Mapping Productivity and Essential Biophysical Parameters of Cultivated Tropical Grasslands from Sentinel-2 Imagery
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Field Area Set-Up and In Situ Measurements
2.2. Sentinel-2 Imagery
2.3. Statistical Analysis
2.3.1. Recommendations for N Fertilization
2.3.2. ARTMO
2.3.3. Spectral Vegetation Indices Analysis
2.3.4. Spatial Analysis of Biophysical Parameters at the Larger Field Scale
3. Results
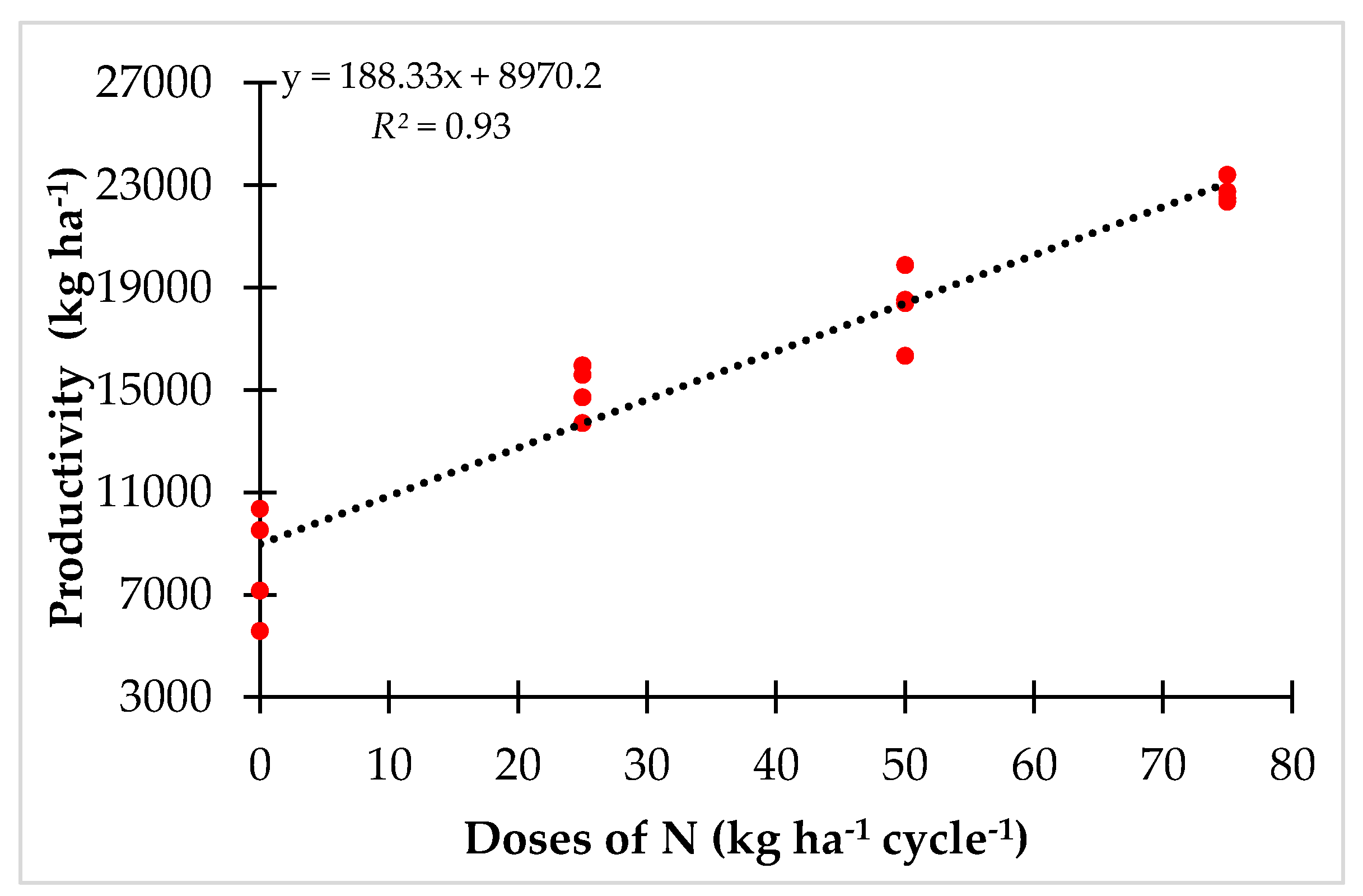
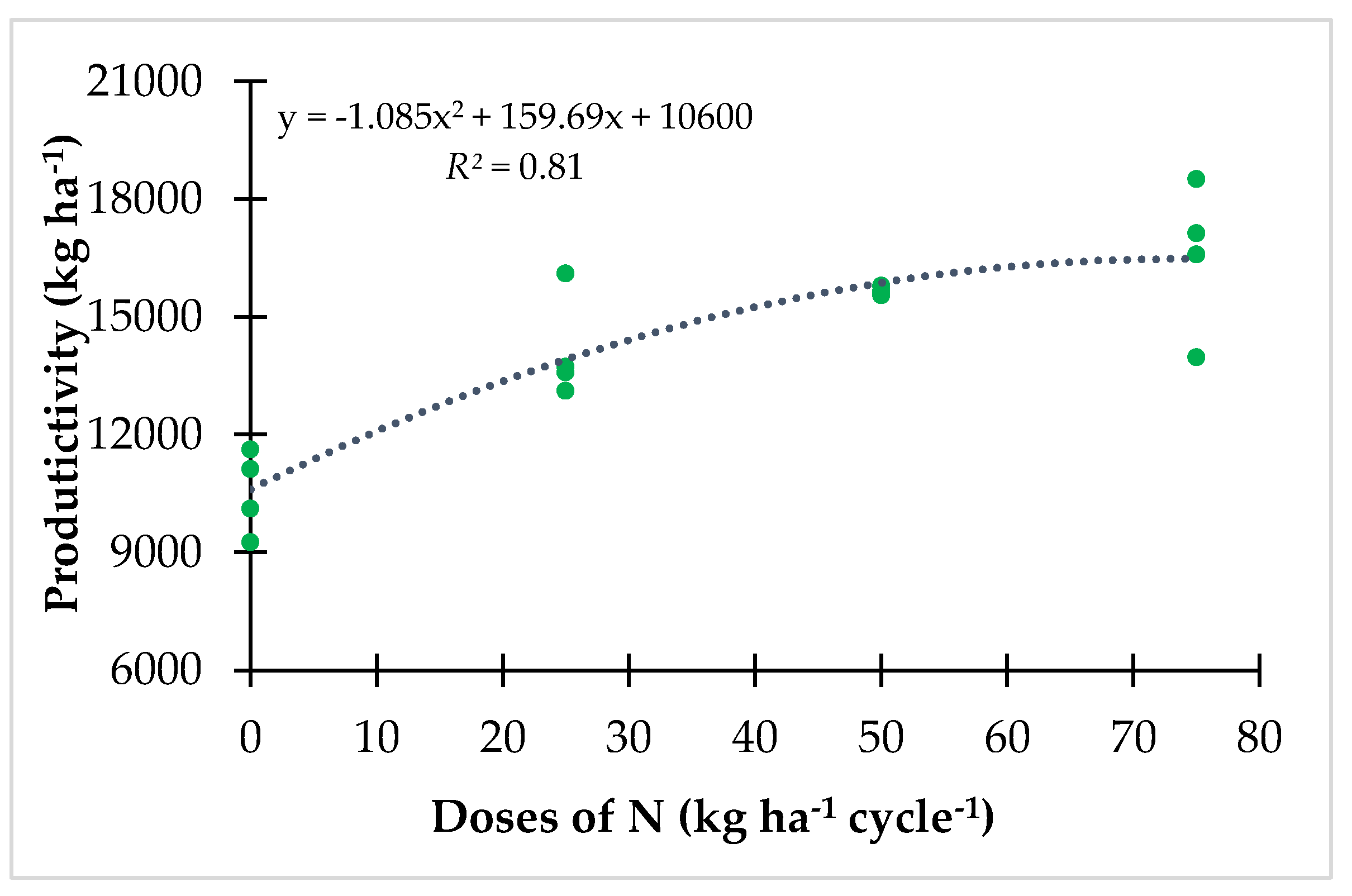
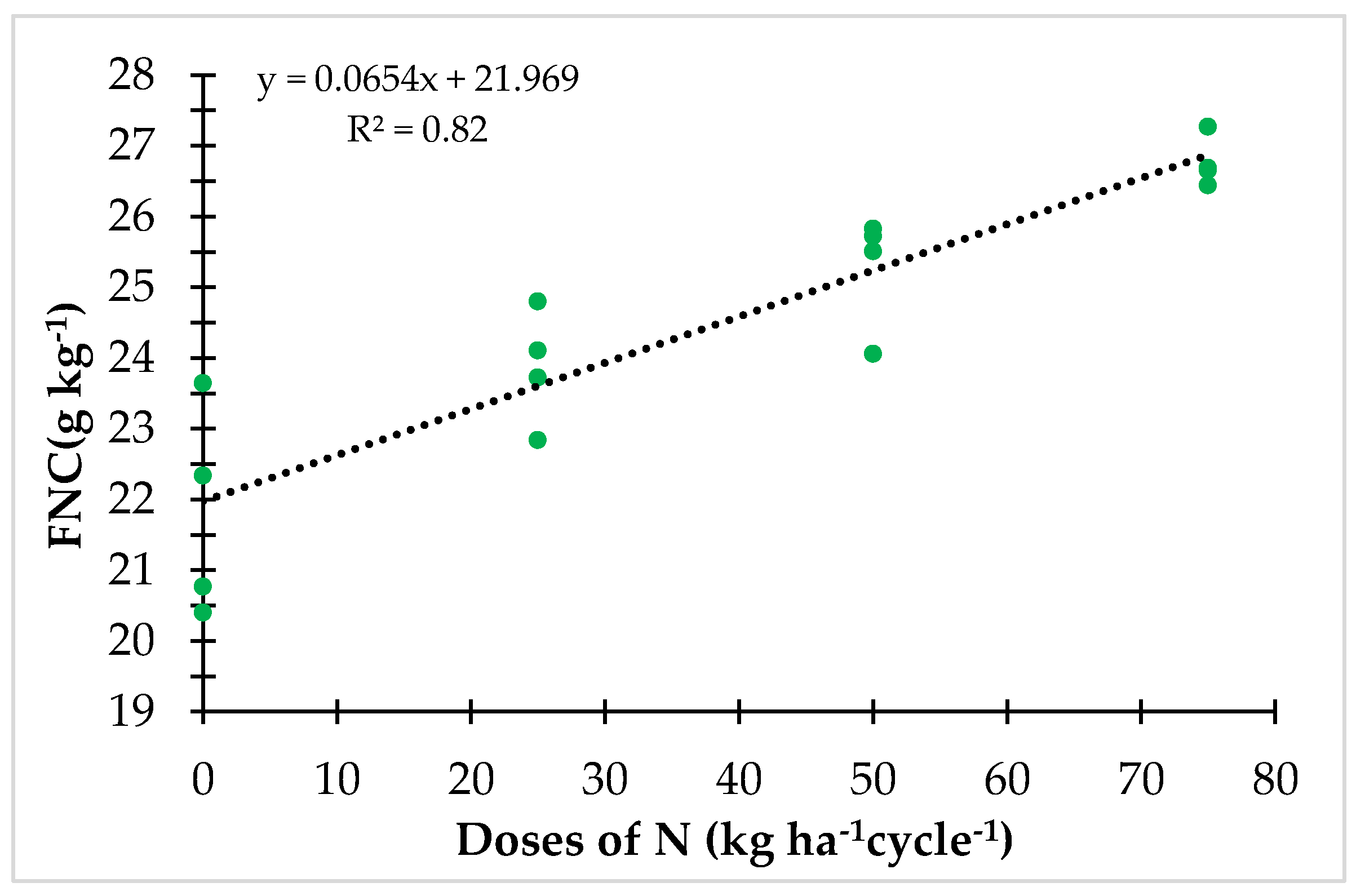
3.1. Productivity and FNC under Controlled N Fertilization
3.2. Performance of Generic Indices
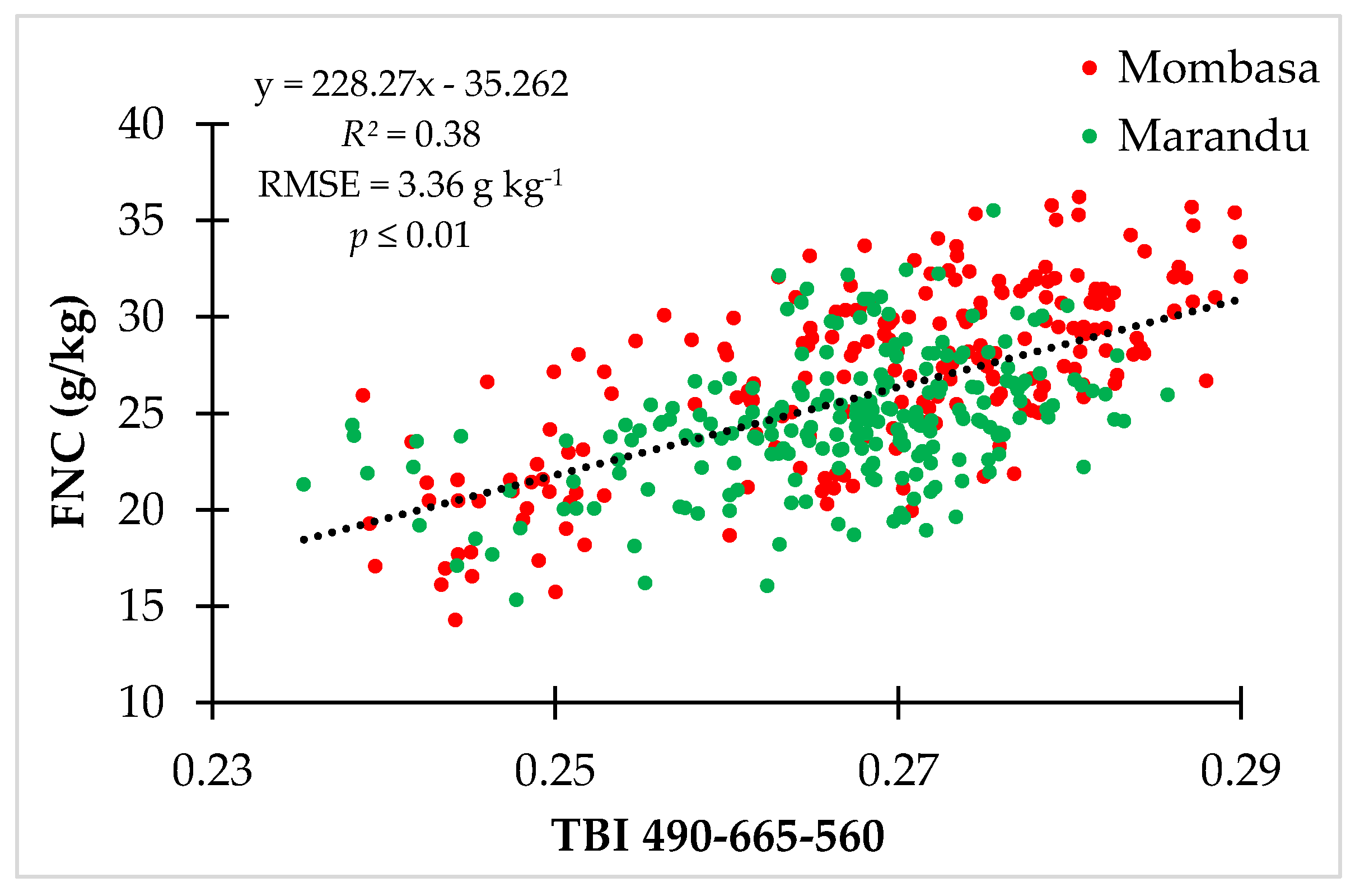
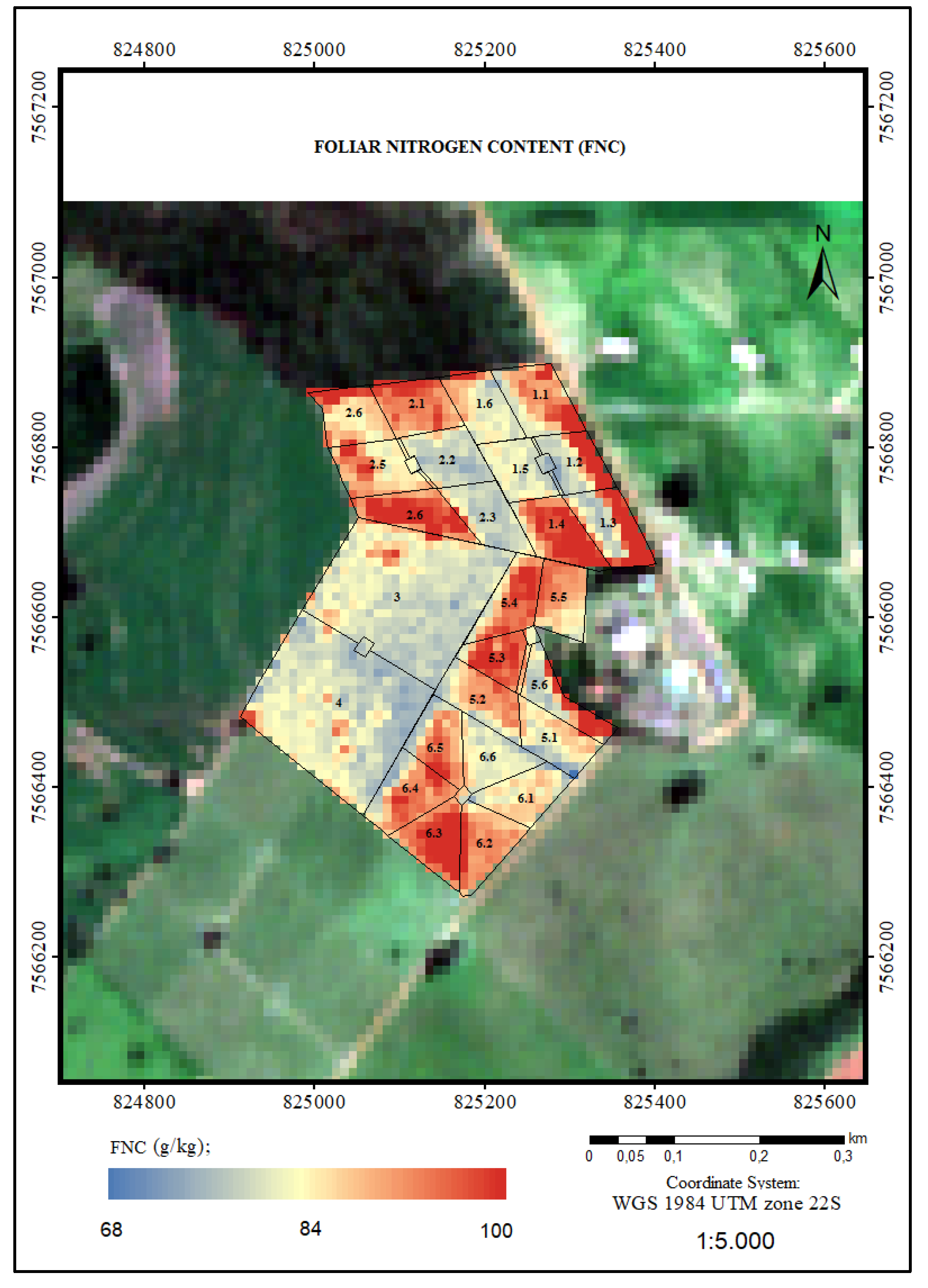
3.2.1. Foliar Nitrogen Content (FNC)
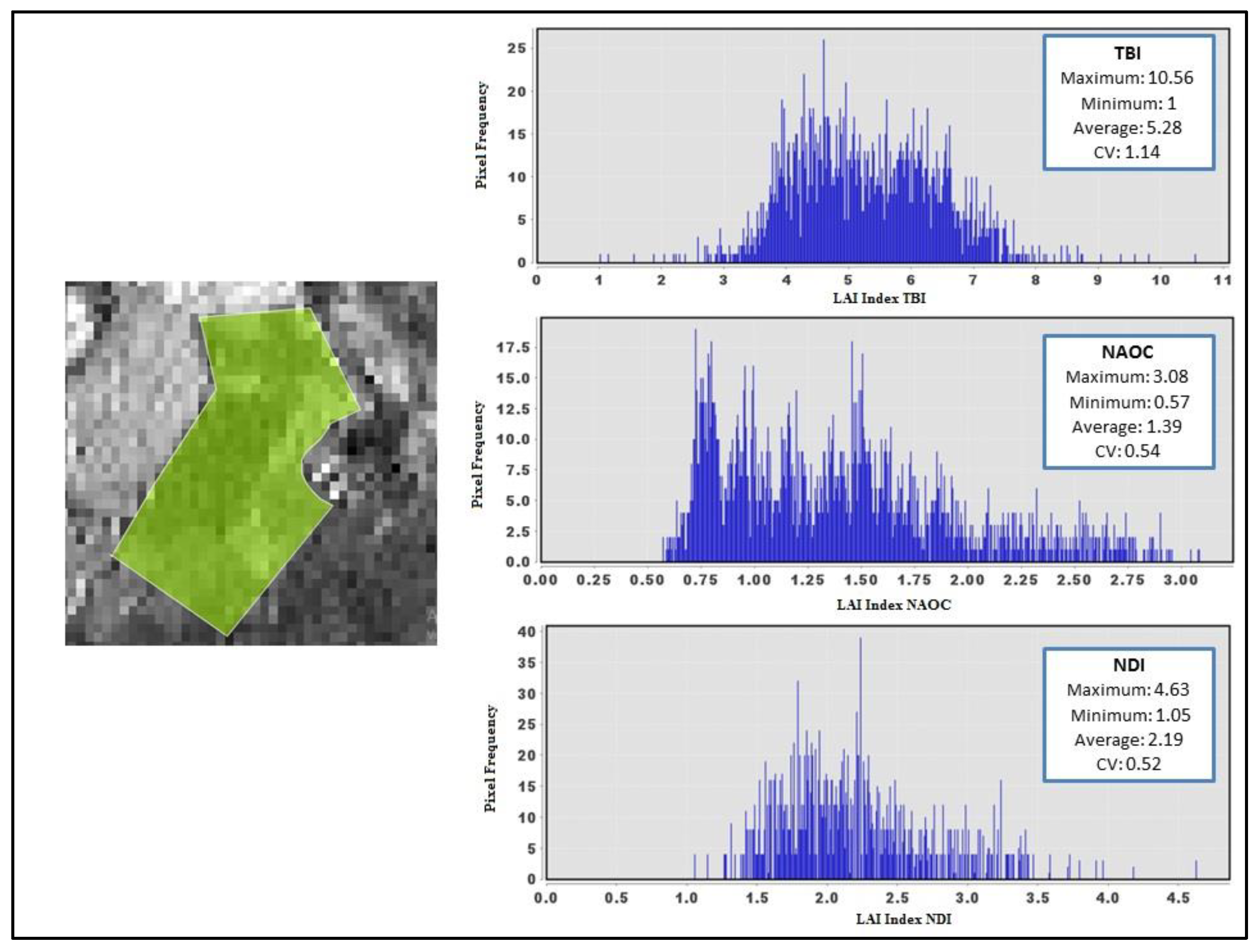
3.2.2. Leaf Area Index (LAI)
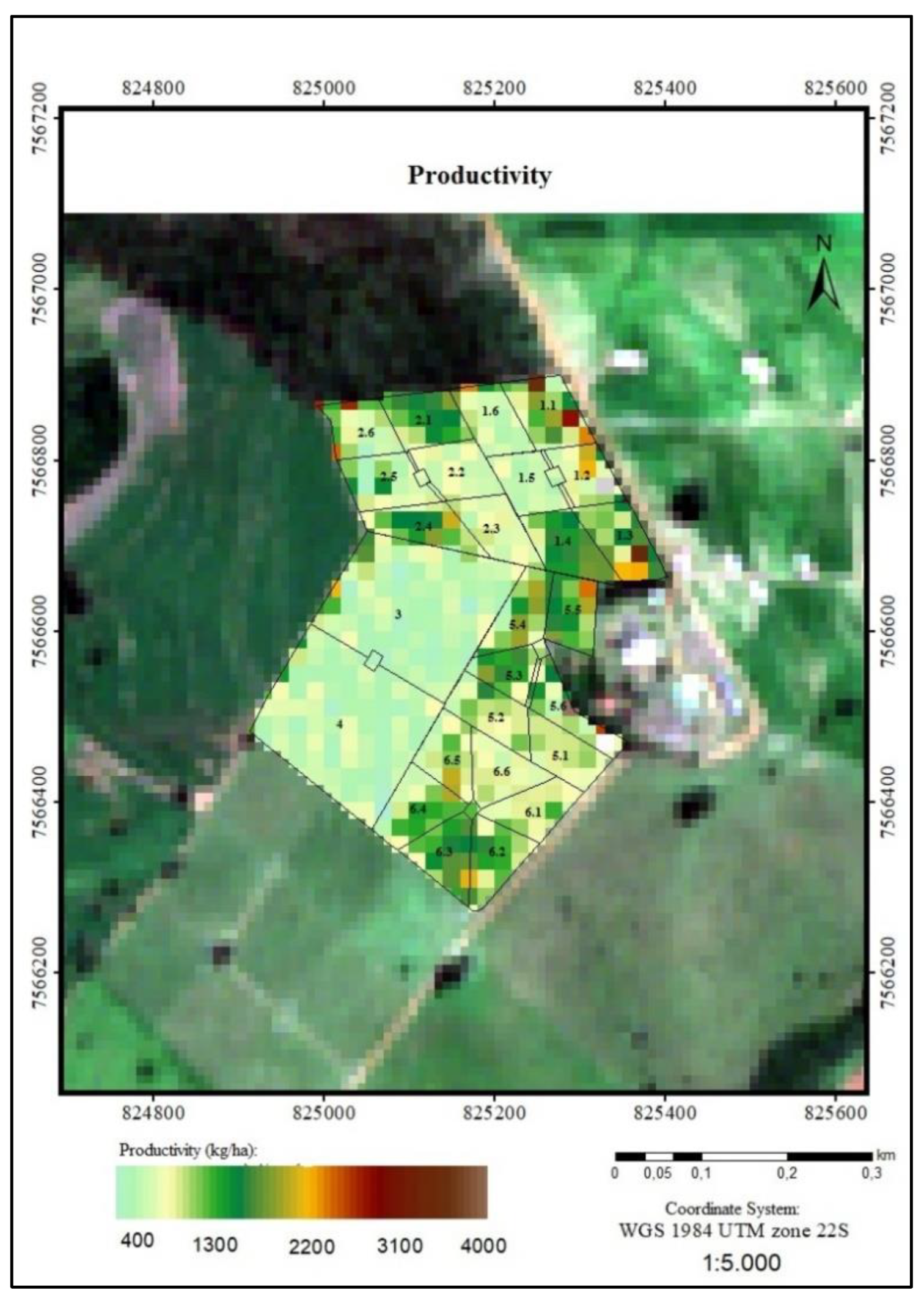
3.2.3. Productivity
4. Discussion
4.1. Foliar Nitrogen Content (FNC)
4.2. Leaf Area Index
4.3. Productivity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martha, G.B.; Alves, E.; Contini, E. Land-saving approaches and beef production growth in Brazil. Agric. Syst. 2012, 110, 173–177. [Google Scholar] [CrossRef]
- Parente, L.; Mesquita, V.; Miziara, F.; Baumann, L.; Ferreira, L. Assessing the pasturelands and livestock dynamics in Brazil, from 1985 to 2017: A novel approach based on high spatial resolution imagery and Google Earth Engine cloud computing. Remote Sens. Environ. 2019, 232, 111301. [Google Scholar] [CrossRef]
- De Alencar, C.A.B.; Cóser, A.C.; Martins, C.E.; De Oliveira, R.A.; Da Cunha, F.F.; Figueiredo, J.L.A. Altura de capins e cobertura do solo sob adubação nitrogenada, irrigação e pastejo nas estações do ano. Acta Sci.-Agron. 2010, 32, 21–27. [Google Scholar] [CrossRef][Green Version]
- Da Silva, S.C. Fundamentos para o manejo do pastejo de plantas forrageiras dos gêneros Brachiaria e Panicum. In Proceedings of the Simpósio Manejo Estratégico Da Pastagem; Anais; UFV, DZO: Viçosa, Brazil, 2004; pp. 347–386. [Google Scholar]
- Zhang, B.; Zhang, L.; Xie, D.; Yin, X.; Liu, C.; Liu, G. Application of synthetic NDVI time series blended from landsat and MODIS data for grassland biomass estimation. Remote Sens. 2016, 8, 10. [Google Scholar] [CrossRef]
- Boddey, R.M.; MacEdo, R.; Tarré, R.M.; Ferreira, E.; De Oliveira, O.C.; De, C.; Cantarutti, R.B.; Pereira, J.M.; Alves, B.J.R.; Urquiaga, S. Nitrogen cycling in Brachiaria pastures: The key to understanding the process of pasture decline. Agric. Ecosyst. Environ. 2004, 103, 389–403. [Google Scholar] [CrossRef]
- Werner, J.C. Adubacao de Pastagens; Boletim té.; Instituto de Zootecnia: Nova Odessa, Brazil, 1986. [Google Scholar]
- Corsi, M.; Nussio, L.G. Manejo do capim-elefante: Correção e adubação do solo. In Simpósio sobre manejo da pastagem; FEALQ: Piracicaba, Brazil, 1993; Volume 10, pp. 87–115. [Google Scholar]
- Andrade, A.C.; Miranda, D.; Fonseca, D.A.; Queiroz, D.S.; Salgado, L.T.; Cecon, P.R. Adubaçao nitrogenada e potássica em capim-elefante (Pennisetum purpureum Schum. cv. Napier). Ciência Agrotec. 2003, edição esp, 1643–1651. [Google Scholar]
- Da Cunha, C.A.H. Relação Entre Comportamento Espectral, índice de área Foliar e produção de matéria seca em capim Tanzânia submetido a diferentes níveis de irrigação e doses de nitrogênio. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2005. [Google Scholar]
- Cunha, O.F.R.; dos Santos, A.C.; de Araújo, L.C.; Ferreira, E.M. PRODUTIVIDADE DO Panicum maximum (MOMBAÇA) EM FUNÇÃO DE DIFERENTES NÍVEIS DE NITROGÊNIO. Rev. FZVA 2010, 17, 136–145. [Google Scholar]
- Fagundes, J.L.; Moreira, A.L.; de Freitas, A.W.P.; Henrichs, R.; Rocha, F.C. Forage production of Tifton 85 fertilized with nitrogen and subjected to continuous stocking. Rev. Bras. Saúde Prod. Anim. 2012, 13, 306–317. [Google Scholar] [CrossRef]
- Bruno, A.; Oliveira, B.; Andrade, A.C.; Magalhães, J.A.; Rodrigues, H.N.; Mehl, H.U.; José, F.; Santos, D. Produtividade do Capim-Digitária (Digitaria spp.) sob Diferentes Doses de Nitrogênio; Embrapa Meio-Norte: Guarapuava-PR, Brazil, 2015; Volume 9. [Google Scholar]
- Region, A.J.; Grande, R.; Sul, D. Determinação de Teores de Nitrogênio Foliar em Azevém (Lolium multiflo-rum Lam.); Nativo na Região do Alto Jacuí: Rio Grande Do Sul, Brazil, 2015. [Google Scholar]
- Foster, A.J.; Kakani, V.G.; Ge, J.; Gregory, M.; Mosali, J. Discriminant analysis of nitrogen treatments in switchgrass and high biomass sorghum using leaf and canopy-scale reflectance spectroscopy. Int. J. Remote Sens. 2016, 37, 2252–2279. [Google Scholar] [CrossRef]
- De Morais, L.F.; de Carvalho, C.A.B.; Anjos, A.N.A.; Viegas, C.R.; da Silva, P.H.F. Avanços na avaliação de pastagens cultivadas com forrageiras tropicais no Brasil. Braz. J. Appl. Technol. Agric. Sci. 2018, 11, 125–136. [Google Scholar]
- Salimon, C.; Anderson, L. How strong is the relationship between rainfall variability and Caatinga productivity? a case study under a changing climate. Ann. Braz. Acad. Sci. 2017, 90, 2121–2127. [Google Scholar] [CrossRef]
- Sudduth, K.A.; Drummond, S.T.; Kitchen, N.R. Operational characteristics of commercial crop canopy sensors for nitrogen application in maize. In Precision Agriculture; Stafford, J.V., Ed.; Wageningen Academic Publishers: Columbia, MO, USA, 2015; pp. 51–58. [Google Scholar]
- Molin, J.P.; Amaral, L.R.; Colaço, A. Agricultura de Precisão; Oficina de Textos: São Paulo, Brazil, 2015. [Google Scholar]
- Colombo, R.; Bellingeri, D.; Fasolini, D.; Marino, C.M. Retrieval of leaf area index in different vegetation types using high resolution satellite data. Remote Sens. Environ. 2003, 86, 120–131. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, 1–4. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; Gitelson, A.A. Remote estimation of crop and grass chlorophyll and nitrogen content using red-edge bands on Sentinel-2 and -3. Int. J. Appl. Earth Obs. Geoinf. 2013, 23, 334–343. [Google Scholar] [CrossRef]
- Gnyp, M.L.; Bareth, G.; Li, F.; Lenz-Wiedemann, V.I.S.; Koppe, W.; Miao, Y.; Hennig, S.D.; Jia, L.; Laudien, R.; Chen, X.; et al. Development and implementation of a multiscale biomass model using hyperspectral vegetation indices for winter wheat in the North China Plain. Int. J. Appl. Earth Obs. Geoinf. 2014, 33, 232–242. [Google Scholar] [CrossRef]
- Gianquinto, G.; Orsini, F.; Fecondini, M.; Mezzetti, M.; Sambo, P.; Bona, S. A methodological approach for defining spectral indices for assessing tomato nitrogen status and yield. Eur. J. Agron. 2011, 35, 135–143. [Google Scholar] [CrossRef]
- Kooistra, L.; Clevers, J.G.P.W. Estimating potato leaf chlorophyll content using ratio vegetation indices. Remote Sens. Lett. 2016, 7, 611–620. [Google Scholar] [CrossRef]
- Vincini, M.; Amaducci, S.; Frazzi, E. Empirical estimation of leaf chlorophyll density in winter wheat canopies using Sentinel-2 spectral resolution. IEEE Trans. Geosci. Remote Sens. 2014, 52, 3220–3235. [Google Scholar] [CrossRef]
- Baret, F.; Guyot, G. Potentials and limits of vegetation indices for LAI and APAR assessment. Remote Sens. Environ. 1991, 35, 161–173. [Google Scholar] [CrossRef]
- Glenn, E.P.; Huete, A.R.; Nagler, P.L.; Nelson, S.G. Relationship between remotely-sensed vegetation indices, canopy attributes and plant physiological processes: What vegetation indices can and cannot tell us about the landscape. Sensors 2008, 8, 2136–2160. [Google Scholar] [CrossRef]
- Van Wittenberghe, S.; Verrelst, J.; Rivera, J.P.; Alonso, L.; Moreno, J.; Samson, R. Gaussian processes retrieval of leaf parameters from a multi-species reflectance, absorbance and fluorescence dataset. J. Photochem. Photobiol. B Biol. 2014, 134, 37–48. [Google Scholar] [CrossRef]
- Mulla, D.J. Twenty five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. Sentinel-2: ESA’s Optical High-Resolution Mission for GMES Operational Services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.Á.D.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; Cunha, T.J.F.; de Oliveira, J.B. Sistema Brasileiro de Classificação de Solos, 3rd ed.; Embrapa: Brasilia, Brazil, 2013. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. eschweizerbartxxx Meteorol. Zeitschrift 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Malavolta, E.; Vitti, G.C.; de Oliveira, S.A. Avaliação do estado nutricional das plantas: Principios e aplicações, 3rd ed.; Associação Brasileira para Pesquisa da Potassa e do Fosfato: Piracicaba, Brazil, 1988. [Google Scholar]
- Louis, J.; Debaecker, V.; Pflug, B.; Main-knorn, M.; Bieniarz, J. Sentinel-2 Sen2Cor: L2A processor for users. In Proceedings of the Living Planet Symposium 2016, Prague, Czech Republic, 9–13 May 2016; Volume 2016. [Google Scholar]
- Verrelst, J.; Romijn, E.; Kooistra, L. Mapping vegetation density in a heterogeneous river floodplain ecosystem using pointable CHRIS/PROBA data. Remote Sens. 2012, 4, 2866–2889. [Google Scholar] [CrossRef]
- Rivera, J.P.; Verrelst, J.; Delegido, J.; Veroustraete, F.; Moreno, J. On the semi-automatic retrieval of biophysical parameters based on spectral index optimization. Remote Sens. 2014, 6, 4927–4951. [Google Scholar] [CrossRef]
- Pasqualotto, N.; Delegido, J.; Van Wittenberghe, S.; Rinaldi, M.; Moreno, J. Multi-crop green LAI estimation with a new simple sentinel-2 LAI index (SeLI). Sensors (Switzerland) 2019, 19, 904. [Google Scholar] [CrossRef]
- Snee, R. Validation and regression models: Methods and examples. Technometrics 1977, 19, 415–428. [Google Scholar] [CrossRef]
- Delegido, J.; Alonso, L.; González, G.; Moreno, J. Estimating chlorophyll content of crops from hyperspectral data using a normalized area over reflectance curve (NAOC). Int. J. Appl. Earth Obs. Geoinf. 2010, 12, 165–174. [Google Scholar] [CrossRef]
- Rouse, J.; Haas, R.; Schell, J.; Deering, D. Monitoring vegetation systems in the Great Plains with ERTS. In Proceedings of the 3rd Earth Resources Technology Satellite-1 Symposium; National Aeronautics and Space Administration: Washington, DC, USA, 1973; pp. 309–317. [Google Scholar]
- Tian, Y.C.; Yao, X.; Yang, J.; Cao, W.X.; Hannaway, D.B.; Zhu, Y. Assessing newly developed and published vegetation indices for estimating rice leaf nitrogen concentration with ground- and space-based hyperspectral reflectance. F. Crop. Res. 2011, 120, 299–310. [Google Scholar] [CrossRef]
- Gower, J.F.R. Observations of in situ fluorescence of chlorophyll-a in Saanich Inlet. Boundary-Layer Meteorol. 1980, 18, 235–245. [Google Scholar] [CrossRef]
- Dall’Olmo, G.; Gitelson, A.A.; Rundquist, D.C. Towards a unified approach for remote estimation of chlorophyll-a in both terrestrial vegetation and turbid productive waters. Geophys. Res. Lett. 2003, 30, 8–11. [Google Scholar] [CrossRef]
- Bayma-silva, G.; Santos, P.M. Protocolo de campo para investigação, calibração e validação de métodos para estimativa de massa de forragem baseados em sensoriamento remoto orbital e proximal de forragem baseados em sensoriamento remoto orbital e proximal 1; Embrapa Informática Agropecuária: Campinas, SP, Brazil, 2019; Volume 133. [Google Scholar]
- da Silva, R.F.; de Fátima Guimarães, M.; de Aquino, A.M.; Mercante, F.M. Análise conjunta de atributos físicos e biológicos do solo sob sistema de integração lavoura-pecuária. Pesqui. Agropecu. Bras. 2011, 46, 1277–1283. [Google Scholar] [CrossRef][Green Version]
- Asrar, G.; Kanemasu, E.T.; Jackson, R.D.; Pinter, P.J. Estimation of total above-ground phytomass production using remotely sensed data. Remote Sens. Environ. 1985, 17, 211–220. [Google Scholar] [CrossRef]
- Pacheco-Labrador, J.; González-Cascón, R.; Pilar Martín, M.; Riaño, D. Understanding the optical responses of leaf nitrogen in mediterranean holm oak (Quercus ilex) using field spectroscopy. Int. J. Appl. Earth Obervation Geoinf. 2014, 26, 105–118. [Google Scholar] [CrossRef]
- Novo, E.M.L. Sensoriamento Remoto: Principios e Aplicações, 4th ed.; Blücher, E., Ed.; Blusher: São Paulo, Brazil, 2010; ISBN 9788521205401. [Google Scholar]
- Rosa, R. Introdução ao Sensoriamento Remoto, 7th ed.; EDUFU, Ed.; EDUFU: Uberlândia, Brazil, 2009; ISBN 9788570781246. [Google Scholar]
- Abrahão, S.A.; De Assis, F.; Pinto, D.C.; Queiroz, D.M. Índices de vegetação de base espectral para discriminar doses de nitrogênio em capim-tanzânia Vegetation spectral indices to discriminate nitrogen rates in tanzania grass. Rev. Bras. Zootec. 2009, 38, 1637–1644. [Google Scholar]
- Parsons, A.J.; Chapman, D.F. The principles of pasture growth and utilization. In Grass: Its Production and Utilization; Hopkins, A., Holmes, W., Eds.; Blackwell Science for the British Grassland Society: Oxford, UK, 2000; ISBN 0632050179. [Google Scholar]
- Curran, P.J.; Dungan, J.L.; Gholz, H.L. Exploring the relationship between reflectance red edge and chlorophyll content in slash pine. Tree Physiol. 1990, 7, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Kira, O.; Nguy-Robertson, A.L.; Arkebauer, T.J.; Linker, R.; Gitelson, A.A. Informative spectral bands for remote green LAI estimation in C3 and C4 crops. Agric. For. Meteorol. 2016, 218–219, 243–249. [Google Scholar] [CrossRef]
- Pasqualotto, N.; Delegido, J.; Van Wittenberghe, S.; Verrelst, J.; Rivera, J.; Moreno, J. Retrieval of canopy water content of different crop types with two new hyperspectral indices: Water Absorption Area Index and Depth Water Index. Int. J. Appl. Earth Obs. Geoinf. 2018, 67, 69–78. [Google Scholar] [CrossRef]
- Malenovský, Z.; Ufer, C.; Lhotáková, Z.; Clevers, J.G.P.; Schaepman, M.; Albrechtová, J.; Cudlín, P. A new hyperspectral index for chlorophyll estimation of a forest canopy: Area under curve normalised to maximal band depth between 650 and 725 nm. EARSeL eProceedings 2006, 5, 161–172. [Google Scholar]
- Mutanga, O.M.C.; Skidmore, A.K.; Kumar, L.; Ferwerda, J. Estimating tropical pasture quality at canopy level using band depth analysis with continuum removal in the visible domain. Int. J. Remote Sens. 2005, 26, 1093–1108. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Skidmore, A.; Schlerf, M.; Atzberger, C.; Corsi, F.; Cho, M. LAI and chlorophyll estimation for a heterogeneous grassland using hyperspectral measurements. ISPRS J. Photogramm. Remote Sens. 2008, 63, 409–426. [Google Scholar] [CrossRef]
- Foster, A.J.; Kakani, V.G.; Mosali, J. Estimation of bioenergy crop yield and N status by hyperspectral canopy reflectance and partial least square regression. Precis. Agric. 2017, 18, 192–209. [Google Scholar] [CrossRef]
- Simões, C.R.; Rossiello, R.O.P.; Graciosa, M.G.; Machado, M.L.; Silva, C.F. da Imagens multiespectrais para avaliação de índice de área foliar e massa seca do capim “Tifton 85”, sob adubação nitrogenada. Ciênc. Rural 2015, 45, 697–703. [Google Scholar] [CrossRef][Green Version]
- Tong, X.; Duan, L.; Liu, T.; Singh, V.P. Combined use of in situ hyperspectral vegetation indices for estimating pasture biomass at peak productive period for harvest decision. Precis. Agric. 2019, 20, 477–495. [Google Scholar] [CrossRef]








| Cultivar | Treatments N (kg ha−1 Cycle) | Name | Description |
|---|---|---|---|
| Panicum maximum cv. ‘Mombasa’ | 0 | M0 | Control |
| 25 | M1 | Lower Dose | |
| 50 | M2 | Medium Dose | |
| 75 | M3 | High Dose | |
| Urochloa brizantha cv. ‘Marandu’ | 0 | B0 | Control |
| 25 | B1 | Lower Dose | |
| 50 | B2 | Medium Dose | |
| 75 | B3 | High Dose |
| Band Number | Mainly Function | Central Wavelength (nm) | Bandwidth (nm) | Spatial Resolution (m) |
|---|---|---|---|---|
| 1 | Coastal aerosol | 443 | 27 | 60 |
| 2 | Blue | 490 | 98 | 10 |
| 3 | Green | 560 | 45 | 10 |
| 4 | Red | 665 | 38 | 10 |
| 5 | Vegetation red-edge | 705 | 19 | 20 |
| 6 | Vegetation red-edge | 740 | 18 | 20 |
| 7 | Vegetation red-edge | 783 | 28 | 20 |
| 8 | Near Infrared (NIR) | 842 | 145 | 10 |
| 8a | Vegetation red-edge | 865 | 33 | 20 |
| 9 | Water vapor | 945 | 26 | 60 |
| 10 | Short Wave Infrared (SWIR)-cirrus | 1380 | 75 | 60 |
| 11 | SWIR | 1610 | 143 | 20 |
| 12 | SWIR | 2190 | 242 | 20 |
| Reference | Formula | Generic Name | Abbreviation | Generic Formula |
|---|---|---|---|---|
| [41] | Normalized Difference Vegetation Index | NDI | ||
| [42] | . | Three Ratio Band Index | TBI | |
| [43] | Difference light Height | DLH | ||
| [44] | Three Band Dall’Olmo | DO | ||
| [40] | Normalized Area Over reflectance Curve | NAOC | Same as the original |
| Systems | Repetition of the Area | Paddock | Fertilization (kg ha−1) | Corn | Growth | Pre Grazing | Grazing | Post Grazing |
|---|---|---|---|---|---|---|---|---|
| A | 1 | 1.1 | 180 | x | ||||
| 1.2 | 180 | x | ||||||
| 1.3 | 180 | x | ||||||
| 1.4 | 180 | x | ||||||
| 1.5 | 180 | x | ||||||
| 1.6 | 180 | x | ||||||
| 2 | 2.1 | 180 | x | |||||
| 2.2 | 180 | x | ||||||
| 2.3 | 180 | x | ||||||
| 2.4 | 180 | x | ||||||
| 2.5 | 180 | x | ||||||
| 2.6 | 180 | x | ||||||
| B | 3 | 3 | 0 | x | ||||
| 4 | 4 | 0 | x | |||||
| C | 5 | 5.1 | 180 | x | ||||
| 5.2 | 180 | x | ||||||
| 5.3 | 180 | x | ||||||
| 5.4 | 180 | x | ||||||
| 5.5 | 180 | x | ||||||
| 5.6 | 180 | x | ||||||
| 6 | 6.1 | 180 | x | |||||
| 6.2 | 180 | x | ||||||
| 6.3 | 180 | x | ||||||
| 6.4 | 180 | x | ||||||
| 6.5 | 180 | x | ||||||
| 6.6 | 180 | x |
| Index | FNC (g/kg) | Productivity (kg ha−1) | Height (cm) | LAI (m2 m−2 or Dimensionless) |
|---|---|---|---|---|
| NDI | ||||
| R2 = 0.18 | R2 = 0.37 | R2 = 0.06 | R2 = 0.54 | |
| TBI | ||||
| R2 = 0.38 | R2 = 0.45 | R2 = 0.15 | R2 = 0.56 | |
| NAOC | ||||
| R2 = 0.13 | R2 = 0.34 | R2 = 0.08 | R2 = 0.57 | |
| DLH | ||||
| R2 = 0.28 | R2 = 0.25 | R2 = 0.002 | R2 = 0.42 | |
| DO | ||||
| R2 = 0.24 | R2 = 0.54 | R2 = 0.006 | R2 = 0.40 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cisneros, A.; Fiorio, P.; Menezes, P.; Pasqualotto, N.; Van Wittenberghe, S.; Bayma, G.; Furlan Nogueira, S. Mapping Productivity and Essential Biophysical Parameters of Cultivated Tropical Grasslands from Sentinel-2 Imagery. Agronomy 2020, 10, 711. https://doi.org/10.3390/agronomy10050711
Cisneros A, Fiorio P, Menezes P, Pasqualotto N, Van Wittenberghe S, Bayma G, Furlan Nogueira S. Mapping Productivity and Essential Biophysical Parameters of Cultivated Tropical Grasslands from Sentinel-2 Imagery. Agronomy. 2020; 10(5):711. https://doi.org/10.3390/agronomy10050711
Chicago/Turabian StyleCisneros, Amparo, Peterson Fiorio, Patricia Menezes, Nieves Pasqualotto, Shari Van Wittenberghe, Gustavo Bayma, and Sandra Furlan Nogueira. 2020. "Mapping Productivity and Essential Biophysical Parameters of Cultivated Tropical Grasslands from Sentinel-2 Imagery" Agronomy 10, no. 5: 711. https://doi.org/10.3390/agronomy10050711
APA StyleCisneros, A., Fiorio, P., Menezes, P., Pasqualotto, N., Van Wittenberghe, S., Bayma, G., & Furlan Nogueira, S. (2020). Mapping Productivity and Essential Biophysical Parameters of Cultivated Tropical Grasslands from Sentinel-2 Imagery. Agronomy, 10(5), 711. https://doi.org/10.3390/agronomy10050711






