
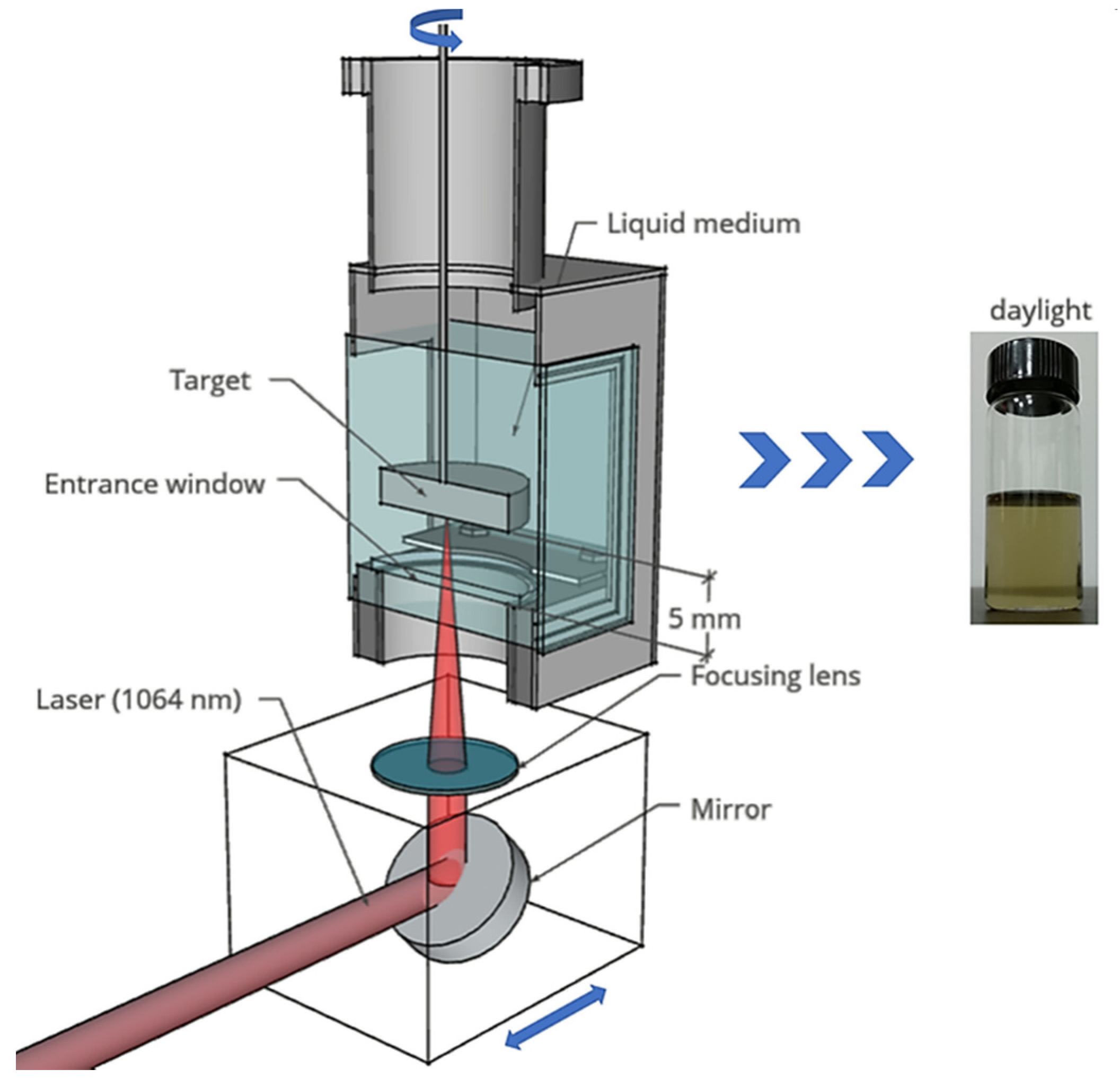
2.2. Synthesis of Carbon Nanoparticles
2.3. Characterization of Nanoparticles
2.4. Nanoparticles Application to Fungi
2.5. Statistical Analysis
3. Results and Discussion
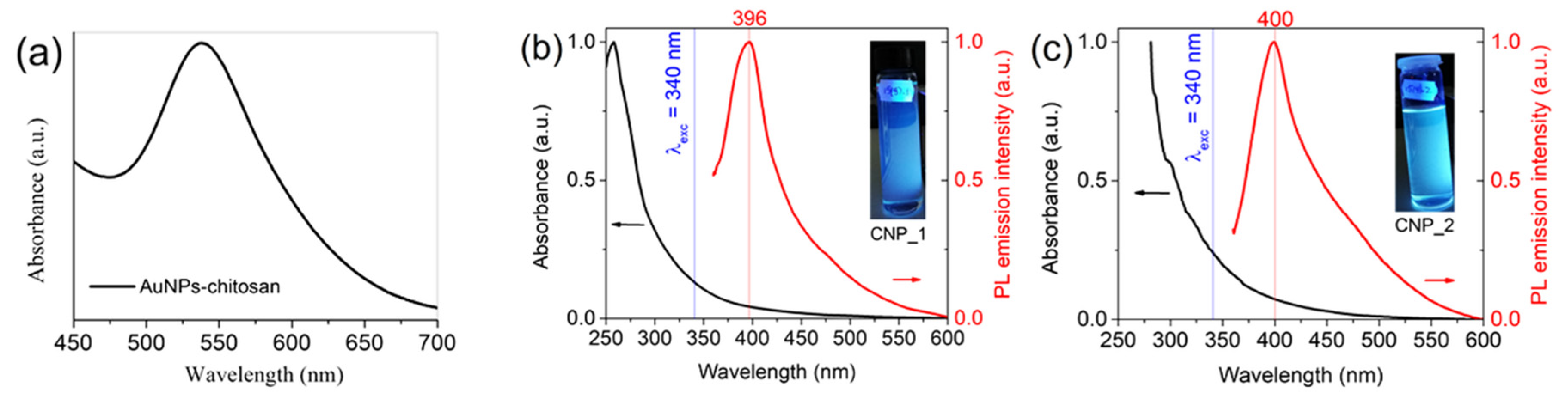
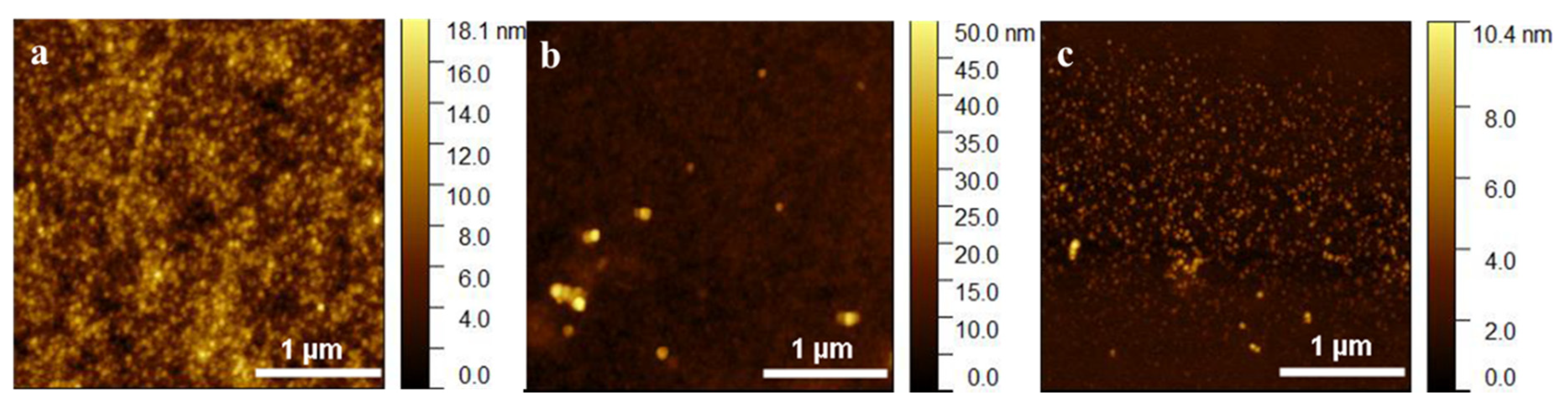
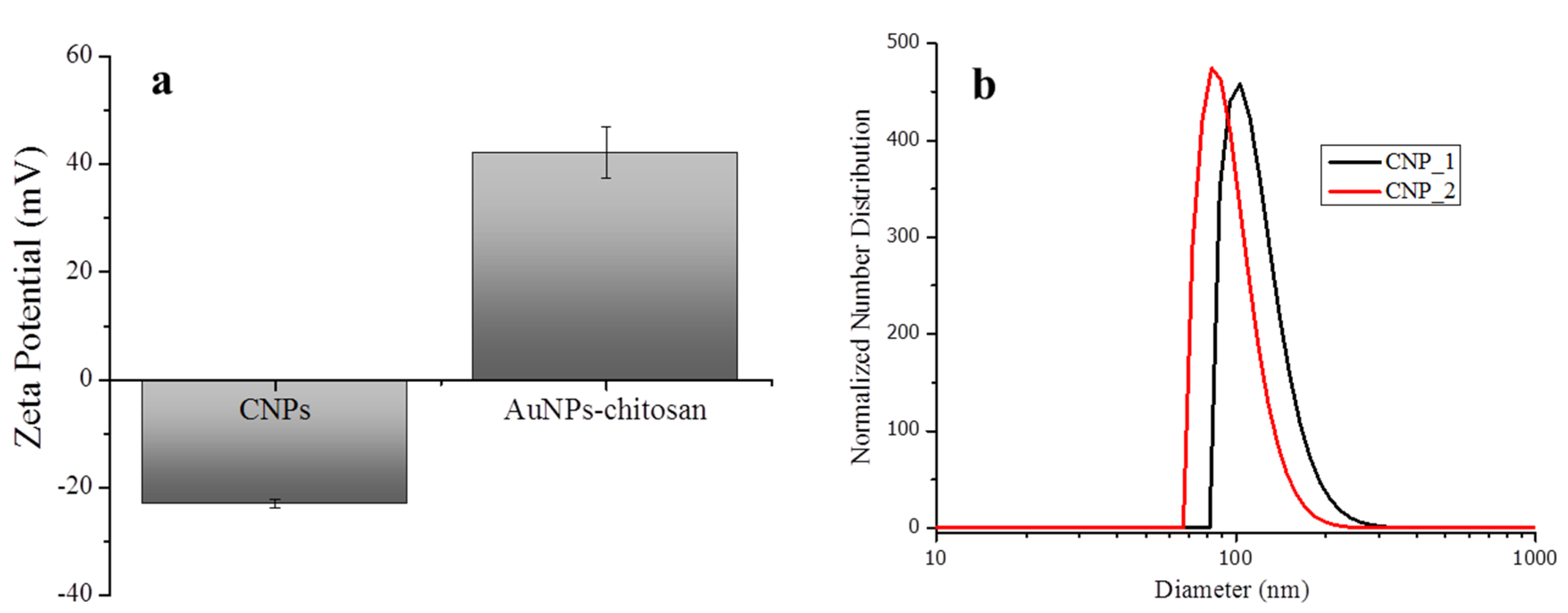
3.1. Synthesis and Characterization of Nanoparticles
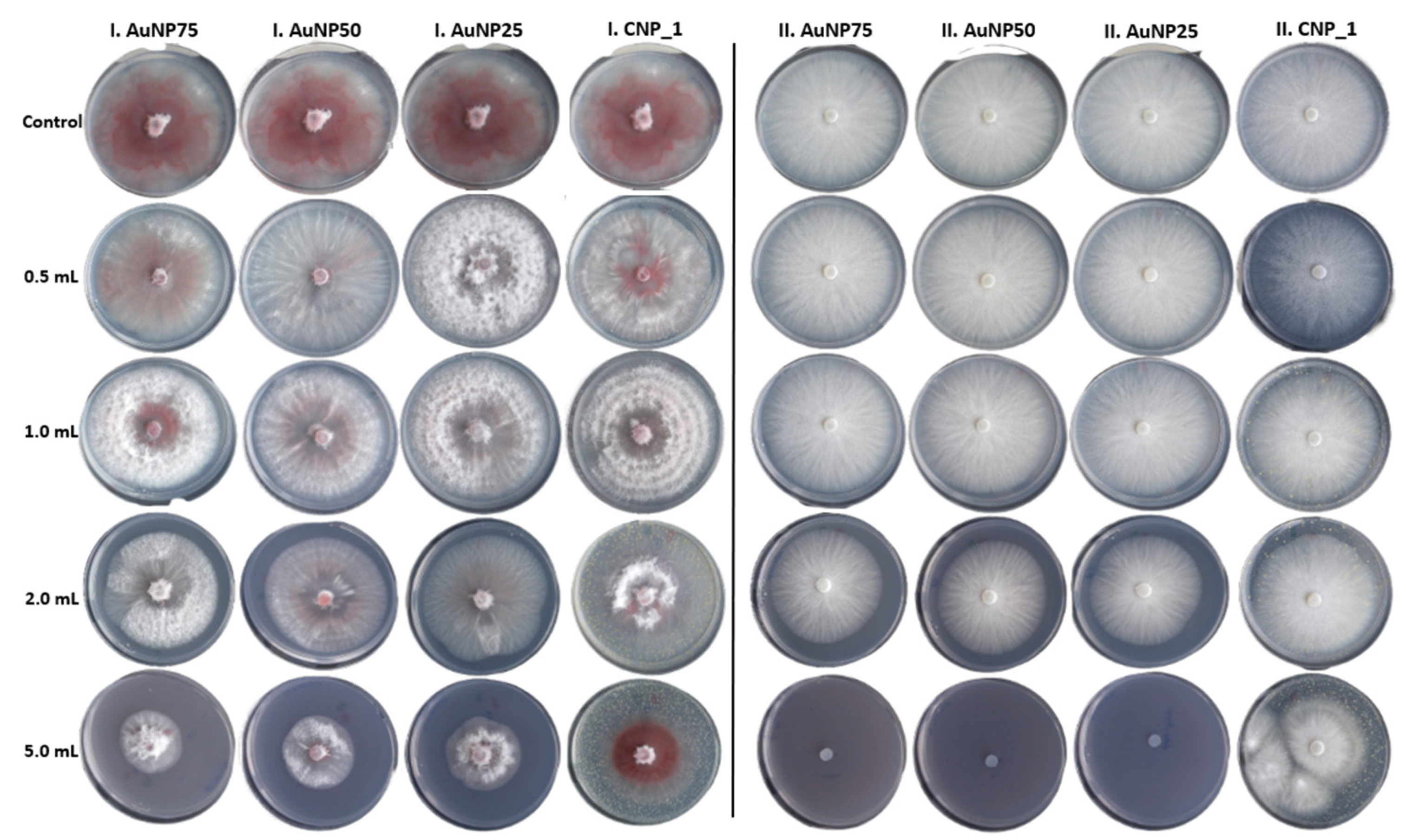
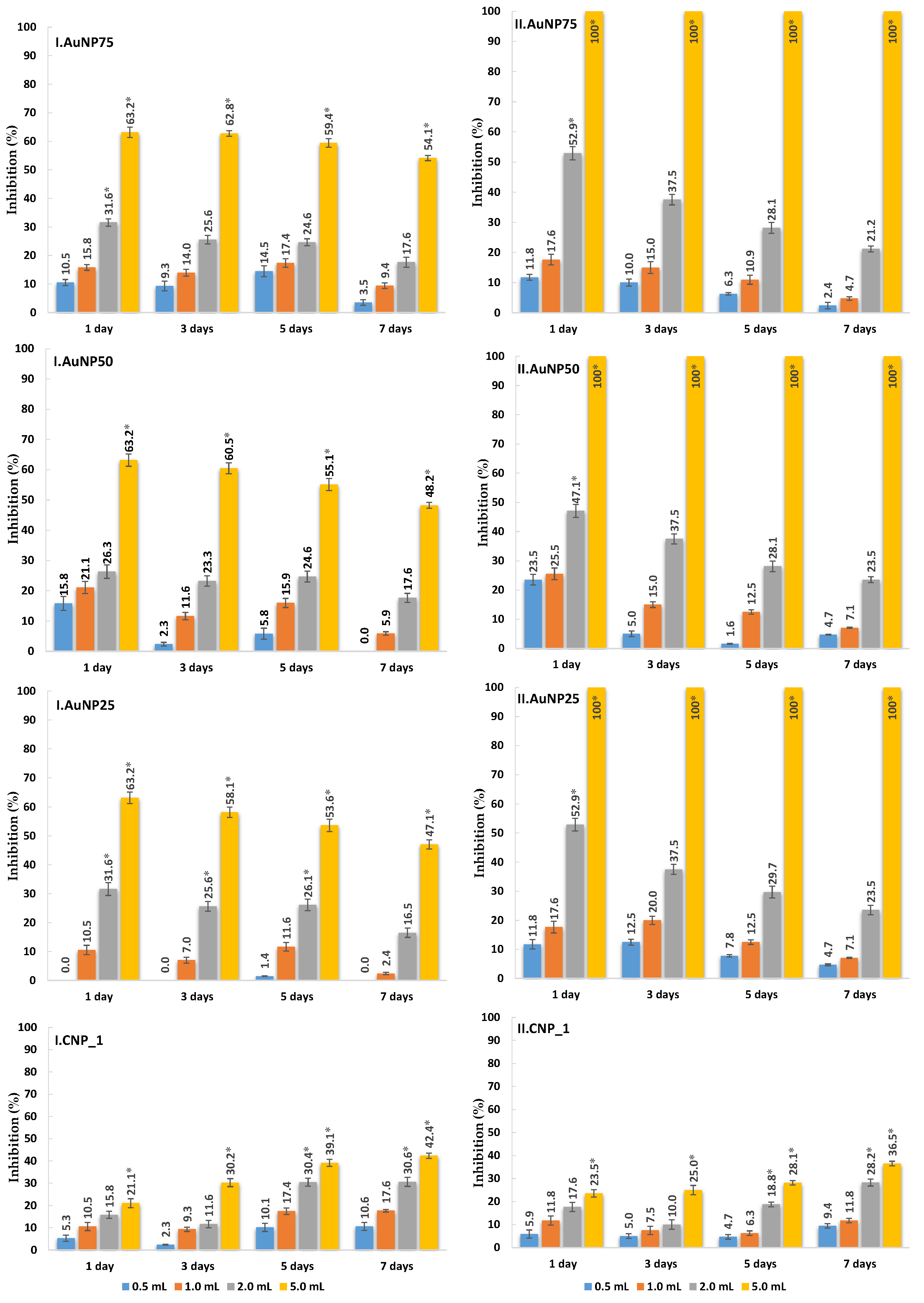
3.2. In Vitro Antifungal Assays
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Llorens, A.; Mateo, R.; Hinojo, M.J.; Valle-Algarra, F.M.; Jiménez, M. Influence of environmental factors on the biosynthesis of type B trichothecenes by isolates of Fusarium spp. from Spanish crops. Int. J. Food Microbiol. 2004, 94, 43–54. [Google Scholar] [CrossRef]
- Mateo, J.J.; Mateo, R.; Jiménez, M. Accumulation of type A trichothecenes in maize, wheat and rice by Fusarium sporotrichioides isolates under diverse culture conditions. Int. J. Food Microbiol. 2002, 72, 115–123. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Morales-Díaz, A.B.; González-Morales, S.; Morelos-Moreno, Á.; Cabrera-De la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and nanomaterials as plant biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef]
- Choi, O.; Deng, K.K.; Kim, N.-J.; Ross, L.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia. coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef]
- Perni, S.; Piccirillo, C.; Pratten, J.; Prokopovich, P.; Chrzanowski, W.; Parkin, I.P.; Wilson, M. The antimicrobial properties of light-activated polymers containing methylene blue and gold nanoparticles. Biomaterials 2009, 30, 89–93. [Google Scholar] [CrossRef]
- Ahmad, T.; Wani, I.A.; Lone, I.H.; Ganguly, A.; Manzoor, N.; Ahmad, A.; Ahmed, J.; Al-Shihri, A.S. Antifungal activity of gold nanoparticles prepared by solvothermal method. Mater. Res. Bull. 2013, 48, 12–20. [Google Scholar] [CrossRef]
- Zeng, H.; Du, X.-W.; Singh, S.C.; Kulinich, S.A.; Yang, S.; He, J.; Cai, W. Nanomaterials via laser ablation/irradiation in liquid: A review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Rahimi, H.; Roudbarmohammadi, S.; Delavari, H.H.; Roudbary, M. Antifungal effects of indolicidin-conjugated gold nanoparticles against fluconazole-resistant strains of Candida albicans isolated from patients with burn infection. Int. J. Nanomedicine 2019, 14, 5323–5338. [Google Scholar] [CrossRef]
- Ursu, C.; Nica, P.; Focsa, C. Excimer laser ablation of graphite: The enhancement of carbon dimer formation. Appl. Surf. Sci. 2018, 456, 717–725. [Google Scholar] [CrossRef]
- Ursu, C.; Nica, P.; Rusu, B.G.; Focsa, C. V-shape plasma generated by excimer laser ablation of graphite in argon: Spectroscopic investigations. Spectrochim. Acta Part B At. Spectrosc. 2020, 163, 105743. [Google Scholar] [CrossRef]
- Walkey, C.; Sykes, E.A.; Chan, W.C.W. Application of semiconductor and metal nanostructures in biology and medicine. Hematology 2009, 2009, 701–707. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chung, H.; Son, Y.; Yoon, T.K.; Kim, S.; Kim, W. The effect of multi-walled carbon nanotubes on soil microbial activity. Ecotoxicol. Environ. Saf. 2011, 74, 569–575. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; Heer, W.A. de Carbon nanotubes--the route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Firme, C.P.; Bandaru, P.R. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 245–256. [Google Scholar] [CrossRef]
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 2016, 3, 17. [Google Scholar] [CrossRef]
- Wang, B.; Chen, K.; Jiang, S.; Reincke, F.; Tong, W.; Wang, D.; Gao, C. Chitosan-mediated synthesis of gold nanoparticles on patterned poly (dimethylsiloxane) surfaces. Biomacromolecules 2006, 7, 1203–1209. [Google Scholar] [CrossRef]
- Esumi, K.; Takei, N.; Yoshimura, T. Antioxidant-potentiality of gold–chitosan nanocomposites. Colloids Surf. B Biointerfaces 2003, 32, 117–123. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Cai, J.; Zhong, L.; Ren, G.; Ma, Q. One pot synthesis of gold nanoparticles using chitosan with varying degree of deacetylation and molecular weight. Carbohydr. Polym. 2017, 178, 105–114. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X. Synthesis of chitosan-stabilized gold nanoparticles in the absence/presence of tripolyphosphate. Biomacromolecules 2004, 5, 2340–2346. [Google Scholar] [CrossRef] [PubMed]
- Guibal, E. Heterogeneous catalysis on chitosan-based materials: A review. Prog. Polym. Sci. 2005, 30, 71–109. [Google Scholar] [CrossRef]
- Zuber, A.; Purdey, M.; Schartner, E.; Forbes, C.; van der Hoek, B.; Giles, D.; Abell, A.; Monro, T.; Ebendorff-Heidepriem, H. Detection of gold nanoparticles with different sizes using absorption and fluorescence based method. Sens. Actuators B Chem. 2016, 227, 117–127. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV−Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Yu, C.; Xuan, T.; Yan, D.; Lou, S.; Hou, X.; Chen, Y.; Wang, J.; Li, H. Sesame-derived ions co-doped fluorescent carbon nanoparticles for bio-imaging, sensing and patterning applications. Sens. Actuators B Chem. 2017, 253, 900–910. [Google Scholar] [CrossRef]
- Control of Colloid Stability through Zeta Potential & its Relationship to Cardiovascular Disease. Available online: http://customers.hbci.com/~wenonah/riddick/index.html (accessed on 25 June 2020).
- Oh, J.-W.; Chun, S.C.; Chandrasekaran, M. Preparation and in vitro characterization of chitosan nanoparticles and their broad-spectrum antifungal action compared to antibacterial activities against phytopathogens of tomato. Agronomy 2019, 9, 21. [Google Scholar] [CrossRef]
- Bell, A.A.; Hubbard, J.C.; Liu, L.; Davis, R.M.; Subbarao, K.V. Effects of chitin and chitosan on the incidence and severity of Fusarium yellows of celery. Plant Dis. 1998, 82, 322–328. [Google Scholar] [CrossRef]
- Beyenbach, K.W.; Wieczorek, H. The V-type H+ ATPase: Molecular structure and function, physiological roles and regulation. J. Exp. Biol. 2006, 209, 577–589. [Google Scholar] [CrossRef]
- Tan, Y.N.; Lee, K.H.; Su, X. Study of single-stranded DNA binding protein–nucleic acids interactions using unmodified gold nanoparticles and its application for detection of single nucleotide polymorphisms. Anal. Chem. 2011, 83, 4251–4257. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Matei, P.M.; Iacomi, B.M.; Martín-Gil, J.; Pérez-Lebeña, E.; Ramos-Sánchez, M.C.; Barrio-Arredondo, M.T.; Martín-Ramos, P. In vitro antifungal activity of composites of AgNPs and polyphenol inclusion compounds against Fusarium culmorum in different dispersion media. Agronomy 2018, 8, 239. [Google Scholar] [CrossRef]
- Al-Hetar, M.Y.; Abidin, M.A.Z.; Sariah, M.; Wong, M.Y. Antifungal activity of chitosan against Fusarium oxysporum f. sp. cubense. J. Appl. Polym. Sci. 2011, 120, 2434–2439. [Google Scholar] [CrossRef]
- Xie, J.; Ming, Z.; Li, H.; Yang, H.; Yu, B.; Wu, R.; Liu, X.; Bai, Y.; Yang, S.-T. Toxicity of graphene oxide to white rot fungus Phanerochaete chrysosporium. Chemosphere 2016, 151, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Chen, J.; Han, H.; Yuan, Z. Evaluation and mechanism of antifungal effects of carbon nanomaterials in controlling plant fungal pathogen. Carbon 2014, 68, 798–806. [Google Scholar] [CrossRef]
- Verma, S.K.; Das, A.K.; Gantait, S.; Kumar, V.; Gurel, E. Applications of carbon nanomaterials in the plant system: A perspective view on the pros and cons. Sci. Total Environ. 2019, 667, 485–499. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipșa, F.-D.; Ursu, E.-L.; Ursu, C.; Ulea, E.; Cazacu, A. Evaluation of the Antifungal Activity of Gold–Chitosan and Carbon Nanoparticles on Fusarium oxysporum. Agronomy 2020, 10, 1143. https://doi.org/10.3390/agronomy10081143
Lipșa F-D, Ursu E-L, Ursu C, Ulea E, Cazacu A. Evaluation of the Antifungal Activity of Gold–Chitosan and Carbon Nanoparticles on Fusarium oxysporum. Agronomy. 2020; 10(8):1143. https://doi.org/10.3390/agronomy10081143
Chicago/Turabian StyleLipșa, Florin-Daniel, Elena-Laura Ursu, Cristian Ursu, Eugen Ulea, and Ana Cazacu. 2020. "Evaluation of the Antifungal Activity of Gold–Chitosan and Carbon Nanoparticles on Fusarium oxysporum" Agronomy 10, no. 8: 1143. https://doi.org/10.3390/agronomy10081143
APA StyleLipșa, F.-D., Ursu, E.-L., Ursu, C., Ulea, E., & Cazacu, A. (2020). Evaluation of the Antifungal Activity of Gold–Chitosan and Carbon Nanoparticles on Fusarium oxysporum. Agronomy, 10(8), 1143. https://doi.org/10.3390/agronomy10081143







