Uniparental Inheritance of Salinity Tolerance and Beneficial Phytochemicals in Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Rice Materials and Salinity Treatment
2.2. Physiological Analysis of Salt Tolerance
2.3. Total Phenols, Total Flavonoids, and Antioxidant Activities
2.4. Identification and Quantification of Momilactones A and B
2.5. Genetic Analysis
2.6. Data Analysis
3. Results
3.1. Salt Tolerance, Agronomical, and Phytochemical Performances of Rice Population
3.2. Correlation of Physiological Parameters between Progeny and Parental Lines
3.3. Genetic Segregation of F2, M2 and M3 Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaiswal, S.; Gautam, R.K.; Singh, R.K.; Krishnamurthy, S.L.; Ali, S.; Sakthivel, K.; Iquebal, M.A.; Rai, A.; Kumar, D. Harmonizing technological advances in phenomics and genomics for enhanced salt tolerance in rice from a practical perspective. Rice 2019, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Menguer, P.K.; Sperotto, R.A.; Ricachenevsky, F.K. A walk on the wild side: Oryza species as source for rice abiotic stress tolerance. Genet. Mol. Biol. 2017, 40, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Linh, H.T.M.; Thach, T.N.; Tien, N.T.K.; Williams, B.; Wurm, P.; Bellairs, S.; Mundree, S. Improvement of salinity stress tolerance in rice: Challenges and opportunities. Agronomy 2016, 6, 54. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Lee, H.S.; Trung, B.H.; Tran, H.-D.; Lall, M.K.; Kakar, K.; Xuan, T.D. Impacts of mainstream hydropower dams on fisheries and agriculture in lower Mekong basin. Sustainability 2020, 12, 2408. [Google Scholar] [CrossRef]
- Negrao, S.N.; Almadanim, M.C.; Pires, I.; Abreu, I.A.; Maroco, J.; Courtois, B.; Gregorio, G.B.; McNally, K.L.; Oliveira, M.M. New allelic variants found in key rice salt-tolerance genes: An association study. Plant Biotechnol. J. 2012, 11, 87–100. [Google Scholar] [CrossRef]
- Thomson, M.J.; Ocampo, M.; Egdane, J.; Rahman, M.A.; Sajise, A.G.; Adorada, D.; Tumimbang-Raiz, E.; Blumwald, E.; Seraj, Z.I.; Singh, R.K.; et al. Characterizing the Saltol quantitative trait locus for salinity tolerance in rice. Rice 2010, 3, 148–160. [Google Scholar] [CrossRef]
- Mardani, Z.; Rabiei, B.; Sabouri, H.; Sabouri, A. Identification of molecular markers linked to salt-tolerant genes at gemination stage of rice. Plant Breed. 2014, 133, 196–202. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; Haritha, G.; Sunitha, T.; Krishnamurthy, S.L.; Divya, B.; Padmavathi, G.; Ram, T.; Sarla, N. Haplotyping of rice genotypes using simple sequence repeat markers associated with salt tolerance. Rice Sci. 2010, 23, 317–325. [Google Scholar] [CrossRef]
- Javed, M.A.; Fahrul, Z.H.; Alina, W.; Faeza, M.S. Identification of QTLs for Morph-Physiological traits related to salinity tolerance at seedling stage in Indica rice. Procedia Environ. Sci. 2011, 8, 389–395. [Google Scholar] [CrossRef]
- Babu, N.N.; Krishnan, S.G.; Vinod, K.K.; Krishnamurthy, S.L.; Singh, V.K.; Singh, M.P.; Singh, R.; Ellur, R.; Rai, V.; Bollinedi, H.B.; et al. Marker aided incorporation of Saltol, a major QTL associated with seedling stage salt tolerance, into Oryza sativa ‘Pusa Basmati 1121′. Front. Plant Sci. 2017, 8, 41. [Google Scholar] [CrossRef]
- Xuan, T.D.; Anh, T.T.T.; Tran, H.-D.; Khanh, T.D.; Dat, T.D. Mutation breeding of a N-methyl-N-nitrosourea (MNU)-induced rice (Oryza sativa L. ssp. Indica) population for the yield attributing traits. Sustainability 2019, 11, 1062. [Google Scholar] [CrossRef]
- Negrao, S.; Courtois, B.; Ahmadi, N.; Abreu, I.; Saibo, N.; Oliveira, M.M. Recent updates on salinity stress in rice: From physiological to molecular responses. Crit. Rev. Plant Sci. 2011, 30, 329–377. [Google Scholar] [CrossRef]
- Rabbani, M.A.; Maruyama, K.; Abe, H.; Khan, M.A.; Katsura, K.; Ito, Y.; Yoshikawa, K.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol. 2003, 133, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Blumwald, E. Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat. Biotechnol. 2001, 19, 765–768. [Google Scholar] [CrossRef]
- Mian, A.A.; Prasad, S.; Maathuis, F.J.M. Improving crop salt tolerance: Anion and cation transporters as genetic engineering targets. In Plant Nutrition and Abiotic Stress Tolerant III Plant Stress 5; Anjum, N.A., Lopez-Lauri, F., Eds.; Global Science Books: York, UK, 2011; pp. 64–72. [Google Scholar]
- Fukuda, A.; Nakamura, A.; Tagiri, A.; Tanaka, H.; Miyao, A.; Hirochika, H.; Tanaka, Y. Function, intracellular localization and the importance in salt tolerance of a vacuolar Na+/H+ antiporter from rice. Plant Cell Physiol. 2004, 45, 146–159. [Google Scholar] [CrossRef]
- Ratho, S.N.; Pradhan, S.B. Cytoplasmically controlled cold tolerance in a cytoplasmic-genetic male sterile line of rice. Euphytica 1992, 58, 241–244. [Google Scholar] [CrossRef]
- Tao, D.; Hu, F.; Yang, J.; Yang, J.; Yang, G.; Yang, Y.; Xu, P.; Li, J.; Ye, C.; Dai, L. Cytoplasm and cytoplasm-nucleus interactions affect agronomic traits in Japonica rice. Euphytica 2004, 135, 129–134. [Google Scholar] [CrossRef]
- Chandraratna, M.F.; Sakai, K.I. A biometrical analysis of matroclinous inheritance of grain weight in rice. Heredity 1960, 14, 365–373. [Google Scholar] [CrossRef][Green Version]
- Chang, T.T.; Liu, F.H. Diallel analysis of protein content in rice. Agron. Abstr. 1974, 65. [Google Scholar]
- Shi, C.H.; Xue, J.M.; Yu, Y.G.; Yang, X.E.; Zhu, J. Analysis of genetic effects on nutrient quality traits in indica rice. Theor. Appl. Genet. 1996, 92, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.B.; Shen, Z.T. Maternal effect on chalkiness in rice kernels. Rice Genet. Newsl. 1988, 5, 111–113. [Google Scholar]
- Shi, C.H.; Zhu, J. Analysis of seed and maternal genetic effects for characters of cooking quality in indica rice. Chin. J. Rice Sci. 1994, 8, 129–134. [Google Scholar]
- Anh, T.T.T.; Khanh, T.D.; Dat, T.D.; Xuan, T.D. Identification of phenotypic variation and genetic diversity in rice (Oryza sativa L.) mutants. Agriculture 2018, 8, 30. [Google Scholar] [CrossRef]
- Gregorio, G.B.; Senadhira, D.; Mendoza, R.D. Screening Rice for Salinity Tolerance, 3rd ed.; International Rice Research Institute: Los Baños, CA, USA, 1997; pp. 1–30. [Google Scholar]
- Quan, N.V.; Xuan, T.D.; Tran, H.-D.; Thuy, N.T.D.; Trang, L.T.; Huong, C.T.; Yusuf, A.; Tuyen, P.T. Antioxidant, α-amylase and α-glucosidase inhibitory activities and potential constituents of Canarium tramdenum Bark. Molecules 2019, 24, 605. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.D.; Minh, T.N.; Anh, L.H.; Khanh, T.D. Allelopathic momilactones A and B are implied in rice drought and salinity tolerance, not weed resistance. Agron. Sustain. Dev. 2016, 36, 52. [Google Scholar] [CrossRef]
- Govindarajan, R.; Rastogi, S.; Vijayakumar, M.; Shirwaikar, A.; Rawat, A.K.; Mehrotra, S.; Pushpangadan, P. Studies on the antioxidant activities of Desmodium gangeticum. Biol. Pharm. Bull. 2003, 26, 1424–1427. [Google Scholar] [CrossRef]
- Quan, N.V.; Thien, D.D.; Khanh, T.D.; Tran, H.-D.; Xuan, T.D. Momilactones A, B, and tricin in rice grain and by-products are potential skin aging inhibitors. Foods 2019, 8, 602. [Google Scholar] [CrossRef]
- Ganie, S.A.; Dey, N.; Mondal, T.K. Differential promoter methylation of salt tolerant and susceptible rice genotypes under salinity stress. Funct. Genom. 2016, 8, 1–11. [Google Scholar] [CrossRef]
- Linh, L.H.; Linh, T.H.; Xuan, T.D.; Ham, L.H.; Ismail, A.M.; Khanh, T.D. Molecular breeding to improve salt tolerance of rice (Oryza sativa L.) in the Red River Delta of Vietnam. Int. J. Plant Genom. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Islam, M.R.; Gregorio, G.B.; Salam, M.A.; Collard, B.C.Y.; Singh, R.K.; Hasan, L. Validation of Saltol linked markers and haplotype diversity on chromosome 1 of rice. Mol. Plant Breed. 2012, 3, 103–114. [Google Scholar] [CrossRef]
- Neelam, S.; Hemant, R.K.; Praveen, S.; Sneh, L.P.; Ashwani, P. A suite of new genes defining salinity stress tolerance in seedlings of contrasting rice genotypes. Funct. Integr. Genom. 2013, 13, 351–365. [Google Scholar] [CrossRef]
- Nejad, M.N.; Arzani, A.; Rezai, A.M.; Singh, R.K.; Gregorio, G.B. Assessment of rice genotypes for salt tolerance using microsatellite markers associated with the Saltol QTL. Afr. J. Biotechnol. 2008, 7, 730–736. [Google Scholar] [CrossRef]
- Fuentes, J.L.; Correa-Victoria, E.J.; Escobar, F.; Prado, G.; Aricapa, G.; Duque, M.C.; Tohme, J. Identification of microsatellite markers linked to the blast resistance gene Pi-1(t) in rice. Euphytica 2008, 160, 295–304. [Google Scholar] [CrossRef]
- Kioko, W.F.; Musyoki, M.A.; Piero, N.M.; Muriira, K.G.; Wavinya, N.D.; Rose, L.; Felix, M.; Ngithi, L.N. Genetic diversity studies on selected rice (Oryza sativa L.) populations based on aroma and cooked kernel elongation. Phylogen. Evol. Biol. 2015, 3, 4. [Google Scholar] [CrossRef]
- Nachimuthu, V.V.; Muthurajan, R.; Sudhakar, D.; Sivakami, R.; Pandian, B.A.; Ponniah, G.; Gunasekaran, K.; Manonmani, S.; Suji, K.K.; Revathi, S.V. Analysis of Population Structure and Genetic Diversity in Rice Germplasm Using SSR Markers: An Initiative Towards Association Mapping of Agronomic Traits in Oryza Sativa. Rice 2015, 8, 30. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Y.; Liang, J.; Luan, X.; Xu, P.; Wang, S.; Zhang, G.; Liu, G. Analysis of QTLs on heading date based on single segment substitution lines in rice (Oryza Sativa L.). Sci. Rep. 2018, 8, 13232. [Google Scholar] [CrossRef]
- Wu, J.L.; Sinha, P.K.; Variar, M.; Zheng, K.L.; Leach, J.E.; Courtois, B.; Leung, H. Association between molecular markers and blast resistance in an advanced backcross population of rice. Theor. Appl. Genet. 2004, 108, 1024–1032. [Google Scholar] [CrossRef]
- Ashkani, S.; Rafii, M.Y.; Sariah, M.; Akmar, A.S.N.; Rusli, I.; Rahim, H.A.; Latif, M.A. Analysis of simple sequence repeat markers linked with blast disease resistance genes in a segregating population of rice (Oryza Sativa). Genet. Mol. Res. 2011, 10, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Wattoo, J.I.; Liaqat, S.; Mubeen, H.; Ashfaq, M.; Shahid, M.N.; Farooq, A.; Sajjad, M.; Arif, M. Genetic mapping of grain nutritional profile in rice using basmati derived segregating population revealed by SSRs. Int. J. Agric. Biol. 2019, 21, 929–935. [Google Scholar] [CrossRef]
- Aliyu, R.E.; Adamu, A.K.; Muazu, S.; Alonge, S.; Gregorio, G.B. Validation of rice markers tagged to salinity stress. Afr. J. Biotechnol. 2013, 12, 32393243. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, P.K.; Arya, M.; Singh, N.K.; Singh, U.S. Molecular screening of blast resistance genes in rice using SSR markers. Plant Pathol. J. 2015, 31, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Boranayaka, M.B.; Lokesha, R.; Diwan, J.R.; Rajendragouda, P. Marker validation in F2 population of rice (Oryza sativa L.) for water and nitrogen use efficiency. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 1275–1278. [Google Scholar] [CrossRef][Green Version]
- Mishra, B.; Singh, R.K.; Jetly, V. Inheritance pattern of salinity stress in rice. J Genet. Breed. 1998, 52, 325–331. [Google Scholar]
- Mohammadi, R.; Merlyn, S.M.; Genaleen, Q.D.; Glenn, B.G.; Rakesh, K.S. Genetic analysis of salt tolerance at seedling and reproductive stages in rice (Oryza sativa). Plant Breed. 2013, 133, 548–559. [Google Scholar] [CrossRef]
- Leon, T.B.; Linscombe, S.; Subudhi, P.K. Identification and validation of QTLs for seedling salinity tolerance in introgression lines of a salt tolerant line landrace ‘Pokkali’. PLoS ONE 2017, 12, e0175361. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Dwivedi, D.K. Inheritance of pattern of salinity tolerance and combining ability in rice (Oryza sativa L.). Int. J. Curr. Microbiol. App. Sci. 2018, 7, 4716–4727. [Google Scholar]
- Pandit, A.; Rai, V.; Bal, S.; Sinha, S.; Kumar, V.; Chauhan, M.; Gautam, R.K.; Singh, R.; Sharma, P.C.; Singh, A.K.; et al. Combining QTL mapping and transcriptome profiling of bulked RILs for identification of functional polymorphism for salt tolerance genes in rice (Oryza sativa L.). Mol. Genet. Genom. 2010, 284, 121–136. [Google Scholar] [CrossRef]
- Tiwari, S.; Krishnamurthy, S.L.; Kumar, V.; Singh, B.; Rao, A.R.; Mithra, S.V.A.; Rai, V.; Singh, A.K.; Singh, N.K. Mapping QTLs for salt tolerance in rice (Oryza sativa L.) by bulked segregant analysis of recombinant inbred lines using 50k SNP chip. PLoS ONE 2016, 11, e0153610. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, B.D.; Kumar, A.; Maurya, S.; Krishnan, S.G.; Vinod, K.K.; Singh, M.P.; Ellur, R.K.; Bhowmick, P.K.; Singh, A.K. Marker-assisted introgression of Satol QTL enhances seedling stage salt tolerance in the rice variety “Pusa Basmati 1”. Intel. J. Genomics 2018. [Google Scholar] [CrossRef]
- Shimura, K.; Okada, A.; Okada, K.; Jikumaru, Y.; Ko, K.W.; Toyomasu, T.; Sassa, T.; Hasegawa, M.; Kodama, O.; Shibuya, N.; et al. Identification of a biosynthetic gene cluster in rice for momilactones. J. Biol. Chem. 2007, 23, 34013–34018. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Kabuto, C.; Sasaki, N.; Tsunagawa, M.; Aizawa, H.; Fujita, K.; Kato, Y.; Kitahara, Y. Momilactones, growth inhibitors from rice, Oryza sativa L. Tetrahedron Lett. 1973, 39, 3861–3864. [Google Scholar] [CrossRef]
- Cartwright, D.W.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Isolation and characterization of two phytoalexins from rice as momilactones A and B. Phytochemistry 1981, 20, 535–537. [Google Scholar] [CrossRef]
- Toyomasu, T.; Kagahara, T.; Okada, K.; Koga, J.; Hasegawa, M.; Mitsuhashi, W.; Sassa, T.; Yamane, H. Diterpene phytoalexins are biosynthesized in and exuded from the roots of rice seedlings. Biosci. Biotechnol. Biochem. 2008, 72, 562–567. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Hasegawa, M.; Ino, T.; Ota, K.; Kujime, H. Contribution of momilactone A and B to rice allelopathy. J. Plant Physiol. 2010, 167, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, M.; Xuan, T.D.; Deba, F.; Tawata, S.; Khanh, T.D.; Chung, I.M. Comparative efficacies in vitro of antibacterial, fungicidal, antioxidant, and herbicidal activities of momilatones A and B. J. Plant Interact. 2007, 2, 245–251. [Google Scholar] [CrossRef]
- Quan, N.T.; Xuan, T.D. Foliar application of vanillic and p-hydroxybenzoic acids enhanced drought tolerance and formation of phytoalexin momilactones in rice. Arch. Agron. Soil Sci. 2018, 64, 1–16. [Google Scholar] [CrossRef]
- Quan, N.V.; Tran, H.-D.; Xuan, T.D.; Ahmad, A.; Dat, T.D.; Khanh, T.D.; Teschke, R. Momilactones A and B are α-Amylase and α-Glucosidase Inhibitors. Molecules 2019, 24, 482. [Google Scholar] [CrossRef]
- Chung, I.M.; Ali, M.; Hahn, S.J.; Siddiqui, N.A.; Lim, Y.H.; Ahmad, A. Chemical constituents from the hulls of Oryza sativa with cytotoxic activity. Chem. Nat. Compd. 2005, 41, 182–189. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, H.R.; Park, E.; Lee, S.C. Cytotoxic and antitumor activity of momilactone B from rice hulls. J. Agric. Food Chem. 2007, 55, 1702–1706. [Google Scholar] [CrossRef]
- Joung, Y.H.; Lim, E.J.; Kim, M.S.; Lim, S.D.; Yoon, S.Y.; Lim, Y.C.; Yoo, Y.B.; Ye, S.K.; Park, T.; Chung, I.M.; et al. Enhancement of hypoxia-induced apoptosis of human breast cancer cells via STAT5b by momilactone B. Int. J. Oncol. 2008, 33, 477–484. [Google Scholar] [CrossRef] [PubMed]
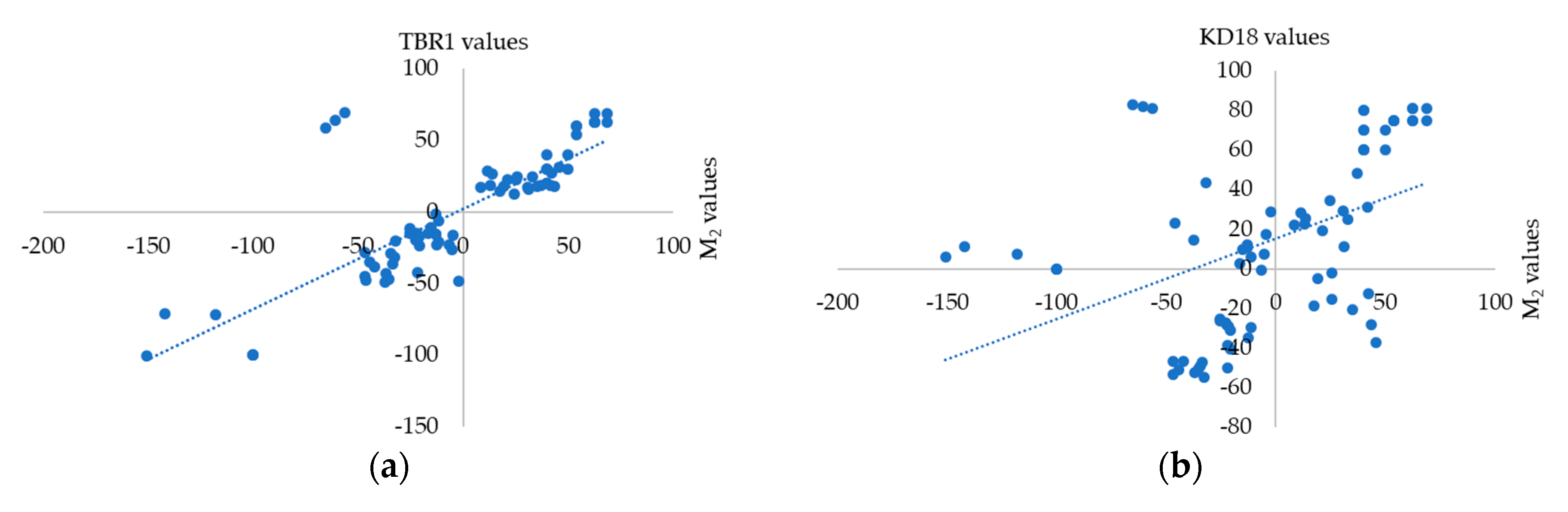
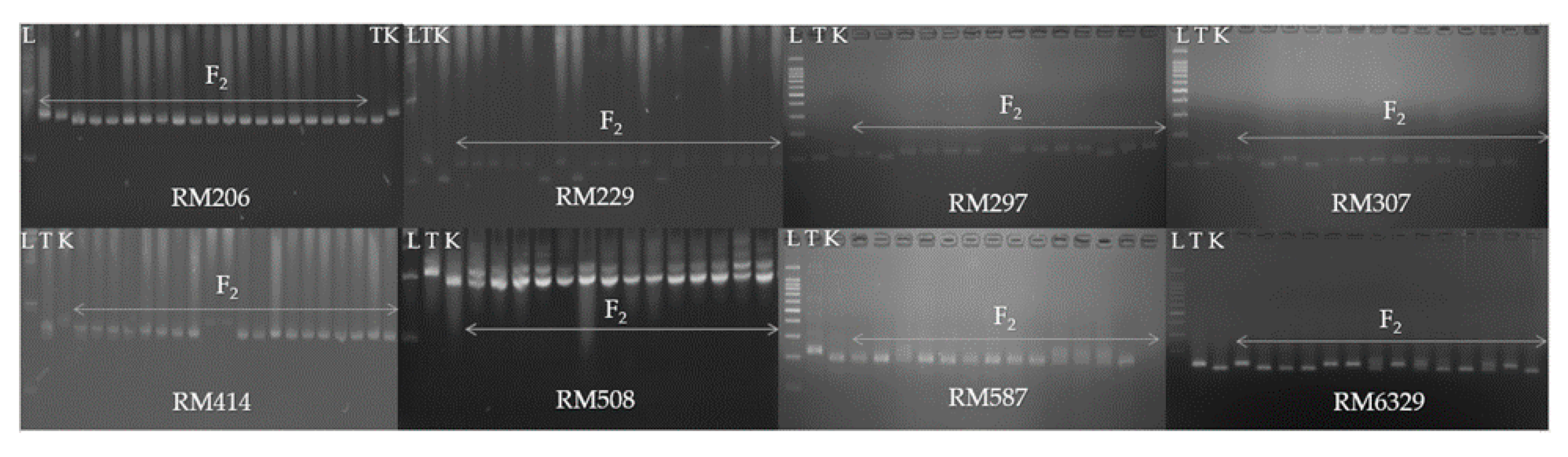
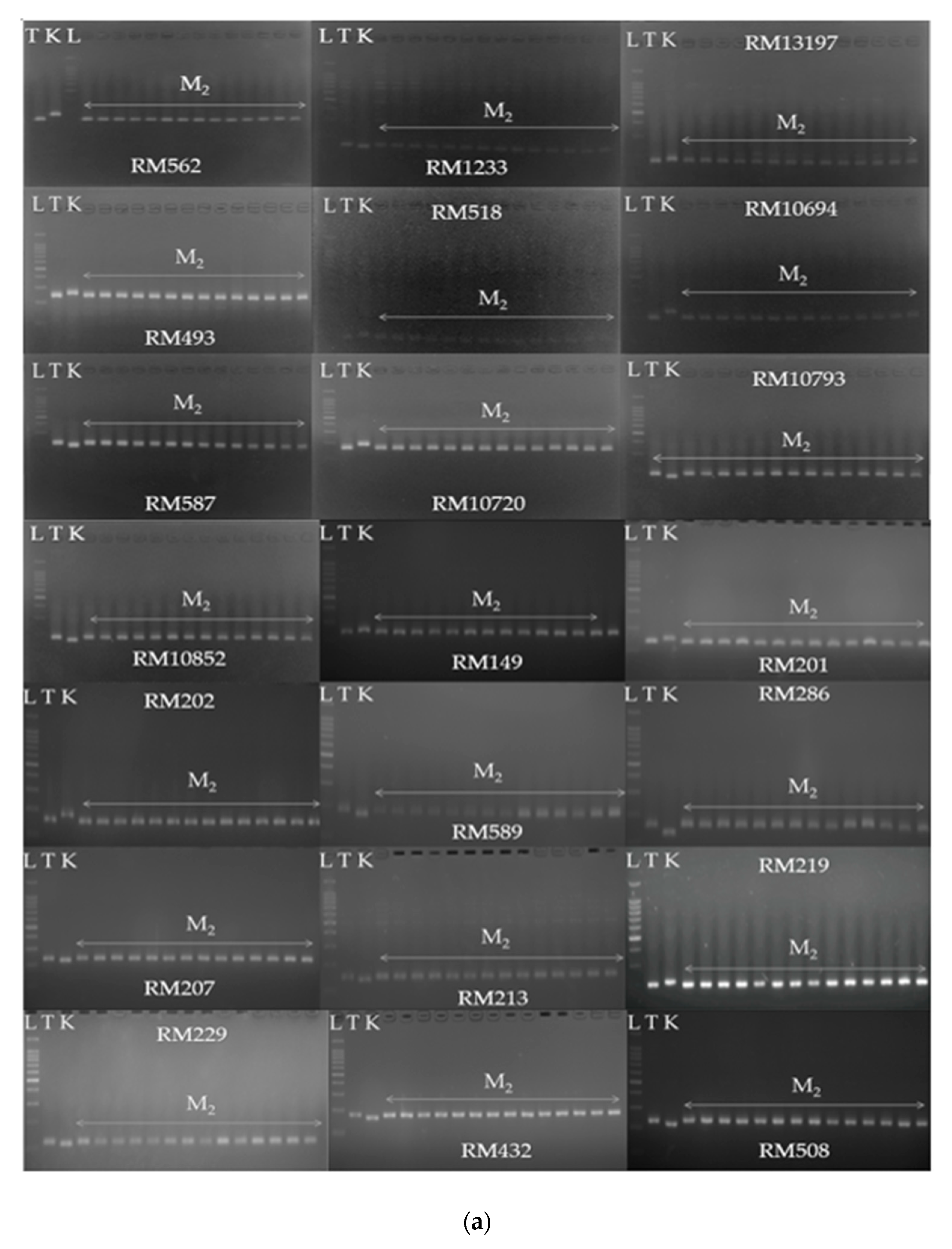

| NC | Samples | Injury Score | SUR (%) | RL (mm) | SL (mm) | FW (mg) | DW (mg) |
|---|---|---|---|---|---|---|---|
| Control | TBR1 | 1.0 ± 0.0 a | 100.0 ± 0.0 a | 58.4 ± 1.5 ab | 188.7 ± 4.0 a | 83.0 ± 3.0 ab | 25.0 ± 1.0 ab |
| KD18 | 1.0 ± 0.0 a | 100.0 ± 0.0 a | 61.1 ± 2.7 ab | 180.7 ± 1.8 ab | 85.0 ± 5.0 ab | 25.0 ± 1.0 ab | |
| F1 | 1.0 ± 0.0 a | 100.0 ± 0.0 a | 61.1 ± 1.8 ab | 181.5 ± 2.5 ab | 86.0 ± 3.0 ab | 25.0 ± 1.0 ab | |
| F2 | 1.0 ± 0.0 a | 100.0 ± 0.0 a | 60.9 ± 2.3 ab | 184.2 ± 1.5 ab | 82.0 ± 1.0 ab | 25.0 ± 0.0 ab | |
| M2 | 1.0 ± 0.0 a | 100.0 ± 0.0 a | 60.9 ± 1.0 ab | 187.6 ± 4.6 a | 97.0 ± 4.0 a | 27.0 ± 1.0 a | |
| M3 | 1.0 ± 0.0 a | 100.0 ± 0.0 a | 62.7 ± 1.5 ab | 185.6 ± 2.3 ab | 92.0 ± 3.0 a | 25.0 ± 1.0 ab | |
| Stress | TBR1 | 5.9 ± 0.2 b | 67.0 ± 1.5 b | 63.1 ± 3.0 ab | 160.0 ± 4.4 b | 74.0 ± 2.0 bc | 21.0 ± 1.0 bc |
| KD18 | 7.2 ± 0.1 c | 32.0 ± 2.5 c | 52.7 ± 2.4 b | 136.8 ± 4.7 d | 60.0 ± 5.0 c | 19.0 ± 1.0 c | |
| F1 | 6.5 ± 0.2 bc | 47.5 ± 2.1 bc | 60.7 ± 1.7 ab | 151.9 ± 4.2 c | 68.2 ± 4.0 bc | 20.0 ± 1.0 bc | |
| F2 | 6.8 ± 0.3 c | 50.8 ± 1.5 bc | 60.5 ± 4.5 ab | 155.6 ± 4.5 c | 70.7 ± 4.0 bc | 19.0 ± 2.0 c | |
| M2 | 6.0 ± 0.2 b | 59.0 ± 1.0 b | 68.0 ± 1.4 a | 165.7 ± 4.0 b | 86.0 ± 4.0 bc | 24.0 ± 1.0 ab | |
| M3 | 6.0 ± 0.5 b | 61.5 ± 2.3 b | 65.1 ± 3.5 a | 167.5 ± 5.2 b | 88.6 ± 3.0 b | 24.0 ± 0.0 ab |
| NC | Samples | TPC (mg GAE g−1 DW) | TFC (mg RE g−1 DW) | DPPH (IC50 mg/mL) | ABTS (IC50 mg/mL) | NO (IC50 mg/mL) | MA (ng/g) | MB (ng/g) |
|---|---|---|---|---|---|---|---|---|
| Control | TBR1 | 1.6 ± 0.1 b | 0.3 ± 0.0 d | 1.5 ± 0.1 b | 1.8 ± 0.0 c | 0.9 ± 0.0 ab | 59.9 ± 1.3 c | Nd |
| KD18 | 0.8 ± 0.2 c | 0.4 ± 0.0 cd | 2.4 ± 0.1 d | 2.1 ± 0.0 d | 1.8 ± 0.1 d | 76.5 ± 2.1 b | Nd | |
| F1 | 1.6 ± 0.2 b | 0.3 ± 0.1 d | 1.9 ± 0.0 bc | 1.9 ± 0.2 cd | 1.5 ± 0.1 c | 61.3 ± 2.5 c | Nd | |
| F2 | 1.5 ± 0.1 b | 0.3 ± 0.0 d | 1.9 ± 0.1 bc | 1.9 ± 0.1 cd | 1.5 ± 0.1 c | 72.3 ± 1.5 bc | Nd | |
| M2 | 0.9 ± 0.1 bc | 1.4 ± 0.1 b | 2.0 ± 0.0 c | 1.7 ± 0.0 c | 1.1 ± 0.1 b | 65.1 ± 0.1 c | Nd | |
| M3 | 0.7 ± 0.2 c | 1.3 ± 0.1 b | 1.9 ± 0.0 bc | 1.7 ± 0.1 c | 1.0 ± 0.2 b | 67.8 ± 0.5 c | Nd | |
| Stress | TBR1 | 2.1 ± 0.0 a | 0.6 ± 0.0 c | 1.1 ± 0.0 a | 1.0 ± 0.0 a | 0.5 ± 0.0 a | 21.4 ± 2.7 d | 46.3 ± 0.6 a |
| KD18 | 0.9 ± 0.1 bc | 0.6 ± 0.0 c | 2.3 ± 0.1 d | 1.5 ± 0.0 b | 1.5 ± 0.1 c | 13.9 ± 0.4 d | Nd | |
| F1 | 1.5 ± 0.0 b | 0.9 ± 0.1 bc | 1.8 ± 0.2 bc | 1.9 ± 0.2 cd | 1.3 ± 0.0 bc | 25.7 ± 1.4 d | Nd | |
| F2 | 1.5 ± 0.2 b | 0.8 ± 0.2 bc | 1.8 ± 0.0 bc | 1.9 ± 0.1 cd | 1.2 ± 0.1 bc | 20.6 ± 3.0 d | Nd | |
| M2 | 1.5 ± 0.1 b | 2.5 ± 0.1 a | 1.1 ± 0.1 a | 1.0 ± 0.0 a | 0.7 ± 0.0 ab | 104.7 ± 3.1 a | 34.9 ± 1.8 b | |
| M3 | 1.0 ± 0.1 bc | 1.6 ± 0.0 ab | 1.5 ± 0.0 b | 1.2 ± 0.1 ab | 0.5 ± 0.2 a | 102.5 ± 2.5 a | 20.5 ± 1.5 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huong, C.T.; Anh, T.T.T.; Dat, T.D.; Dang Khanh, T.; Dang Xuan, T. Uniparental Inheritance of Salinity Tolerance and Beneficial Phytochemicals in Rice. Agronomy 2020, 10, 1032. https://doi.org/10.3390/agronomy10071032
Huong CT, Anh TTT, Dat TD, Dang Khanh T, Dang Xuan T. Uniparental Inheritance of Salinity Tolerance and Beneficial Phytochemicals in Rice. Agronomy. 2020; 10(7):1032. https://doi.org/10.3390/agronomy10071032
Chicago/Turabian StyleHuong, Can Thu, Truong Thi Tu Anh, Tran Dang Dat, Tran Dang Khanh, and Tran Dang Xuan. 2020. "Uniparental Inheritance of Salinity Tolerance and Beneficial Phytochemicals in Rice" Agronomy 10, no. 7: 1032. https://doi.org/10.3390/agronomy10071032
APA StyleHuong, C. T., Anh, T. T. T., Dat, T. D., Dang Khanh, T., & Dang Xuan, T. (2020). Uniparental Inheritance of Salinity Tolerance and Beneficial Phytochemicals in Rice. Agronomy, 10(7), 1032. https://doi.org/10.3390/agronomy10071032







