Seasonal Functional Partitioning of Carbohydrates and Proline among Plant Parts of the Sand Daffodil
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Plant Material
2.2. Soluble Sugars and Starch
2.3. Proline
2.4. Statistical Analysis
3. Results
3.1. Soluble Sugars
3.2. Starch
3.3. Proline
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- De Castro, O.; Brullo, S.; Colombo, P.; Jury, S.; De Luca, P.; Di Maio, A. Phylogenetic and biogeographical inferences for Pancratium (Amaryllidaceae), with an emphasis on the Mediterranean species based on plastid sequence data. Bot. J. Linn. Soc. 2012, 170, 12–28. [Google Scholar] [CrossRef][Green Version]
- Pouris, J.; Rhizopoulou, S. On Pancratium maritimum (sea daffodil, sea lily, sand lily). Hortic. Int. J. 2018, 2, 1. [Google Scholar]
- Rhizopoulou, S.; Pantazi, H. Constraints on floral water status of successively blossoming Mediterranean plants under natural conditions. Acta Bot. Gallica 2015, 162, 97–102. [Google Scholar] [CrossRef]
- Curr, R.H.F.; Koh, A.; Edwards, E.; Williams, A.T.; Davies, P. Assessing anthropogenic impact on Mediterranean sand dunes from aerial digital photography. J. Coast. Conserv. 2000, 6, 15–22. [Google Scholar] [CrossRef]
- Zahreddine, H.; Clubbe, C.; Baalbaki, R.; Ghalayini, A.; Talhouk, S. Status of native species in threatened Mediterranean habitats: The case of Pancratium maritimum L. (sea daffodil) in Lebanon. Boil. Conserv. 2004, 120, 11–18. [Google Scholar] [CrossRef]
- Grassi, F.; Cazzaniga, E.; Minuto, L.; Peccenini, S.; Barberis, G.; Basso, B. Evaluation of biodiversity and conservation strategies in Pancratium maritimum L. for the Northern Tyrrhenian Sea. Biodivers. Conserv. 2005, 14, 2159–2169. [Google Scholar] [CrossRef]
- De Castro, O.; Di Maio, A.; Di Febbraro, M.; Imparato, G.; Innangi, M.; Véla, E.; Menale, B. A Multi-Faceted Approach to Analyse the Effects of Environmental Variables on Geographic Range and Genetic Structure of a Perennial Psammophilous Geophyte: The Case of the Sea Daffodil Pancratium maritimum L. in the Mediterranean Basin. PLoS ONE 2016, 11, e0164816. [Google Scholar] [CrossRef]
- Nafea, E.M.A. Impacts of anthropogenic activities on the habitats and flora at the coastal Nile Delta Mediterranean Region, Egypt. J. Mediter. Ecol. 2019, 17, 23–28. [Google Scholar]
- Farris, E.; Pisanu, S.; Ceccherelli, G.; Filigheddu, R. Human trampling effects on Mediterranean coastal dune plants. Plant Biosyst. Int. J. Deal. All Asp. Plant Boil. 2013, 147, 1043–1051. [Google Scholar] [CrossRef]
- Médail, F. The specific vulnerability of plant biodiversity and vegetation on Mediterranean islands in the face of global change. Reg. Environ. Chang. 2017, 17, 1775–1790. [Google Scholar] [CrossRef]
- Paradiso, R.; Buonomo, R.; De Pascale, S.; Cardarelli, M. Evaluation of spontaneous species for the innovation in floriculture: Pancratium maritimum L. as ornamental plant. Acta Hort. 2009, 881, 563–566. [Google Scholar] [CrossRef]
- De Pascale, S.; Romano, D. Potential use of wild plants in floriculture. Acta Hortic. 2019, 87–98. [Google Scholar] [CrossRef]
- Shmida, A.; Dafni, A. Blooming strategies, flower size and advertising in the lily-group geophytes in Israel. Herbertia 1989, 45, 111–122. [Google Scholar]
- Medrano, M.; Guitián, P.; Guitián, J. Breeding system and temporal variation in fecundity of Pancratium maritimum L. (Amaryllidaceae). Flora - Morphol. Distrib. Funct. Ecol. Plants 1999, 194, 13–19. [Google Scholar] [CrossRef]
- Nikopoulos, D.; Nikopoulou, D.; Papadopoulou, K.; Alexopoulos, A. Pancratium maritimum ecosystems in Greece. In Proceedings of the Naxos International Conference on Sustainable Management and Development of Mountainous and Island Areas, Heraklion, Greece, 29 September–1 October 2006. [Google Scholar]
- Hesp, P.A. Ecological processes and plant adaptations on coastal dunes. J. Arid Environ. 1991, 21, 165–191. [Google Scholar] [CrossRef]
- Sperandii, M.G.; Bazzichetto, M.; Acosta, A.T.R.; Barták, V.; Malavasi, M. Multiple drivers of plant diversity on coastal dunes: A Mediterranean experience. Sci. Total Environ. 2019, 652, 1435–1444. [Google Scholar] [CrossRef]
- Baumann, H. Greek Wildflowers and Plant Lore in Ancient Greece; The Herbert Press: London, UK, 1996; pp. 170–184. [Google Scholar]
- De Cleene, M.; Lejeune, M.C. Compendium of Symbolic and Ritual Plans in Europe; Man and Culture Publishers: Ghent, Belgium, 2003; p. 325. [Google Scholar]
- Tucker, A.O. Identification of the rose, sage, iris, and lily in the “Blue Bird Fresco” from Knossos, Crete (ca. 1450 BC). Econ. Bot. 2004, 58, 733–736. [Google Scholar] [CrossRef]
- Mavromati, A. Landscape and wood-fuel in Akrotiri (Thera, Greece) during the Bronze Age. Quatern. Int. 2017, 458, 44–55. [Google Scholar] [CrossRef]
- Danylova, T. Between the land, sea, and sky: Some words on the art of the Minoan Civilization of Bronze Age Crete. INDECS 2018, 13, 107–116. [Google Scholar]
- Negbi, M. Theophrastus on geophytes. Bot. J. Linn. Soc. 1989, 100, 15–43. [Google Scholar] [CrossRef]
- Janick, J.; Stolarczyk, J. Ancient Greek illustrated Dioscoridean herbals: Origins and impact of the Juliana Anicia Codex and the Codex Neopolitanus. Not. Bot. Horti. Agrobo. 2012, 40, 9–17. [Google Scholar] [CrossRef][Green Version]
- Harris, S.A. Sibthorp, Bauer and the Flora Graeca. Oxford Plant Syst. 2008, 15, 7–8. [Google Scholar]
- Radcliffe Science Library Digital Flora Graeca, published version (vol. 4, tabula 309), drawings (vol. 5, folio 74). Available online: http://www.bodley.ox.ac.uk/users/millsr/isbes/FG/FGE2/ (accessed on 18 February 2020).
- Harris, S. The Magnificent Flora Graeca; Bodleian Library, University of Oxford: Oxford, UK, 2007; p. 189. [Google Scholar]
- Lack, H.W. Oxford, Greek revival and John Sibthorp. Oxford Plant Syst. 2002, 9, 8–10. [Google Scholar]
- Gledhill, D. The Names of Plants; Cambridge Univesity Press: Cambridge, UK, 2008; p. 289. [Google Scholar]
- De Felice, B.; Manfellotto, F.; D’Alessandro, R.; De Castro, O.; Di Maio, A.; Trifuoggi, M. Comparative transcriptional analysis reveals differential gene expression between sand daffodil tissues. Genetica 2013, 141, 443–452. [Google Scholar] [CrossRef][Green Version]
- Giovino, A.; Domina, G.; Bazan, G.; Campisi, P.; Scibetta, S. Taxonomy and conservation of Pancratium maritimum (Amaryllidaceae) and relatives in the Central Mediterranean. Acta Bot. Gallica 2015, 162, 289–299. [Google Scholar] [CrossRef]
- Konyar, S.T. Ultrastructural aspects of pollen ontogeny in an endangered plant species, Pancratium maritimum L. (Amaryllidaceae). Protoplasma 2017, 254, 881–900. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Frahm, A.W. Alkaloids of the flowers of Pancratium maritimum. Planta Med. 1998, 64, 669–670. [Google Scholar] [CrossRef]
- Asolkar, R.N.; Kamat, V.P.; Kirtany, J.K. Synthesis of maritimin, a chromone from Pancratium maritimum. J. Chem. Res. 2001, 12, 549–550. [Google Scholar] [CrossRef]
- Georgiev, V.; Ivanov, I.; Berkov, S.; Pavlov, A. Alkaloids biosynthesis by Pancratium maritimum L. shoots in liquid culture. Acta Physiol. Plant. 2011, 33, 927–933. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Shaala, L.A.; Youssef, D.T.A.; El-Sayed, K.A. New alkaloids from Pancratium maritimum. Planta Med. 2013, 79, 1480–1484. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kaya, G.I.; Somer, N.U. Chemical composition and enzyme inhibitory activities of Turkish Pancratium maritimum bulbs. Nat. Prod. Commun. 2019, 14, 1–4. [Google Scholar] [CrossRef]
- Moeini, A.; Cimmino, A.; Masi, M.; Evidente, A.; Van Reenen, A. The incorporation and release of ungeremine, an antifungal Amaryllidaceae alkaloid, in poly (lactic acid)/poly (ethylene glycol) nanofibers. J. Appl. Polym. Sci. 2020, 49098. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 4, 1627–1629. [Google Scholar] [CrossRef]
- Meletiou-Christou, M.S.; Rhizopoulou, S. Leaf functional traits of four evergreen species growing in Mediterranean environmental conditions. Acta Physiol. Plant. 2017, 39, 34. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Ain-Lhout, F.; Zunzunegui, M.; Barradas, M.D.; Tirado, R.; Clavijo, A.; Novo, F.G. Comparison of proline accumulation in two Mediterranean shrubs subjected to natural and experimental water deficit. Plant Soil 2001, 230, 175–183. [Google Scholar] [CrossRef]
- Dafni, A.; Cohen, D.; Noy-Mier, I. Life-cycle variation in geophytes. Ann. Missouri Bot. Gard. 1981, 68, 652–660. [Google Scholar] [CrossRef]
- Lundgren, M.R.; Des Marais, D.L. Life history variation as a model for understanding trade-offs in plant–environment interactions. Curr. Biol. 2020, 30, R180–R189. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Ettinger, A.K. Back to the future for plant phenology research. New Phytol. 2014, 203, 1021–1024. [Google Scholar] [CrossRef]
- Gratani, L.; Varone, L.; Crescente, M.F. Photosynthetic activity and water use efficiency of dune species: The influence of air temperature on functioning. Photosynthetica 2009, 47, 575–585. [Google Scholar] [CrossRef]
- Mooney, H.A.; Winner, W.E.; Pett, E.J. Response of Plants to Multiple Stresses, 2nd ed.; Academic Press: Cambridge, MA, USA, 1991; pp. 161–188. [Google Scholar]
- Jensen, K.H.; Savage, J.A.; Holbrook, N.M. Optimal concentration for sugar transport in plants. J. R. Soc. Interface 2013. [Google Scholar] [CrossRef]
- Villarino, G.H.; Mattson, N.S. Assessing tolerance to sodium chloride salinity in fourteen floriculture species. HortTechnology 2011, 21, 539–545. [Google Scholar] [CrossRef]
- Medrano, M.N.; Guitián, P.; Guitián, J. Patterns of fruit and seed set within inflorescences of Pancratium maritimum (Amaryllidaceae): Non-uniform pollination, resource limitation, or architectural effects? Am. J. Bot. 2000, 87, 493–501. [Google Scholar] [CrossRef]
- Sánchez, F.J.; Manzanares, M.; de Andres, E.F.; Tenorio, J.L.; Ayerbe, L. Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field Crops Res. 1998, 59, 225–235. [Google Scholar] [CrossRef]
- Rhizopoulou, S.; Diamantoglou, S.T.; Passiakou, L. Free proline accumulation in leaves, stems and roots of four Mediterranean native phrygana species. Acta Oecol. 1990, 11, 585–593. [Google Scholar]
- Lansac, A.R.; Zaballos, J.P.; Martin, A. Seasonal water potential changes and proline accumulation in Mediterranean shrubland species. Vegetatio 1994, 113, 141–154. [Google Scholar]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Xie, F. Effect of drought stress at reproductive stages on growth and nitrogen metabolism in soybean. Agronomy 2020, 10, 302. [Google Scholar] [CrossRef]
- Khedr, A.H.A.; Abbas, M.A.; Wahid, A.A.A.; Quick, W.P.; Abogadallah, G.M. Proline induces the expression of salt-stress-responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. J. Exp. Bot. 2003, 54, 2553–2562. [Google Scholar] [CrossRef]
- Orthen, B. Sprouting of the fructan-and starch-storing geophyte Lachenalia minima: Effects on carbohydrate and water content within the bulbs. Physiol. Plantarum 2001, 113, 308–314. [Google Scholar] [CrossRef]
- Darras, A.I. Novel Elicitors Induce Defense Responses in Cut Flowers; INTECH Open Access Publisher: London, UK, 2012; pp. 85–115. [Google Scholar]
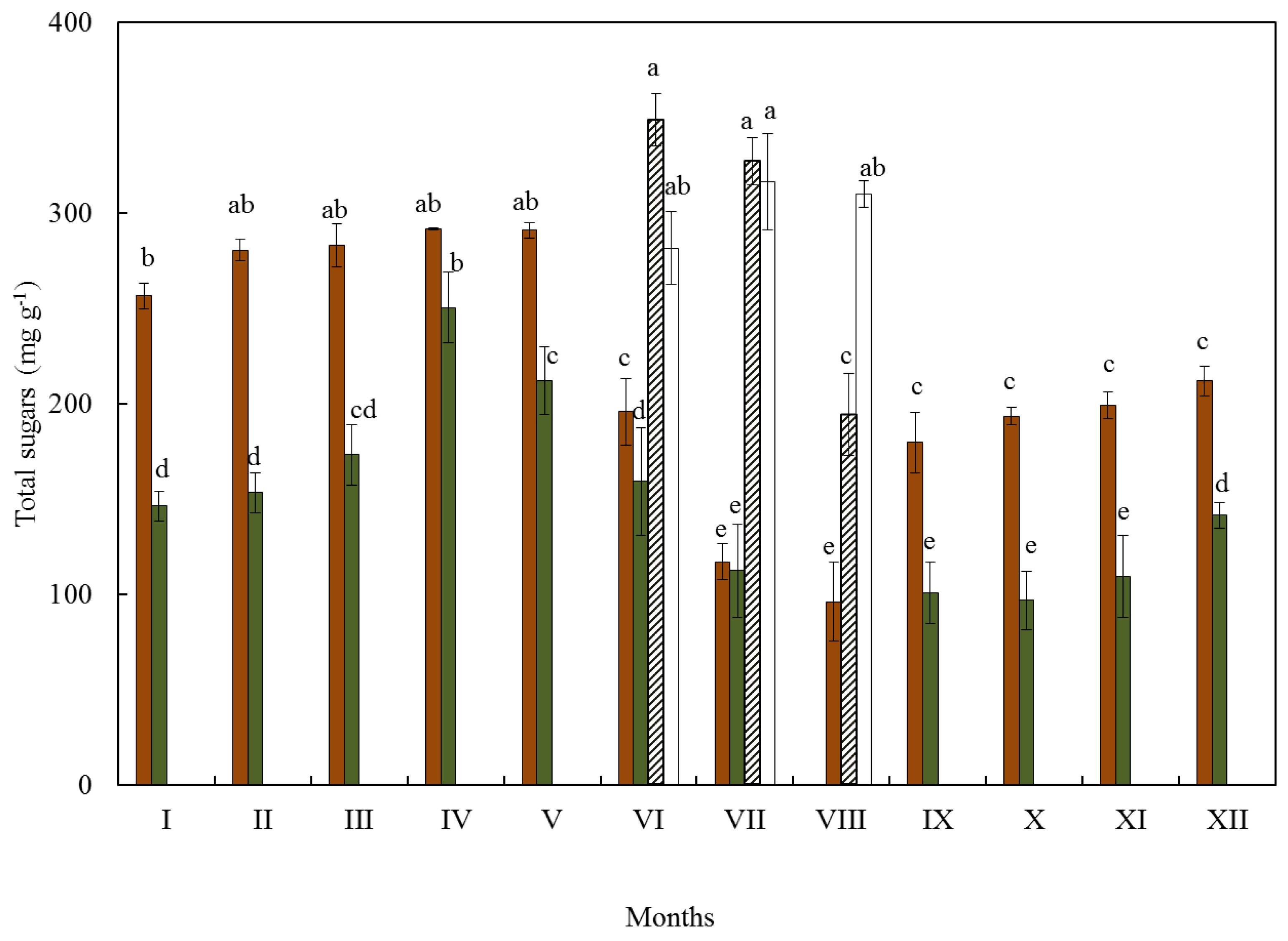

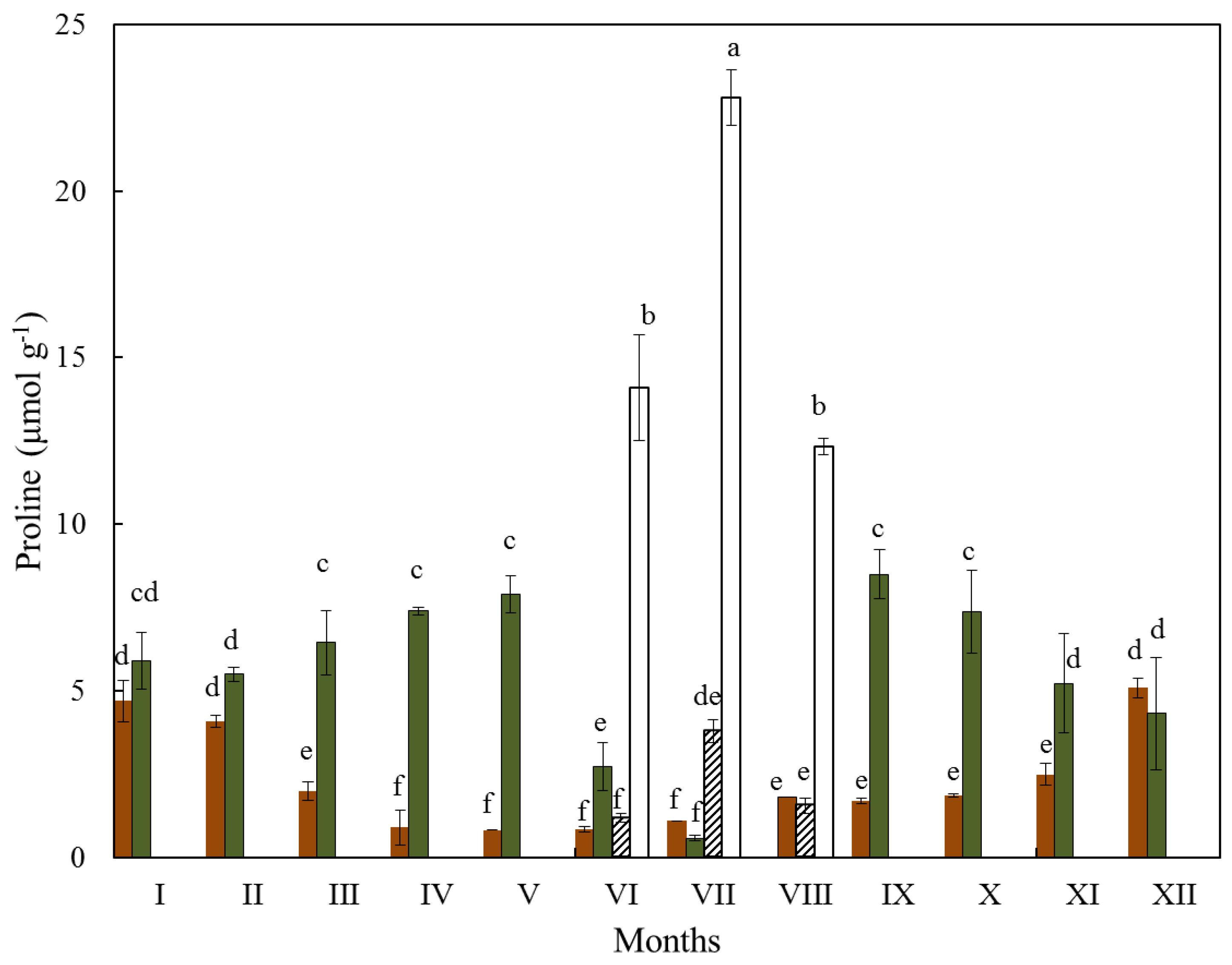
| Months | Ι | ΙΙ | ΙΙΙ | IV | V | VI | VII | VIII | IX | X | XI | XII |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | 5.4 | 8.6 | 10.5 | 14.8 | 18.3 | 24.3 | 27.0 | 25.5 | 21.8 | 17.0 | 13.2 | 7.3 |
| R (mm) | 155.6 | 98.0 | 95.6 | 30.6 | 22.2 | 10.2 | 5.6 | 3.0 | 25.2 | 56.0 | 137.6 | 139.8 |
| Source | Parameter | MS | F | Source | Parameter | MS | F |
|---|---|---|---|---|---|---|---|
| Tissues | Sugars | 28,131.71 | 8.18 | Substances | Leaves | 55,575.67 | *56.14 |
| Starch | 128,342.21 | 10.93 | Bulbs | 251,016.60 | 25.35 | ||
| Proline | 169.35 | 28.56 | Petals | 79,213.60 | 620.21 | ||
| Scapes | 64,789.33 | 22.54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pouris, J.; Meletiou-Christou, M.-S.; Chimona, C.; Rhizopoulou, S. Seasonal Functional Partitioning of Carbohydrates and Proline among Plant Parts of the Sand Daffodil. Agronomy 2020, 10, 539. https://doi.org/10.3390/agronomy10040539
Pouris J, Meletiou-Christou M-S, Chimona C, Rhizopoulou S. Seasonal Functional Partitioning of Carbohydrates and Proline among Plant Parts of the Sand Daffodil. Agronomy. 2020; 10(4):539. https://doi.org/10.3390/agronomy10040539
Chicago/Turabian StylePouris, John, Maria-Sonia Meletiou-Christou, Chrysanthi Chimona, and Sophia Rhizopoulou. 2020. "Seasonal Functional Partitioning of Carbohydrates and Proline among Plant Parts of the Sand Daffodil" Agronomy 10, no. 4: 539. https://doi.org/10.3390/agronomy10040539
APA StylePouris, J., Meletiou-Christou, M.-S., Chimona, C., & Rhizopoulou, S. (2020). Seasonal Functional Partitioning of Carbohydrates and Proline among Plant Parts of the Sand Daffodil. Agronomy, 10(4), 539. https://doi.org/10.3390/agronomy10040539






