Co-Inoculation of Rhizobacteria and Biochar Application Improves Growth and Nutrientsin Soybean and Enriches Soil Nutrients and Enzymes
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture, Soybean, and Biochar
2.2. Screening for the Production of PGP Metabolites
2.3. Surface Sterilization, Germination, and Bacterization of Seeds
2.4. Experimental Design
2.5. Measurement of Plant Growth Parameters and Plant Nutrients
2.6. Analysis of Soil Nutrient and Soil Enzymes
2.7. Statistical Analyses
3. Results
3.1. Analysis of Maize Biochar
3.2. Screening for the Production of PGP Metabolites
3.3. Measurement of Plant Growth Parameters and Plant Nutrients
3.4. Estimation of Soil Nutrient Content and Soil Enzymes
4. Discussion
4.1. Screening for the Production of PGP Metabolites
4.2. Measurement of Plant Growth Parameters and Plant Nutrients
4.3. Estimation of Soil Nutrients and Soil Enzymes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarfraz, R.; Hussain, A.; Sabir, A.; Fekih, I.B.; Ditta, A.; Xing, S. Role of biochar and plant growth promoting rhizobacteria to enhance soil carbon sequestration—A review. Environ. Monit. Assess. 2019, 191, 251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Bian, R.; Pan, G.; Cui, L.; Hussain, Q.; Li, L.; Zheng, J.; Zheng, J.; Zhang, X.; Han, X. Effects of biochar amendment on soil quality, crop yield and greenhouse gas emission in a Chinese rice paddy: A field study of 2 consecutive rice growing cycles. Field Crops Res. 2012, 127, 153–160. [Google Scholar] [CrossRef]
- Asai, H.; Samson, B.K.; Stephan, H.M.; Songyikhangsuthor, K.; Homma, K.; Kiyono, Y.; Inoue, Y.; Shiraiwa, T.; Horie, T. Biochar amendment techniques for upland rice production in Northern Laos: 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Res. 2009, 111, 81–84. [Google Scholar] [CrossRef]
- Saxena, J.; Rana, G.; Pandey, M. Impact of addition of biochar along with Bacillus sp. on growth and yield of French beans. Sci. Hortic. 2013, 162, 351–356. [Google Scholar] [CrossRef]
- Agboola, K.; Moses, S. Effect of biochar and cowdung on nodulation, growth and yield of soybean (Glycine max L. Merrill). Int. J. Agric. Biosci. 2015, 4, 154–160. [Google Scholar]
- Głodowska, M.; Schwinghamer, T.; Husk, B.; Smith, D. Biochar based inoculants improve soybean growth and nodulation. Agric. Sci. 2017, 8, 1048–1064. [Google Scholar] [CrossRef]
- Pandit, N.; Mulder, J.; Hale, S.; Martinsen, V.; Schmidt, H.; Cornelissen, G. Biochar improves maize growth by alleviation of nutrient stress in a moderately acidic low-input Nepalese soil. Sci. Total Environ. 2018, 625, 1380–1389. [Google Scholar] [CrossRef]
- Chen, H.; Ma, J.; Wei, J.; Gong, X.; Yu, X.; Guo, H.; Zhao, Y. Biochar increases plant growth and alters microbial communities via regulating the moisture and temperature of green roof substrates. Sci. Total Environ. 2018, 635, 333–342. [Google Scholar] [CrossRef]
- Laird, D. The charcoal vision: A win–win–win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agron. J. 2008, 100, 178–181. [Google Scholar] [CrossRef]
- Hossain, M.; Strezov, V.; Chan, K.; Nelson, P. Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicone sculentum). Chemosphere 2010, 78, 1167–1171. [Google Scholar] [CrossRef]
- Zhang, H.; Prithiviraj, B.; Charles, T.; Driscoll, B.; Smith, D. Low-temperature tolerant Bradyrhizobium japonicum strains allowing improved nodulation and nitrogen fixation of soybean in a short season (cool spring) area. Eur. J. Agron. 2003, 19, 205–213. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabborova, D.; Wirth, S. Alleviation of salt stress in legumes by co-inoculation with Pseudomonas and Rhizobium. In Plant-Microbe Symbiosis: Fundamentals and Advances; Springer: Singapore, 2013; pp. 291–303. [Google Scholar] [CrossRef]
- Sayyed, R.; Gangurde, N.; Patel, P.; Josh, S.; Chincholkar, S. Siderophore production by Alcaligenes faecalis and its application for growth promotion in Arachis hypogaea. Indian J. Biotechnol. 2010, 9, 302–307. [Google Scholar]
- Jabborova, D.; Annapurna, K.; Fayzullaeva, M.; Sulaymonov, K.; Kadirova, D.; Jabbarov, Z.; Sayyed, R. Isolation and characterization of endophytic bacteria from ginger (Zingiberofficinale Rosc.). Ann. Phytomed. 2020, 9, 116–121. [Google Scholar] [CrossRef]
- Buckley, S.; Allen, D.; Brackin, R.; Jämtgård, S.; Näsholm, T.; Schmidt, S. Microdialysis as an in situ technique for sampling soil enzymes. Soil Biol. Biochem. 2019, 135, 20–27. [Google Scholar] [CrossRef]
- Sayyed, R.; Patel, P.; Shaikh, S. Plant growth promotion and root colonization by EPS producing Enterobacter sp. RZS5 under heavy metal contaminated soil. Indian J. Exp. Biol. 2015, 53, 116–123. [Google Scholar]
- Sagar, A.; Riyazuddin, R.; Shukla, P.; Ramteke, P.; Sayyed, R. Heavy metal stress tolerance in Enterobacter sp. PR14 is mediated by plasmid. Indian J. Exp. Biol. 2020, 58, 115–121. [Google Scholar]
- Elkoca, E.; Kantar, F.; Sahin, F. Influence of nitrogen-fixing and phosphorus solubilizing bacteria on the nodulation, plant growth, and yield of chickpea. J. Plant Nutr. 2007, 31, 157–171. [Google Scholar] [CrossRef]
- Jabborova, D.; Davranov, K. Effect of phosphorus and nitrogen concentrations on root colonization of soybean (Glycine max L.) by Bradyrhizobium japonicum and Pseudomonas putida. Int. J. Adv. Biotechnol. Res. 2015, 6, 418–424. [Google Scholar]
- Egamberdieva, D.; Jabborova, D.; Berg, G. Synergistic interactions between Bradyrhizobium japonicum and the endophyte Stenotrophomonas rhizophila and their effects on growth, and nodulation of soybean under salt stress. Plant Soil 2016, 405, 35–45. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Jabborova, D.; Räsänen, L.; Liao, H. Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. J. Plant Interact. 2017, 12, 100–107. [Google Scholar] [CrossRef]
- Jabborova, D.; Enakiev, Y.; Davranov, K.; Begmatov, S. Effect of co-inoculation with Bradyrhizobiumjaponicum and Pseudomonas putida on root morph-architecture traits, nodulation and growth of soybean in response to phosphorus supply under hydroponic conditions. Bulg. J. Agric. Sci. 2018, 24, 1004–1011. [Google Scholar]
- Egamberdieva, D.; Jabborova, D.; Wirth, S.; Alam, P.; Alyemeni, M.; Ahmad, P. Interaction of magnesium with nitrogen and phosphorus modulates symbiotic performance of soybean with Bradyrhizobiumjaponicum and its root architecture. Front. Microbiol. 2018, 9, 1. [Google Scholar]
- Holik, L.; Vranová, V. Proteolytic activity in meadow soil after the Application of phytohormones. Biomolecules 2019, 9, 507. [Google Scholar] [CrossRef] [PubMed]
- Reibe, K.; Götz, K.; Roß, C.; Döring, T.; Ellmer, F.; Ruess, L. Impact of quality and quantity of biochar and hydrochar on soil Collembola and growth of spring wheat. Soil Biol. Biochem. 2015, 83, 84–87. [Google Scholar] [CrossRef]
- Nautiyal, C. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Bric, J.; Bostock, R.; Silverstone, S. Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef]
- Patel, P.; Shaikh, S.; Sayyed, R. Modified chrome azurol S method for detection and estimation of siderophores having affinity for metal ions other than iron. Environ. Sustain. 2018, 1, 81–87. [Google Scholar] [CrossRef]
- Payne, S. Detection, isolation, and characterization of siderophores. In Methods in Enzymol; Elsevier: Amsterdam, The Netherlands, 1994; pp. 329–344. [Google Scholar] [CrossRef]
- Penrose, D.; Glick, B. Methods for isolating and characterizing ACC deaminase--containing plant growth--promoting rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef]
- Labconco, C. A Guide to Kjeldahl Nitrogen Determination Methods and Apparatus; Labconco Corporation: Houston, TX, USA, 1998. [Google Scholar]
- Upadhyay, A.; Sahu, R. Determination of total nitrogen in soil and plant. In Laboratory Manual on Advances in Agro-Technologies for Improving Soil, Plant and Atmosphere Systems; CAFT: Jabalpur, India, 2012; pp. 18–19. [Google Scholar]
- Upadhyay, A.; Sahu, R. Determination of potassium in soil and plant. In Laboratory Manual on Advances in Agro-Technologies for Improving Soil, Plant and Atmosphere Systems; CAFT: Jabalpur, India, 2012; pp. 23–35. [Google Scholar]
- Sahrawat, K. Determination of calcium, magnesium, zinc and manganese in plant tissue using a dilute HCl extraction method. Commun. Soil Sci. Plant Anal. 1987, 18, 947–962. [Google Scholar] [CrossRef]
- Sims, J. Soil test phosphorus: Principles and methods. Methods of Phosphorus Analysis for Soils, Sediments, Residuals and Waters. Southern Coop. Ser. Bull. 2009, 408, 9–19. [Google Scholar]
- Nelson, D.; Sommers, L. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1983, 9, 539–579. [Google Scholar] [CrossRef]
- Tabatabai, M.; Bremner, J. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Ladd, J.; Butler, J. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Patel, P.; Shaikh, S.; Sayyed, R. Dynamism of PGPR in bioremediation and plant growth promotion in heavy metal contaminated soil. Indian J. Exp. Biol. 2016, 54, 286–290. [Google Scholar]
- Wani, S.; Shaikh, S.; Sayyed, R. Statistical-based optimization and scale-up of siderophore production process on laboratory bioreactor. 3Biotech 2016, 6, 69. [Google Scholar]
- Saxena, B.; Rani, A.; Sayyed, R.; El-Enshasy, H.A. Analysis of Nutrients, Heavy Metals and Microbial Content In Organic and Non-Organic Agriculture Fields of Bareilly Region-Western Uttar Pradesh, India. Biosci. Biotechnol. Res. Asia 2020, 17, 399–406. [Google Scholar] [CrossRef]
- Sagar, A.; Sayyed, R.; Ramteke, P.; Sharma, S.; Najat Marraiki Elgorban, A.; Syed, A. ACC deaminase and antioxidant enzymes producing halophilic Enterobacter sp. PR14 promotes the growth of rice and millets under salinity stress. Plant Physiol. Mol. Biol. 2020. [Google Scholar] [CrossRef]
- Seneviratne, M.; Gunaratne, S.; Bandara, T.; Weerasundara, L.; Rajakaruna, N.; Seneviratne, G.; Vithanage, M. Plant growth promotion by Bradyrhizobium japonicum under heavy metal stress. S. Afr. J. Bot. 2016, 105, 19–24. [Google Scholar] [CrossRef]
- Mubarik, N.; Mahagiani, I.; Wahyudi, A. Production of IAA by Bradyrhizobium sp. World Acad. Sci. Eng. Technol. 2013, 152–155. [Google Scholar] [CrossRef]
- Masciarelli, O.; Llanes, A.; Luna, V. A new PGPR co-inoculated with Bradyrhizobium japonicum enhances soybean nodulation. Microbiol. Res. 2014, 169, 609–615. [Google Scholar] [CrossRef]
- Meliani, A.; Bensoltane, A.; Benidire, L.; Oufdou, K. Plant growth-promotion and IAA secretion with Pseudomonas fluorescens and Pseudomonas putida. Res. Rev. J. Bot. Sci. 2017, 6, 16–24. [Google Scholar]
- Genesio, L.; Miglietta, F.; Baronti, S.; Vaccari, F.P. Biochar increases vineyard productivity without affecting grape quality: Results from a four years field experiment in Tuscany. Agric. Ecosyst. Environ. 2015, 201, 20–25. [Google Scholar] [CrossRef]
- Sayyed, R.; Naphade, B.; Joshi, S.; Gangurde, N.; Bhamare, H.; Chincholkar, S. Consortium of A. feacalis and P. fluorescens promoted the growth of Arachis hypogea (Groundnut). Asian J. Microbiol. Biotechnol. Environ. Sci. 2009, 48, 83–86. [Google Scholar]
- Shaikh, S.; Patel, P.; Patel, S.; Nikam, S.; Rane, T.; Sayyed, R. Production of biocontrol traits by banana field fluorescent Pseudomonads and comparison with chemical fungicide. Indian J. Exp. Biol. 2014, 52, 917–920. [Google Scholar] [PubMed]
- Pandya, N.; Desai, P.; Sayyed, R. Antifungal, and phytohormone production ability of plant growth-promoting rhizobacteria associated with the rhizosphere of sugarcane. World J. Microbiol. Biotechnol. 2011, 13, 112–116. [Google Scholar]
- Sayyed, R.; Badgujar, M.; Sonawane, H.; Mhaske, M.; Chincholkar, S. Production of microbial iron chelators (siderophores) by fluorescent Pseudomonads. Indian J. Biotechnol. 2005, 4, 484–490. [Google Scholar]
- Uzoma, K.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Iijima, M.; Yamane, K.; Izumi, Y.; Daimon, H.; Motonaga, T. Continuous application of biochar inoculated with root nodule bacteria to subsoil enhances yield of soybean by the nodulation control using crack fertilization technique. Plant Prod. Sci. 2015, 18, 197–208. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota–a review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Li, L.; Ma, H.; Wirth, S.; Bellingrath-Kimura, S. Soil amendment with different maize biochars improves chickpea growth under different moisture levels by improving symbiotic performance with Mesorhizobium ciceri and soil biochemical properties to varying degrees. Front. Microbiol. 2019, 10, 2423. [Google Scholar] [CrossRef]
- Bruun, E.; Petersen, C.; Hansen, E.; Holm, J.; Hauggaard-Nielsen, H. Biochar amendment to coarse sandy subsoil improves root growth and increases water retention. Soil Use Manag. 2014, 30, 109–118. [Google Scholar] [CrossRef]
- Shen, Q.; Hedley, M.; Camps Arbestain, M.; Kirschbaum, M. Can biochar increase the bioavailability of phosphorus? J. Soil Sci. Plant Nutr. 2016, 16, 268–286. [Google Scholar] [CrossRef][Green Version]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S. Localisation of nitrate in the rhizosphere of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 2243–2246. [Google Scholar] [CrossRef]
- Wang, C.; Alidoust, D.; Yang, X.; Isoda, A. Effects of bamboo biochar on soybean root nodulation in multi-elements contaminated soils. Ecotoxicol. Environ. Saf. 2018, 150, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D. Effect of biochar and irrigation on soybean-Rhizobium symbiotic performance and soil enzymatic activity in field rhizosphere. Agronomy 2019, 9, 626. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, R.; Liu, R. Characterization of biochar from fast pyrolysis and its effect on chemical properties of the tea garden soil. J. Anal. Appl. Pyrolysis 2014, 110, 375–381. [Google Scholar] [CrossRef]
- Ouyang, L.; Tang, Q.; Yu, L.; Zhang, R. Effects of amendment of different biochars on soil enzyme activities related to carbon mineralisation. Soil Res. 2014, 52, 706–716. [Google Scholar] [CrossRef]
- Fall, D.; Bakhoum, N.; NourouSall, S.; Zoubeirou, A.; Sylla, S.; Diouf, D. Rhizobial inoculation increases soil microbial functioning and gum arabic production of 13-year-old senegaliasenegal (L.) britton, trees in the north part of Senegal. Front. Plant Sci. 2016, 7, 1355. [Google Scholar] [CrossRef]
- Oladele, S. Effect of biochar amendment on soil enzymatic activities, carboxylate secretions and upland rice performance in a sandy clay loam Alfisol of Southwest Nigeria. Sci. Afr. 2019, 4, e00107. [Google Scholar] [CrossRef]
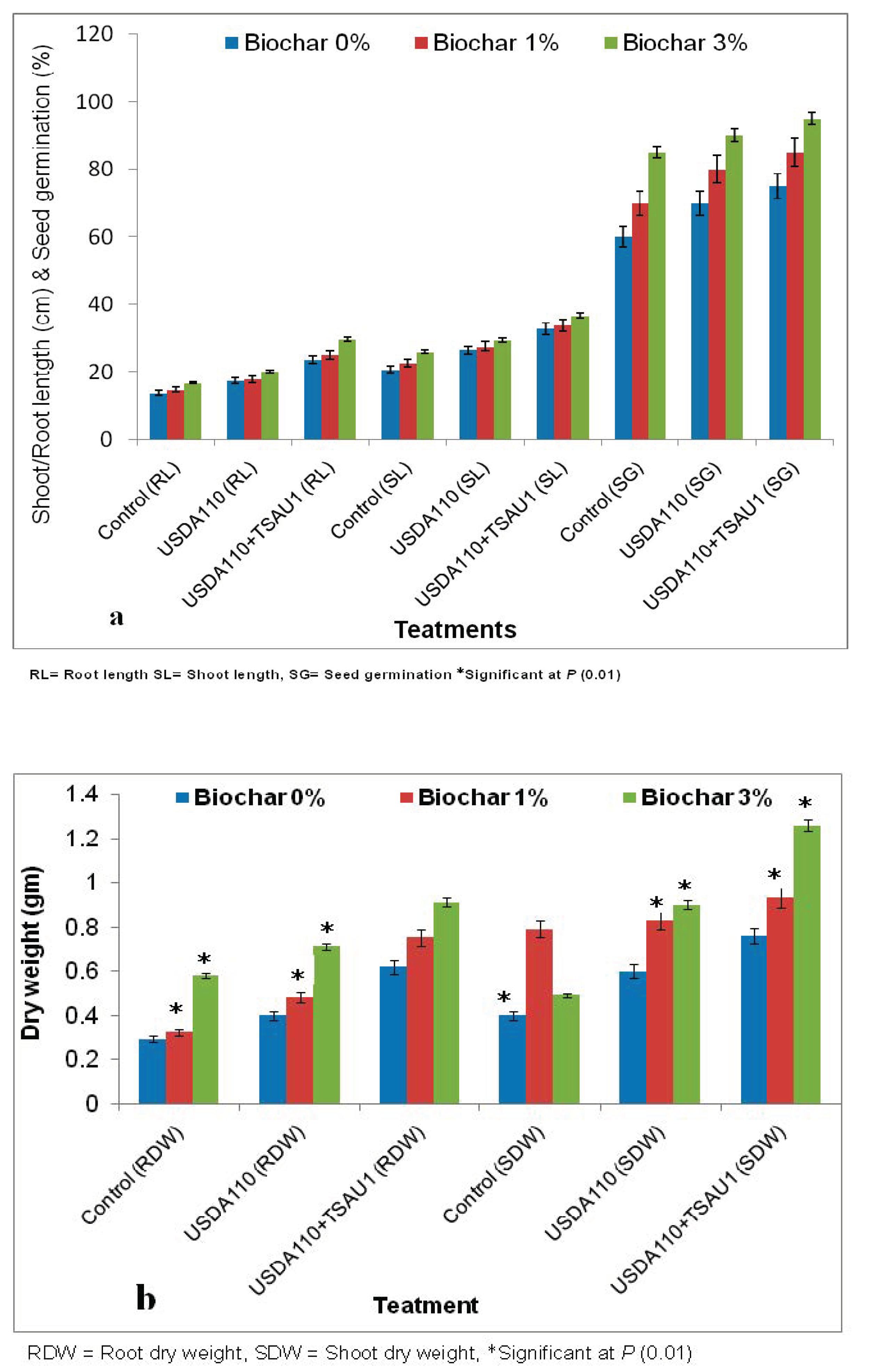
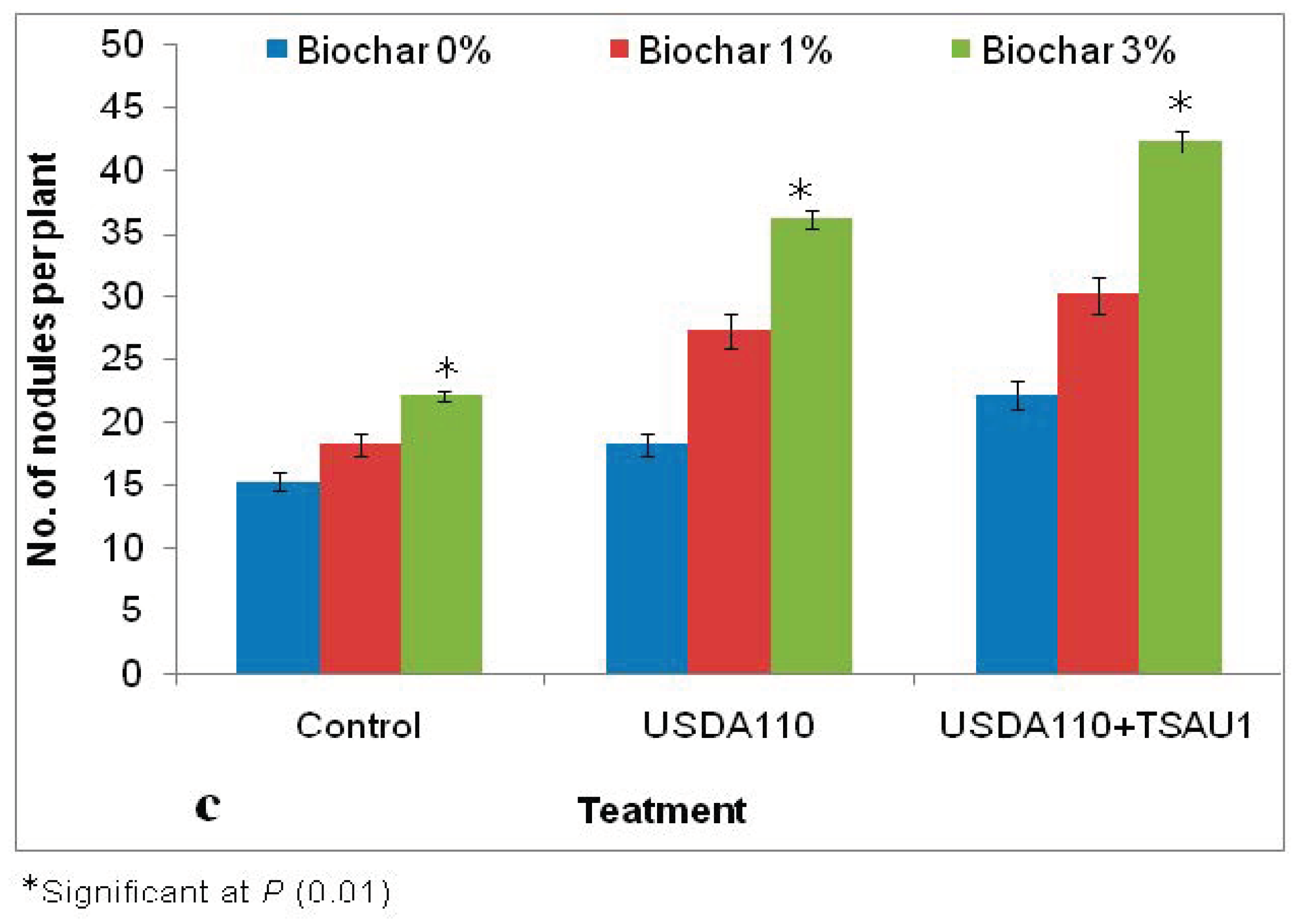
| Biochar Application | Treatments | N (%) | P (%) | K (%) | Mg (%) | Na (%) | Ca (%) |
|---|---|---|---|---|---|---|---|
| 0% | Control | 1.75 + 0.01 | 0.24 + 0.01 | 1.40 + 0.04 | 0.39 + 0.10 | 0.02 + 0.00 | 0.82 + 0.03 |
| TSAU1 | 2.00 + 0.02 * | 0.25 + 0.04 | 1.41 + 0.02 | 0.43 + 0.02 | 0.06 + 0.01 * | 0.91 + 0.03 | |
| USDA 110 | 2.39 + 0.02 * | 0.26 + 0.04 | 1.49 + 0.02 | 0.47 + 0.02 | 0.08 + 0.01 * | 1.07 + 0.03 | |
| USDA110+TSAU1 | 2.60 + 0.02 * | 0.27 + 0.02 | 2.09 + 0.15 * | 0.62 + 0.01 * | 0.09 + 0.01 * | 1.17 + 0.01 * | |
| 1% | Control | 1.77 + 0.02 | 0.27 + 0.03 | 2.33 + 0.02 | 0.66 + 0.02 | 0.03 + 0.01 | 0.95 + 0.03 |
| USDA 110 | 2.51 + 0.02 * | 0.28 + 0.02 | 2.52 + 0.04 | 0.52 + 0.02 | 0.07 + 0.01 * | 1.25 + 0.03 | |
| USDA+TSAU 1 | 2.64 + 0.02 * | 0.32 + 0.02 | 2.33 + 0.03 | 0.68 + 0.02 | 0.13 + 0.04 * | 1.21 + 0.02 | |
| 3% | Control | 1.91 + 0.02 | 0.28 + 0.01 | 2.41 + 0.02 | 0.64 + 0.02 | 0.03 + 0.03 | 1.09 + 0.02 |
| USDA 110 | 2.27 + 0.01 | 0.37 + 0.01 | 3.64 * + 0.01 | 0.48 + 0.01 | 0.02 + 0.01 | 0.99 + 0.01 | |
| USDA+TSAU1 | 2.85 * + 0.01 | 0.35 + 0.01 * | 3.72 * + 0.01 | 0.39 + 0.01 | 0.03 + 0.01 | 1.04 + 0.01 |
| Biochar Application | Treatments | SOC (%) | Total N (%) | P (mg) | K (mg) |
|---|---|---|---|---|---|
| 0% | Control | 21.09 ± 0.01 | 0.080 ± 0.01 | 4.29 ± 0.03 | 2.95 ± 0.02 |
| TSAU1 | 23.06 ± 0.01 | 0.082 ± 0.01 | 4.43 ± 0.03 | 3.05 ± 0.02 | |
| USDA 110 | 27.08 ± 0.01 | 0.083 ± 0.01 | 4.60 ± 0.02 * | 3.27 ± 0.03 * | |
| USDA+TSAU1 | 29.04 ± 0.02 * | 0.094 ± 0.8 * | 4.88 ± 0.02 * | 5.58 ± 0.03 * | |
| 1% | Control | 25.09 ± 0.01 | 0.091 ± 0.01 | 4.22 ± 0.03 | 4.83 ± 0.02 |
| USDA 110 | 29.06 ± 0.01 | 0.101 ± 0.02 * | 6.14 ± 0.01 * | 5.44 ± 0.01 * | |
| USDA+TSAU1 | 32.07 ± 0.8 * | 0.164 ± 0.03 * | 16.67 ± 0.05 * | 5.68 ± 0.02 * | |
| 3% | Control | 25.09 ± 0.01 | 0.094 ± 0.01 | 6.02 ± 0.01 | 5.35 ± 0.03 |
| USDA 110 | 33.05 ± 0.01 | 0.163 ± 0.01 * | 16.47 ± 0.01 * | 6.30 ± 0.01 * | |
| USDA+TSAU1 | 41.08 ± 0.01 * | 0.170 ± 0.01 * | 18.33 ± 0.01 * | 8.49 ± 0.01 * |
| Biochar Application | Treatments | Protease Activity (µg NH4+-N g−1h−1) | Acid Phosphomonoesterase Activity (µg pNPg−1h−1) | Alkaline Phosphomonoesterase Activity (µg pNPg−1r−1) |
|---|---|---|---|---|
| 0% | Control | 19.2 ± 0.05 | 650.3 ± 30.1 | 300.1 ± 16.3 |
| TSUA1 | 20.1 ± 0.05 | 697.1 ± 20.1 | 317.1 ± 12.3 | |
| USDA 110 | 23.5 ± 0.10 | 703.3 ± 34.5 | 365.6 ± 18.1 | |
| USDA+TSAU 1 | 25.8 ± 0.19 * | 780.6 ± 38.8 * | 380.2 ± 20.4 * | |
| 1% | Control | 21.4 ± 0.07 | 766.3 ± 35.7 | 370.5 ± 19.5 |
| USDA 110 | 25.8 ± 0.20 * | 820.9 ± 45.3 * | 425.3 ± 21.6 * | |
| USDA+TSAU 1 | 27.7 ± 0.18 * | 940.6 ± 43.2 * | 482.2 ± 20.8 * | |
| 3% | Control | 24.3 ± 0.09 | 810.3 ± 37.6 | 420.6 ± 19.5 |
| USDA 110 | 28.5 ± 0.11 * | 911.8 ± 46.3 * | 483.5 ± 21.2 * | |
| USDA+TSAU 1 | 30.8 ± 0.15 * | 1020.4 ± 48.6 * | 535.7 ± 25.2 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabborova, D.; Wirth, S.; Kannepalli, A.; Narimanov, A.; Desouky, S.; Davranov, K.; Sayyed, R.Z.; El Enshasy, H.; Malek, R.A.; Syed, A.; et al. Co-Inoculation of Rhizobacteria and Biochar Application Improves Growth and Nutrientsin Soybean and Enriches Soil Nutrients and Enzymes. Agronomy 2020, 10, 1142. https://doi.org/10.3390/agronomy10081142
Jabborova D, Wirth S, Kannepalli A, Narimanov A, Desouky S, Davranov K, Sayyed RZ, El Enshasy H, Malek RA, Syed A, et al. Co-Inoculation of Rhizobacteria and Biochar Application Improves Growth and Nutrientsin Soybean and Enriches Soil Nutrients and Enzymes. Agronomy. 2020; 10(8):1142. https://doi.org/10.3390/agronomy10081142
Chicago/Turabian StyleJabborova, Dilfuza, Stephan Wirth, Annapurna Kannepalli, Abdujalil Narimanov, Said Desouky, Kakhramon Davranov, R. Z. Sayyed, Hesham El Enshasy, Roslinda Abd Malek, Asad Syed, and et al. 2020. "Co-Inoculation of Rhizobacteria and Biochar Application Improves Growth and Nutrientsin Soybean and Enriches Soil Nutrients and Enzymes" Agronomy 10, no. 8: 1142. https://doi.org/10.3390/agronomy10081142
APA StyleJabborova, D., Wirth, S., Kannepalli, A., Narimanov, A., Desouky, S., Davranov, K., Sayyed, R. Z., El Enshasy, H., Malek, R. A., Syed, A., & Bahkali, A. H. (2020). Co-Inoculation of Rhizobacteria and Biochar Application Improves Growth and Nutrientsin Soybean and Enriches Soil Nutrients and Enzymes. Agronomy, 10(8), 1142. https://doi.org/10.3390/agronomy10081142








