Establishment and Application of Critical Nitrogen Dilution Curve for Rice Based on Leaf Dry Matter
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Plant Sampling and Determination of N Content in Tissues
2.2.1. Plant Sampling
2.2.2. Determination of Biomass and N Content in Leaves and Determination of Yields
2.2.3. Plant N Accumulation
2.3. Data Analysis
3. Results
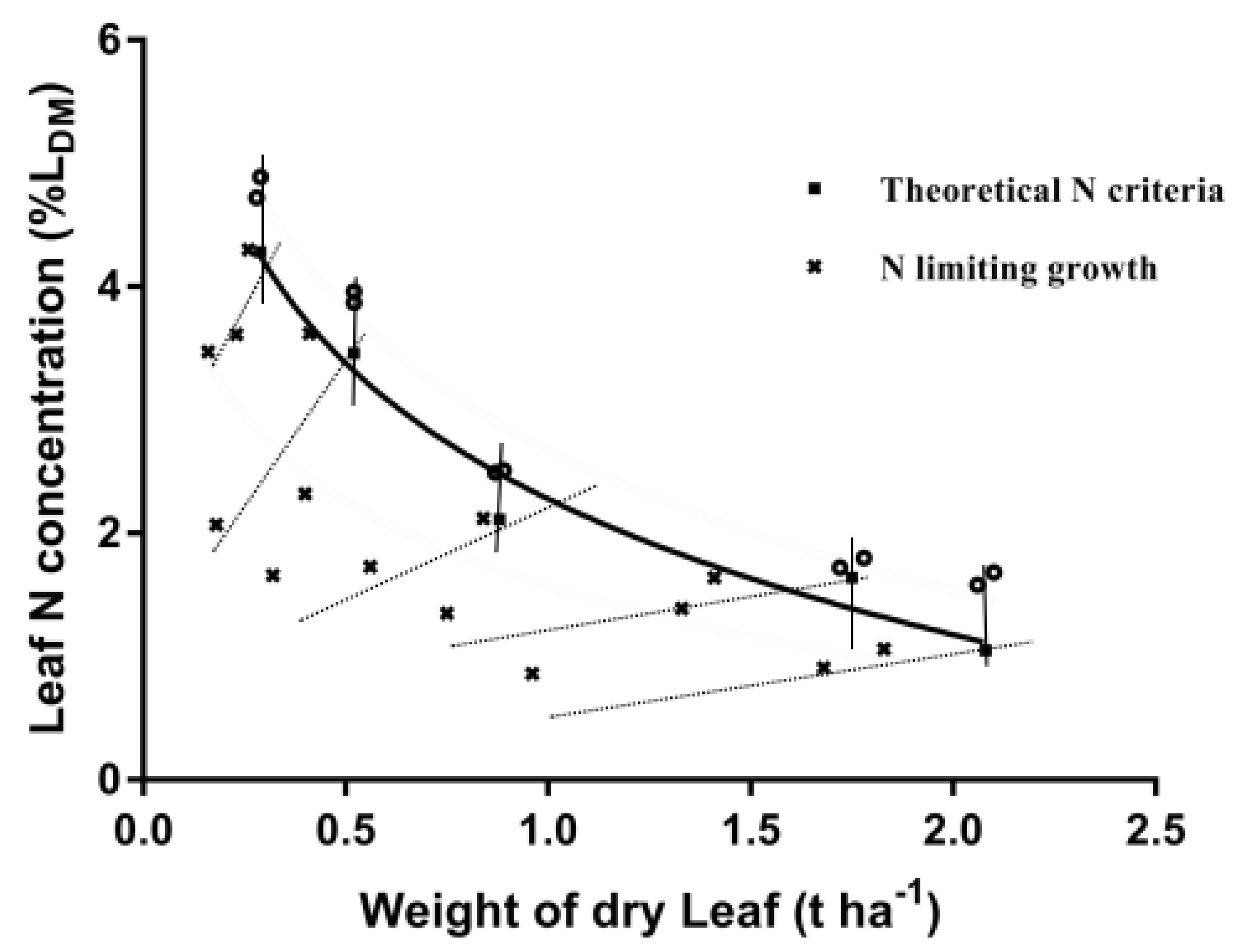
3.1. Leaf Dry Matter and N Concentration
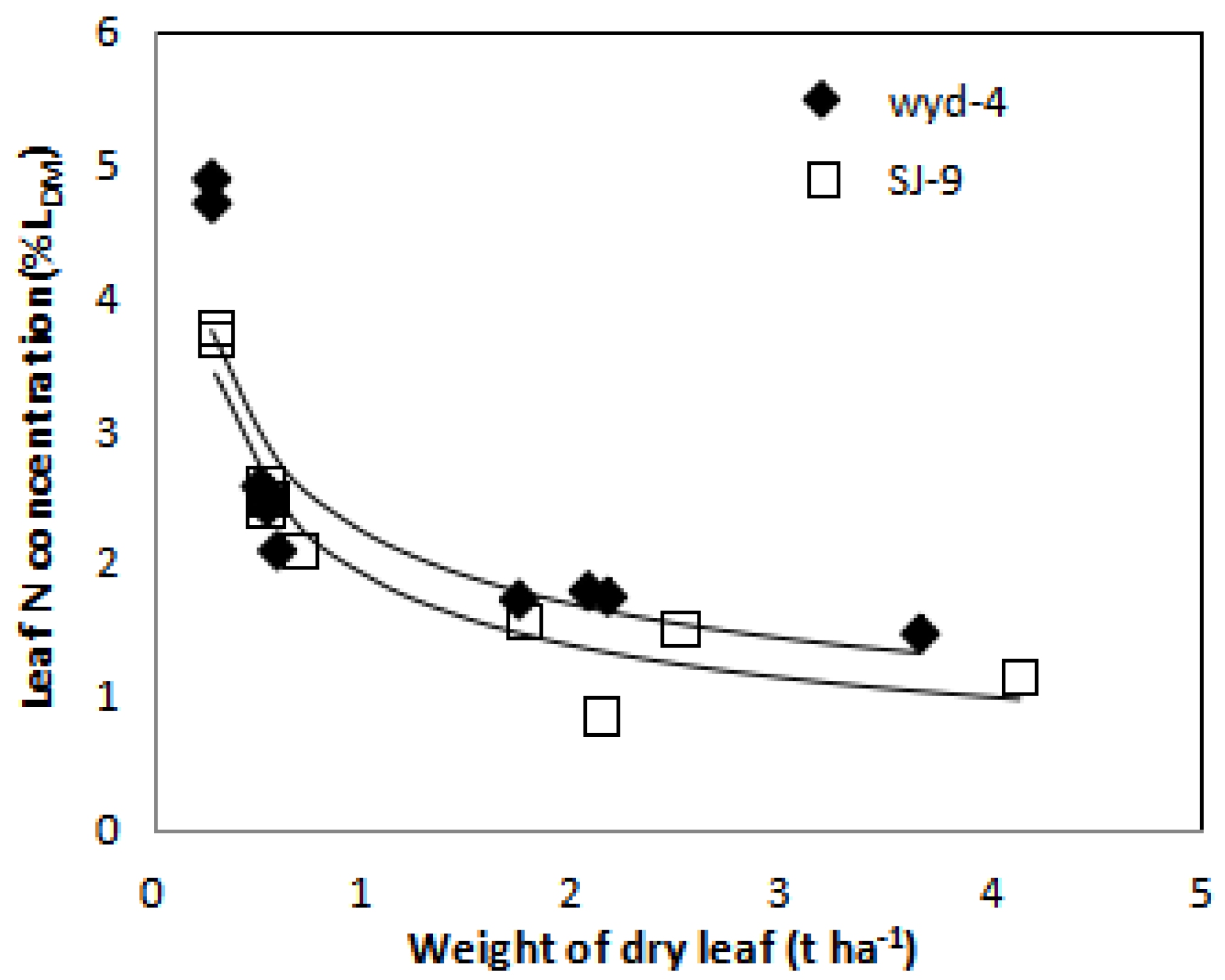
3.2. Determination of Dilution Curves for Nc Concentration
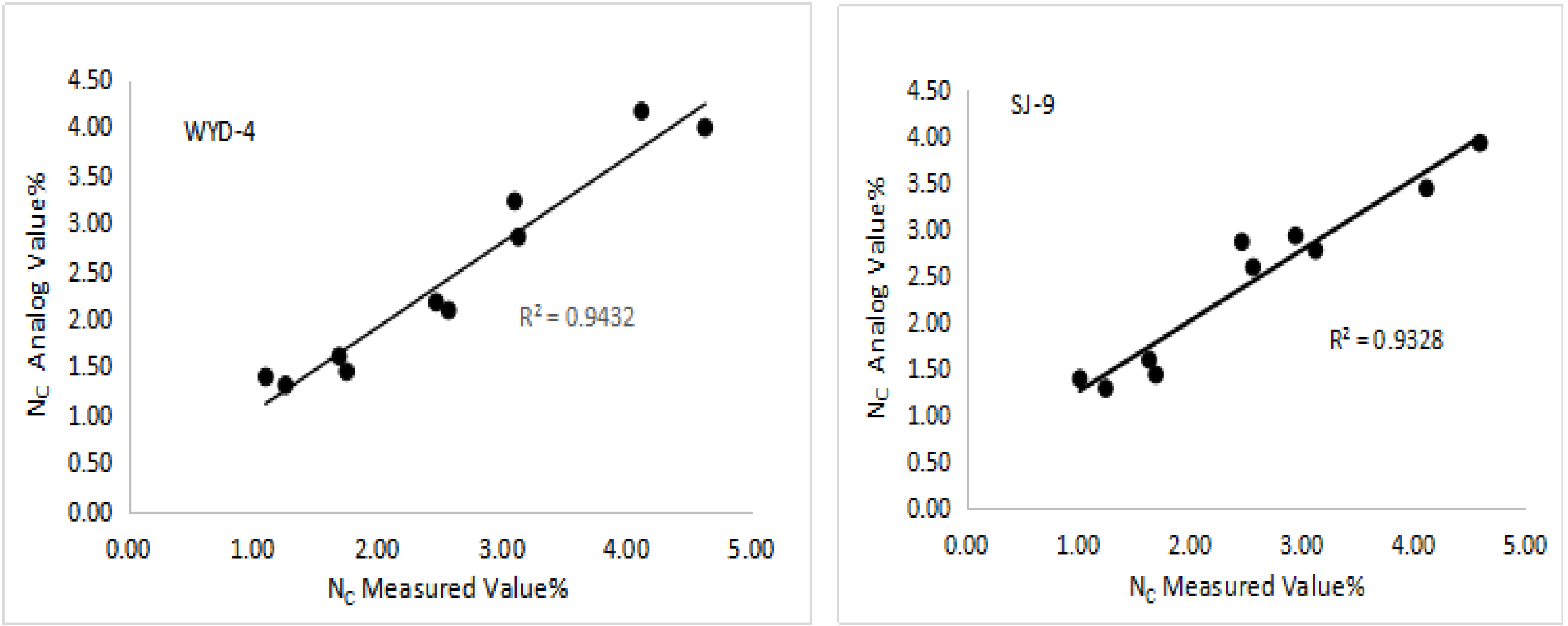
3.3. Validation of the Nc Dilution Curve
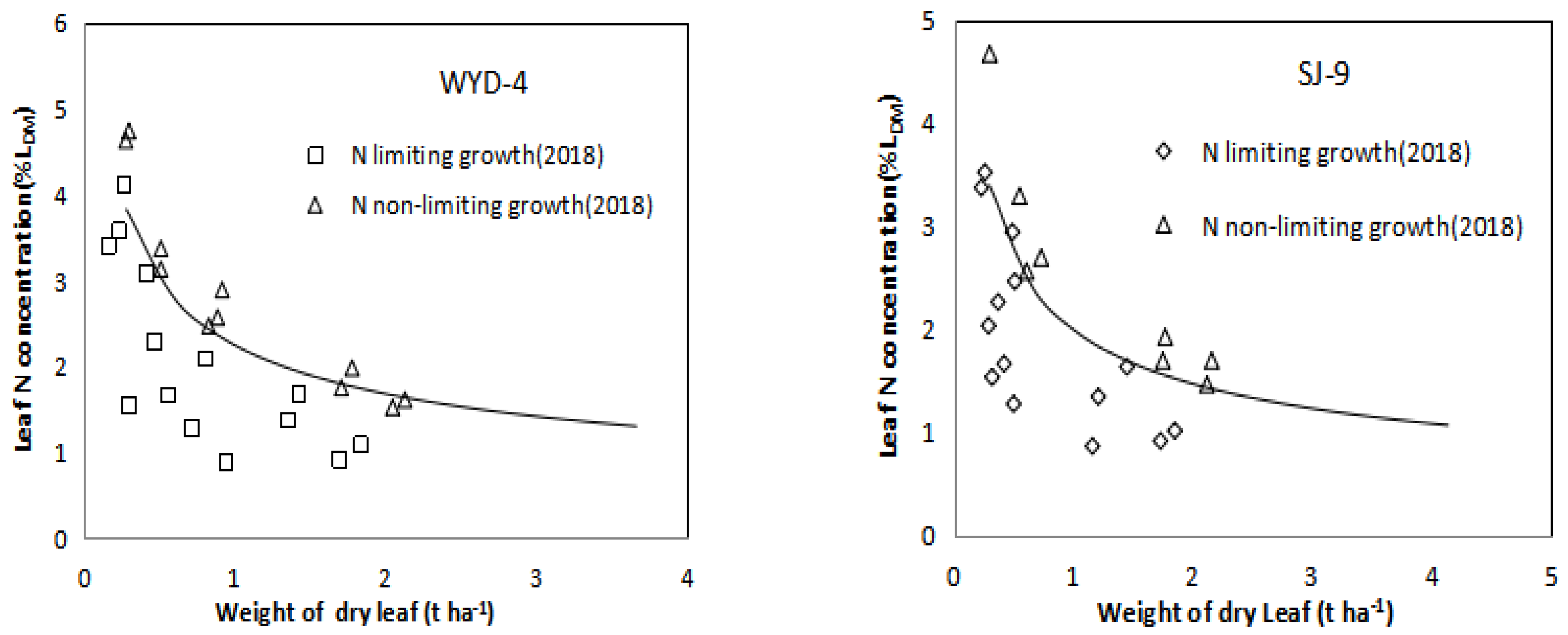
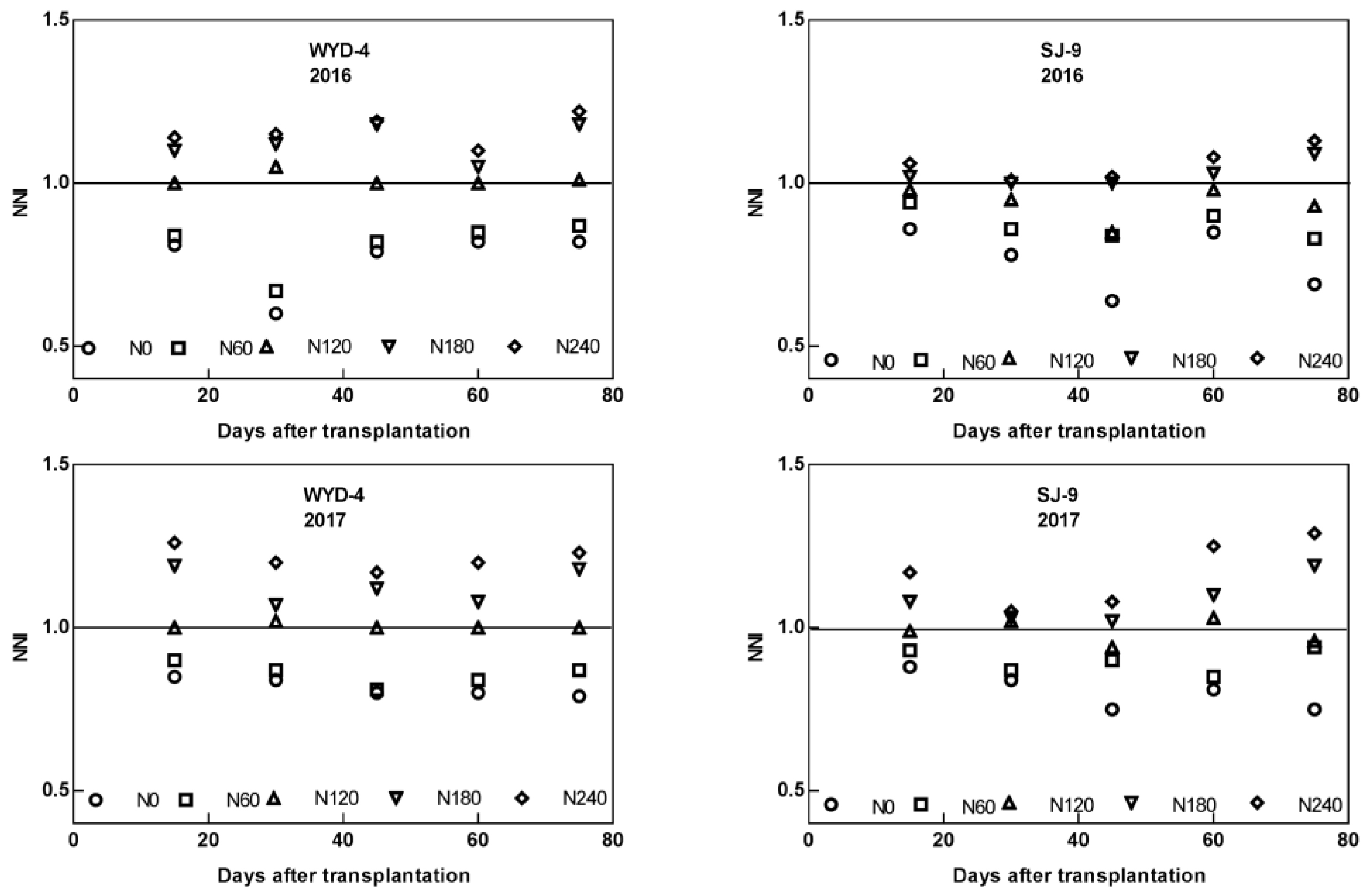
3.4. Effect of Different Nitrogen Application Levels on Nitrogen Nutrition Index (NNI) in Rice Leaves
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Yang, J.C. Research progress on the effects of nitrogen fertilizer on the yield of super rice and its biological basis. Crop J. 2012, 6, 19–24. [Google Scholar]
- Gong, J.L.; Hu, Y.J.; Ge, M.J.; Long, H.Y.; Chang, Y.; Ma, Q.; Yang, X.; Zhang, H.C.; Dai, Q.G.; Huo, Z.Y. Study on the highest productivity and formation characteristics of the southern rice-type super rice nitrogen fertilizer group. J. Nucl. Agric. 2012, 26, 558–572. [Google Scholar]
- Xi, X.H.; Shi, F.Z.; Yan, X.M.; Luo, Y.X.; Ma, T.C.; Luo, Z.X. Effects of nitrogen levels on nitrogen use efficiency, yield and quality of different genotypes of rice. J. Appl. Ecol. 2017, 28, 1219–1226. [Google Scholar]
- Ma, J.H.; Liu, Y.; Yang, X.G.; Wang, W.F.; Xue, C.Y.; Zhang, X.Y. Trends in climate resources in the North China Plain under the background of global climate change. Acta Ecol. Sin. 2010, 30, 3818–3827. [Google Scholar]
- Grohs, D.S.; Bredemeier, C.; Mundstock, C.M.; Poletto, N. Model for yield potential estimation in wheat and barley using the GreenSeeker sensor. Eng. Agr. 2009, 29, 101–112. [Google Scholar] [CrossRef]
- Cai, H.G.; Mi, G.H.; Chen, F.J.; Zhang, X.Z.; Gao, Q. Inter-species variation of SPAD value, total nitrogen and nitrate nitrogen content in maize leaves. Plant Nutr. Fertil. 2010, 16, 866–873. [Google Scholar]
- Ghasemi, N.; Sahebi, M.R.; Mohammadzadeh, A. A review on biomass estimation methods using synthetic aperture radar data. Int. J. Geomat. Geosci. 2011, 1, 776–788. [Google Scholar]
- Greenwood, D.J.; Lemaire, G.; Gosse, G.; Cruz, P.; Draycott, A.; Neeteson, J.J. Decline in Percentage N of C3 and C4 Crops with Increasing Plant Mass. Ann. Bot. 1990, 66, 425–436. [Google Scholar] [CrossRef]
- Colnenne, C.; Meynard, J.M.; Reau, R.; Justes, E.; Merrien, A. Determination of a Critical Nitrogen Dilution Curve for Winter Oilseed Rape. Ann. Bot. 1998, 81, 311–317. [Google Scholar] [CrossRef]
- Xue, X.P.; Zhou, Z.G.; Zhang, L.J.; Wang, Y.L.; Guo, W.Q.; Chen, B.L. Development and application of critical nitrogen concentration Dilution Model for cotton after flowering. Acta Ecol. Sin. 2006, 26, 1781–1791. [Google Scholar]
- Giletto, C.M.; Echeverría, H.E. Critical Nitrogen Dilution Curve for Processing Potato in Argentinean Humid Pampas. Am. J. Potato Res. 2012, 89, 102–110. [Google Scholar] [CrossRef]
- Yue, S.; Meng, Q.; Zhao, R.; Li, F.; Chen, X.; Zhang, F.; Cui, Z. Critical Nitrogen Dilution Curve for Optimizing Nitrogen Management of Winter Wheat Production in the North China Plain. Agron. J. 2012, 104, 523. [Google Scholar] [CrossRef]
- Yao, X.; Ata-Ul-Karim, S.T.; Zhu, Y.; Tian, Y.C.; Liu, X.J.; Cao, W.X. Development of critical nitrogen dilution curve in rice based on leaf dry matter. Eur. J. Agro. 2014, 55, 20–28. [Google Scholar] [CrossRef]
- Kage, H.; Alt, C.; Stutzel, H. Nitrogen concentration of cauliflower organs as determined by organ size, N supply, and radiation environment. Plant Soil 2002, 246, 201–209. [Google Scholar] [CrossRef]
- Vouillot, M.O.; Huet, P.; Boissard, P. Early detection of N deficiency in a wheat crop using physiological and radiometric methods. Agronomie 1998, 18, 117–130. [Google Scholar] [CrossRef]
- Ma X., J.; Zhang, X.T.; Huang, Y.F.; Ye, Y.L. Establishment and application of critical nitrogen dilution curve for wheat based on leaf dry matter. Plant Physiol. J. 2017, 53, 1313–1321. [Google Scholar]
- Liu, X.J.; Tahir, S.; Ata-Ul-Karim, S.T. Critical nitrogen concentration diluted rice model and nitrogen nutrition diagnosis. China Rice 2013, 4, 149. [Google Scholar]
- Xu, R.L.; Dai, Q.G.; Wang, X.Q.; Huang, Y.Z.; Lü, X.T. Effects of nitrogen application rate, application period and operation on nitrogen use efficiency of rice. Js. Agric. Scis. 2005, 2, 19–22. [Google Scholar]
- Zhong, Y.M. Rapid Diagnosis of Nitrogen Nutrition in Rice Leaves and Characteristics of Greenhouse Gas Emissions in Paddy Fields. Ph.D. Thesis, Zhejiang University, Hangzhou, China, June 2016. [Google Scholar]
- Debruin, J.; Messina, C.D.; Munaro, E.; Thompson, K.; Conlon-Beckner, C.; Fallis, L.; Sevenich, D.M.; Gupta, R.; Dhugga, K.S. N distribution in maize plant as a marker for grain yield and limits on its remobilization after flowering. Plant Breed. 2013, 132, 500–505. [Google Scholar] [CrossRef]
- Sheehy, J.E.; Dionora, M.J.A.; Mitchell, P.L.; Peng, S.; Cassman, K.G.; Lemaire, G.; Williams, R.L. Critical nitrogen concentrations: Implications for high-yielding rice ( Oryza sativa L.) cultivars in the tropics. Field Crop. Res. 1998, 59, 31–41. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Yao, X.; Liu, X.; Cao, W.; Zhu, Y. Development of critical nitrogen dilution curve of Japonica rice in Yangtze River Reaches. Field Crop. Res. 2013, 149, 149–158. [Google Scholar] [CrossRef]
- Lu, Z.Z. Study on critical nitrogen concentration and spectral index model of rice in the lower reaches of the Yangtze River. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, June 2015. [Google Scholar]
- Justes, E.; Mary, B.; Meynard, J.M.; Machet, J.M.; Thelierhuche, L. Determination of a Critical Nitrogen Dilution Curve for Winter Wheat Crops. Ann. Bot. 1994, 74, 397–407. [Google Scholar] [CrossRef]
- Lemaire, G.; Van, O.E.; Sheehy, J.; Jeuffroy, M.H.; Massignam, A.; Rossato, L. Is crop N demand more closely related to dry matter accumulation or leaf area expansion during vegetative growth. Field Crop. Res. 2007, 100, 91–106. [Google Scholar] [CrossRef]
- Gastal, F.; Lemaire, G. N uptake and distribution in crops: An agronomical and ecophysiological perspective. J. Exp. Bot. 2002, 53, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Willmott, C.J. Some Comments on the Evaluation of Model Performance. B. Am. Meteorol. Soc. 1982, 63, 1309–1369. [Google Scholar] [CrossRef]
- Yang, J.; Greenwood, D.J.; Rowell, D.L.; Wadsworth, G.A.; Burns, I.G. Statistical methods for evaluating a crop nitrogen simulation model. Agr. Syst. 2000, 64, 37–53. [Google Scholar] [CrossRef]
- Jamieson, P.D.; Porter, J.R.; Wilson, D.R. A test of the computer simulation model ARCWHEAT1 on wheat crops grown in New Zealand. Field Crop. Res. 1991, 27, 337–350. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Yao, X.; Liu, X.J.; Cao, W.X.; Zhu, Y. Determination of Critical Nitrogen Dilution Curve Basedon Stem Dry Matter in Rice. Plos One 2014, 9, e104540. [Google Scholar] [CrossRef]
- Huang, S.Y.; Miao, Y.X.; Cao, Q.; Yao, Y.K.; Zhao, G.M.; Yu, W.F.; Shen, J.N.; Yu, K.; Georg, B. A new critical nitrogen dilution curve for rice nitrogen status diagnosis in Northeast China. Pedosphere 2018, 28, 814–822. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Zhu, Y.; Liu, X.J.; Cao, Q.; Tian, Y.C.; Cao, W.X. Comparison of different critical nitrogen dilution curves for nitrogen diagnosis in rice. Sci. Rep. 2017, 7, 42679. [Google Scholar] [CrossRef]
- Novoa, R.; Loomis, R. Nitrogen and plant production. Plant Soil 1981, 58, 177–204. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Y.; Qiu, X.L.; Ata-Ul-Karim, S.T.; Li, Y.D.; Liu, X.J.; Cao, Q. Development of a critical nitrogen dilution curve of double cropping rice in South China. Front. Plant Sci. 2017, 8, 638. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Rice nitrogen nutrition diagnosis and recommended fertilization based on visible light image. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, June 2015. [Google Scholar]
- Lv, R.J.; Shang, Q.Y.; Chen, L.; Zeng, Y.J.; Hu, S.X.; Yang, X.H. Plant Study on diagnosis of nitrogen nutrition in rice based on critical nitrogen concentration. Plant Nutr. Fertil. Sci. 2018, 5, 1396–1405. [Google Scholar]
- Yang, H.; Yang, J.P.; Lv, Y.M.; He, J.J. SPAD Values and Nitrogen Nutrition Index for the Evaluation of Rice Nitrogen Status. Plant Prod. Sci. 2014, 17, 81–92. [Google Scholar] [CrossRef]
- Ziadi, N.; Belanger, G.; Claessens, A.; Lefebvre, L.; Tremblay, N.; Cambouris, A.N.; Nolin, M.C.; Parent, L.E. Plant-based diagnostic tools for evaluating wheat nitrogen status. Crop Sci. 2010, 50, 2580–2590. [Google Scholar] [CrossRef]


| Experiment No. | Transplanting/Harvesting Date | Location | Cultivar | N Rate (kg ha−1) | Sampling Stage | Sampling Date | Soil Characteristics |
|---|---|---|---|---|---|---|---|
| Experiment 1 in 2016 | 20-May/ 25-Sep | Wuchang (44°92′ N, 127°15′ E) | Wuyoudao4 Songjing9 | N0(0) N60(60) N120(120) N180(180) N240(240) | Active tillering Panicle initiation Stem elongation Booting Heading | 13-Jun 27-Jun 8-Jul 23-Jul 13-Aug | Soil type = Brunisolic Soil pH = 6.59 Total P = 2.15g kg−1 Total K = 17.5g kg−1 Available N = 114ppm Available P = 37.8ppm Available K = 156ppm |
| Experiment 2 in 2017 | 18-May/ 24-Sep | Wuchang (44°92′ N, 127°15′ E) | Wuyoudao4 Songjing9 | N0(0) N60(60) N120(120) N180(180) N240(240) | Active tillering Panicle initiation Stem elongation Booting Heading | 10-Jun 24-Jun 6-Jul 22-Jul 6-Aug | Same as above |
| Experiment 3 in 2018 | 22-May/ 25-Sep | Wuchang (44°92′ N, 127°15′ E) | Wuyoudao4 Songjing9 | N0(0) N60(60) N120(120) N180(180) N240(240) | Active tillering Panicle initiation Stem elongation Booting Heading | 15-Jun 28-Jun 9-Jul 21-Jul 8-Aug | Same as above |
| Region | Cultivar | Model | Researcher |
|---|---|---|---|
| Torrid zone | Cultivars in different regions | Nc = 5.2 PDM−0.5 | Sheehy et al. [21] |
| South China | Zhongjiazao17, Tanliangyou83 Tianyouhuazhan, Yueyou9113, Xiangyou186 etc. | Early rice: Nc = 3.37 PDM−0.44 Late rice: Nc = 3.69 PDM−0.34 | He et al. [35] |
| South China | Nanjing46, Nangan48, Wuyujing24 etc. | Nc = 3.33 PDM−0.26 | Wang et al. [36] |
| South China | Yliangyou1, Chaoyouqianhao etc. Jinnongsimiao, Yuenongsimiao etc. | Hybrid rice: Nc = 3.36 PDM−0.31 Conventional rice: Nc = 2.96 PDM−0.25 | Lv et al. [37] |
| South China | Xiushui63, Hang43 | Xiushui63: Nc = 5.31 PDM−0.5 Hang43: Nc = 5.38 PDM−0.49 | Yang et al. [38] |
| South China | Hang43 | 2013-Hang43: Nc = 5.38 PDM−0.49 2015-Hang43: Nc = 4.29 PDM−0.55 | Zhong [19] |
| Northern China | Kongyu131, Longjing31 | Nc = 2.77 PDM−0.34 | Huang et al. [31] |
| South China | Lingxiangyou18, Wuxiangjing14 etc. | Nc = 3.53 PDM−0.28 | Ata-Ul-Karim et al. [22] |
| South China | Lingxiangyou18, Wuxiangjing14 etc. | Nc = 3.76 LDM−0.22 | Yao et al. [13] |
| South China | Lingxiangyou18, Wuxiangjing14 etc. | Nc = 2.17 SDM−0.27 | Ata-Ul-Karim et al [30] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Wang, S.; Ye, W. Establishment and Application of Critical Nitrogen Dilution Curve for Rice Based on Leaf Dry Matter. Agronomy 2020, 10, 367. https://doi.org/10.3390/agronomy10030367
Song L, Wang S, Ye W. Establishment and Application of Critical Nitrogen Dilution Curve for Rice Based on Leaf Dry Matter. Agronomy. 2020; 10(3):367. https://doi.org/10.3390/agronomy10030367
Chicago/Turabian StyleSong, Lijuan, Shu Wang, and Wanjun Ye. 2020. "Establishment and Application of Critical Nitrogen Dilution Curve for Rice Based on Leaf Dry Matter" Agronomy 10, no. 3: 367. https://doi.org/10.3390/agronomy10030367
APA StyleSong, L., Wang, S., & Ye, W. (2020). Establishment and Application of Critical Nitrogen Dilution Curve for Rice Based on Leaf Dry Matter. Agronomy, 10(3), 367. https://doi.org/10.3390/agronomy10030367




