Abstract
Phosphorus (P) is an essential element for plant growth and development. Finding new P sources and ways to improve crop P utilization are necessary due to the depletion of phosphate reserves. Five crop species, buckwheat (Fagopyrum esculentum L.), maize (Zea mays L.), oilseed rape (Brassica napus L. ssp. oleifera (Moench) Metzg.), spelt wheat (Triticum spelta L.), and white lupine (Lupinus albus L.), were grown in pots containing sandy soil with chemical nutrients, digestate, and meat bone meal (MBM) without added nutrients. Thirty days after the seeding plants were harvested, the growth stage, soil-plant analysis development (SPAD) value, biomass, P content of the plants, colonization of the roots with endomycorrhiza, and soil pH were analyzed, and the number of fungal spores in the soil was counted. All species showed interaction with the P sources for measured traits, except for the rhizosphere pH. A high biomass was recorded in all species fertilized with various P sources compared to the unfertilized treatment. Buckwheat and spelt wheat showed a higher P uptake with MBM, and the mycorrhizal symbiosis improved with digestate or MBM compared to synthetic P. The results indicate that different species have adaptative mechanisms to various P sources which could improve the resilience and sustainability of cropping systems.
1. Introduction
Phosphorus (P) is one of the main nutrients needed for plant growth [1]. Due to the globally limited availability of P reserves based on rock phosphate, attention has been paid to the possibilities of utilizing organic sources of P such as digestate, meat bone meal, and manure. Although the P content in these organic sources is high, not all P is in soluble form and thus is not directly available for plants. Rock phosphates and meat bone meal contain approximately 20–30% P as hydroxyl apatite, the bioavailability of which is low. Hydroxyl apatite is more available in acid soils than in calcareous or alkaline soils and in the presence of mycorrhizal soil fungi [2]. The origin of the feedstock of digestate affects the P content and its solubility. For example, sewage sludge-based digestate can contain, for example, aluminum oxide and ferrosulphate P adsorbents, which both decrease the bioavailability of P [3].
Added P may also build up in agricultural soils. P ions can be adsorbed and precipitate onto positively charged minerals, such as calcium (Ca), iron (Fe), and aluminum (Al) oxides, which are affected by soil pH and the concentration of anions that compete with P ions for ligand exchange reactions [4]. According to Lambers et al. [1], 80–90% of applied P is under retention and sorption by soil particles. The possibility of improving the utilization of soil P reserves, including apatite, for plant growth has therefore also become important. The nutrient flux in the soil-plant system is controlled by complex interactions among plant roots, soil microorganisms, chemical reactions, and pathways for loss. The availability of soil P for plant roots is controlled by the concentration of phosphate ions in the soil solution and the ability of the soil to replenish these ions when plant roots remove them [5].
Many important crops, for example, cereals and oilseeds, are sensitive to P availability at the early growth stages [6,7]. The most critical period is typically from the second to the fourth week of growth when the seedlings are running out of seed P storage [8]. P deficiency, for example, delays phenological development, limits tiller production and secondary root development, and decreases both plant size and biomass accumulation, which are mainly irreversible [6]. In a P limited environment, plants have adaptive responses for enhancing P mobility in the soil to increase its uptake [9]. These responses include the modification of rhizosphere pH by the exudation of P-mobilizing compounds, such as protons, organic acids, and phosphatases from the roots [4,10], changes in root architecture and morphology, such as the length and number of adventitious roots, root hair proliferation [9], and symbioses with microbes such as mycorrhizal fungi and bacteria [10,11]. Various mechanisms have been suggested to increase P uptake by mycorrhizal plants such as the exploration of a larger soil volume, more rapid movement of P into mycorrhizal hyphae, and solubilization of soil P [11,12]. As an example, maize (Zea mays L.) is known to develop intraradical mycorrhiza rapidly [13], but also to enhance the lateral rooting as a response to P limitation [14]. Similarly, buckwheat (Fagopyrum esculentum L.) has a great ability to proliferate its roots under P limitation [6]. It has increased root surface phosphatase activity as a response to P limitation and root exudates, organic acids and phenolics, to lower the soil pH for the increased availability of P [15,16]. White lupine (Lupinus albus L.) is able to adjust its root architecture by forming specialized root structures, i.e., proteoid roots, adjust the physiological processes, and exude citric acid from the clusters to maximize P ion acquisition [1,17,18]. Maize, wheat (Triticum aestivum L.), and oilseed rape (Brassica napus L. ssp. oleifera (Moench) Metzg.) are known to dominantly exude malic acid from their roots under nutrient deficiency [4]. Oilseed rape is also able to release phosphatases to break down organic phosphatases and increase the availability of P as well as proliferate its root system [6]. However, it is also known to synthesize soluble and volatile compounds that are considered to reduce spore germination and hyphae extension of mycorrhizal fungi [19,20], and thus it is considered a non-mycorrhizal plant [12,20]. Spelt wheat (Triticum spelta L.) is suggested to have a greater ability than wheat to acquire P from soil due to its root system architecture [21] and a higher root phosphatase activity [22].
The aim of this preliminary research was to evaluate the early growth and P uptake response of the aforementioned crops, buckwheat, maize, oilseed rape, spelt wheat, and white lupin, known to differ in their P acquisition abilities to different agricultural P sources. The additional aim was to evaluate the occurrence of mycorrhiza in the roots. The P sources used in this study were chosen based on the different bioavailability of P; chemical P, meat bone meal and digestate based on ferrosulphate-treated sludge. The hypothesis was that crop species with different P uptake strategies, especially with root exudates and mycorrhiza, can utilize the recycled P in meat bone meal and digestate for their early growth.
2. Materials and Methods
2.1. Experimental Design, Plant Material and Growth Conditions
A pot experiment with four replicates was conducted in an environmentally controlled glasshouse at the University of Helsinki, Finland. Pots (1 L) were filled with 1 kg of sandy soil (pH 6.2, soluble P 16.5 mg/L, reserve P 560 mg/L, more detailed description of soil characteristics in [23]). The treatments were four different P sources: synthetic fertilizer (liquid nutrient mix: NH4NO3, Merck KGaA, Darmstadt, Germany; K2SO4, Riedel-de Haën AG, Seelze, Germany; Ca(H2PO4)2∙H2O, Sigma–Aldrich, Steinheim, Germany), meat bone meal (N-P-K:7-7-0.3, pH 6.9; Honkajoki Oy, Finland), digestate (N-P-K:3-3-0.2, pH 7.2; HSY, Finland) and unfertilized soil. The P level was set to 50 mg kg−1 soil based on the P content of the P source, and ammonium nitrate (NH4NO3) and potassium sulfate (K2SO4) were used to adjust potassium to 150 mg kg−1 soil and nitrogen to 100 mg kg−1 soil in all treatments. Ten seeds each of buckwheat (landrace), white lupine (cv. Boruta), maize (cv. Ronaldinio), oilseed rape (cv. Apollo), and spelt wheat (landrace) were placed at a depth of 1 cm in each pot and 100 mL of potable water (pH 8.4, more detailed description of chemical characteristics in [24]) was added. Each treatment combination included four replicates. During the experiment, day/night temperatures were set to 20/16 °C and relative humidity to 45%. High-pressure sodium lamps (Master son-t; Philips Lighting N.V., Eindhoven, The Netherlands) provided an 18 h photoperiod with a photosynthetic flux density of 250 µmol m−2 s−1. After germination, seedlings were thinned to five per pot. Water was supplied daily.
2.2. Sampling and Measurements
All plants were harvested at 30 days after seeding (DAS). The growth stages of all plants were determined concurrently according to the Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie (BBCH) scale [25], and the soil-plant analysis development (SPAD) values (SPAD-502, Konica Minolta Sensing, Osaka, Japan) were measured from ten leaves per pot. Whole plants were sampled without damaging the roots. Plants were placed on paper sheets and the soil from roots was removed by gentle shaking and brushing. They were collected in plastic tubes and stored at −20 °C until further analysis. Shoots and roots were separated, and the roots were washed under potable water. A 1-g root sample was collected and used immediately for mycorrhizal colonization determination. Shoots were oven-dried at 60 °C for two days and weighed. Dried plant samples were ground into fine powder (0.5-mm sieve) and stored at room temperature until further analysis. A representative soil sample was collected from the remaining soil in each pot (bulk soil), sieved (1 and 63 µm), and stored at −20 °C until further analysis. The bulk soil pHH2O (IQ160 pH meter, IQ Scientific Instruments Inc., Carlsbad, CA, USA) and rhizosphere soil pHH2O were measured from each pot.
2.3. P Analysis
Phosphorus was analyzed from ground plant samples according to Seleiman et al. [26]. A subsample of 250 mg was weighed into acid-washed Teflon tubes (CEM Corp., Matthews, NC, USA), and 6 mL nitric acid (67–70%, VWR International Oy, Helsinki, Finland) and 1 mL hydrogen peroxide (H2O2 (30%, Merck KGaA, Darmstadt, Germany)) were added for microwave digestion (MARSXpress, MARS 240/50, CEM Corp., Matthews, NC, USA). Digested samples were filtered through ash-free paper (Whatman, Grade No. 42, pore size 2.5 µm, GE Lifesciences, Helsinki, Finland) diluted in purified water and stored at −20 °C. Phosphorus analysis was run with inductively coupled plasma-optical Emission spectrometry (iCAP 6200, Thermo Fischer Scientific, Cambridge, UK).
2.4. Mycorrhiza Analyses
The mycorrhizal spore density in the bulk soil was counted according to the slightly modified method of Allen et al. [27]. A soil subsample of 10 g was weighed into a centrifuge tube containing 20 mL of distilled water. Samples were allowed to hydrate for 15 min and centrifuged for 10 min at 2000 rpm at 10 °C to remove organic matter. Samples were resuspended in 20 mL of 2M sucrose in sodium hexametaphosphate ((NaPO3)6, Merck KGaA, Darmstadt, Germany), solution and centrifuged. The supernatant containing the spores was poured into a separatory funnel and allowed to settle for 10 min. The liquid was then slowly drained out (10 mL min−1). Spores were washed carefully from the funnel walls with 2 mL of distilled water into a Petri dish (Tissue Culture Plate 6-Well Flat Bottom, Sarstedt Inc., Newton, NC, USA). The number of spores was counted immediately under a stereomicroscope (Leica MZ FL III, Fluorescent Stereo Microscope 420C, Leica microsystems, Heerbrugg, Germany). Spores were classified according to Giovannetti and Mosse [28] into Type 1 (black, brownish, or translucent) and Type 2 (hairy, small, and shiny).
The mycorrhizal colonization was evaluated with the gridline intersect method according to Brundrett [29]. Thereafter, a fresh root subsample of 1 g was cut into 2-cm pieces, placed in bottles containing 40 mL of 10% potassium hydroxide (KOH, Merck KGaA, Darmstadt, Germany), and kept in a water bath at 60–90 °C for 2–4 h [30]. Samples were rinsed with 10 mL of 10% hydrogen chloride (HCl, Sigma–Aldrich, Steinheim, Germany), followed by distilled water, then stained with cotton blue (Riedel-de Haën AG, Seelze, Germany) according to Grace and Stribley [31]. Stained root pieces were dispensed in the Petri dish (Ø9 cm) with grid lines. Colonized (discolored with cotton blue) and non-colonized root parts intersecting lines were counted from vertical and horizontal lines under a stereomicroscope. The proportion of colonized root parts to non-colonized indicates the percentage of root samples colonized by arbuscular mycorrhizal (AM). Root pieces were viewed under a light microscope (Leitz Laborlux S, Wetzlar, Germany) with an attached charge coupled device (CDD) camera (Olympus DP 50, Olympus Europa, Hamburg, Germany) to visualize the arbuscular mycorrhizal (AM) hyphae, arbuscules, and vesicles as well as zoosporangia, dark septate endophyte (DSE) microsclerotia and hyphae in the roots.
2.5. Statistics
The data were subjected to a two-way ANOVA to show the effects of the various crop species, P sources, and their interaction as fixed effects on the measured parameters. Statistical significance was established when the p-values were <0.05, and means were compared using Tukey’s multiple range test. All statistical analyses were carried out using R program (version 4.0.2; R Development Core Team, Vienna, Austria).
3. Results
3.1. Growth-Related Traits and P Content of Shoots
At the time of harvest, i.e., 30 DAS, the growth stages of crops did not vary between the treatments, except in buckwheat. The BBCH growth stage of unfertilized buckwheat was 36, whereas the growth stage of fertilized individuals ranged from 61 to 64. The growth stages of white lupine, maize, oilseed rape, and spelt wheat were 16, 13, 13, and 14, respectively.
An interaction was observed between the crop species and the P source in plant biomasses. Buckwheat, oilseed rape, and spelt wheat had the lowest biomasses when crops were grown in soil without added fertilizer, whereas no differences were observed in the biomass of plants grown with meat bone meal, digestate, and synthetic fertilizer-treated soil (Table 1). Furthermore, no significant differences were found in the biomasses of white lupine and maize when grown with different P sources.

Table 1.
Single plant shoot biomass (g/plant), phosphorus (P) content in shoots (g/kg DM), P uptake (mg/shoot), and soil-plant analysis development (SPAD) value of the studied crop species grown with different P sources. Data shown are means, n = 3–4.
An interaction also existed between the crop species and P source in the P content. In general, the P content was lowest in plants grown in soil treated with meat bone meal and digestate, and highest when grown in soil without added fertilizer (Table 1). Maize and spelt wheat had the lowest P contents, whereas white lupine and buckwheat had the highest P contents. Buckwheat was the most responsive crop species towards the different P sources.
Differences were observed in the P uptake between plant species, with buckwheat and white lupine having the highest P uptakes and spelt wheat having the lowest P uptake. Interestingly, digestate treatment resulted in the lowest P uptake of crops, whereas meat bone meal resulted in the highest P uptake of crops. No differences occurred in the P uptake following the use of synthetic fertilizer and control treatments.
The interaction between crop species and P sources was also observed in the SPAD values. The soil-plant analysis development value was lowest in plants grown without added fertilizer (Table 1). No differences in the SPAD value between the meat bone meal, digestate, and synthetic fertilizer-grown white lupine, maize, and spelt wheat were observed. Overall, the highest SPAD value was recorded in white lupine, followed by oilseed rape, whereas maize had the lowest SPAD value.
3.2. Soil pH and Mycorrhiza
Interaction effect was observed between the crop species and P source on the soil pH but not on the rhizosphere soil pH (Table 2). The changes in the soil pH were more evident in maize and spelt wheat treated with the various P sources, but no changes were observed in buckwheat, white lupine and oilseed rape treated with the various P sources. The soil pH and rhizosphere soil pH were generally lower in soil treated with synthetic fertilizer and higher in soil treated with digestate and meat bone meal (Table 2). The plant species affected the rhizosphere soil pH. The highest rhizosphere soil pH was obtained with oilseed rape and the lowest with buckwheat. The highest soil pH was obtained with white lupine and the lowest with buckwheat. Notably, no difference was found in the soil pH with white lupine, oilseed rape, spelt wheat, and maize (Table 2).

Table 2.
Bulk soil and rhizosphere soil pHH2O, number of mycorrhizal spores in 10 g of rhizosphere soil, and the percent of root mycorrhizal colonization in the studied crop species grown under different P sources. Data are shown are means, n = 3–4.
Soil fungal spores were identified into Type 1 (AM-type spores) and 2 (no AM-type spores) based on their appearance. There was an interaction between the crop species and P source in the total number of spores and in the number of Type 2 spores (Table 2). The number of Type 1 spores in the soil was markedly higher compared to the number of Type 2 spores, which were small, black, hairy or shiny, and seed-shaped (Table 2). The number of Type 1 spores did not vary markedly in soil treated with different P sources, except in maize and spelt wheat. In buckwheat, the greatest number of Type 1 spores was obtained with digestate, meat bone meal, and without P treatment, whereas the lowest was obtained with synthetic fertilizer. In contrast, the greatest number of Type 2 spores was obtained with synthetic fertilizer in maize. Generally, the total number of spores, along with the number of Type 1 and 2 spores, was lowest in soil with oilseed rape and greatest in soil with maize and white lupine (Table 2).
The roots of all the studied crops were colonized by mycorrhizal hyphae and vesicles, while treelike invaginations, i.e., arbuscules, were observed only in maize and buckwheat root cells (Table 2, Figure 1). Dark septate endophytic fungi, and microsclerotia (MS) and zoosporic plant pathogenic Olpidium brassicae (Woronin) P.A. Dang. were observed in addition to endo-mycorrhizal structures (Figure 1). In general, the highest colonization rates were in meat bone meal-treated seedlings. The highest colonization rate of endomycorrhiza was observed in spelt wheat fertilized with digestate. White lupine had the lowest rate of mycorrhizal colonization in all P treatments.
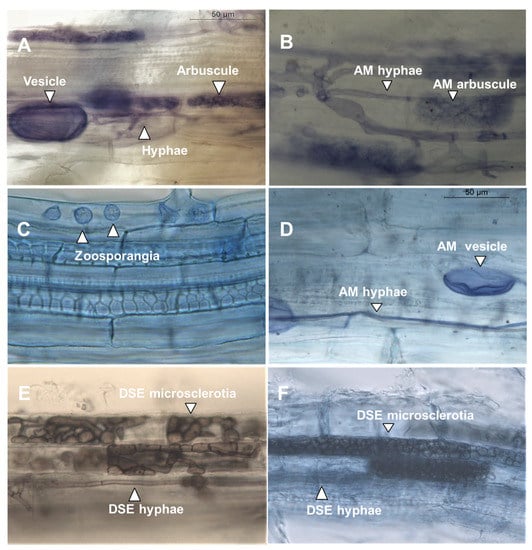
Figure 1.
(A) Maize root cells when fertilized with digestate. Arbuscular mycorrhizal (AM) vesicle, hyphae, and arbuscule. (B) Maize root cells when fertilized with meat bone meal. Arbuscular mycorrhiza (AM) hyphae and arbuscule. (C) Oilseed rape root cells when fertilized with digestate. Olpidium brassicae zoosporangia in rhizodermal cells and (D) AM hyphae and vesicle. (E) Oilseed rape root cells without added fertilizer. Dark septate endophyte (DSE) hyphae and microslerotia. (F) Spelt wheat root cells when fertilized with synthetic fertilizer. DSE fungal hyphae and microslerotia. All figures are in the same scale and the bar indicates 50 µm.
4. Discussion
Fertilization affected the growth and phenology of buckwheat only, even though buckwheat has a five to eight times greater ability than wheat to proliferate its roots under P limitation [10]. As a typical response to P limited growing conditions [10], fertilized buckwheat was at BBCH stages 61 to 64, while the unfertilized buckwheat was still at stage 36. The fertilized buckwheat had concurrently accumulated 23% to 41% more biomass than unfertilized buckwheat. However, the low SPAD values of unfertilized buckwheat suggest the plants were also N-deficient, and thus, the observed delay in phenology cannot be related solely to P. Similarly to buckwheat, the biomass of oilseed rape and spelt wheat was lowest without added fertilizer. The low biomass accumulation of oilseed rape, especially in the unfertilized treatment, may at least partly be related to a low P uptake. According to Grant et al. [7], the need and uptake of P in oilseed rape is rapid in the early growth stages and P deficiency thus frequently limits its growth.
The P uptakes and shoot P contents of buckwheat and white lupine were the highest, indicating that they were able to utilize soil apatite P reserves. Buckwheat has adaptive root traits, and root exudates, enabling P uptake even in low soil P conditions [15]. Inamullah et al. [32] reported that unfertilized white lupine had a 50% higher biomass and 43% higher P uptake when grown without fertilizer. White lupine has cluster roots and the ability to release carboxylates, mainly citrate and malate, into the rhizosphere to solubilize the immobile P reserves [1,33]. Cluster roots also release phenolic compounds, which inhibit the active growth of mycelia and induce fungal sporulation, thus preventing the degradation of carboxylates by the growing mycelia [34]. However, cluster root formation is suppressed when plants are grown with a higher P supply [35]. This partly explains the low shoot biomass, low P uptake and low P content observed in white lupine fertilized with various P sources compared with the unfertilized treatment.
Legumes require P for the establishment of symbiosis and symbiotic N2 fixation [36,37], and P deficiency slows down the processes markedly [38]. Due to its ability to affect the solubility of the soil P reserves, buckwheat is recommended to be included in intercropping and crop rotation systems to activate P sources in calcareous soils and to increase the soil P availability for the following crop [39]. White lupine should also be given a similar status. The high P uptake observed in buckwheat and spelt wheat when grown with meat bone meal can be associated with the slow mineralization of P from meat bone meal [40]. In agreement, [41] found bone meals to be a richer source of available phosphorus for crops than phosphates.
The decrease in the pH of the soil and rhizosphere treated with synthetic fertilizer could be related to the acidic nature of the fertilizer, which contains ammonium ions. In contrast, digestate and meat bone meal contain organic matter which buffers the soil pH [1,5]. This was evident in this study as no pH changes were observed between the digestate and meat bone meal in relation to the unfertilized treatment. The differences in the crop species’ rhizosphere soil pH were most evident between buckwheat (4.92) and oilseed rape (5.72). Buckwheat responds to P limitation both with root system proliferation [6] as well as an increased phosphatase activity and exuding phenolics and organic acids, which all lower soil pH [15,16]. This was also evident at a high P uptake. Even though oilseed rape also has similar mechanisms as a response to P limitation [6], the adaptive mechanisms might not have been expressed and thus, the rhizosphere soil pH remained high and P uptake low.
The total number and number of Type 2 spores observed in the soil were related to the P source, especially in buckwheat. Similarly, Toljander et al. [42] observed the highest richness of AM with organic fertilizer treatments when compared with mineral fertilizers in maize. Type 2 spores, which were not classified as AM spores in the present study, were at their highest levels in the soil with an application of synthetic fertilizer, followed by a digestate application. Earlier, Seleiman et al. [43] also reported that the application of digestate increased the sporulation of mycorrhizal fungi. Interestingly, mycorrhizal colonization in the roots and fungal sporulation in the soil were observed in all crop species. However, a lack of functional arbuscules in oilseed rape and white lupine root cells indicates that likely no functional symbiosis was formed. Both AM symbiosis and spore numbers are generally accepted to be at their lowest in soil with oilseed rape. Oilseed rape is known to synthesize soluble and volatile compounds that are considered to reduce spore germination and hyphae extension of mycorrhizal fungi [19]. Brassicas, including oilseed rape, are considered non-mycorrhizal plants [12]. Demars and Boerner [44] inoculated 646 Brassica species with Rhizophagus irregularis (Błaszk, Wubet, Renker & Buscot) C. Walker & A. Schüßler, yet they did not observe arbuscules in any of the examined root samples. However, Tommerup [19] has previously reported a minor colonization by vesicular-AM fungi in oilseed rape. Unidentified vesicle-like structures in oilseed rape roots may be the zoospores of Olpidium spp. [45]. Moreover, AM strains have displayed various colonization rates, suggesting a certain selectivity towards their host plants [10]. A specific, perhaps unsuitable fungal type may explain the non-functional AM symbiosis. Furthermore, high soluble P content in soil also inhibits the establishment of AM symbiosis, whereas it is stimulated by P deficiency [46].
Dark septate endophytes hyphae (DES) and MS were observed in root cells in addition to AM hyphae, vesicles, arbuscules, and possible O. brassicae spores, especially in digestate. These fungi did not stain with cotton blue similarly to AM fungi. Instead, the cell walls were either translucent or dark. According to Ban et al. [47], melanin, an essential antioxidant, accumulates in fungal cell walls when in contact with heavy metals to protect root cells against heavy metals. DSE colonization has also been shown to promote plant growth and allow the utilization of nutrients from organic sources and nutrient uptake in poor soils [48].
Interestingly, soil with white lupine had the greatest number of spores, yet the colonization percentage in white lupine roots was the lowest. According to Douds and Schenck [49], a large number of spores is not necessarily related to a high degree of the host root colonization by mycorrhizal fungi. Similarly to earlier observations by Seleiman et al. [43] in comparison to synthetic fertilizer, digestate increased the mycorrhizal colonization in maize and spelt wheat, and meat bone meal increased it in all species. In this study, no difference in maize biomasses between different P sources were observed. The associations of crop plants with mycorrhizal fungi may improve P uptake when it is in a poorly soluble or organic form but has little effect when it is in a soluble form, such as in synthetic fertilizers [50,51]. The root AM colonization was slightly higher in spelt wheat than in maize, but the number of Type 1 spores was largest in the rhizosphere of digestate-fertilized maize. In addition, maize roots were rich in arbuscules, which were not observed in spelt wheat roots. Additionally, a high P supply in the early growth stages of wheat is known to be associated with high grain yields, probably due to P mobilization within the plant during the grain-filling period when the P requirement is high [52].
In conclusion, this study demonstrates that organic P sources, such as digestate and meat bone meal, can promote functional mycorrhizal symbiosis with roots to improve the P uptake and utilization efficiency in various crop species. The species differences in the P uptake varied markedly depending on the P source. Therefore, the organic P sources may represent a viable alternative for the replacement of inorganic P fertilizers in the nutrition of crops with the proper designing of cropping systems. However, field studies are needed to verify the findings in this study.
Author Contributions
Investigation, A.S.H., A.S.; writing, P.S.A.M. A.S., D.O.W., A.S.H.; data analysis D.O.W.; supervision, P.S.A.M., A.S.; funding acquisition, P.S.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NIEMI säätiö, Finland.
Acknowledgments
The authors are grateful to Marjo Kilpinen and Markku Tykkyläinen for technical assistance while conducting the experiment. Helsingin Seudun Ympäristöpalvelut-kuntayhtymä, Vesihuolto, and Jukka Kivelä are acknowledged for providing the digestate and meat bone meal, respectively. Open access funding provided by the University of Helsinki Libraries.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Lambers, H.; Shane, M.W.; Cramber, M.D.; Pearse, S.J.; Veneklaas, E.J. Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Hable, M. Mycorrhizal fungi and plant nutrition. In Plant Nutrient Management in Hawaii’s Soils, Approaches for Tropical and Subtropical Agriculture; Silva, J.A., Uchida, R., Eds.; College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa: Honolulu, HI, USA, 2000; pp. 127–132. [Google Scholar]
- Seleiman, M.F.; Santanen, A.; Mäkelä, P.S.A. Recycling sludge on cropland as fertilizer—Advantages and risks. Resour. Conserv. Recycl. 2020, 155, 104647. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of soil and fertilizer phosphorus use. In FAO Fertilizer and Plant Nutrition Bulletin 18; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008. [Google Scholar]
- Grant, C.A.; Flaten, D.N.; Tomasiewicz, D.J.; Sheppard, S.C. The importance of early season phosphorus nutrition. Can. J. Plant Sci. 2001, 81, 211–224. [Google Scholar] [CrossRef]
- Grant, C.A.; Bailey, L.D. Fertility management in canola production. Can. J. Plant Sci. 1993, 73, 651–670. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J.; et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. Topsoil foraging—An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Chen, Y.P.; Rekha, P.D.; Arun, A.B.; Shen, F.T.; Lai, W.-A.; Young, C.C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Grant, C.; Bittman, S.; Montreal, M.; Plenchette, C.; Morel, C. Soil and fertilizer phosphorus: Effects on plant P supply and mycorrhizal development. Can. J. Plant Sci. 2005, 85, 3–14. [Google Scholar] [CrossRef]
- Bolan, N.S. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 1991, 134, 189–207. [Google Scholar] [CrossRef]
- Gavito, M.E.; Miller, M.H. Early phosphorus nutrition, mycorrhizae development, dry matter partitioning and yield of maize. Plant Soil 1998, 199, 177–186. [Google Scholar] [CrossRef]
- Zhu, J.; Lynch, J.P. The contribution of lateral rooting to phosphorus acquisition efficiency in maize (Zea mays) seedlings. Funct. Plant Biol. 2004, 31, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Amann, C.; Amberger, A. Phosphorus efficiency of buckwheat (Fagopyrum esculentum). J. Plant Nutr. Soil Sci. 1989, 152, 181–189. [Google Scholar] [CrossRef]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J. Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef]
- Watt, M.; Evans, J.R. Proteoid roots. Physiology and development. Plant Phys. 1999, 121, 317–323. [Google Scholar] [CrossRef]
- Müller, J.; Gödde, V.; Niehaus, K.; Zörb, C. Metabolic adaptations of white lupin roots and shoots under phosphorus deficiency. Front. Plant Sci. 2015, 6, 1014. [Google Scholar] [CrossRef]
- Tommerup, I.C. Development of infection by a vesicular-arbuscular mycorrhizal fungus in Brassica napus L. and Trifolium subterraneum L. New Phytol. 1984, 98, 487–495. [Google Scholar] [CrossRef]
- Miller, M.H. Arbuscular mycorrhizae and the phosphorus nutrition of maize: A review of Guelph studies. Can. J. Plant Sci. 2000, 80, 47–52. [Google Scholar] [CrossRef]
- Evans, J.; Neeson, R.; Burnett, V.; Luckett, D.J.; Fettell, N.A. Phosphorus-use efficiency, growth and yield of spelt wheat (Triticum aestivum ssp. spelta) compared with standard wheat (T. aestivum ssp. vulgare) in south-eastern Australia. J. Org. Syst. 2014, 9, 63–78. [Google Scholar]
- Sarapatka, B.; Dudová, L.; Kršková, M.E. Effect of pH and phosphate supply on acid phosphatase activity in cereal roots. Biologia 2003, 59, 127–131. [Google Scholar]
- Tammeorg, P.; Simojoki, A.; Mäkelä, P.; Stoddard, F.L.; Alakukku, L.; Helenius, J. Short-term effects of biochar on soil properties and wheat yield formation with meat bone meal and inorganic fertiliser on a boreal loamy sand. Agric. Ecosyst. Environ. 2014, 191, 108–116. [Google Scholar] [CrossRef]
- HSY Water Treatment Laboratory. Average Water Quality at Pitkäkoski and Vanhankaupunki Water Treatment Plants. Available online: https://vanha.hsy.fi/en/experts/water-services/drinking-water-quality/Documents/Water%20quality%202020_1.pdf (accessed on 25 May 2020).
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants. BBCH Monograph, 2nd ed.; Federal Biological Research Centre for Agriculture and Forestry: Berlin, Germany, 2001. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Santanen, A.; Stoddard, F.L.; Mäkelä, P.S.A. Feedstock quality and growth of bioenergy crops fertilized with sewage sludge. Chemosphere 2012, 89, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.F.; Moore, T.S.; Christensen, J.R.M. Growth of vesicular-arbuscular mycorrhizal and non-mycorrhizal Bouteloua gracilis in a defined medium. Mycologia 1979, 71, 666–669. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Brundrett, M. Mycorrhizal Associations. The Web Resource. Available online: http://mycorrhizas.info/index.html (accessed on 29 May 2020).
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–163. [Google Scholar] [CrossRef]
- Grace, C.; Stribley, D.P. A safer procedure for routine staining of vesicular-arbuscular mycorrhizal fungi. Mycol. Res. 1991, 95, 1160–1162. [Google Scholar] [CrossRef]
- Inamullah, S.G.; Ayub, M.; Ali Khan, A.; Anwar, S.; Alam Khan, S. Response of common buckwheat to nitrogen and phosphorus fertilization. Sarhad J. Agric 2012, 28, 171–178. [Google Scholar]
- Neumann, G.G.; Massonneau, A.; Martinoia, E.; Römheld, V. Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta 1999, 208, 373–382. [Google Scholar] [CrossRef]
- Weisskoppf, L.; Abou-Mansour, E.; Fromin, N.; Tomasi, N.; Santelia, D.; Edelkott, I.; Neumann, G.; Arango, M.; Tabacchi, R.; Martinoia, E. White lupin has developed a complex strategy to limit microbial degradation of secreted citrate required for phosphate acquisition. Plant Cell Environ. 2006, 29, 919–927. [Google Scholar] [CrossRef]
- Lambers, H.; Clements, J.C.; Nelson, M.N. How a phosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am. J. Bot. 2013, 100, 263–288. [Google Scholar] [CrossRef]
- Sulieman, S.; Ha, C.V.; Schulze, I.; Tran, I.S.P. Growth and nodulation of symbiotic Medicago truncatula at different levels of phosphorus availability. J. Exp. Bot. 2013, 64, 2701–2712. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, S.; Tran, I.S.P. Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Sci. 2015, 239, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Schulze, I.; Temple, G.; Temple, S.I.; Beschow, H.; Vance, C.P. Nitrogen fixation by white lupin under phosphorus deficiency. Ann. Bot. 2006, 98, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-G.; He, Y.-Q.; Smith, S.E.; Smith, F.A. Buckwheat (Fagopyrum esculentum Moench) has high capacity to take up phosphorus (P) from a calcium (Ca)-bound source. Plant Soil 2001, 239, 1–8. [Google Scholar] [CrossRef]
- Nogalska, A.; Zalewska, M. The effect of meat and bone meal on phosphorus concentrations in soil and crop plants. Plant Soil Environ. 2013, 59, 575–580. [Google Scholar] [CrossRef]
- Warren, G.P.; Robinson, J.S.; Someus, E. Dissolution of phosphorus from animal bone char in 12 soils. Nutr. Cycl. Agroecosyst. 2009, 84, 167–178. [Google Scholar] [CrossRef]
- Toljander, J.F.; Santos-González, J.C.; Tehler, A.; Finlay, R.D. Community analysis of arbuscular mycorrhizal fungi and bacteria in the maize mycorrhizosphere in a long-term fertilization trial. FEMS Microb. Ecol. 2008, 65, 323–338. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Santanen, A.; Kleemola, J.; Stoddard, F.L.; Mäkelä, P.S.A. Improved sustainability of feedstock production with sludge and interacting mycorrhiza. Chemosphere 2013, 91, 1236–1242. [Google Scholar] [CrossRef]
- Demars, B.G.; Boerner, R.E.J. Vesicular arbuscular mycorrhizal development in the Brassicacea in relation to plant life span. Flora 1996, 191, 179–189. [Google Scholar] [CrossRef]
- Lay, C.-Y.; Hamel, C.; St-Arnaud, M. Taxonomy and pathogenity of Olpidium brassicae and its allied species. Fungal Biol. 2018, 122, 837–846. [Google Scholar] [CrossRef]
- McArthur, D.A.I.; Knowles, N.R. Resistance responses of potato to vesicular arbuscular mycorrhizal fungi under varying abiotic phosphorus levels. Plant Physiol. 1992, 100, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Tang, M.; Chen, H.; Xu, Z.; Zhang, H.; Yang, H. The response of dark septate endophytes (DES) to heavy metals in pure culture. PLoS ONE 2012, 7, e47968. [Google Scholar] [CrossRef] [PubMed]
- Yakti, W.; Kowacs, G.M.; Vagi, P.; Franken, P. Impact of dark septate endophytes on tomato growth and nutrient uptake. Plant Ecol. Divers. 2018, 11, 637–648. [Google Scholar] [CrossRef]
- Douds, D.D.; Schenck, N.C. Relationship of colonization and sporulation by VA mycorrhizal fungi to plant nutrient and carbohydrate contents. New Phytol. 1990, 116, 621–627. [Google Scholar] [CrossRef]
- Vance, C.P. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Phys. 2001, 127, 390–397. [Google Scholar] [CrossRef]
- Römer, W.; Schilling, G. Phosphorus requirements of the wheat plant in various stages of its life cycle. Plant Soil 1986, 91, 221–229. [Google Scholar] [CrossRef]
- Makádi, M.; Tomócsik, A.; Orosz, V. Digestate: A new nutrient source—Review. In Biogas; Kumar, S., Ed.; IntechOpen: London, UK, 2012; pp. 295–310. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).