Insights into the Genetic Architecture of Phenotypic Stability Traits in Winter Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Collection and Analysis of Phenotypic Data
2.3. Genotype by Environment Interactions and Stability Indices
2.4. SNP Genotyping and Genomewide Association Study
2.5. Genomic Selection
3. Results
3.1. Genotype-by-Environment Interactions, Trait Heritability, and Correlations
3.2. Marker-Trait Associations for Trait Values and Stability Indices
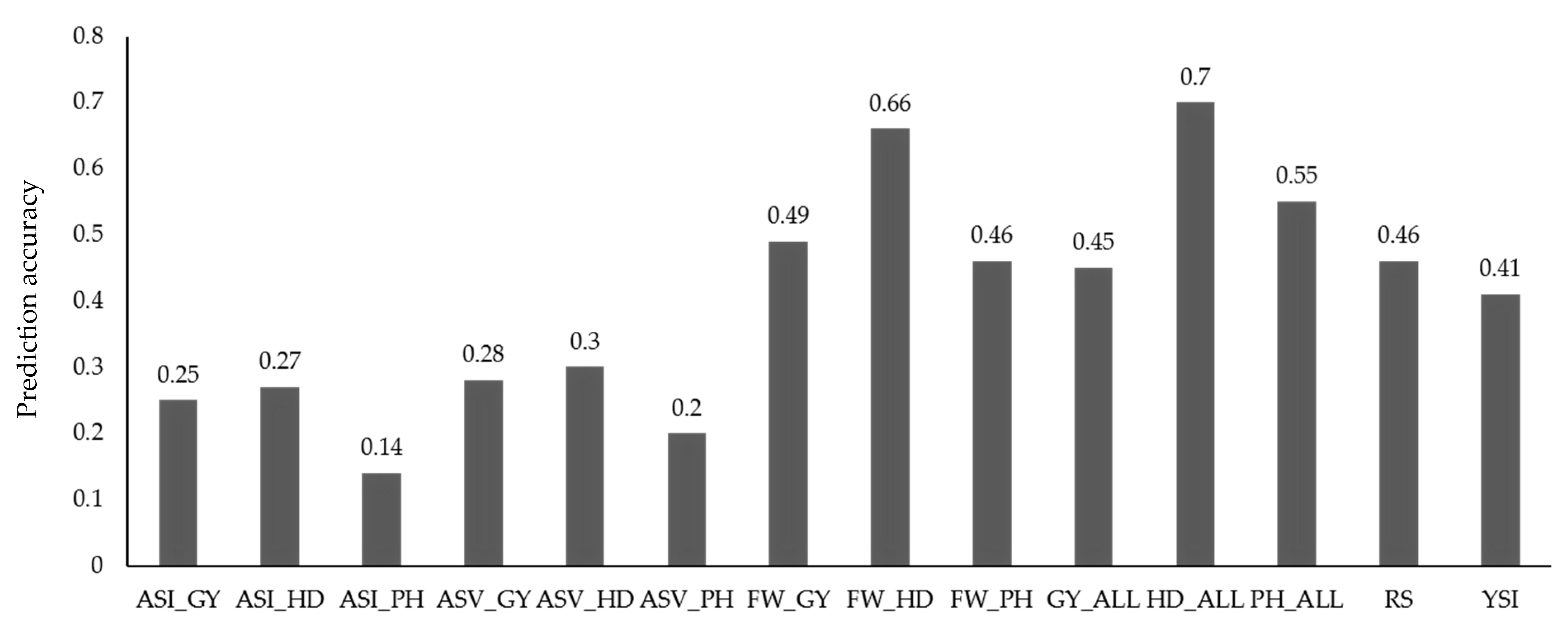
3.3. Prediction Accuracy for Trait Values and Stability Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability Statement
References
- Laitinen, R.A.E.; Nikoloski, Z. Genetic basis of plasticity in plants. J. Exp. Bot. 2018, 70, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Van Eeuwijk, F.A.; Bustos-Korts, D.V.; Malosetti, M. What should students in plant breeding know about the statistical aspects of genotype× environment interactions? Crop. Sci. 2016, 56, 2119–2140. [Google Scholar] [CrossRef]
- Huang, M.; Cabrera, A.; Hoffstetter, A.; Griffey, C.; Van Sanford, D.; Costa, J.; McKendry, A.; Chao, S.; Sneller, C. Genomic selection for wheat traits and trait stability. Theor. Appl. Genet. 2016, 129, 1697–1710. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhao, Y.; Mirdita, V.; Reif, J.C. Efficient strategies to assess yield stability in winter wheat. Theor. Appl. Genet. 2017, 130, 1587–1599. [Google Scholar] [CrossRef]
- Wang, Y.; Mette, M.F.; Miedaner, T.; Wilde, P.; Reif, J.C.; Zhao, Y. First insights into the genotype–phenotype map of phenotypic stability in rye. J. Exp. Bot. 2015, 66, 3275–3284. [Google Scholar] [CrossRef]
- Sukumaran, S.; Dreisigacker, S.; Lopes, M.; Chavez, P.; Reynolds, M.P. Genome-wide association study for grain yield and related traits in an elite spring wheat population grown in temperate irrigated environments. Theor. Appl. Genet. 2015, 128, 353–363. [Google Scholar] [CrossRef]
- Lozada, D.N.; Mason, R.E.; Babar, M.A.; Carver, B.F.; Guedira, G.B.; Merrill, K.; Arguello, M.N.; Acuna, A.; Vieira, L.; Holder, A.; et al. Association mapping reveals loci associated with multiple traits that affect grain yield and adaptation in soft winter wheat. Euphytica 2017, 213, 222. [Google Scholar] [CrossRef]
- Bhatta, M.; Morgounov, A.; Belamkar, V.; Baenziger, P.S. Genome-Wide Association Study Reveals Novel Genomic Regions for Grain Yield and Yield-Related Traits in Drought-Stressed Synthetic Hexaploid Wheat. Int. J. Mol. Sci. 2018, 19, 3011. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, Y.; Rodemann, B.; Plieske, J.; Kollers, S.; Korzun, V.; Ebmeyer, E.; Argillier, O.; Hinze, M.; Ling, J.; et al. Potential and limits to unravel the genetic architecture and predict the variation of Fusarium head blight resistance in European winter wheat (Triticum aestivum L.). Heredity. 2014, 114, 318. [Google Scholar] [CrossRef]
- Zhu, C.; Gore, M.; Buckler, E.S.; Yu, J. Status and prospects of association mapping in plants. Plant. Genome 2008, 1, 5–20. [Google Scholar] [CrossRef]
- Lozada, D.; Godoy, J.V.; Murray, T.D.; Ward, B.P.; Carter, A.H. Genetic Dissection of Snow Mold Tolerance in US Pacific Northwest Winter Wheat Through Genome-Wide Association Study and Genomic Selection. Front. Plant. Sci. 2019, 10, 1337. [Google Scholar] [CrossRef] [PubMed]
- Arruda, M.P.; Brown, P.; Brown-Guedira, G.; Krill, A.M.; Thurber, C.; Merrill, K.R.; Foresman, B.J.; Kolb, F.L. Genome-Wide Association Mapping of Fusarium Head Blight Resistance in Wheat using Genotyping-by-Sequencing. Plant. Genome 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Zanke, C.; Ling, J.; Plieske, J.; Kollers, S.; Ebmeyer, E.; Korzun, V.; Argillier, O.; Stiewe, G.; Hinze, M.; Neumann, F.; et al. Analysis of main effect QTL for thousand grain weight in European winter wheat (Triticum aestivum L.) by genome-wide association mapping. Front. Plant. Sci. 2015, 6, 644. [Google Scholar] [CrossRef] [PubMed]
- Zanke, C.; Ling, J.; Plieske, J.; Kollers, S.; Ebmeyer, E.; Korzun, V.; Argillier, O.; Stiewe, G.; Hinze, M.; Beier, S. Genetic architecture of main effect QTL for heading date in European winter wheat. Front. Plant. Sci. 2014, 5, 217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, F.; Wen, W.; Liu, J.; Zhang, Y.; Cao, S.; He, Z.; Rasheed, A.; Jin, H.; Zhang, C.; Yan, J.; et al. Genetic architecture of grain yield in bread wheat based on genome-wide association studies. BMC Plant. Biol. 2019, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Neumann, K.; Kobiljski, B.; Denčić, S.; Varshney, R.K.; Börner, A. Genome-wide association mapping: A case study in bread wheat (Triticum aestivum L.). Mol. Breed. 2011, 27, 37–58. [Google Scholar] [CrossRef]
- Tian, F.; Bradbury, P.J.; Brown, P.J.; Hung, H.; Sun, Q.; Flint-Garcia, S.; Rocheford, T.R.; McMullen, M.D.; Holland, J.B.; Buckler, E.S. Genome-wide association study of leaf architecture in the maize nested association mapping population. Nat. Genet. 2011, 43, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Breseghello, F.; Sorrells, M.E. Association analysis as a strategy for improvement of quantitative traits in plants. Crop. Sci. 2006, 46, 1323–1330. [Google Scholar] [CrossRef]
- Korte, A.; Farlow, A. The advantages and limitations of trait analysis with GWAS: A review. Plant. Methods 2013, 9, 29. [Google Scholar] [CrossRef]
- Newell, M.A.; Jannink, J.L. Genomic Selection in Plant Breeding In. Crop Breeding: Methods and Protocols; Fleury, D., Whitford, R., Eds.; Springer: New York, NY, USA, 2014; pp. 117–130. [Google Scholar]
- Crossa, J.; Pérez-Rodríguez, P.; Cuevas, J.; Montesinos-López, O.; Jarquín, D.; de los Campos, G.; Burgueño, J.; Camacho-González, J.M.; Pérez-Elizalde, S.; Beyene, Y. Genomic selection in plant breeding: Methods, models, and perspectives. Trends Plant. Sci. 2017, 22, 11–961. [Google Scholar] [CrossRef]
- Lozada, D.N.; Mason, R.E.; Sarinelli, J.M.; Guedira, G.B. Accuracy of genomic selection for grain yield and agronomic traits in soft red winter wheat. BMC Genet. 2019, 20, 82. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Dekkers, J.C.M.; Fernando, R.L.; Jannink, J.L. Factors Affecting Accuracy From Genomic Selection in Populations Derived From Multiple Inbred Lines: A Barley Case Study. Genetics 2009, 182, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Spindel, J.; Begum, H.; Akdemir, D.; Virk, P.; Collard, B.; Redoña, E.; Atlin, G.; Jannink, J.L.; McCouch, S.R. Genomic Selection and Association Mapping in Rice (Oryza sativa): Effect of Trait Genetic Architecture, Training Population Composition, Marker Number and Statistical Model on Accuracy of Rice Genomic Selection in Elite, Tropical Rice Breeding Lines. PLoS Genet. 2015, 11, 1–25. [Google Scholar] [CrossRef]
- Cericola, F.; Jahoor, A.; Orabi, J.; Andersen, J.R.; Janss, L.L.; Jensen, J. Optimizing Training Population Size and Genotyping Strategy for Genomic Prediction Using Association Study Results and Pedigree Information. A Case of Study in Advanced Wheat Breeding Lines. PLoS ONE 2017, 12, e0169606. [Google Scholar] [CrossRef] [PubMed]
- Gauch, H.G., Jr. Model selection and validation for yield trials with interaction. Biometrics 1988, 705–715. [Google Scholar] [CrossRef]
- Gauch, H.G. A simple protocol for AMMI analysis of yield trials. Crop. Sci. 2013, 53, 1860–1869. [Google Scholar] [CrossRef]
- Gauch, H.G. Statistical analysis of yield trials by AMMI and GGE. Crop. Sci. 2006, 46, 1488–1500. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S.; Ma, B.; Woods, S.; Cornelius, P.L. GGE biplot vs. AMMI analysis of genotype-by-environment data. Crop. Sci. 2007, 47, 643–653. [Google Scholar] [CrossRef]
- Purchase, J.L.; Hatting, H.; Van Deventer, C.S. Genotype× environment interaction of winter wheat (Triticum aestivum L.) in South Africa: II. Stability analysis of yield performance. S. Afr. J. Plant. Soil 2000, 17, 101–107. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, L.; Zhonghuai, X. Analysis of Variety Stability Based on AMMI Model. Acta Agronomica Sinica 1998, 3, 304–309. [Google Scholar]
- Bose, L.K.; Jambhulkar, N.N.; Pande, K.; Singh, O.N. Use of AMMI and other stability statistics in the simultaneous selection of rice genotypes for yield and stability under direct-seeded conditions. Chil. J. Agric. Res. 2014, 74, 3–9. [Google Scholar] [CrossRef]
- Finlay, K.W.; Wilkinson, G.N. The analysis of adaptation in a plant-breeding programme. Aust. J. Agric. Res. 1963, 14, 742–754. [Google Scholar] [CrossRef]
- Lian, L.; de Los Campos, G. FW: An R Package for Finlay-Wilkinson Regression that Incorporates Genomic/Pedigree Information and Covariance Structures Between Environments. G3: Genes Genom Genet 2015, 6, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Lozada, D.N.; Carter, A.H. Accuracy of single and multi-trait genomic prediction models for grain yield in US Pacific Northwest winter wheat. Crop. Breed. Genet. Genomics 2019. [Google Scholar] [CrossRef]
- Lewien, M.J.; Murray, T.D.; Jernigan, K.L.; Garland-Campbell, K.A.; Carter, A.H. Genome-wide association mapping for eyespot disease in US Pacific Northwest winter wheat. PLoS ONE 2018, 13, 1–19. [Google Scholar] [CrossRef]
- Jernigan, K.L.; Godoy, J.V.; Huang, M.; Zhou, Y.; Morris, C.F.; Garland-Campbell, K.A.; Zhang, Z.; Carter, A.H. Genetic Dissection of End-Use Quality Traits in Adapted Soft White Winter Wheat. Front. Plant. Sci. 2018, 9, 271. [Google Scholar] [CrossRef]
- Peterson, C.J.; Allan, R.E.; Rubenthaler, G.L.; Line, R.F. Registration of ‘Eltan’ Wheat. Crop. Sci. 1991, 31, 1704. [Google Scholar] [CrossRef]
- Allan, R.E.; Peterson, C.J.; Rubenthaler, G.L.; Line, R.F.; Roberts, D.E. Registration of ‘Madsen’wheat. Crop. Sci. 1989, 29, 1575–1576. [Google Scholar] [CrossRef]
- Rodríguez, F.; Alvarado, G.; Pacheco, Á.; Burgueño, J.; ACBD-R. Augmented Complete Block Design with R for Windows. Version 4.0. CIMMYT Research Data & Software Repository Network. 2018. Available online: hdl:11529/10855 (accessed on 24 September 2019).
- Wang, Y.; Mette, M.F.; Miedaner, T.; Gottwald, M.; Wilde, P.; Reif, J.C.; Zhao, Y. The accuracy of prediction of genomic selection in elite hybrid rye populations surpasses the accuracy of marker-assisted selection and is equally augmented by multiple field evaluation locations and test years. BMC Genomics 2014, 15, 556. [Google Scholar] [CrossRef][Green Version]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package. 1.3-1. 2019. Available online: https://cran.r-project.org/web/packages/agricolae/index.html (accessed on 19 January 2020).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http.//www.R-project.org.
- Zobel, R.W.; Wright, M.J.; Gauch, H.G. Statistical analysis of a yield trial. Agron. J. 1988, 80, 388–393. [Google Scholar] [CrossRef]
- Amiri, E.; Farshadfar, E.; Jowkar, M.M. AMMI analysis of wheat substitution lines for detecting genes controlling adaptability. Int. J. Adv. Biol Biomed. Res. 2013, 1, 1112–1123. [Google Scholar]
- Wang, S.; Wong, D.; Forrest, K.; Allen, A.; Chao, S.; Huang, B.E.; Maccaferri, M.; Salvi, S.; Milner, S.G.; Cattivelli, L.; et al. Characterization of polyploid wheat genomic diversity using a high-density 90,000 single nucleotide polymorphism array. Plant. Biotechnol. J. 2014, 12, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, M.; Fan, B.; Buckler, E.S.; Zhang, Z. Iterative Usage of Fixed and Random Effect Models for Powerful and Efficient Genome-Wide Association Studies. PLoS Genet. 2016, 12, e1005767. [Google Scholar] [CrossRef] [PubMed]
- Lipka, A.E.; Tian, F.; Wang, Q.; Peiffer, J.; Li, M.; Bradbury, P.J.; Gore, M.A.; Buckler, E.S.; Zhang, Z. GAPIT: Genome association and prediction integrated tool. Bioinformatics 2012, 28, 2397–2399. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 289–300. [Google Scholar] [CrossRef]
- JMP Pro v.11® SAS Institute. Cary, NC,,USA 1989-2019. Available online: https://www.jmp.com/en_us/home.html.
- Endelman, J.B. Ridge regression and other kernels for genomic selection with R package rrBLUP. Plant. Genome 2011, 4, 250–255. [Google Scholar] [CrossRef]
- Chen, C.J.; Zhang, Z. iPat: Intelligent prediction and association tool for genomic research. Bioinformatics. 2018, 34, 1925–1927. [Google Scholar] [CrossRef]
- Gupta, P.K.; Kulwal, P.L.; Jaiswal, V. Chapter Two—Association mapping in plants in the post-GWAS genomics era. In Advances in Genetics; Kumar, D., Ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Liu, H.-J.; Yan, J. Crop genome-wide association study: A harvest of biological relevance. Plant. J. 2019, 97, 8–18. [Google Scholar] [CrossRef]
- Miedaner, T.; Hübner, M.; Korzun, V.; Schmiedchen, B.; Bauer, E.; Haseneyer, G.; Wilde, P.; Reif, J.C. Genetic architecture of complex agronomic traits examined in two testcross populations of rye (Secale cereale L.). BMC Genomics 2012, 13, 706. [Google Scholar] [CrossRef]
- Lacaze, X.; Hayes, P.M.; Korol, A. Genetics of phenotypic plasticity: QTL analysis in barley, Hordeum vulgare. Hered. 2009, 102, 163–173. [Google Scholar] [CrossRef]
- Frashadfar, E.; Safari, H.; Jamshidi, B. GGE biplot analysis of adaptation in wheat substitution lines. Int. J. Agric. Crop. Sci. 2012, 4, 877–881. [Google Scholar]
- Lozada, D.N.; Godoy, J.V.; Ward, B.P.; Carter, A.H. Genomic Prediction and Indirect Selection for Grain Yield in US Pacific Northwest Winter Wheat Using Spectral Reflectance Indices from High-Throughput Phenotyping. Int. J. Mol. Sci. 2020, 21, 165. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.B.; Lynch, M. Selection and G.x.E: Evolution and Selection of Quantitative Traits II: Advanced Topics. In Breeding and Evolution; University Press: Oxford, UK, 2014. [Google Scholar]
- Cober, E.R.; Morrison, M.J. Genetic Improvement Estimates from Cultivar × Crop Management Trials, Are Larger in High-Yield Cropping Environments. Crop. Sci. 2015, 55, 1425–1434. [Google Scholar] [CrossRef]
- Tollenaar, M.; Lee, E.A. Yield potential, yield stability and stress tolerance in maize. F. Crop. Res. 2002, 75, 161–169. [Google Scholar] [CrossRef]
- Xavier, A.; Jarquin, D.; Howard, R.; Ramasubramanian, V.; Specht, J.E.; Graef, G.L.; Beavis, W.D.; Diers, B.W.; Song, Q.; Cregan, P.B.; et al. Genome-Wide Analysis of Grain Yield Stability and Environmental Interactions in a Multiparental Soybean Population. G3: Genes Genom Genet. 2018, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
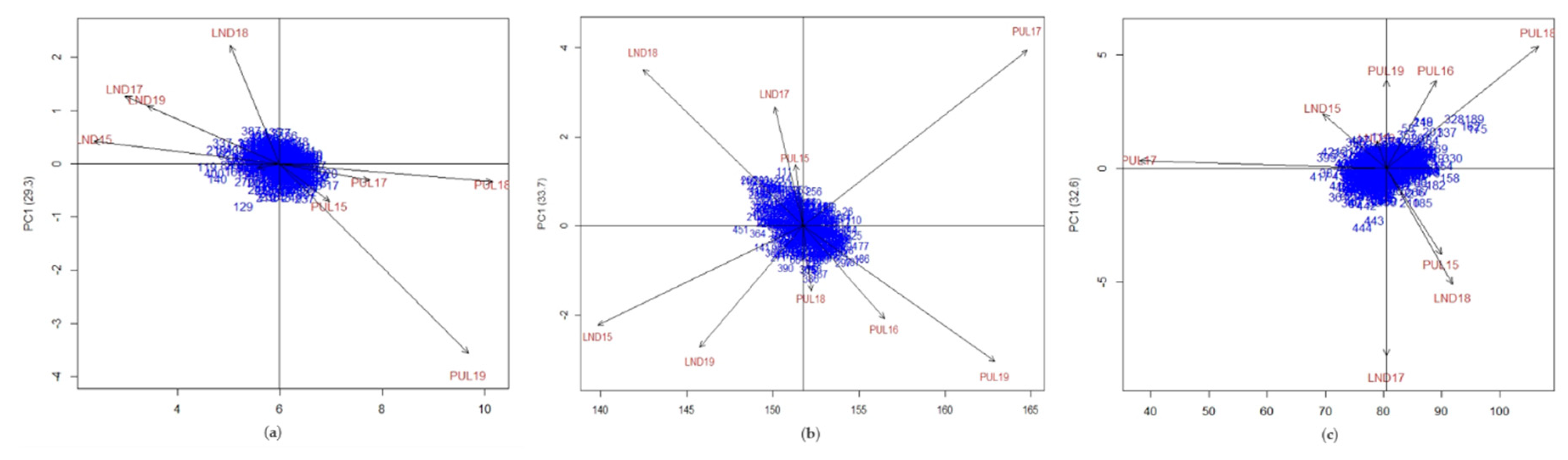
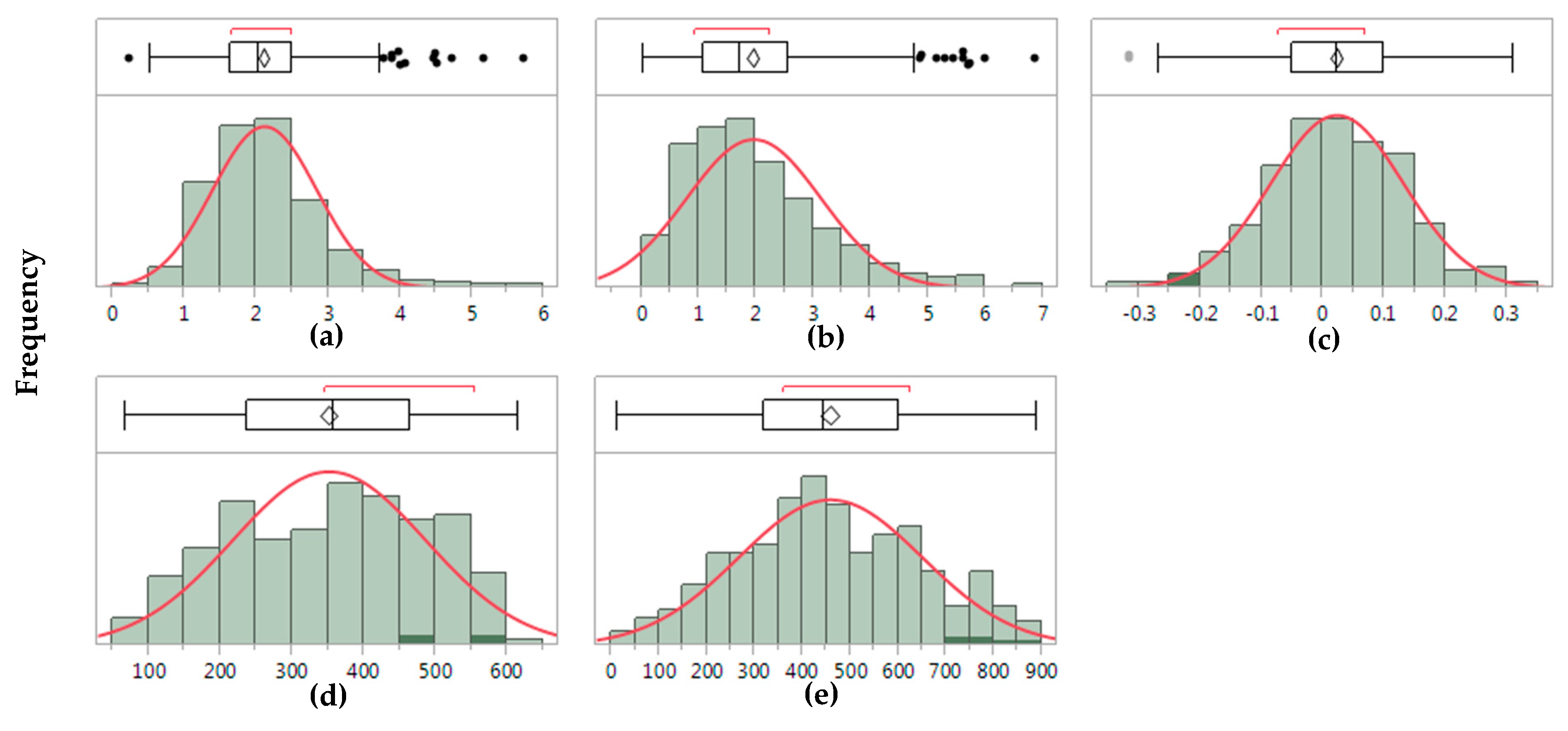
| Trait | Genotype (G) | Environment (E) | GE | Residuals | No. of Sig. IPC | % Variation for IPC1 | Ratio (GEsignal/G) |
|---|---|---|---|---|---|---|---|
| Grain yield | 3.90 | 196.61 | 15.65 | 12.78 | 7 | 29.30 | 2.55 |
| Heading date | 44.31 | 1753.47 | 126.23 | 112.09 | 5 | 33.70 | 1.73 |
| Plant height | 474.09 | 8725.56 | 948.72 | 909.79 | 5 | 32.60 | 1.15 |
| Trait 1 | Heritability | Heritability, Trait Stability 2 | Mean (Adj. Values) | Stability Index (Mean) | ||||
|---|---|---|---|---|---|---|---|---|
| ASI 3 | ASV | FW | RS | YSI | ||||
| GY (t ha−1) | 0.56 | 0.55 | 6.10 | 2.10 | 1.95 | 0.02 | 350.85 | 457 |
| HD (Julian Days) | 0.83 | 0.63 | 151.74 | 5.71 | 7.11 | 0.07 | - | - |
| PH (cm) | 0.79 | 0.70 | 87.1 | 15.43 | 15.59 | 0.15 | - | - |
| Stability Index | Grain Yield | Heading Date | Plant Height | |||
|---|---|---|---|---|---|---|
| Adjusted Values | GEBV | Adjusted Values | GEBV | Adjusted Values | GEBV | |
| ASI | −0.11 * | −0.11 * | −0.12 * | −0.12 * | 0.05 | 0.01 |
| ASV | −0.05 | −0.08 | −0.10 * | −0.10 * | −0.02 | −0.04 |
| FW | 0.41 *** | 0.35 *** | 0.74 *** | 0.75 *** | 0.84 *** | 0.77 *** |
| RS | −0.92 *** | −0.81 *** | - | - | - | - |
| YSI | −0.66 *** | −0.61 *** | - | - | - | - |
| SNP ID | SNP Name | Chr. | Position (cM) | Allele | Minor Allele Frequency | Positive Allele 1 | Trait 2 | Model | Effect | FDR-Adjusted p-Value | R2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IWB9588 | BS00065601_51 | 1A | 84.63 | [A/G] | 0.17 | A | PH_ALL | K | 1.18 | 2.2E-04 | 0.02 |
| A | PH_ALL | K-PC | 1.15 | 4.08E-05 | 0.03 | ||||||
| A | PH_FW | K | 0.03 | 4.0E-04 | 0.04 | ||||||
| IWB72787 | Tdurum_contig61410_542 | 1B | 53.49 | [A/G] | 0.28 | G | GY_ALL | K-PC | 0.06 | 0.003 | 0.03 |
| A | GY_YSI | K | −61.66 | 7.8E-04 | 0.08 | ||||||
| A | GY_RS | K-PC | −44.14 | 0.003 | 0.06 | ||||||
| A | GY_YSI | K-PC | −79.59 | 2.98E-06 | 0.02 | ||||||
| IWB7202 | BS00022666_51 | 2A | 115.08 | [A/G] | 0.27 | G | HD_PUL | K | 0.34 | 1.14E-03 | 0.03 |
| G | HD_FW | K | 0.022 | 0.007 | 0.01 | ||||||
| IWB72096 | Tdurum_contig50589_867 | 2B | 114.09 | [T/C] | 0.27 | T | HD_PUL | K | −0.29 | 1.14E-05 | 0.02 |
| T | HD_PUL | K-PC | −0.22 | 0.015 | 0.02 | ||||||
| T | HD_FW | K-PC | −0.02 | 0.010 | 0.01 | ||||||
| IWB7001 | BS00022276_51 | 2D | 18.22 | [T/G] | 0.20 | G | HD_ALL | K-PC | 0.27 | 4.21E-04 | 0.07 |
| G | HD_PUL | K-PC | 0.37 | 5.08E-04 | 0.06 | ||||||
| G | HD_FW | K | 0.03 | 0.006 | 0.05 | ||||||
| G | HD_FW | K-PC | 0.03 | 3.29E-04 | 0.11 | ||||||
| IWB75368 | wsnp_BE405275A_Ta_1_1 | 4A | 29.86 | [T/G] | 0.09 | T | GY_ALL | K | −0.08 | 3.05E-05 | 0.02 |
| T | GY_ALL | K-PC | −0.07 | 0.002 | 0.02 | ||||||
| G | GY_RS | K-PC | 37.42 | 0.037 | 0.02 | ||||||
| IWB12343 | BS00108019_51 | 5B | 51.16 | [T/C] | 0.47 | T | PH_ALL | K | −0.63 | 0.003 | 0.02 |
| T | PH_ALL | K-PC | −0.56 | 0.029 | 0.05 | ||||||
| T | PH_FW | K | −0.02 | 0.003 | 0.03 | ||||||
| IWB27416 | Excalibur_c53772_302 | 5B | 68.36 | [T/C] | 0.05 | C | GY_ALL | K-PC | 0.06 | 0.032 | 0.01 |
| T | HD_LND | K-PC | −0.19 | 0.012 | 0.01 | ||||||
| T | GY_RS | K-PC | −55.62 | 0.001 | 0.03 | ||||||
| IWB69770 | Tdurum_contig29357_338 | 6A | 118.07 | [A/C] | 0.22 | A | GY_ASI | K | −0.20 | 0.005 | 0.02 |
| A | PH_FW | K | −0.02 | 0.036 | 0.06 | ||||||
| IWB77743 | wsnp_Ex_c31955_40681185 | 7A | 216.28 | [T/C] | 0.25 | T | GY_ALL | K | −0.04 | 0.010 | 0.02 |
| C | GY_YSI | K | 38.92 | 0.004 | 0.01 | ||||||
| IWB80605 | wsnp_Ku_c5693_10079278 | 7A | 208.71 | [A/C] | 0.28 | A | PH_ALL | K | −0.59 | 0.028 | 0.01 |
| A | PH_FW | K | −0.02 | 0.001 | 0.04 | ||||||
| IWB7147 | BS00022542_51 | 7B | 76.31 | [T/C] | 0.49 | T | GY_ALL | K-PC | −0.04 | 0.011 | 0.03 |
| C | GY_RS | K | 28.88 | 0.004 | 0.10 | ||||||
| C | GY_YSI | K | 42.28 | 0.003 | 0.05 | ||||||
| C | GY_RS | K-PC | 32.22 | 0.001 | 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozada, D.N.; Carter, A.H. Insights into the Genetic Architecture of Phenotypic Stability Traits in Winter Wheat. Agronomy 2020, 10, 368. https://doi.org/10.3390/agronomy10030368
Lozada DN, Carter AH. Insights into the Genetic Architecture of Phenotypic Stability Traits in Winter Wheat. Agronomy. 2020; 10(3):368. https://doi.org/10.3390/agronomy10030368
Chicago/Turabian StyleLozada, Dennis N., and Arron H. Carter. 2020. "Insights into the Genetic Architecture of Phenotypic Stability Traits in Winter Wheat" Agronomy 10, no. 3: 368. https://doi.org/10.3390/agronomy10030368
APA StyleLozada, D. N., & Carter, A. H. (2020). Insights into the Genetic Architecture of Phenotypic Stability Traits in Winter Wheat. Agronomy, 10(3), 368. https://doi.org/10.3390/agronomy10030368





