Resistance Evolution to EPSPS Inhibiting Herbicides in False Barley (Hordeum murinum) Harvested in Southern Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Dose–Response Curves
2.3. Shikimic Acid Accumulation
2.4. Adjuvant Effectiveness Assays
2.5. Foliar Retention Assay
2.6. Alternative Chemical Control
2.7. Data Analysis
3. Results
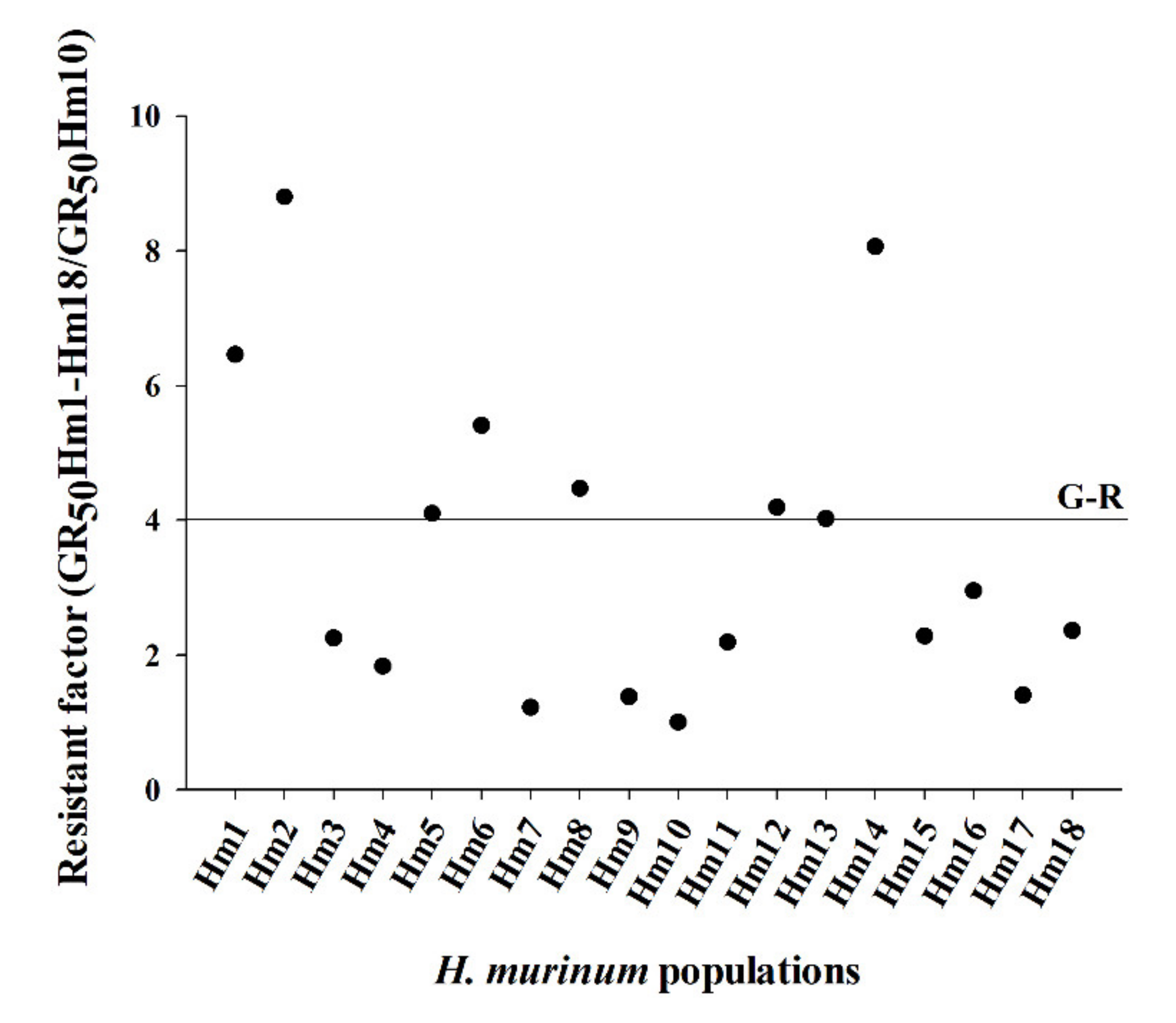
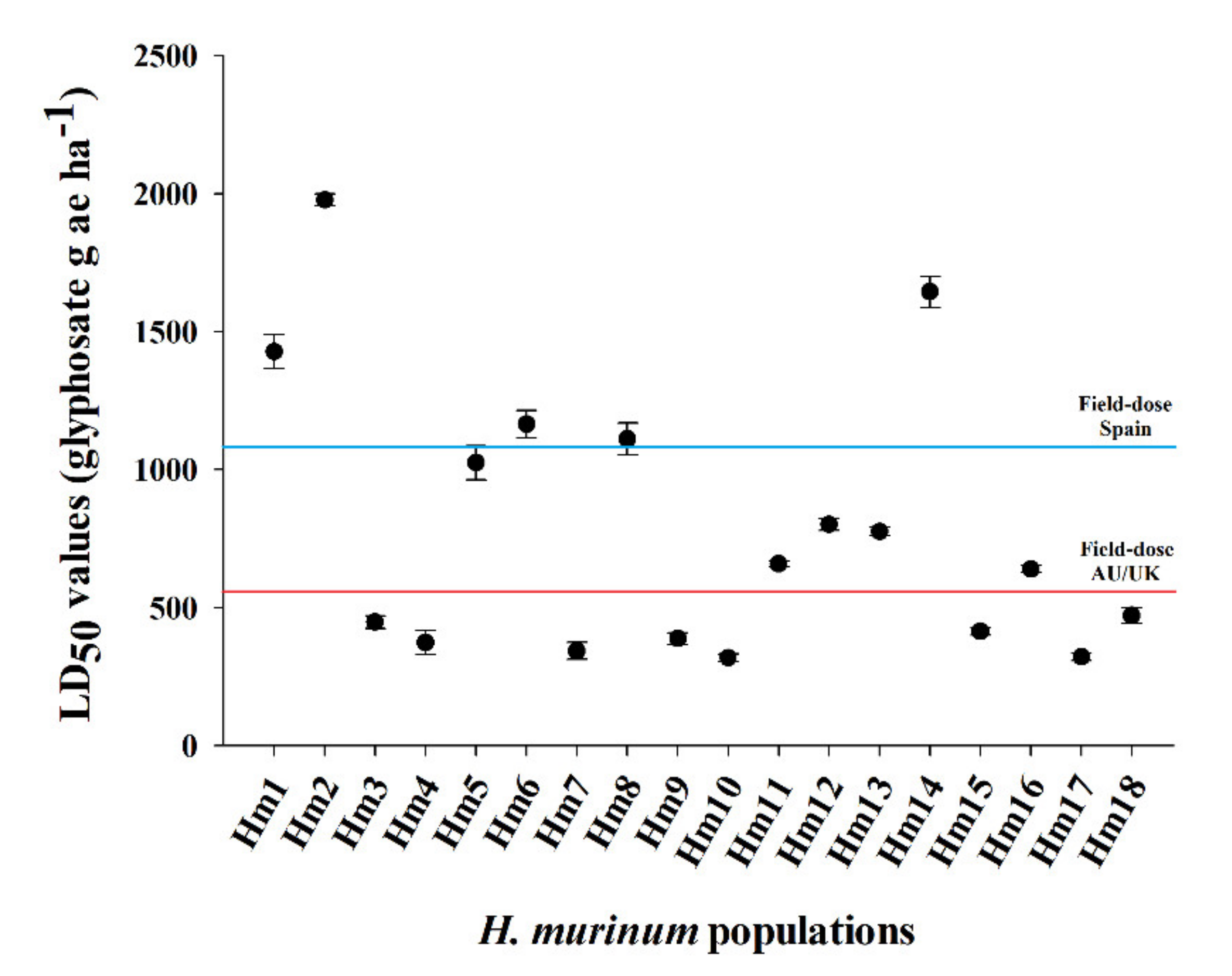
3.1. Dose–Response Assays
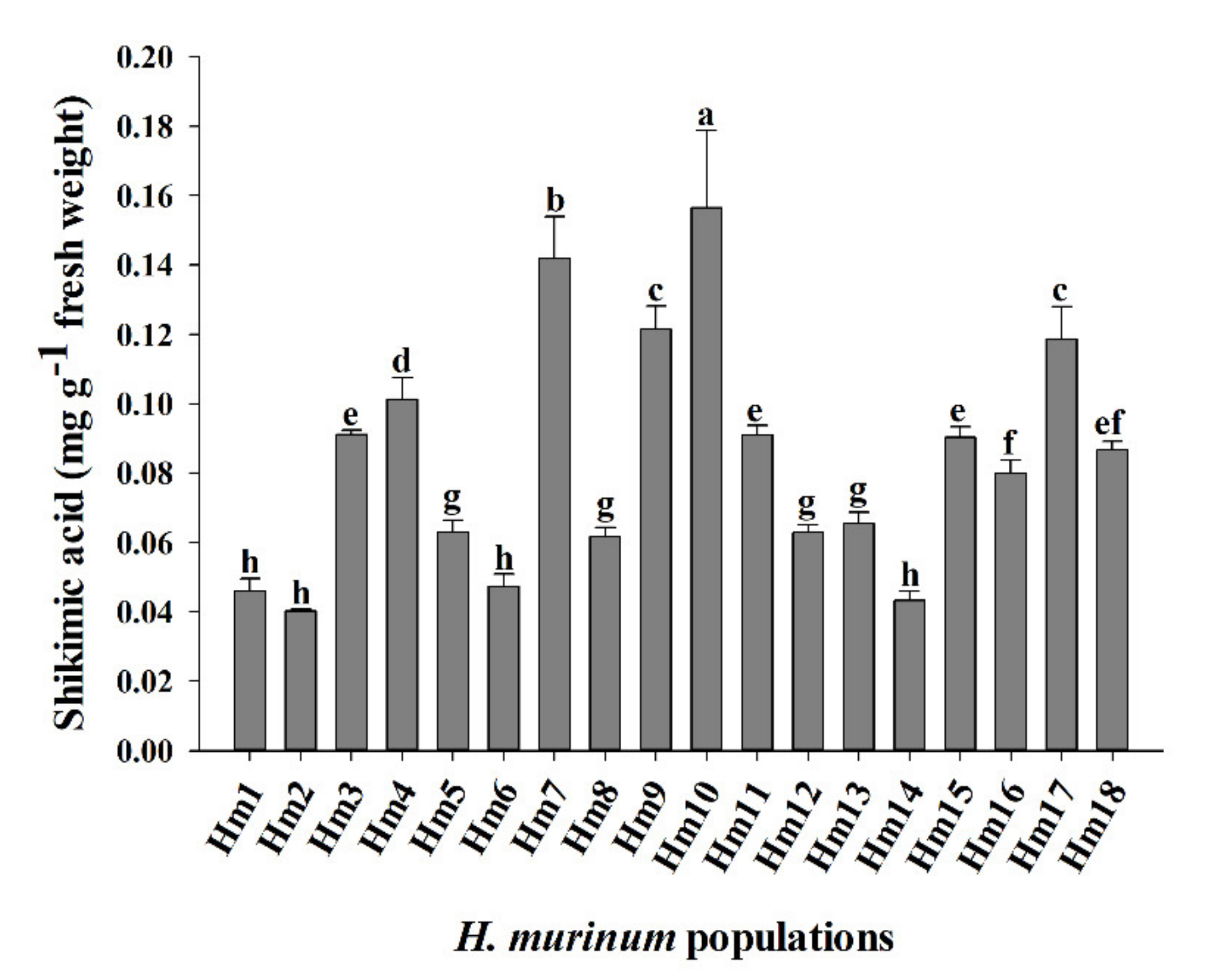
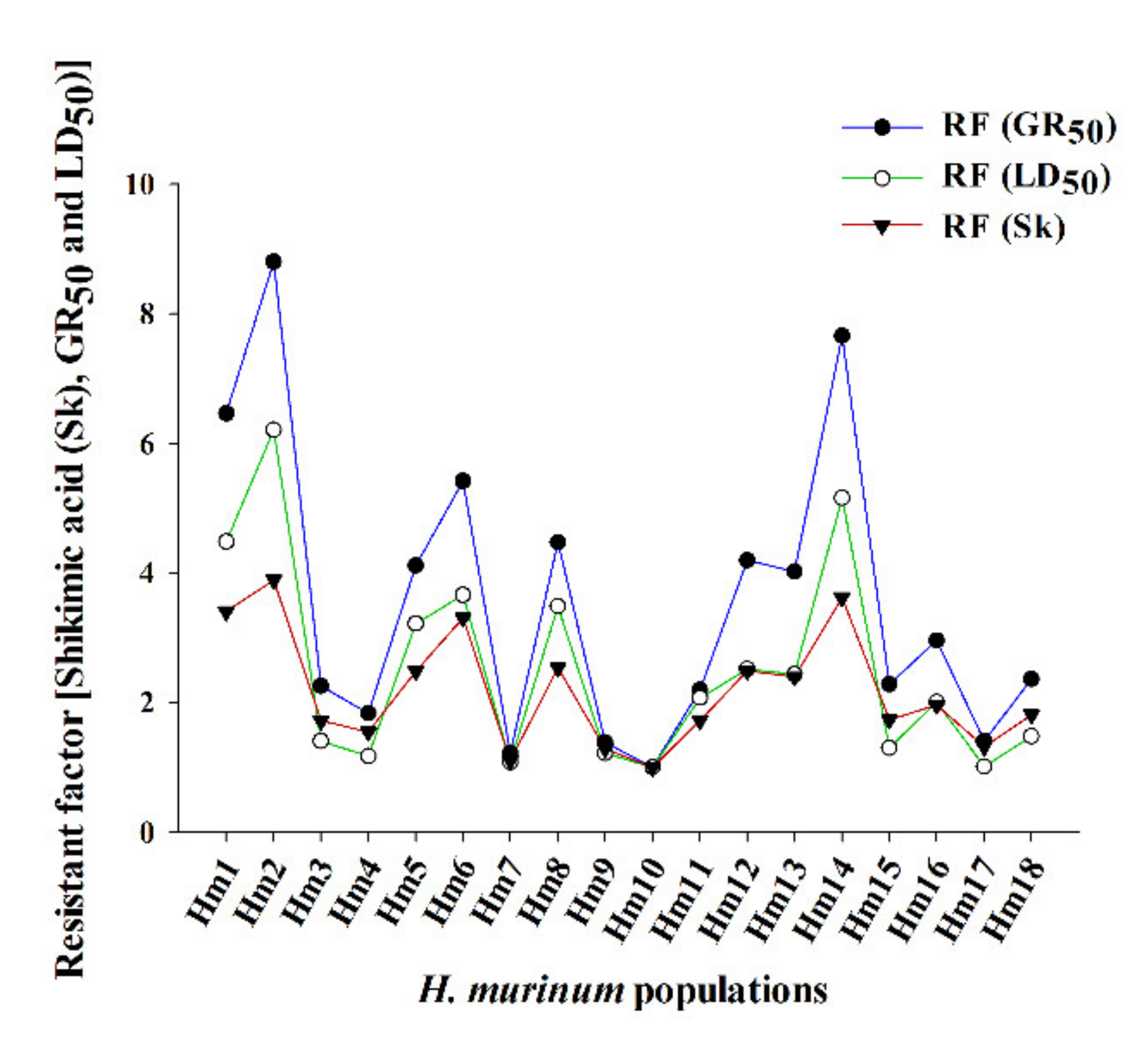
3.2. Shikimic Acid Accumulation
3.3. Adjuvant Effectiveness Assays
3.4. Foliar Retention Assays
3.5. Alternative Chemical Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruíz-Ferna, J.; Soler, C. Distribution and habitat of Spanish populations of the subtribe Hordeineae; improved views following germplasm collecting activities. Genet. Resour. Crop. Evol. 1997, 44, 33–41. [Google Scholar] [CrossRef]
- León, E.; Nieto, E.L.; Martínez, M.L.; Salvá, A.J.P. El agregado de Hordeum murinum (Poaceae) en “Flora Iberica”. Acta Bot. Malacit. 2019, 39, 311–319. [Google Scholar] [CrossRef]
- Barkworth, M.E.; Von Bothmer, R.; Jacobsen, N.; Baden, C.; Jorgensen, R.B.; Linde-Laursen, I. An Ecogeographical Study of the Genus Hordeum. In Systematic and Ecogeographic Studies on Crop Genepools, 2nd ed.; International Plant Genetic Resources Institute: Rome, Italy, 1995; p. 129. [Google Scholar]
- Iqbal, N.; Bajwa, A.A.; Manalil, S.; Khan, A.M.; Kebaso, L.; Frimpong, D.; Ali, H.H.; Jha, P.; Chauhan, B.S. Biology and management of two Hordeum weedy species: A review. Crop. Prot. 2019, 125, 104908. [Google Scholar] [CrossRef]
- Powles, S. Appearance of a biotype of the weed, Hordeum glaucum Steud., resistant to the herbicide paraquat. Weed Res. 1986, 26, 167–172. [Google Scholar] [CrossRef]
- Tucker, E.S.; Powles, S. A Biotype of Hare Barley (Hordeum leporinum) Resistant to Paraquat and Diquat. Weed Sci. 1991, 39, 159–162. [Google Scholar] [CrossRef]
- Davis, R.C. Agrichemical Industry Initiatives to Combat Development of Herbicide Resistance. In Proceedings of the 1st Internat Weed Control Cong, Melbourne, Australia, 17–21 February 1992; Volume 2, pp. 144–147. [Google Scholar]
- Purba, E.; Preston, C.; Powles, S. The mechanism of resistance to paraquat is strongly temperature dependent in resistant Hordeum leporinum Link and H. glaucum Steud. Planta 1995, 196, 464–468. [Google Scholar] [CrossRef]
- Alizadeh, H.; Preston, C.; Powles, S. Paraquat-resistant biotypes of Hordeum glaucum from zero-tillage wheat. Weed Res. 1998, 38, 139–142. [Google Scholar] [CrossRef]
- Matthews, N.; Powles, S.B.; Preston, C. Mechanisms of resistance to acetyl-coenzyme A carboxylase-inhibiting herbicides in a Hordeum leporinum population. Pest Manag. Sci. 2000, 56, 441–447. [Google Scholar] [CrossRef]
- Yu, Q.; Nelson, J.K.; Zheng, M.Q.; Jackson, M.; Powles, S.B. Molecular characterization of resistance to ALS-inhibiting herbicides in Hordeum leporinum biotypes. Pest Manag. Sci. 2007, 63, 918–927. [Google Scholar] [CrossRef]
- Owen, M.J.; Goggin, D.E.; Powles, S.B. Identification of resistance to either paraquat or ALS-inhibiting herbicides in two Western Australian Hordeum leporinum biotypes. Pest Manag. Sci. 2012, 68, 757–763. [Google Scholar] [CrossRef]
- Adu-Yeboah, P.; Malone, J.M.; Fleet, B.; Gill, G.; Preston, C. EPSPS gene amplification confers resistance to glyphosate resistant populations of Hordeum glaucum Stued (northern barley grass) in South Australia. Pest Manag. Sci. 2019, 76, 1214–1221. [Google Scholar] [CrossRef]
- Heap, I. International Survey of Herbicide Resistant Weeds. Available online: http://weedscience.org/ (accessed on 26 March 2020).
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. Glyphosate: The world’s most successful herbicide under intense scientific scrutiny. Pest Manag. Sci. 2018, 74, 1025–1026. [Google Scholar] [CrossRef] [PubMed]
- Boocock, M.R.; Coggins, J.R. Kinetics of 5-enolpyruvylshikimate-3-phosphate synthase inhibition by glyphosate. FEBS Lett. 1983, 154, 127–133. [Google Scholar] [CrossRef]
- Velini, E.D. Modo de Acao Do Glyphosate. In Glyphosate; Velini, E.D., Meschede, D.K., Carbonari, C.A., Trindade, M.L.B., Eds.; Fepaf: Botucato, Brazil, 2009; pp. 113–133. [Google Scholar]
- Sammons, R.D.; Gaines, T.A. Glyphosate resistance: State of knowledge. Pest Manag. Sci. 2014, 70, 1367–1377. [Google Scholar] [CrossRef]
- Moss, S. Herbicide Resistance in Weeds. In Weed Research: Expanding Horizons; Hatcher, P.E., Froud-Williams, R.J., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 181–214. [Google Scholar]
- Beffa, R.; Menne, H.; Köcher, H. Herbicide Resistance Action Committee (HRAC). Available online: https://www.hracglobal.com (accessed on 6 April 2020).
- Powles, S.; Yu, Q. Evolution in Action: Plants Resistant to Herbicides. Annu. Rev. Plant Boil. 2010, 61, 317–347. [Google Scholar] [CrossRef]
- Michitte, P.; De Prado, R.; Espinosa, N.; Gauvrit, C. Glyphosate resistance in a Chilean Lolium multiflorum. Commun. Agric. Appl. Boil. Sci. 2005, 70, 507–513. [Google Scholar]
- Fernández-Moreno, P.T.; La Cruz, R.A.-D.; Smeda, R.J.; De Prado, R. Differential Resistance Mechanisms to Glyphosate Result in Fitness Cost for Lolium perenne and L. multiflorum. Front. Plant Sci. 2017, 8, 8. [Google Scholar] [CrossRef]
- Brunharo, C.; Patterson, E.; Carrijo, D.; De Melo, M.S.C.; Nicolai, M.; A Gaines, T.; Nissen, S.J.; Christoffoleti, P. Confirmation and mechanism of glyphosate resistance in tall windmill grass (Chloris elata) from Brazil. Pest Manag. Sci. 2016, 72, 1758–1764. [Google Scholar] [CrossRef]
- Ngo, T.D.; Krishnan, M.; Boutsalis, P.; Gill, G.; Preston, C. Target-site mutations conferring resistance to glyphosate in feathertop Rhodes grass (Chloris virgata) populations in Australia. Pest Manag. Sci. 2017, 74, 1094–1100. [Google Scholar] [CrossRef]
- Bracamonte, E.; Da Silveira, H.M.; La Cruz, R.A.-D.; Domínguez-Valenzuela, J.A.; Cruz-Hipolito, H.E.; De Prado, R. From tolerance to resistance: Mechanisms governing the differential response to glyphosate in Chloris barbata. Pest Manag. Sci. 2018, 74, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Garcia, J.G.; Golmohammadzadeh, S.; Palma-Bautista, C.; Rojano-Delgado, A.M.; Domínguez-Valenzuela, J.A.; Cruz-Hipólito, H.E.; De Prado, R. New Case of False-Star-Grass (Chloris distichophylla) Population Evolving Glyphosate Resistance. Agronomy 2020, 10, 377. [Google Scholar] [CrossRef]
- Gaines, T.A.; Cripps, A.; Powles, S.B. Evolved Resistance to Glyphosate in Junglerice (Echinochloa colona) from the Tropical Ord River Region in Australia. Weed Technol. 2012, 26, 480–484. [Google Scholar] [CrossRef]
- Alarcón-Reverte, R.; Garcia, A.; Urzúa, J.; Fischer, A.J. Resistance to Glyphosate in Junglerice (Echinochloa colona) from California. Weed Sci. 2013, 61, 48–54. [Google Scholar] [CrossRef]
- Nandula, V.K.; Montgomery, G.B.; Vennapusa, A.R.; Jugulam, M.; Giacomini, D.A.; Ray, J.D.; Bond, J.A.; Steckel, L.E.; Tranel, P. Glyphosate-Resistant Junglerice (Echinochloa colona) from Mississippi and Tennessee: Magnitude and Resistance Mechanisms. Weed Sci. 2018, 66, 603–610. [Google Scholar] [CrossRef]
- De Carvalho, L.B.; Alves, P.L.C.A.; González-Torralva, F.; Cruz-Hipolito, H.E.; Rojano-Delgado, A.M.; De Prado, R.; Gil-Humanes, J.; Barro, F.; De Castro, M.D.L. Pool of Resistance Mechanisms to Glyphosate in Digitaria insularis. J. Agric. Food Chem. 2012, 60, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Vila-Aiub, M.M.; Balbi, M.C.; Gundel, P.E.; Ghersa, C.M.; Powles, S.B. Evolution of Glyphosate-Resistant Johnsongrass (Sorghum halepense) in Glyphosate-Resistant Soybean. Weed Sci. 2007, 55, 566–571. [Google Scholar] [CrossRef]
- Riar, D.S.; Norsworthy, J.K.; Johnson, D.B.; Scott, R.C.; Bagavathiannan, M. Glyphosate Resistance in a Johnsongrass (Sorghum halepense) Biotype from Arkansas. Weed Sci. 2011, 59, 299–304. [Google Scholar] [CrossRef]
- Vazquez-Garcia, J.G.; Palma-Bautista, C.; Rojano-Delgado, A.M.; De Prado, R.; Menendez, J. The First Case of Glyphosate Resistance in Johnsongrass (Sorghum halepense (L.) Pers.) in Europe. Plants 2020, 9, 313. [Google Scholar] [CrossRef]
- Infante-Amate, J.; Villa, I.; Aguilera, E.; Torremocha, E.; Guzmán, G.; Cid, A.; Emanueli, F. The Making of Olive Landscapes in the South of Spain. A History of Continuous Expansion and Intensification. In Biocultural Diversity in Europe; Agnoletti, M., Emanueli, F., Eds.; Springer: New York, NY, USA, 2017; pp. 157–159. [Google Scholar]
- Márquez-García, F.; González-Sánchez, E.J.; Castro-Garcia, S.; Ordóñez-Fernández, R. Improvement of soil carbon sink by cover crops in olive orchards under semiarid conditions. Influence of the type of soil and weed. Span. J. Agric. Res. 2013, 11, 335. [Google Scholar] [CrossRef]
- Galán, C.; García-Mozo, H.; Vázquez, L.; Ruiz, L.; de la Guardia, C.D.; Domínguez-Vilches, E. Modeling Olive Crop Yield in Andalusia, Spain. Agron. J. 2008, 100, 98–104. [Google Scholar] [CrossRef]
- Shaner, D.L.; Nadler-Hassar, T.; Henry, W.B.; Koger, C.H. A rapid in vivo shikimate accumulation assay with excised leaf discs. Weed Sci. 2005, 53, 769–774. [Google Scholar] [CrossRef]
- Palma-Bautista, C.; Vazquez-Garcia, J.G.; Travlos, I.; Tataridas, A.; Kanatas, P.; Domínguez-Valenzuela, J.A.; De Prado, R. Effect of Adjuvant on Glyphosate Effectiveness, Retention, Absorption and Translocation in Lolium rigidum and Conyza canadensis. Plants 2020, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Gauvrit, C. Glyphosate Response to Calcium, Ethoxylated Amine Surfactant, and Ammonium Sulfate. Weed Technol. 2003, 17, 799–804. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Travlos, I.; Cheimona, N.; Bilalis, D. Glyphosate Efficacy of Different Salt Formulations and Adjuvant Additives on Various Weeds. Agronomy 2017, 7, 60. [Google Scholar] [CrossRef]
- De Oliveira, R.B.; Antuniassi, U.R.; Mota, A.A.B.; Chechetto, R.G. Potential of adjuvants to reduce drift in agricultural spraying. Eng. Agrícola 2013, 33, 986–992. [Google Scholar] [CrossRef]
- Evans, J.A.; Tranel, P.J.; Hager, A.G.; Schutte, B.; Wu, C.; Chatham, L.A.; Davis, A.S. Managing the evolution of herbicide resistance. Pest Manag. Sci. 2015, 72, 74–80. [Google Scholar] [CrossRef]
- Shergill, L.; Fleet, B.; Preston, C.; Gill, G.; Gill, G. Incidence of Herbicide Resistance, Seedling Emergence, and Seed Persistence of Smooth Barley (Hordeum glaucum) in South Australia. Weed Technol. 2015, 29, 782–792. [Google Scholar] [CrossRef]
- Beckie, H.J.; Tardif, F.J. Herbicide cross resistance in weeds. Crop. Prot. 2012, 35, 15–28. [Google Scholar] [CrossRef]
| Code | Location | Crops | H. Application | Coordinates |
|---|---|---|---|---|
| Hm1 | Cordoba | Orchard | Many years using glyphosate | 37.708194, −4.789167 |
| Hm2 | Cordoba | Orchard | Many years using glyphosate | 37.709861, −4.788778 |
| Hm3 | Cordoba | No crop | Tank mix a | 37.695903, −4.504091 |
| Hm4 | Cordoba | Orchard | Many years using glyphosate | 37.548358, −4.275374 |
| Hm5 | Cordoba | Orchard | Many years using glyphosate | 37.709694, −4.806389 |
| Hm6 | Cordoba | Orchard | Many years using glyphosate | 37.696306, −4.816556 |
| Hm7 | Cordoba | No crop | Mechanical control | 37.914798, −4.714411 |
| Hm8 | Cordoba | No crop | Tank mix a | 37.646018, −4.3771 |
| Hm9 | Lleida | Vineyard | Mechanical control | 41.679000, 0.474111 |
| Hm10 | Cordoba | No crop | Tank mix a | 37.506787, −4.847001 |
| Hm11 | Sevilla | Olive | Many years using glyphosate | 37.511594, −4.842501 |
| Hm12 | Sevilla | Olive | Many years using glyphosate | 37.513054, −4.841346 |
| Hm13 | Sevilla | Olive | Many years using glyphosate | 37.509776, −4.838388 |
| Hm14 | Sevilla | Olive | Many years using glyphosate | 37.575327, −4.985865 |
| Hm15 | Sevilla | Olive | Many years using glyphosate | 37.536481, −4.959472 |
| Hm16 | Malaga | Olive | Many years using glyphosate | 36.974889, −4.918069 |
| Hm17 | Sevilla | Cereal | ACCase inhibitors | 37.511594, −4.842501 |
| Hm18 | Malaga | Olive | Many years using glyphosate | 37.039433, −4.553680 |
| Active Ingredient | SoA a | HRAC (WSSA Group) | Doses (g ai ha−1) | Timing |
|---|---|---|---|---|
| Propaquizafop | ACCase | A (1) | 62.5 and 125 | Post |
| Quizalofop | ACCase | A (1) | 50 and 100 | Post |
| Iodosulfuron | ALS | B (2) | 2.5 and 5 | Post |
| Flazasulfuron | ALS | B (2) | 25 and 50 | Pre |
| Paraquat | PS I | D (22) | 200 and 400 | Post |
| Oxyfluorfen | PPO | E (14) | 250 and 500 | Pre |
| Glufosinate | GS | H (10) | 250 and 500 | Post |
| Tembotrione | HPPD | F2 (27) | 60 and 120 | Post |
| Diuron | PS II | C2 (7) | 900 and 1800 | Pre |
| Atrazine | PS II | C1 (5) | 1000 and 2000 | Pre |
| Parameters a Calculated Using Non-Linear Regression b | ||||||||
|---|---|---|---|---|---|---|---|---|
| Population | b | d | GR50 | RF c | b | d | LD50 | RF c |
| Hm1 | 1.5 | 98.1 | 794.1 ± 62.9 | 6.5 | 5.3 | 98.8 | 1427.8 ± 61.7 | 4.5 |
| Hm2 | 2.4 | 93.4 | 1081.6 ± 56.5 | 8.8 | 7.2 | 100.1 | 1977.9 ± 21.8 | 6.2 |
| Hm3 | 1.4 | 97.2 | 277.0 ± 26.4 | 2.3 | 2.8 | 99.5 | 447.7 ± 22.3 | 1.4 |
| Hm4 | 3.2 | 97.2 | 225.1 ± 12.1 | 1.8 | 2.0 | 97.9 | 373.0 ± 44.2 | 1.2 |
| Hm5 | 1.8 | 93.2 | 504.8 ± 49.9 | 4.1 | 3.3 | 100.2 | 1024.7 ± 61.6 | 3.2 |
| Hm6 | 1.5 | 96.7 | 665.7 ± 65.7 | 5.4 | 4.9 | 98.8 | 1165.1 ± 50.0 | 3.7 |
| Hm7 | 2.5 | 99.4 | 150.0 ± 9.0 | 1.2 | 1.9 | 101.3 | 343.4 ± 30.7 | 1.1 |
| Hm8 | 1.6 | 98.5 | 549.4 ± 42.9 | 4.5 | 4.5 | 97.0 | 1111.3 ± 59.3 | 3.5 |
| Hm9 | 2.5 | 99.7 | 170.0 ± 10.1 | 1.4 | 3.3 | 100.5 | 388.0 ± 21.1 | 1.2 |
| Hm10 | 1.5 | 99.1 | 122.8 ± 10.7 | - | 1.9 | 100.0 | 318.6 ± 14.1 | - |
| Hm11 | 1.7 | 97.6 | 269.5 ± 19.4 | 2.2 | 2.1 | 99.9 | 659.2 ± 9.9 | 2.1 |
| Hm12 | 3.3 | 95.0 | 515.3 ± 20.8 | 4.2 | 2.7 | 100.0 | 802.0 ± 21.8 | 2.5 |
| Hm13 | 3.1 | 91.7 | 494.0 ± 24.3 | 4.0 | 2.6 | 100.0 | 775.5 ± 15.0 | 2.4 |
| Hm14 | 3.4 | 95.8 | 940.9 ± 30.7 | 7.7 | 3.8 | 100.4 | 1644.7 ± 55.8 | 5.2 |
| Hm15 | 7.7 | 95.9 | 280.1 ± 12.1 | 2.3 | 4.6 | 99.6 | 413.8 ± 13.2 | 1.3 |
| Hm16 | 6.4 | 98.0 | 363.0 ± 15.3 | 3.0 | 6.4 | 97.6 | 639.7 ± 13.1 | 2.0 |
| Hm17 | 2.5 | 99.9 | 172.6 ± 5.8 | 1.4 | 3.2 | 100.1 | 322.1 ± 13.4 | 1.01 |
| Hm18 | 7.1 | 97.8 | 290.5 ± 6.2 | 2.4 | 9.8 | 99.2 | 471.8 ± 28.2 | 1.5 |
| Treatment | dw Reduction (%) | |||
|---|---|---|---|---|
| R a (750 g ae ha−1)a | IE (%) | S a (100 g ae ha−1) | IE (%) | |
| Gly | 36.6 ± 4.5 b | - | 40.3 ± 1.7 b | - |
| Gly + Retenol | 70.3 ± 3.3 a | 91.9 | 59.7± 4.1 a | 48.3 |
| Gly + Trend 90 | 75.0 ± 5.4 a | 104.9 | 69.5 ± 3.3 a | 72.7 |
| Treatment | Mean a (μL g−1 dry weight) | |||
|---|---|---|---|---|
| R | IE (%) | S | IE (%) | |
| Gly | 447.5 ± 14.4 c | - | 467.25 ± 13.2 c | - |
| Gly + Retenol | 638.75 ± 12.1 b | 42.7 | 631.75 ± 7.8 b | 35.2 |
| Gly + Trend 90 | 1133.75 ± 12.5 a | 153.4 | 1222.5 ± 15.5 a | 161.6 |
| Herbicides | Doses (g ai ha−1) | Visual Evaluation a | % Survival Plant b | % fw Reduction c | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | R | S | R | S | R | ||||||||
| Check (untreated) | 0 | D | 0 | C | 100 | A | 100 | A | 0 | D | 0 | I | |
| Propaquizafop | 62.5 | 100 | A | 100 | A | 0 | D | 0 | F | 100 | A | 100 | A |
| 125 | 100 | A | 100 | A | 0 | D | 0 | F | 100 | A | 100 | A | |
| Quizalofop | 50 | 100 | A | 100 | A | 0 | D | 0 | F | 100 | A | 100 | A |
| 100 | 100 | A | 100 | A | 0 | D | 0 | F | 100 | A | 100 | A | |
| Iodosulfuron | 2.5 | 0 | D | 0 | C | 100 | A | 100 | A | 5.83 | D | 0 | I |
| 5 | 0 | D | 0 | C | 100 | A | 100 | A | 5 | D | 10.36 | H | |
| Flazasulfuron | 25 | 100 | A | 100 | A | 0 | D | 100 | A | 100 | A | 57.31 | E |
| 50 | 100 | A | 100 | A | 0 | D | 50 | D | 100 | A | 93.29 | B | |
| Paraquat | 200 | 100 | A | 100 | A | 0 | D | 0 | F | 100 | A | 100 | A |
| 400 | 100 | A | 100 | A | 0 | D | 0 | F | 100 | A | 100 | A | |
| Oxyfluorfen | 250 | 100 | A | 100 | A | 100 | A | 100 | A | 78.5 | B | 66.46 | D |
| 500 | 100 | A | 100 | A | 65 | C | 75 | B | 95.83 | A | 87.80 | C | |
| Glufosinate | 250 | 100 | A | 100 | A | 85 | B | 60 | C | 80 | B | 83.53 | C |
| 500 | 100 | A | 100 | A | 65 | C | 10 | E | 81.6 | B | 98.78 | A | |
| Tembotrione | 60 | 0 | D | 0 | C | 100 | A | 100 | A | 28.33 | C | 18.29 | G |
| 120 | 30 | C | 25 | B | 100 | A | 100 | A | 30.83 | C | 25.60 | F | |
| Diuron | 900 | 85 | B | 100 | A | 0 | D | 75 | B | 100 | A | 68.29 | D |
| 1800 | 85 | B | 100 | A | 0 | D | 60 | C | 100 | A | 93.9 | B | |
| Atrazine | 1000 | 100 | A | 100 | A | 0 | D | 0 | F | 100 | A | 100 | A |
| 2000 | 100 | A | 100 | A | 0 | D | 0 | F | 100 | A | 100 | A | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-García, J.G.; Castro, P.; Torra, J.; Cruz, R.A.-d.l.; Prado, R.D. Resistance Evolution to EPSPS Inhibiting Herbicides in False Barley (Hordeum murinum) Harvested in Southern Spain. Agronomy 2020, 10, 992. https://doi.org/10.3390/agronomy10070992
Vázquez-García JG, Castro P, Torra J, Cruz RA-dl, Prado RD. Resistance Evolution to EPSPS Inhibiting Herbicides in False Barley (Hordeum murinum) Harvested in Southern Spain. Agronomy. 2020; 10(7):992. https://doi.org/10.3390/agronomy10070992
Chicago/Turabian StyleVázquez-García, José G., Patricia Castro, Joel Torra, Ricardo Alcántara-de la Cruz, and Rafael De Prado. 2020. "Resistance Evolution to EPSPS Inhibiting Herbicides in False Barley (Hordeum murinum) Harvested in Southern Spain" Agronomy 10, no. 7: 992. https://doi.org/10.3390/agronomy10070992
APA StyleVázquez-García, J. G., Castro, P., Torra, J., Cruz, R. A.-d. l., & Prado, R. D. (2020). Resistance Evolution to EPSPS Inhibiting Herbicides in False Barley (Hordeum murinum) Harvested in Southern Spain. Agronomy, 10(7), 992. https://doi.org/10.3390/agronomy10070992







