Effect of Bead Composition, PVS Type, and Recovery Medium in Cryopreservation of Bleeding Heart ‘Valentine’—Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and General In-Vitro Culture Conditions
2.2. Preculture
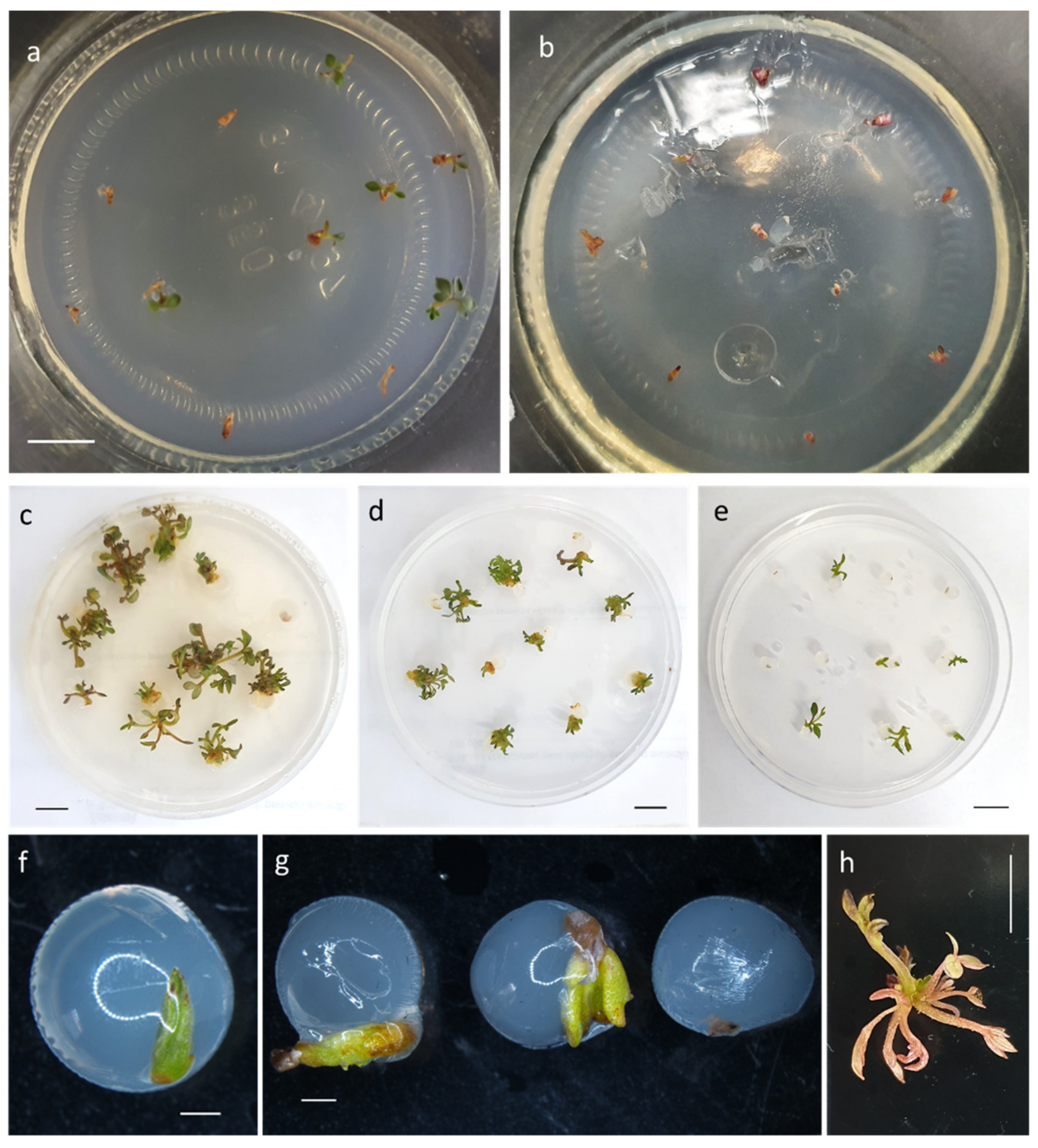
2.3. Experiment I: Comparison of Bead Matrix and Culture Medium Composition on the Recovery of Non-LN-Stored Shoot Tips of Bleeding Heart
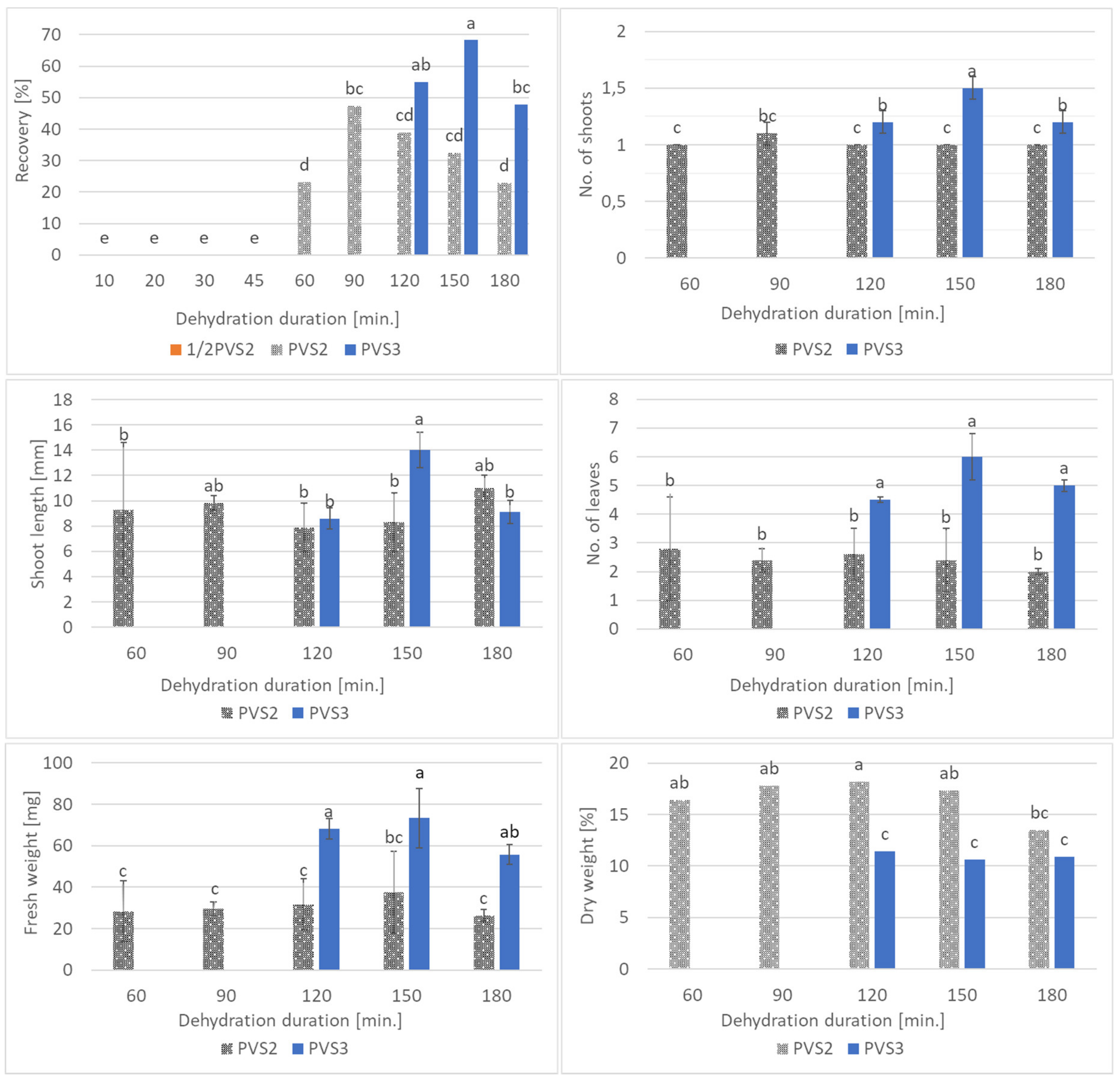
2.4. Experiment II: Comparison of PVS2 and PVS3 Effectiveness in Cryopreservation of Bleeding Heart
2.5. Survival and Biometrical Analyses
2.6. Statistical Analysis
3. Results
3.1. Experiment I: Comparison of Bead Matrix and Culture Medium Composition on the Recovery of Non-LN-Stored Shoot Tips of Bleeding Heart
3.2. Experiment II: Comparison of PVS2 and PVS3 Effectiveness in Cryopreservation of Bleeding Heart
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Roberts, C.M.; Serek, M.; Andersen, A.S. Supplemental irradiance and STS improve the display life of Dicentra species forced as flowering potted plants. Sci. Hortic. 1995, 62, 121–128. [Google Scholar] [CrossRef]
- Hodges, L. Bleeding heart: A review for growers. Hort. Technol. 2012, 22, 517–522. [Google Scholar] [CrossRef]
- Petruczynik, A.; Plech, T.; Tuzimski, T.; Misiurek, J.; Kaproń, B.; Misiurek, D.; Szultka-Młyńska, M.; Buszewski, B.; Waksmundzka-Hajnos, M. Determination of selected isoquinoline alkaloids from Mahonia aquifolia; Meconopsis cambrica; Corydalis lutea; Dicentra spectabilis; Fumaria officinalis; Macleaya cordata extracts by HPLC-DAD and comparison of their cytotoxic activity. Toxins 2019, 11, 575. [Google Scholar] [CrossRef]
- Lee, K.P.; Lee, D.W. Somatic embryogenesis and plant regeneration from seeds of wild Dicentra spectabilis (L.) Lem. Plant Cell Rep. 2003, 22, 105–109. [Google Scholar] [CrossRef]
- Lee, K.-S.; Sim, O.-K.; Shin, J.-S.; Choi, Y.-E.; Kim, E.-Y. Mass propagation of Dicentra spectabilis L. Lemaire through in vitro suspension culture. J. Plant Biotechnol. 2004, 31, 121–126. [Google Scholar] [CrossRef]
- Kulus, D. Influence of growth regulators on the development, quality, and physiological state of in vitro-propagated Lamprocapnos spectabilis (L.) Fukuhara. In Vitro Cell. Develop. Biol. Plant 2020. [Google Scholar] [CrossRef]
- FAO. Climate Change and Food Security: Risks and Responses; FAO: Rome, Italy, 2016; p. 110. [Google Scholar]
- Deno, N.C. Seed Germination Theory and Practice, 2nd ed.; Pennsylvania State College: State College, PA, USA, 1993; p. 53. [Google Scholar]
- Hammond, H.S.D.; Viehmannova, I.; Zamecnik, J.; Panis, B.; Cepkova, P.H. Efficient slow-growth conservation and assessment of clonal fidelity of Ullucus tuberosus Caldas microshoots. Plant Cell Tiss. Organ Cult. 2019, 138, 559–570. [Google Scholar] [CrossRef]
- Muñoz-Miranda, L.A.; Rodríguez-Sahagún, A.; Acevedo Hernández, G.J.; Cruz-Martínez, V.O.; Torres-Morán, M.I.; Lépiz-Ildefonso, R.; Aarland, R.C.; Castellanos-Hernández, O.A. Evaluation of somaclonal and ethyl methane sulfonate-induced genetic variation of Mexican oregano (Lippia graveolens H.B.K.). Agronomy 2019, 9, 166. [Google Scholar] [CrossRef]
- Kulus, D. Cryopreservation of bleeding heart (Lamprocapnos spectabilis (L.) Fukuhara) shoot tips using encapsulation-dehydration. CryoLetters 2020, 41, 75–85. [Google Scholar]
- Kim, H.H.; Lee, Y.G.; Shin, D.J.; Ko, H.C.; Gwag, J.G.; Cho, E.G.; Engelmann, F. Development of alternative plant vitrification solutions in droplet-vitrification procedures. CryoLetters 2009, 30, 320–334. [Google Scholar] [CrossRef]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Aronen, T.S.; Krajnakova, J.; Haggman, H.; Ryynänen, L. Genetic fidelity of cryopreserved embryonic cultures of open-pollinated Abies cephalonica. Plant Sci. 1999, 142, 163–172. [Google Scholar] [CrossRef]
- Hosoki, T. In vitro storage of Chrysanthemum morifolium at room temperature. Plant Tissue Cult. Lett. 1989, 6, 86–87. [Google Scholar] [CrossRef]
- Nishizawa, S.; Sakai, A.; Amano, Y.; Matsuzawa, T. Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci. 1993, 91, 67–73. [Google Scholar] [CrossRef]
- Kulus, D.; Zalewska, M. Cryopreservation as a tool used in long-term storage of ornamental species—A review. Sci. Hortic. 2014, 168, 88–107. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kulus, D.; Zalewska, M. In vitro plant recovery from alginate encapsulated Chrysanthemum × grandiflorum /Ramat./Kitam. shoot tips. Prop. Ornam. Plants 2014, 14, 3–12. [Google Scholar]
- Dobránszki, J.; Mendler-Drienyovszk, N. Cytokinin-induced changes in the chlorophyll content and fluorescence of in vitro apple leaves. J. Plant Physiol. 2014, 171, 1472–1478. [Google Scholar] [CrossRef]
- Ivanova, M.; Van Staden, J. Influence of gelling agent and cytokinins on the control of hyperhydricity in Aloe polyphylla. Plant Cell Tiss. Organ Cult. 2011, 104, 13–21. [Google Scholar] [CrossRef]
- Kulus, D. Application of synthetic seeds in propagation, storage and cryopreservation of Asteraceae plant species. In Synthetic Seeds Germplasm Regeneration, Preservation and Prospects; Faisal, A., Alatar, A., Eds.; Springer: Berlin, Germany, 2019; pp. 155–179. [Google Scholar]
- Lineberger, R.D. Shoot proliferation, rooting and transplant survival of tissue-culture ‘Hally Jolivette’ cherry. HortScience 1983, 18, 182–185. [Google Scholar]
- Kulus, D.; Abratowska, A. Cryoconservation of Ajania pacifica (Nakai) Bremer et Humphries via encapsulation-dehydration technique. CryoLetters 2017, 38, 387–398. [Google Scholar] [PubMed]
- Popova, E.; Bukhov, N.; Popov, A.; Kim, H.-H. Cryopreservation of protocorm-like bodies of the hybrid orchid Bratonia (Miltonia flavescens × Brassia longissimi). CryoLetters 2010, 31, 426–437. [Google Scholar] [PubMed]
- Jitsupakul, N.; Thammasiri, K.; Ishikawa, K. Cryopreservation of Vanda coerulea protocorm-like bodies by droplet-vitrification. Acta Hort. 2011, 908, 207–214. [Google Scholar] [CrossRef]
- Kulus, D.; Serocka, M.; Mikuła, A. Effect of various preculture and osmotic dehydration conditions on cryopreservation efficiency and morphogenetic response of chrysanthemum shoot tips. Acta Sci. Pol. Hort. Cult. 2018, 17, 139–147. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Z.; Zámečník, J.; Bilavčík, A.; Blystad, D.-R.; Haugslien, S.; Wang, Q.-C. Droplet-vitrification for shoot tip cryopreservation of shallot (Allium cepa var. aggregatum): Effects of PVS3 and PVS2 on shoot regrowth. Plant Cell Tiss. Organ Cult. 2020, 140, 185–195. [Google Scholar] [CrossRef]
- Vianna, M.G.; Ferreira, A.L.; Garcia, R.O.; Falcão, E.; Pacheco, G.; Mansur, E. Comparison of vitrification-based techniques in the efficacy of cryopreservation of Passiflora suberosa L. and P. foetida L. shoot tips. Cryobiology 2012, 65, 346. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, E335. [Google Scholar] [CrossRef]
- Mikuła, A.; Tykarska, T.; Kuraś, M. Ultrastructure of Gentiana tibetica proembryogenic cells before and after cooling treatments. CryoLetters 2005, 26, 367–378. [Google Scholar]
- Pawłowska, B.; Szewczyk-Taranek, B. Efficient cryopreservation by droplet vitrification of pentaploid roses and the phenotype of regenerated plants. Acta Soc. Bot. Pol. 2015, 84, 439–442. [Google Scholar] [CrossRef]
| Bead Composition | Recovery Medium Composition | ||
|---|---|---|---|
| BA | KIN | MS0 | |
| Recovery [%] | |||
| alginate + MS | 75.8 a | 81.3 a | 52.4 bc |
| alginate + H2O | 57.2 b | 58.8 b | 43.6 c |
| Number of shoots | |||
| alginate + MS | 1.8 ± 0.2 ab | 2.0 ± 0.2 a | 1.0 ± 0.0 c |
| alginate + H2O | 1.5 ± 0.1 b | 1.9 ± 0.1 a | 1.0 ± 0.0 c |
| Shoot length [mm] | |||
| alginate + MS | 14.0 ± 1.3 a | 9.0 ± 0.9 c | 11.9 ± 0.3 ab |
| alginate + H2O | 10.2 ± 0.8 bc | 8.6 ± 0.6 c | 11.8 ± 0.5 ab |
| Number of leaves | |||
| alginate + MS | 6.9 ± 0.4 a | 5.8 ± 0.3 ab | 3.7 ± 0.1 c |
| alginate + H2O | 5.1 ± 1.1 a–c | 3.8 ± 0.1 c | 4.0 ± 0.4 bc |
| Fresh weight [mg] | |||
| alginate + MS | 102.8 ± 12.2 a | 34.1 ± 2.2 bc | 10.2 ± 1.2 d |
| alginate + H2O | 83.6 ± 8.7 a | 46.1 ± 2.2 b | 16.8 ± 0.9 cd |
| Share of DW in FW [%] | |||
| alginate + MS | 9.4 b | 12.5 a | 12.6 a |
| alginate + H2O | 11.6 ab | 11.6 ab | 8.6 b |
| Rooting [%] | |||
| alginate + MS | 33.6 a | 0.0 b | 0.0 b |
| alginate + H2O | 20.0 ab | 0.0 b | 0.0 b 1 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulus, D. Effect of Bead Composition, PVS Type, and Recovery Medium in Cryopreservation of Bleeding Heart ‘Valentine’—Preliminary Study. Agronomy 2020, 10, 891. https://doi.org/10.3390/agronomy10060891
Kulus D. Effect of Bead Composition, PVS Type, and Recovery Medium in Cryopreservation of Bleeding Heart ‘Valentine’—Preliminary Study. Agronomy. 2020; 10(6):891. https://doi.org/10.3390/agronomy10060891
Chicago/Turabian StyleKulus, Dariusz. 2020. "Effect of Bead Composition, PVS Type, and Recovery Medium in Cryopreservation of Bleeding Heart ‘Valentine’—Preliminary Study" Agronomy 10, no. 6: 891. https://doi.org/10.3390/agronomy10060891
APA StyleKulus, D. (2020). Effect of Bead Composition, PVS Type, and Recovery Medium in Cryopreservation of Bleeding Heart ‘Valentine’—Preliminary Study. Agronomy, 10(6), 891. https://doi.org/10.3390/agronomy10060891





