Production of Cellulose Nanofibers from Olive Tree Harvest—A Residue with Wide Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Pulping Process
2.3. Raw Material and Cellulosic Pulp Characterization
2.4. Cellulose Nanofiber Production
2.5. Cellulose Nanofiber Characterization
2.6. Viscosity, Degree of Polymerization and Length
2.7. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.8. X-Ray Diffraction (XRD) Analysis
2.9. Thermogravimetric Analysis (TGA)
3. Results and Discussion
3.1. Cellulosic Fiber Production and Characterization
3.2. Cellulose Nanofiber Isolation and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gurría, P.; Ronzon, T.; Tamosiunas, S.; López, R.; García Condado, S.; Guillén, J.; Cazzaniga, N.E.; Jonsson, R.; Banja, M.; Fiore, G.; et al. Biomass Flows in the European Union; Publications Office of the European Union: Luxembourg, Luxembourg, 2017. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). 2018 FAOSTAT. Available online: http://www.fao.org/faostat/en/#data (accessed on 2 April 2020).
- Miranda, I.; Simões, R.; Medeiros, B.; Nampoothiri, K.M.; Sukumaran, R.K.; Rajan, D.; Pereira, H.; Ferreira-Dias, S. Valorization of lignocellulosic residues from the olive oil industry by production of lignin, glucose and functional sugars. Bioresource Technol. 2019, 292, 121936. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Klajn, F.; Romero-García, J.M.; Díaz, M.J.; Castro, E. Comparison of fermentation strategies for ethanol production from olive tree pruning biomass. Ind. Crop. Prod. 2018, 122, 98–106. [Google Scholar] [CrossRef]
- Santos, J.I.; Fillat, Ú.; Martín-Sampedro, R.; Eugenio, M.E.; Negro, M.J.; Ballesteros, I.; Rodríguez, A.; Ibarra, D. Evaluation of lignins from side-streams generated in an olive tree pruning-based biorefinery: Bioethanol production and alkaline pulping. Int. J. Biol. Macromol. 2017, 105, 238–251. [Google Scholar] [CrossRef]
- Suardi, A.; Latterini, F.; Alfano, V.; Palmieri, N.; Bergonzoli, S.; Pari, L. Analysis of the Work Productivity and Costs of a Stationary Chipper Applied to the Harvesting of Olive Tree Pruning for Bio-Energy Production. Energies 2020, 13, 1359. [Google Scholar] [CrossRef] [Green Version]
- Liuzzi, S.; Rubino, C.; Martellotta, F.; Stefanizzi, P.; Casavola, C.; Pappalettera, G. Characterization of biomass-based materials for building applications: The case of straw and olive tree waste. Ind. Crop. Prod. 2020, 147, 112229. [Google Scholar] [CrossRef]
- Mutjé, P.; Pèlach, M.A.; Vilaseca, F.; García, J.C.; Jiménez, L. A comparative study of the effect of refining on organosolv pulp from olive trimmings and kraft pulp from eucalyptus wood. Bioresource Technol. 2005, 96, 1125–1129. [Google Scholar] [CrossRef]
- García, A.; Gandini, A.; Labidi, J.; Belgacem, N.; Bras, J. Industrial and crop wastes: A new source for nanocellulose biorefinery. Ind. Crop. Prod. 2016, 93, 26–38. [Google Scholar] [CrossRef]
- Balea, A.; Fuente, E.; Monte, C.M.; Merayo, N.; Campano, C.; Negro, C.; Blanco, A. Industrial Application of Nanocelluloses in Papermaking: A Review of Challenges, Technical Solutions, and Market Perspectives. Molecules 2020, 25, 526. [Google Scholar] [CrossRef] [Green Version]
- Inshakova, E.; Inshakov, O. World market for nanomaterials: Structure and trends. MATEC Web Conf. 2017, 129, 02013. [Google Scholar] [CrossRef]
- Boufi, S.; González, I.; Delgado-Aguilar, M.; Tarrès, Q.; Pèlach, M.À.; Mutjé, P. Nanofibrillated cellulose as an additive in papermaking process: A review. Carbohyd. Polym. 2016, 154, 151–166. [Google Scholar] [CrossRef]
- Tayeb, P.H.; Tayeb, A. Nanocellulose applications in sustainable electrochemical and piezoelectric systems: A review. Carbohyd. Polym. 2019, 224, 115149. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.H.; Zhong, W. Nanocellulose-Based Conductive Membranes for Free-Standing Supercapacitors: A Review. Membranes 2019, 9, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongao, H.C.; Gabatino, R.R.A.; Arias, C.F.H.; Magdaluyo, E.R. Micro/nanocellulose from waste Pili (Canarium ovatum) pulp as a potential anti-ageing ingredient for cosmetic formulations. Mater. Today-Proc. 2020, 22, 275–280. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef] [Green Version]
- Putro, J.N.; Kurniawan, A.; Ismadji, S.; Ju, Y.-H. Nanocellulose based biosorbents for wastewater treatment: Study of isotherm, kinetic, thermodynamic and reusability. Environ. Nanotechnol. Monit. Manage. 2017, 8, 134–149. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, X.; Wang, B.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Chen, X.; Sui, X. Nanocellulose sponges as efficient continuous flow reactors. Carbohyd. Polym. 2019, 224, 115184. [Google Scholar] [CrossRef]
- Barnat-Hunek, D.; Grzegorczyk-Frańczak, M.; Szymańska-Chargot, M.; Łagód, G. Effect of Eco-Friendly Cellulose Nanocrystals on Physical Properties of Cement Mortars. Polymers 2019, 11, 2088. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sui, Y.; Liu, C.; Liu, C.; Wu, M.; Li, B.; Li, Y. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohyd. Polym. 2018, 188, 27–36. [Google Scholar] [CrossRef]
- Moohan, J.; Stewart, A.S.; Espinosa, E.; Rosal, A.; Rodríguez, A.; Larrañeta, E.; Donnelly, F.R.; Domínguez-Robles, J. Cellulose Nanofibers and Other Biopolymers for Biomedical Applications. A Review. Appl. Sci. 2019, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Dufresne, A. Nanocellulose, from Nature to High Performance Tailored Materials; Walter de Gruyter GmbH.: Berlin, Germany, 2012. [Google Scholar]
- Espinosa, E.; Tarres, Q.; Delgado-Aguilar, M.; Gonzalez, I.; Mutje, P.; Rodriguez, A. Suitability of wheat straw semichemical pulp for the fabrication of lignocellulosic nanofibres and their application to papermaking slurries. Cellulose 2016, 23, 837–852. [Google Scholar] [CrossRef]
- Rol, F.; Karakashov, B.; Nechyporchuk, O.; Terrien, M.; Meyer, V.; Dufresne, A.; Belgacem, M.N.; Bras, J. Pilot-Scale Twin Screw Extrusion and Chemical Pretreatment as an Energy-Efficient Method for the Production of Nanofibrillated Cellulose at High Solid Content. ACS Sustain. Chem. Eng. 2017, 5, 6524–6531. [Google Scholar] [CrossRef]
- Wang, W.; Mozuch, M.D.; Sabo, R.C.; Kersten, P.; Zhu, J.Y.; Jin, Y. Production of cellulose nanofibrils from bleached eucalyptus fibers by hyperthermostable endoglucanase treatment and subsequent microfluidization. Cellulose 2015, 22, 351–361. [Google Scholar] [CrossRef]
- Espinosa, E.; Rol, F.; Bras, J.; Rodríguez, A. Production of lignocellulose nanofibers from wheat straw by different fibrillation methods. Comparison of its viability in cardboard recycling process. J. Clean. Prod. 2019, 239, 118083. [Google Scholar] [CrossRef]
- Espinosa, E.; Dominguez-Robles, J.; Sanchez, R.; Tarres, Q.; Rodriguez, A. The effect of pre-treatment on the production of lignocellulosic nanofibers and their application as a reinforcing agent in paper. Cellulose 2017, 24, 2605–2618. [Google Scholar] [CrossRef]
- Tarres, Q.; Saguer, E.; Pelach, M.A.; Alcala, M.; Delgado-Aguilar, M.; Mutje, P. The feasibility of incorporating cellulose micro/nanofibers in papermaking processes: The relevance of enzymatic hydrolysis. Cellulose 2016, 23, 1433–1445. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Rol, F.; Saini, S.; Meyer, V.; Petit-Conil, M.; Bras, J. Production of cationic nanofibrils of cellulose by twin-screw extrusion. Ind. Crop. Prod. 2019, 137, 81–88. [Google Scholar] [CrossRef]
- Jiménez, L.; Pérez, I.; de la Torre, J.; García, J.C. The effect of processing variables on the soda pulping of olive tree wood. Bioresource Technol. 1999, 69, 95–102. [Google Scholar] [CrossRef]
- Martin-Sampedro, R.; Rodríguez, A.; Requejo, A.; Eugenio, M.E. Improvement of TCF bleaching of olive tree pruning residue pulp by addition of a laccase and/or xylanase pre-treatment. Bioresources 2012, 7, 1488–1505. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, E.; Bascón-Villegas, I.; Rosal, A.; Pérez-Rodríguez, F.; Chinga-Carrasco, G.; Rodríguez, A. PVA/(ligno)nanocellulose biocomposite films. Effect of residual lingin content on the structural, mechanical, barrier and antioxidant properties. Int. J. Biol. Macromol. 2019, 141, 197–206. [Google Scholar] [CrossRef]
- Espinosa, E.; Sánchez, R.; González, Z.; Domínguez-Robles, J.; Ferrari, B.; Rodríguez, A. Rapidly growing vegetables as new sources for lignocellulose nanofibre isolation: Physicochemical, thermal and rheological characterisation. Carbohyd. Polym. 2017, 175, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohyd. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Marx-Figini, M. The acid-catalyzed degradation of cellulose linters in distinct ranges of degree of polymerization. J. Appl. Polym. Sci. 1987, 33, 2097–2105. [Google Scholar] [CrossRef]
- Shinoda, R.; Saito, T.; Okita, Y.; Isogai, A. Relationship between Length and Degree of Polymerization of TEMPO-Oxidized Cellulose Nanofibrils. Biomacromolecules 2012, 13, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallintiy of native cellulose using X-ray diffractometer. Text. Res. J. 1959, 29, 786–974. [Google Scholar] [CrossRef]
- Requejo, A.; Rodríguez, A.; Colodette, J.L.; Gomide, J.L.; Jiménez, L. Optimization of ECF bleaching and refining of kraft pulping from olive tree pruning. BioResources 2012, 7, 4046–4055. [Google Scholar]
- Fillat, Ú.; Wicklein, B.; Martín-Sampedro, R.; Ibarra, D.; Ruiz-Hitzky, E.; Valencia, C.; Sarrión, A.; Castro, E.; Eugenio, M.E. Assessing cellulose nanofiber production from olive tree pruning residue. Carbohyd. Polym. 2018, 179, 252–261. [Google Scholar] [CrossRef]
- Chaker, A.; Alila, S.; Mutje, P.; Vilar, M.R.; Boufi, S. Key role of the hemicellulose content and the cell morphology on the nanofibrillation effectiveness of cellulose pulps. Cellulose 2013, 20, 2863–2875. [Google Scholar] [CrossRef]
- Rajan, K.; Djioleu, A.; Kandhola, G.; Labbé, N.; Sakon, J.; Carrier, D.J.; Kim, J.-W. Investigating the effects of hemicellulose pre-extraction on the production and characterization of loblolly pine nanocellulose. Cellulose 2020. [Google Scholar] [CrossRef]
- Kassab, Z.; Kassem, I.; Hannache, H.; Bouhfid, R.; Qaiss, A.E.K.; El Achaby, M. Tomato plant residue as new renewable source for cellulose production: Extraction of cellulose nanocrystals with different surface functionalities. Cellulose 2020. [Google Scholar] [CrossRef]
- Jongaroontaprangsee, S.; Chiewchan, N.; Devahastin, S. Production of nanocellulose from lime residues using chemical-free technology. Mater. Today-Proc. 2018, 5, 11095–11100. [Google Scholar] [CrossRef]
- Septevani, A.A.; Rifathin, A.; Sari, A.A.; Sampora, Y.; Ariani, G.N.; Sudiyarmanto; Sondari, D. Oil palm empty fruit bunch-based nanocellulose as a super-adsorbent for water remediation. Carbohyd. Polym. 2020, 229, 115433. [Google Scholar] [CrossRef] [PubMed]
- Benini, K.C.C.d.C.; Voorwald, H.J.C.; Cioffi, M.O.H.; Rezende, M.C.; Arantes, V. Preparation of nanocellulose from Imperata brasiliensis grass using Taguchi method. Carbohyd. Polym. 2018, 192, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues - Wheat straw and soy hulls. Bioresource Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Delgado-Aguilar, M.; González Tovar, I.; Tarrés, Q.; Alcalá, M.; Pèlach, M.À.; Mutjé, P. Approaching a Low-Cost Production of Cellulose Nanofibers for Papermaking Applications. BioResources 2015, 10, 5435–5455. [Google Scholar] [CrossRef]
- González, I.; Boufi, S.; Pèlach, M.A.; Alcalà, M.; Vilaseca, F.; Mutjé, P. Nanofibrillated cellulose as papper additive in eucalyptus pulp. BioResources 2012, 7, 5167–5180. [Google Scholar] [CrossRef] [Green Version]
- Vallejos, M.E.; Felissia, F.E.; Area, M.C.; Ehman, N.V.; Tarrés, Q.; Mutjé, P. Nanofibrillated cellulose (CNF) from eucalyptus sawdust as a dry strength agent of unrefined eucalyptus handsheets. Carbohyd. Polym. 2016, 139, 99–105. [Google Scholar] [CrossRef]
- Tarrés, Q.; Ehman, N.V.; Vallejos, M.E.; Area, M.C.; Delgado-Aguilar, M.; Mutjé, P. Lignocellulosic nanofibers from triticale straw: The influence of hemicelluloses and lignin in their production and properties. Carbohyd. Polym. 2017, 163, 20–27. [Google Scholar] [CrossRef]
- Espinosa, E.; Sánchez, R.; Otero, R.; Domínguez-Robles, J.; Rodríguez, A. A comparative study of the suitability of different cereal straws for lignocellulose nanofibers isolation. Int. J. Biol. Macromol. 2017, 103, 990–999. [Google Scholar] [CrossRef]
- Tarrés, Q.; Espinosa, E.; Domínguez-Robles, J.; Rodríguez, A.; Mutjé, P.; Delgado-Aguilar, M. The suitability of banana leaf residue as raw material for the production of high lignin content micro/nano fibers: From residue to value-added products. Ind. Crop. Prod. 2017, 99, 27–33. [Google Scholar] [CrossRef]
- Saito, T.; Shibata, I.; Isogai, A.; Suguri, N.; Sumikawa, N. Distribution of carboxylate groups introduced into cotton linters by the TEMPO-mediated oxidation. Carbohyd. Polym. 2005, 61, 414–419. [Google Scholar] [CrossRef]
- Theng, D.; Arbat, G.; Delgado-Aguilar, M.; Vilaseca, F.; Ngo, B.; Mutjé, P. All-lignocellulosic fiberboard from corn biomass and cellulose nanofibers. Ind. Crop. Prod. 2015, 76, 166–173. [Google Scholar] [CrossRef]
- Solala, I.; Iglesias, M.C.; Peresin, M.S. On the potential of lignin-containing cellulose nanofibrils (LCNFs): A review on properties and applications. Cellulose 2019. [Google Scholar] [CrossRef]
- Sang, X.K.; Qin, C.R.; Tong, Z.F.; Kong, S.; Jia, Z.; Wan, G.C.; Liu, X.L. Mechanism and kinetics studies of carboxyl group formation on the surface of cellulose fiber in a TEMPO-mediated system. Cellulose 2017, 24, 2415–2425. [Google Scholar] [CrossRef]
- Zimmermann, T.; Bordeanu, N.; Strub, E. Properties of nanofibrillated cellulose from different raw materials and its reinforcement potential. Carbohyd. Polym. 2010, 79, 1086–1093. [Google Scholar] [CrossRef]
- Puangsin, B.; Yang, Q.L.; Saito, T.; Isogai, A. Comparative characterization of TEMPO-oxidized cellulose nanofibril films prepared from non-wood resources. Int. J. Biol. Macromol. 2013, 59, 208–213. [Google Scholar] [CrossRef]
- Sharma, P.R.; Varma, A.J. Thermal stability of cellulose and their nanoparticles: Effect of incremental increases in carboxyl and aldehyde groups. Carbohyd. Polym. 2014, 114, 339–343. [Google Scholar] [CrossRef]
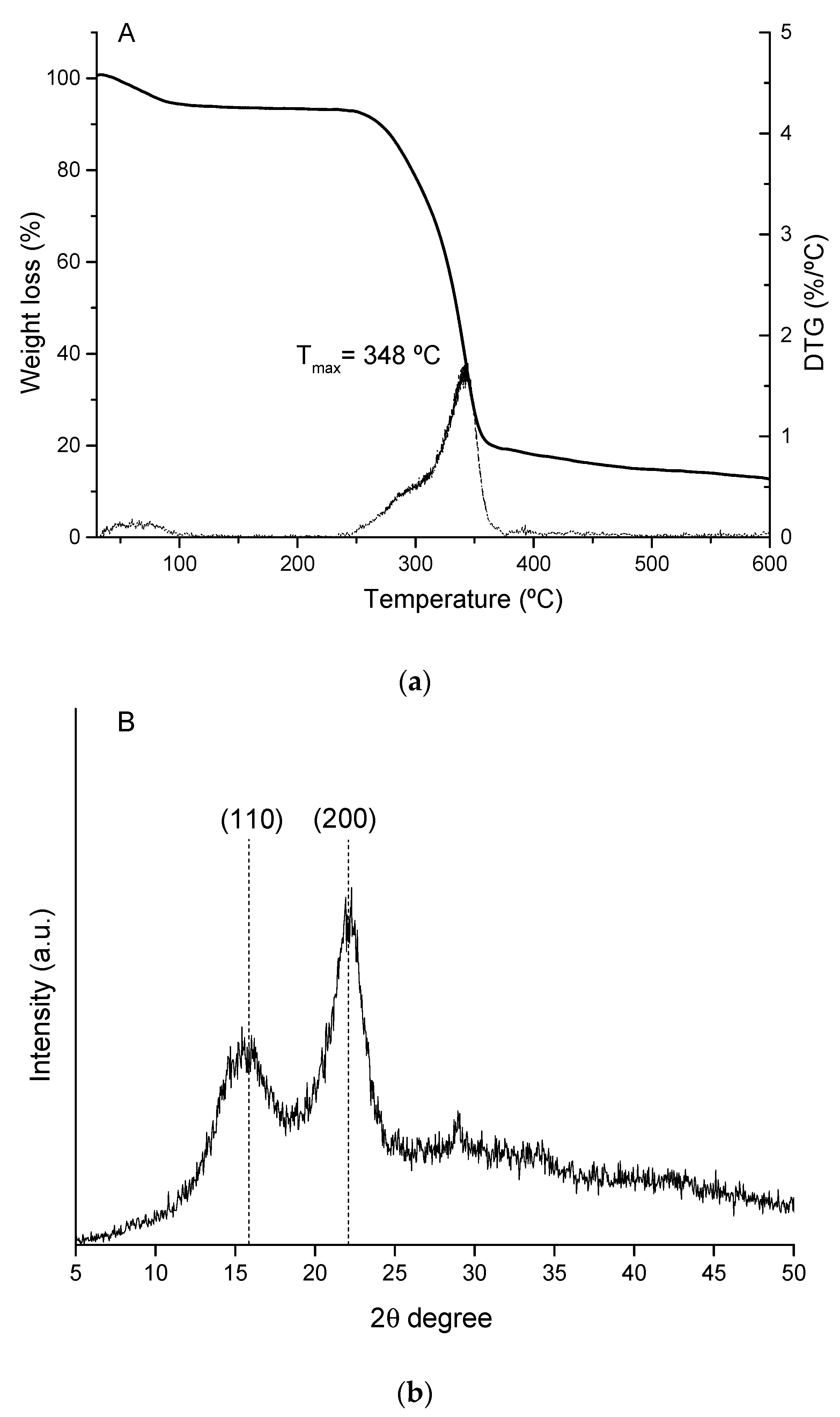
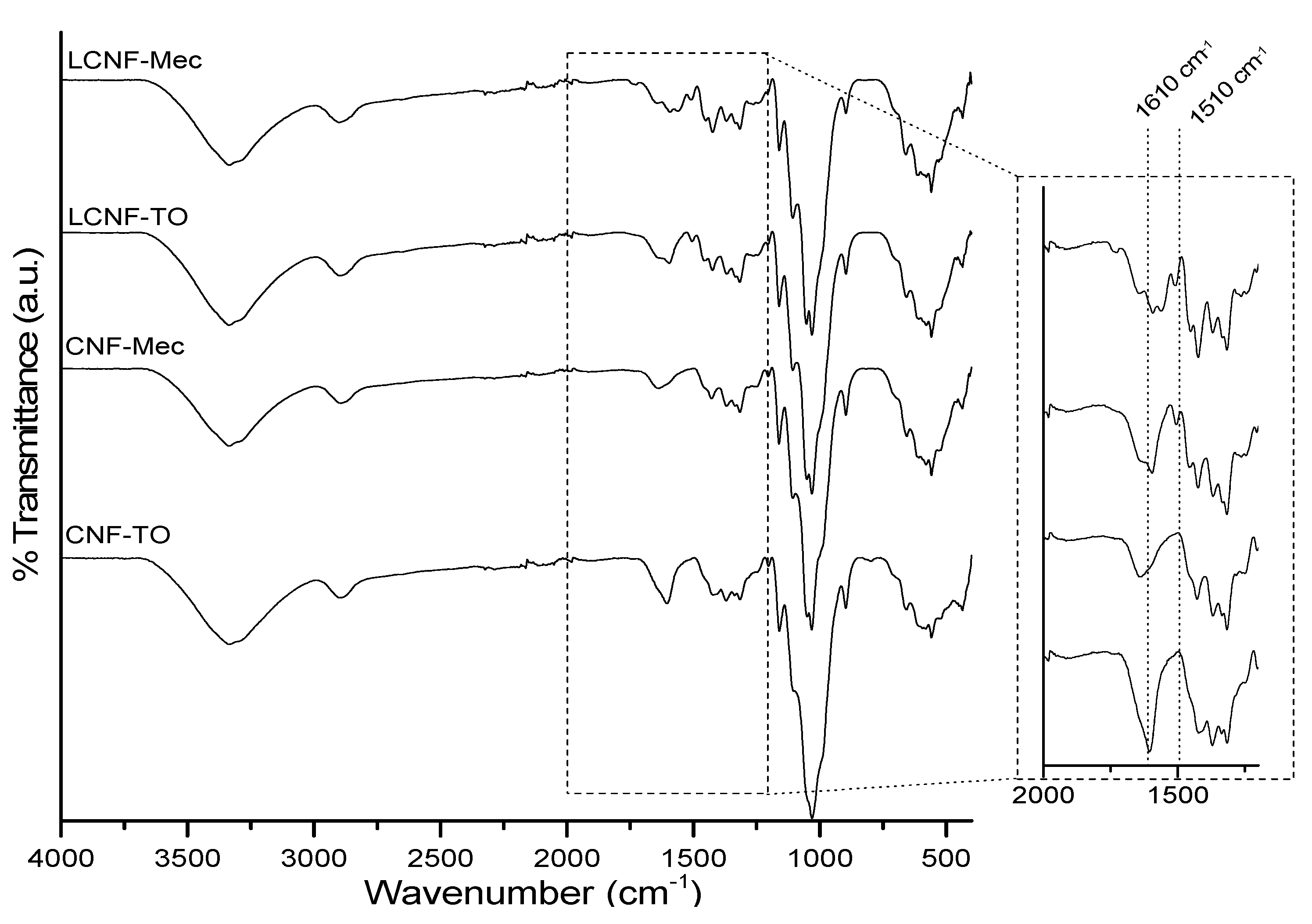
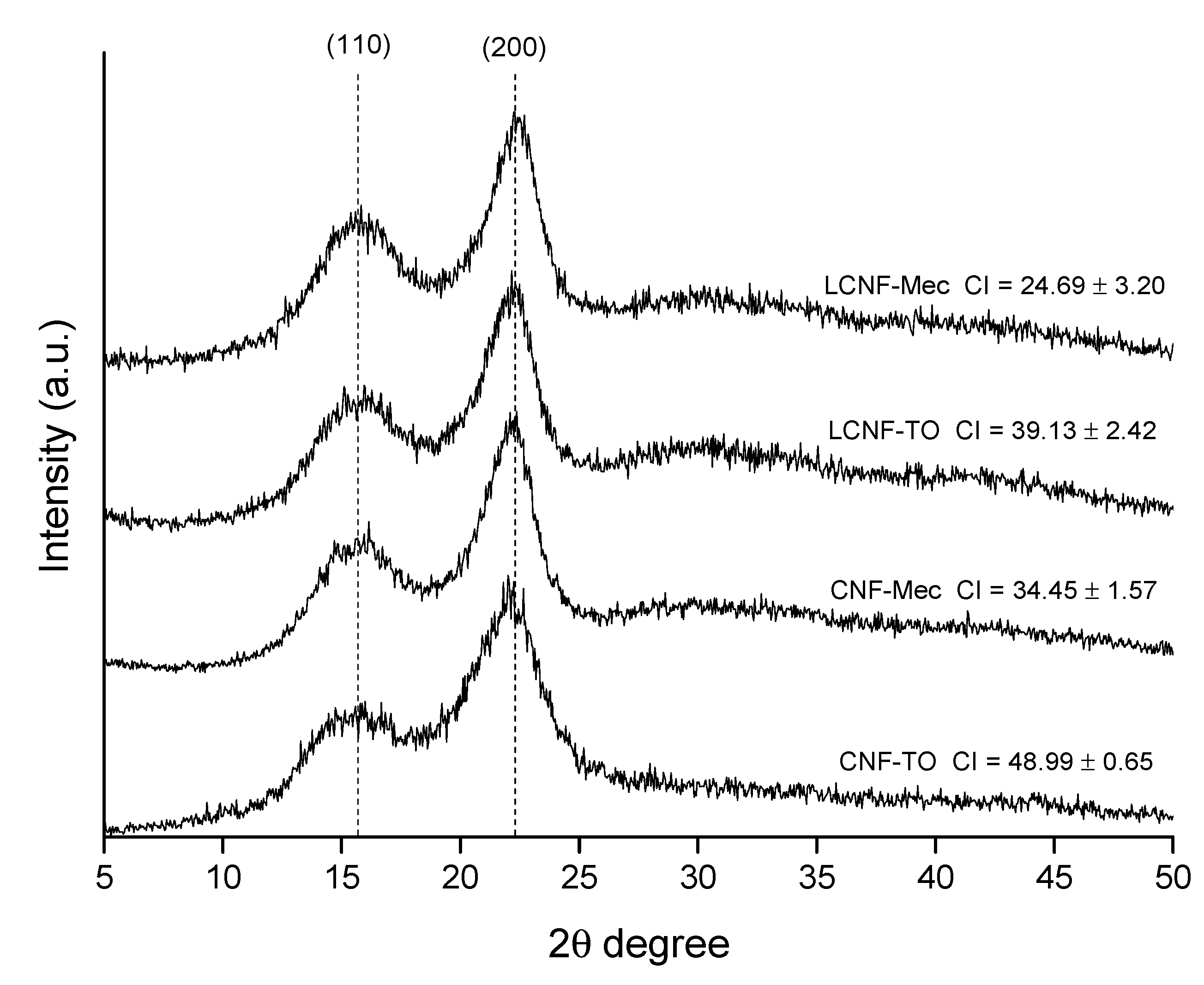
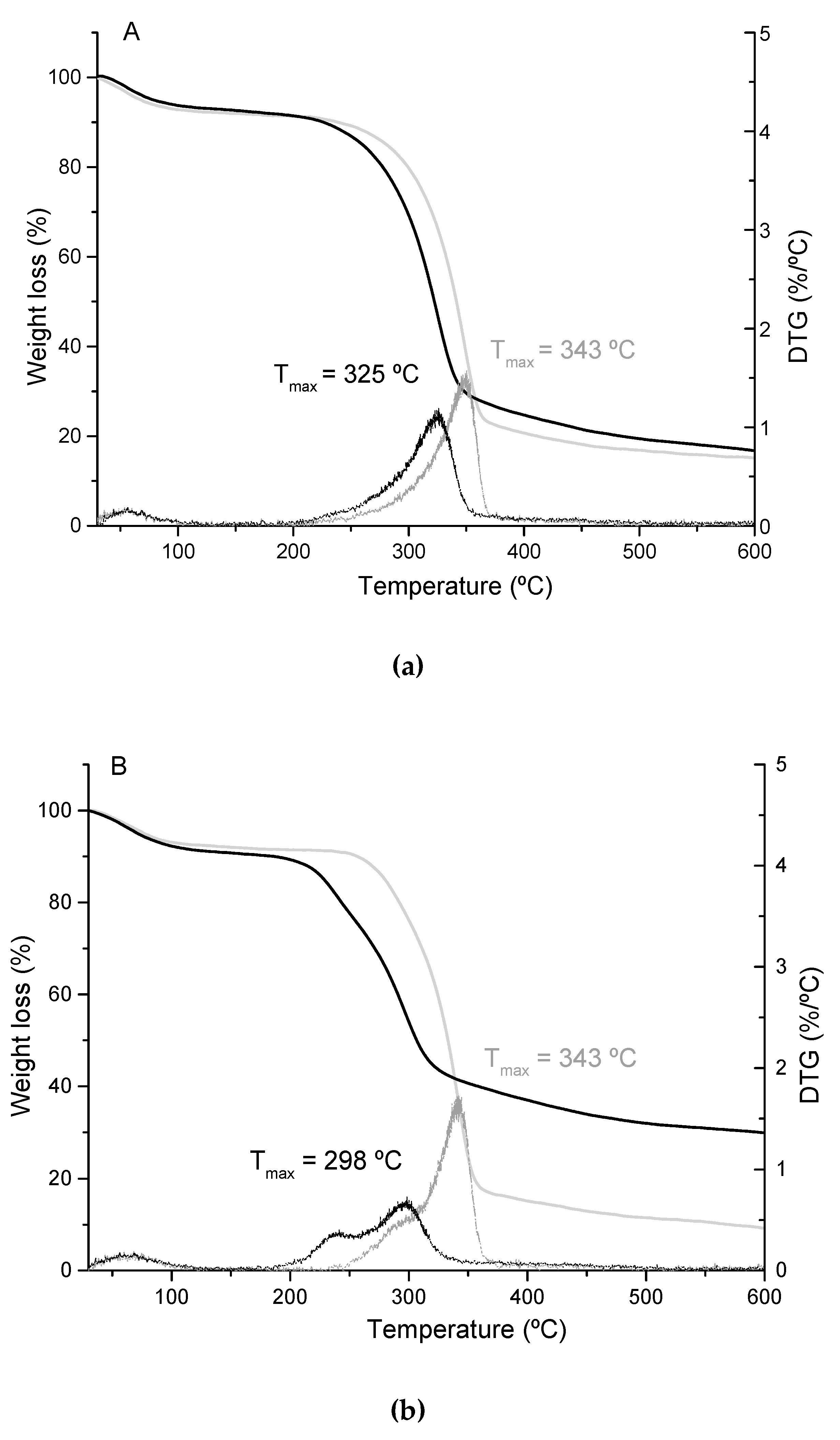
| Ext. EtOH (%) | Ext. AQ (%) | Ashes (%) | Lignin (%) | Hemicellulose (%) | α-Cellulose (%) | |
|---|---|---|---|---|---|---|
| Olive tree pruning biomass | 10.11 ± 0.74 | 6.2 ± 0.46 | 1.20 ± 0.04 | 21.80 ± 1.10 | 25.70 ± 0.47 | 41.41 ± 0.76 |
| Olive cellulosic pulp | 1.18 ± 0.02 | 0.02 ± 0.01 | 2.20 ± 0.01 | 14.60 ± 0.52 | 25.68 ± 0.08 | 59.67 ± 0.02 |
| Sample | ɳ (%) | T800 (%) | CD (µeq/g) | CC (µmols/g) | σ (m2/g) | Width (nm) | Length (nm) |
|---|---|---|---|---|---|---|---|
| LCNF-Mec | 15.33 ± 0.47 | 9.12 | 253.33 ± 18.64 | 150.72 ± 15.17 | 49.97 | 50 | 4671 |
| LCNF-TO | 17.98 ± 0.89 | 13.74 | 223.85 ± 18.62 | 152.43 ± 6.63 | 34.78 | 71 | 1478 |
| CNF-Mec | 13.34 ± 0.02 | 18.27 | 240.06 ± 18.86 | 147.83 ± 3.63 | 44.78 | 55 | 3331 |
| CNF-TO | 26.44 ± 4.15 | 50.59 | 521.27 ± 9.33 | 311.95 ± 19.02 | 101.93 | 24 | 705 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Gutiérrez, M.; Espinosa, E.; Bascón-Villegas, I.; Pérez-Rodríguez, F.; Carrasco, E.; Rodríguez, A. Production of Cellulose Nanofibers from Olive Tree Harvest—A Residue with Wide Applications. Agronomy 2020, 10, 696. https://doi.org/10.3390/agronomy10050696
Sánchez-Gutiérrez M, Espinosa E, Bascón-Villegas I, Pérez-Rodríguez F, Carrasco E, Rodríguez A. Production of Cellulose Nanofibers from Olive Tree Harvest—A Residue with Wide Applications. Agronomy. 2020; 10(5):696. https://doi.org/10.3390/agronomy10050696
Chicago/Turabian StyleSánchez-Gutiérrez, Mónica, Eduardo Espinosa, Isabel Bascón-Villegas, Fernando Pérez-Rodríguez, Elena Carrasco, and Alejandro Rodríguez. 2020. "Production of Cellulose Nanofibers from Olive Tree Harvest—A Residue with Wide Applications" Agronomy 10, no. 5: 696. https://doi.org/10.3390/agronomy10050696






