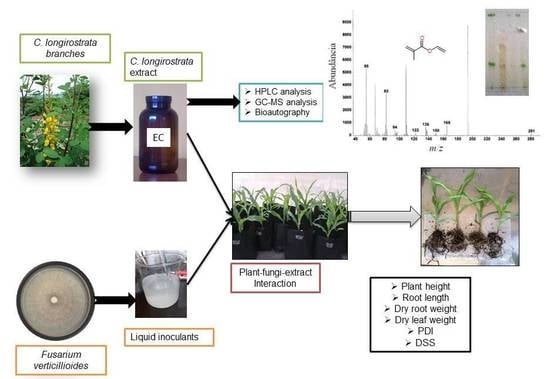
Potential Application of Crotalaria longirostrata Branch Extract to Reduce the Severity of Disease Caused by Fusarium
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Preparation of Extract
2.4. Fungal Strains
2.5. In Vivo Greenhouse Experiment
2.6. High Performance Liquid Chromatography (HPLC) Analysis
2.7. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.8. Bioautography
2.9. Experimental Design and Statistical Analysis
3. Results
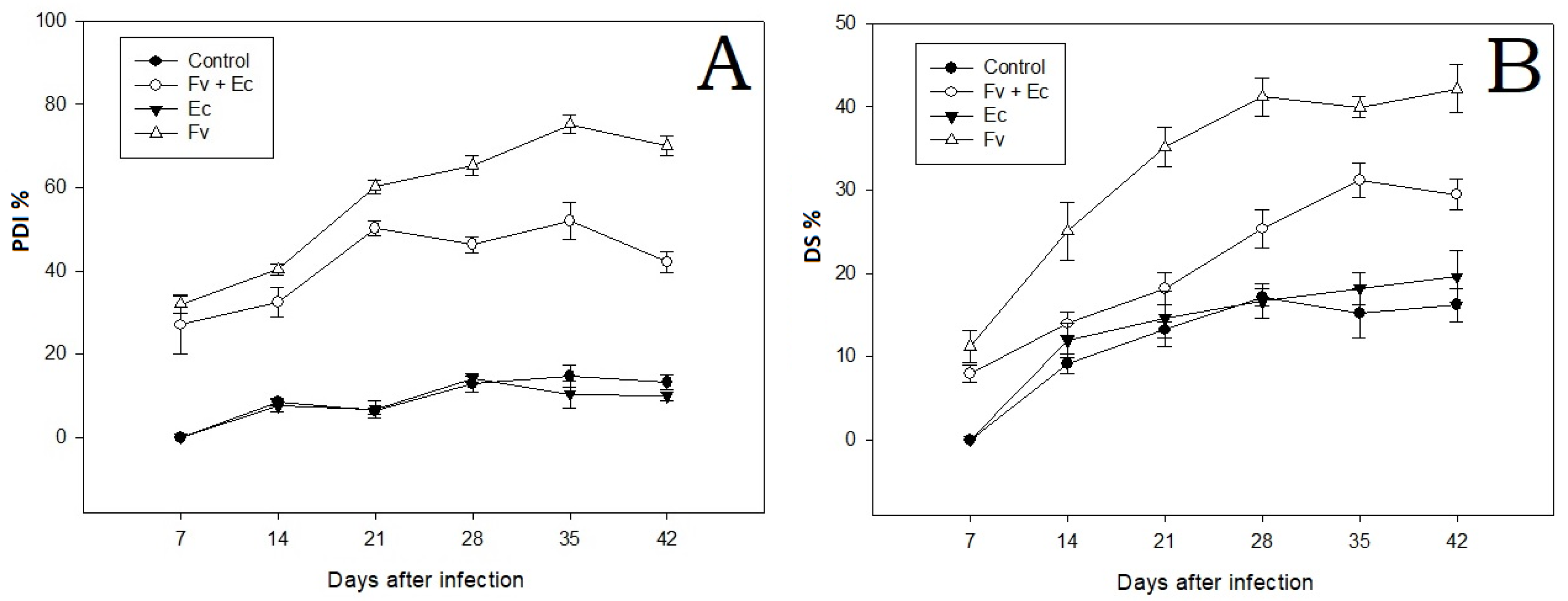
3.1. Effects on Plant Growth Parameters
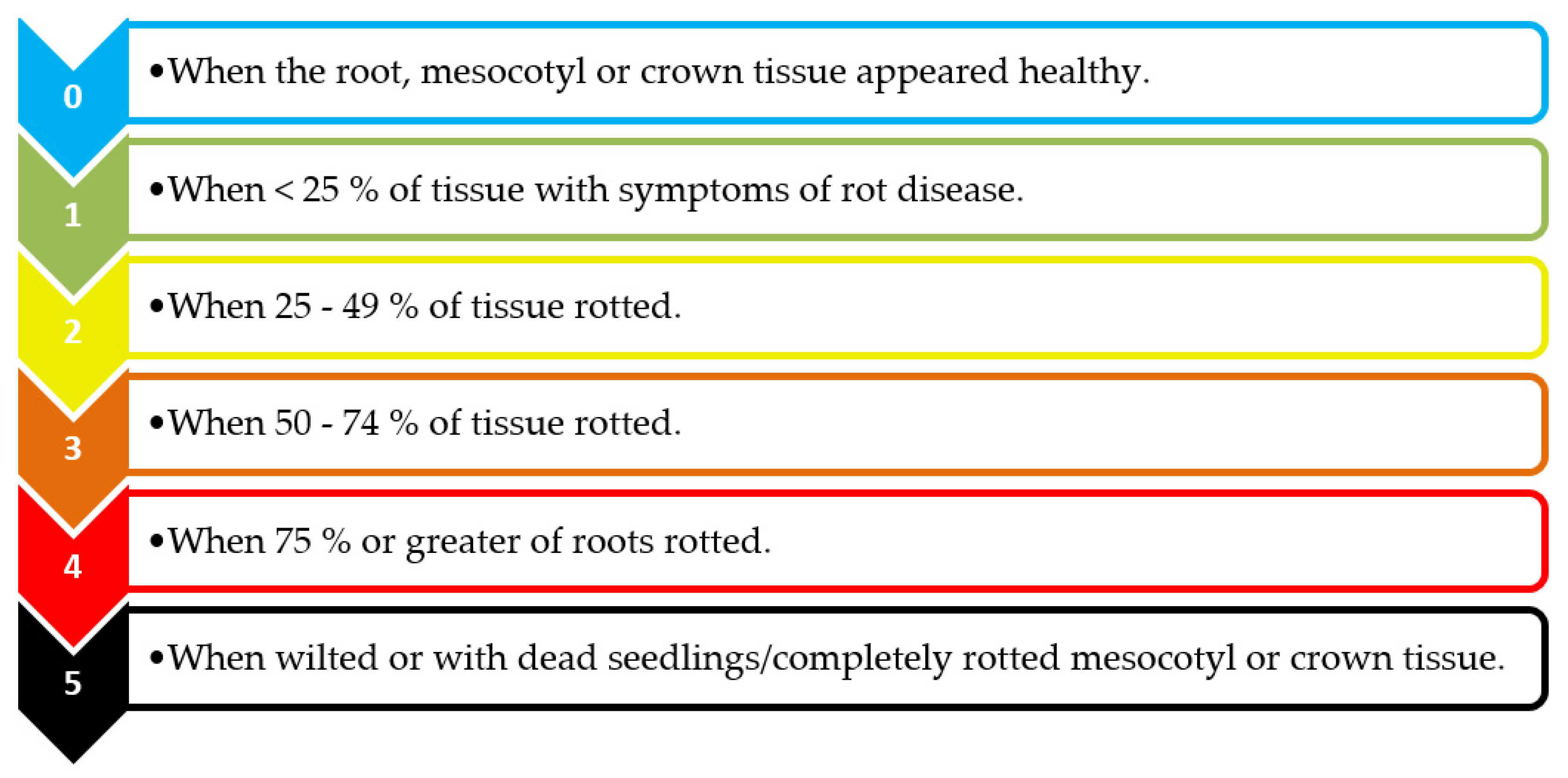
3.2. Disease Assessment
3.3. Phytochemical Analysis of C. longirostrata Branch Extract
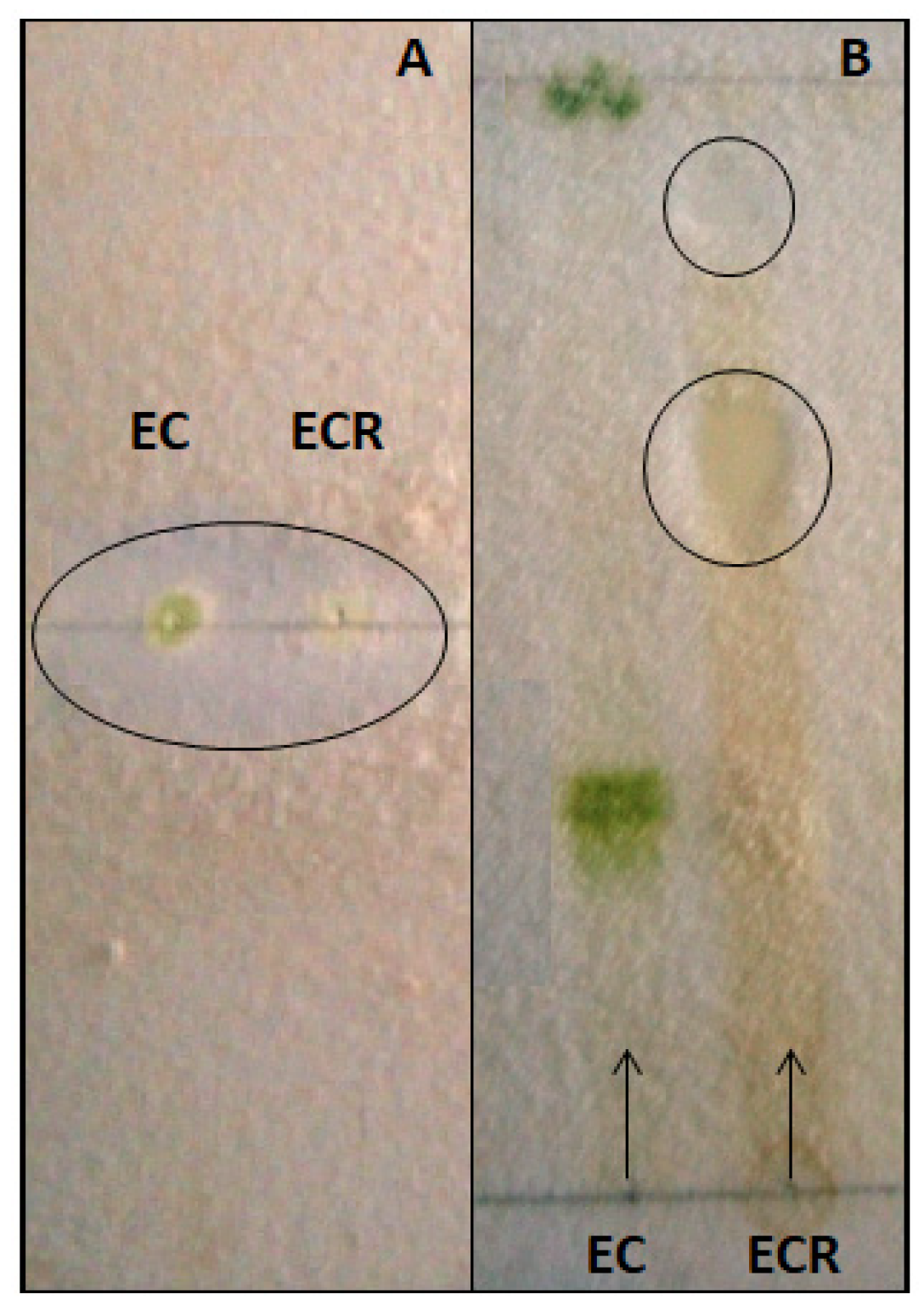
3.4. Fungitoxicity Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agrios, G. Plant Pathology, 5th ed.; Elsevier Academic Press: New York, NY, USA, 2005; p. 922. [Google Scholar]
- Wagacha, J.; Muthomi, J.W. Fusarium culmorum: Infection process, mechanisms of mycotoxin production and their role in pathogenesis in wheat. Crop. Prot. 2007, 26, 877–885. [Google Scholar] [CrossRef]
- Nelson, P.E. Taxonomy and biology of Fusarium moniliforme. Mycopathol. 1992, 117, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Oren, L.; Ezrati, S.; Cohen, D.; Sharon, A. Early Events in the Fusarium verticillioides-Maize Interaction Characterized by Using a Green Fluorescent Protein-Expressing Transgenic Isolate. Appl. Environ. Microbiol. 2003, 69, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Pantelides, I.; Tjamos, S.; Pappa, S.; Kargakis, M.; Paplomatas, E.J. The ethylene receptor ETR1 is required for Fusarium oxysporum pathogenicity. Plant Pathol. 2013, 62, 1302–1309. [Google Scholar] [CrossRef]
- Patil, M.K.M.; Nimbaragi, Y. Management of Stalk Rot of Maize Caused by Fusarium moniliforme (Sheldon). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3546–3552. [Google Scholar] [CrossRef]
- Katan, J. Physical and cultural methods for the management of soil-borne pathogens. Crop. Prot. 2000, 19, 725–731. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Dürr, C.; Schwanck, A.A.; Robin, M.-H.; Sarthou, J.-P.; Cellier, V.; Messéan, A.; Aubertot, J.-N. Integrated management of damping-off diseases. A review. Agron. Sustain. Dev. 2017, 37, 420. [Google Scholar] [CrossRef]
- Javaid, A.; Rauf, S. Management of basal rot disease of onion with dry leaf biomass of Chenopodium album as soil amendment. Int. J. Agric. Biol. 2015, 17, 142–148. [Google Scholar]
- Prieto, J.A.; Patiño, O.J.; Plazas, E.A.; Pabón, L.C.; Ávila, M.C.; Guzmán, J.D.; Cuca, L.E. Natural Products from Plants as Potential Source Agents for Controlling Fusarium. In Fungicides—Showcases of Integrated Plant Disease Management from around the World; IntechOpen: London, UK, 2013. [Google Scholar]
- Paul, P.; Sharma, P. Azadirachta indica leaf extract induces resistance in barley against leaf stripe disease. Physiol. Mol. Plant Pathol. 2002, 61, 3–13. [Google Scholar] [CrossRef]
- Guleria, S.; Kumar, A. Azadirachta indica leaf extract induces resistance in sesame against Alternaria leaf spot disease. J. Cell Mol. Biol. 2006, 5, 81–86. [Google Scholar]
- Trilogy, A Product of Neem (Azadirachta indica) Induces Resistance in Cucumber Against Podosphaera xanthii. Res. J. Microbiol. 2007, 2, 402–414. [CrossRef]
- McGovern, R. Management of tomato diseases caused by Fusarium oxysporum. Crop. Prot. 2015, 73, 78–92. [Google Scholar] [CrossRef]
- Akanmu, A.O.; Odebode, A.C.; Abiala, M.A.; Aiyelaagbe, O.O.; Olaoluwa, O.O. Inhibition of Fusarium pathogens in millet by extracts of Jatropha curcas and Magnifera indica. Int. J. Plant Biol. Res. 2014, 2, 1007. [Google Scholar]
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal activity of pomegranate peel extract against fusarium wilt of tomato. Eur. J. Plant Pathol. 2016, 147, 229–238. [Google Scholar] [CrossRef]
- Cruz-Rodríguez, R.I.; Meza-Gordillo, R.; Rodríguez-Mendiola, M.A.; Arias-Castro, C.; Mancilla-Margalli, N.A.; Ávila-Miranda, M.E.; Ricaldi, J.M.C.; Gutiérrez-Miceli, F.A.; Ruíz-Valdiviezo, V.M.; Ayora-Talavera, T.D.R. Antifungal activity of Crotalaria longirostrata Hook. & Arn. extracts against phytopathogen fungi from maize. Gayana Bot. 2017, 74, 167–175. [Google Scholar]
- Miranda-Granados, J.; Chacón, C.; Ruiz-Lau, N.; Vargas, M.E.; Zepeda, L.G.; Gutiérrez, P.E. Álvarez; Meza-Gordillo, R.; Lagunas-Rivera, S. Alternative Use of Extracts of Chipilín Leaves (Crotalaria longirostrata Hook. & Arn) as Antimicrobial. Sustainability 2018, 10, 883. [Google Scholar]
- Grunberger, D.; Banerjee, R.; Eisinger, K.; Oltz, E.M.; Efros, L.; Caldwell, M.; Estevez, V.; Nakanishi, K. Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Cell. Mol. Life Sci. 1988, 44, 230–232. [Google Scholar] [CrossRef]
- Figueroa-López, A.M.; Cordero-Ramírez, J.D.; Quiroz-Figueroa, F.; Maldonado-Mendoza, I.E. A high-throughput screening assay to identify bacterial antagonists against Fusarium verticillioides. J. Basic Microbiol. 2013, 54, 125. [Google Scholar] [CrossRef]
- Abiala, M.O.; Akanmu, A.O.; Onanuga, O.E.; Odebode, A.C. Azadirachta indica inhibited phytopathogenic fungi of sorghum (Sorghum bicolor. L. Moench). Advan. Biol. Res. 2013, 7, 241–247. [Google Scholar]
- Miftakhurohmah Hidayat, S.H.; Mutaqin, K.H.; Soekarno, B.P.W.; Wahyuno, D. Incidence and severity of mottle disease in black pepper plants (piper nigrum) in Sukamulya Research Station, Sukabumi Regency, West Java. IOP Conf. Ser. Earth Environ. Sci. 2020, 418, 012054. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Cheng, J.; Suh, J.-W.; Yang, S.; Meng, L. Biological control of anthracnose (Colletotrichum gloeosporioides) in yam by Streptomyces sp. MJM5763. J. Appl. Microbiol. 2011, 111, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B. Analysis of US EPA Method 8270D. Semivolatile Organic Compounds by GC/MS. Lc Gc N AM. 2002, 20, 8. [Google Scholar]
- Masoko, P.; Picard, J.; Eloff, J.N. Antifungal activities of six South African Terminalia species (Combretaceae). J. Ethnopharmacol. 2005, 99, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Statgraphics Plus for Windows. Design of Experiments Analyses Software; Manugistics, Inc.: Rockville, MD, USA, 1999. [Google Scholar]
- Ngo, M.T.; Han, J.W.; Yoon, S.; Bae, S.; Kim, S.-Y.; Kim, H.; Choi, G.J. Discovery of New Triterpenoid Saponins Isolated from Maesa japonica with Antifungal Activity against Rice Blast Fungus Magnaporthe oryzae. J. Agric. Food Chem. 2019, 67, 7706–7715. [Google Scholar] [CrossRef]
- Tomazoni, E.Z.; Ribeiro, R.T.S.; Pauletti, G.F.; Soares, G.L.G.; Schwambach, J. Inhibition of Alternaria stem canker on tomato by essential oils from Baccharis species. J. Environ. Sci. Heal. Part B 2019, 54, 781–790. [Google Scholar] [CrossRef]
- Akila, R.; Rajendran, L.; Harish, S.; Saveetha, K.; Raguchander, T.; Samiyappan, R. Combined application of botanical formulations and biocontrol agents for the management of Fusarium oxysporum f. sp. cubense (Foc) causing Fusarium wilt in banana. Biol. Control. 2011, 57, 175–183. [Google Scholar] [CrossRef]
- Sangeetha, G.; Thangavelu, R.; Rani, S.U.; Muthukumar, A. Antimicrobial activity of medicinal plants and induction of defense related compounds in banana fruits cv. Robusta against crown rot pathogens. Biol. Control. 2013, 64, 16–25. [Google Scholar] [CrossRef]
- Lagopodi, A.; Ram, A.F.; Lamers, G.E.M.; Punt, P.J.; Hondel, C.A.M.J.J.V.D.; Lugtenberg, B.J.J.; Bloemberg, G.V. Novel Aspects of Tomato Root Colonization and Infection by Fusarium oxysporum f. sp. radicis-lycopersici Revealed by Confocal Laser Scanning Microscopic Analysis Using the Green Fluorescent Protein as a Marker. Mol. Plant Microbe Interact. 2002, 15, 172–179. [Google Scholar] [CrossRef]
- Jetiyanon, K. Defensive-related enzyme response in plants treated with a mixture of Bacillus strains (IN937a and IN937b) against different pathogens. Biol. Control. 2007, 42, 178–185. [Google Scholar] [CrossRef]
- Shanmugam, V.; Kanoujia, N. Biological management of vascular wilt of tomato caused by Fusarium oxysporum f.sp. lycospersici by plant growth-promoting rhizobacterial mixture. Biol. Control. 2011, 57, 85–93. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.; Cruz, R.P.; Menezes, I.R.; et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Suzuki, R.; Koide, T.; Sakaguchi, N.; Ogihara, Y.; Yabu, Y. Antioxidant, Gallic Acid, Induces Apoptosis in HL-60RG Cells. Biochem. Biophys. Res. Commun. 1994, 204, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-J.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.-G.; Aibai, S. Antifungal Activity of Gallic Acid In Vitro and In Vivo. Phytotherapy Res. 2017, 31, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Campbell, B.C.; Mahoney, N.E.; Chan, K.L.; Molyneux, R.J. Identification of Phenolics for Control ofAspergillus flavusUsingSaccharomyces cerevisiaein a Model Target-Gene Bioassay. J. Agric. Food Chem. 2004, 52, 7814–7821. [Google Scholar] [CrossRef] [PubMed]
- Velluti, A. Inhibitory effect of cinnamon, clove, lemongrass, oregano and palmarose essential oils on growth and fumonisin B1 production by Fusarium proliferatum in maize grain. Int. J. Food Microbiol. 2003, 89, 145–154. [Google Scholar] [CrossRef]
- Alma, M.H.; Ertaş, M.; Nitz, S.; Kollmannsberger, H. chemical composition an content of essential oil from the bud of cultivated Turkish clove (Syzygium aromaticum L.). BioResources 2007, 2, 265–269. [Google Scholar]
- Choma, I.M.; Grzelak, E.M. Bioautography detection in thin-layer chromatography. J. Chromatogr. A 2011, 1218, 2684–2691. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, X.J.; Jiao, G.; Yuan, Y.; Sun, W. Simultaneous in vivo RP-HPLC-DAD quantification of multiple-component and drug-drug interaction by pharmacokinetics, using 6,7-dimethylesculetin, geniposide and rhein as examples. Biomed. Chromatogr. 2011, 26, 844–850. [Google Scholar] [CrossRef]
- Romero-Cortes, T.; Pérez-España, V.H.; López-Pérez, P.A.; Rodríguez-Jimenes, G.C.; Robles-Olvera, V.J.; Aparicio-Burgos, J.E.; Cuervo-Parra, J.A. Antifungal activity of vanilla juice and vanillin against Alternaria alternata. CyTA-J Food 2019, 17, 375–383. [Google Scholar] [CrossRef]

| No. | Treatment | Plant Height (cm) | Root Length (cm) | Vegetative Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|
| 1 | Control | 54.0 ± 5.66 a | 82.3 ± 3.32 a | 8.9 ± 1.13 a | 3.2 ± 0.93 a |
| 2 | Fv + EC | 43.7 ± 3.21 b | 75.2 ± 2.87 b | 7.8 ± 1.78 b | 3.1 ± 1.12 a |
| 3 | EC | 46.0 ± 4.08 ab | 83.7 ± 4.31 a | 8.5 ± 2.97 a | 3.2 ± 1.04 a |
| 4 | Fv | 40.3 ± 4.04 b | 70.3 ± 2.25 c | 7.5 ± 0.97 c | 2.8 ± 0.98 b |
| Treatment | AUDPC Percent/Days | |
|---|---|---|
| Incidence in Roots | Severity of Leaves | |
| Control | 834.4 a | 870.8 b |
| Fv + EC | 1210.9 b | 875.9 b |
| EC | 875.1 a | 679.3 a |
| Fv | 2057.8 c | 1180.9 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Rodríguez, R.I.; Cruz-Salomón, A.; Ruiz-Lau, N.; Pérez-Villatoro, J.I.; Esquinca-Avilés, H.A.; Meza-Gordillo, R. Potential Application of Crotalaria longirostrata Branch Extract to Reduce the Severity of Disease Caused by Fusarium. Agronomy 2020, 10, 524. https://doi.org/10.3390/agronomy10040524
Cruz-Rodríguez RI, Cruz-Salomón A, Ruiz-Lau N, Pérez-Villatoro JI, Esquinca-Avilés HA, Meza-Gordillo R. Potential Application of Crotalaria longirostrata Branch Extract to Reduce the Severity of Disease Caused by Fusarium. Agronomy. 2020; 10(4):524. https://doi.org/10.3390/agronomy10040524
Chicago/Turabian StyleCruz-Rodríguez, Rosa Isela, Abumalé Cruz-Salomón, Nancy Ruiz-Lau, José Iván Pérez-Villatoro, Hector Armando Esquinca-Avilés, and Rocío Meza-Gordillo. 2020. "Potential Application of Crotalaria longirostrata Branch Extract to Reduce the Severity of Disease Caused by Fusarium" Agronomy 10, no. 4: 524. https://doi.org/10.3390/agronomy10040524
APA StyleCruz-Rodríguez, R. I., Cruz-Salomón, A., Ruiz-Lau, N., Pérez-Villatoro, J. I., Esquinca-Avilés, H. A., & Meza-Gordillo, R. (2020). Potential Application of Crotalaria longirostrata Branch Extract to Reduce the Severity of Disease Caused by Fusarium. Agronomy, 10(4), 524. https://doi.org/10.3390/agronomy10040524