Biochar and Vermicompost Amendments Affect Substrate Properties and Plant Growth of Basil and Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Container Substrates Treatments
2.2. Substrate Physical Properties and Substrate Leachate pH
2.3. Plant Growth and Development
2.4. Statistical Analysis
3. Results and Discussion
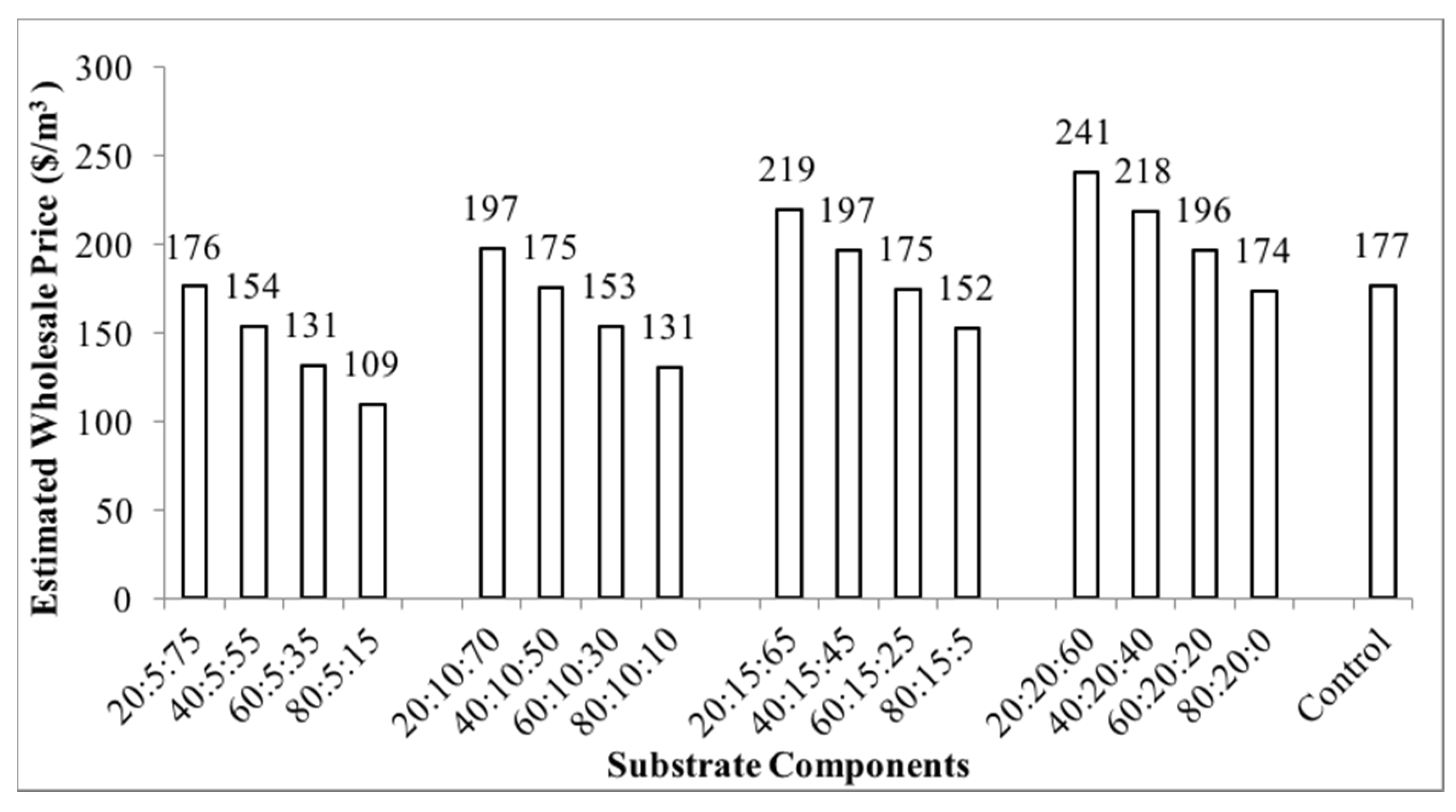
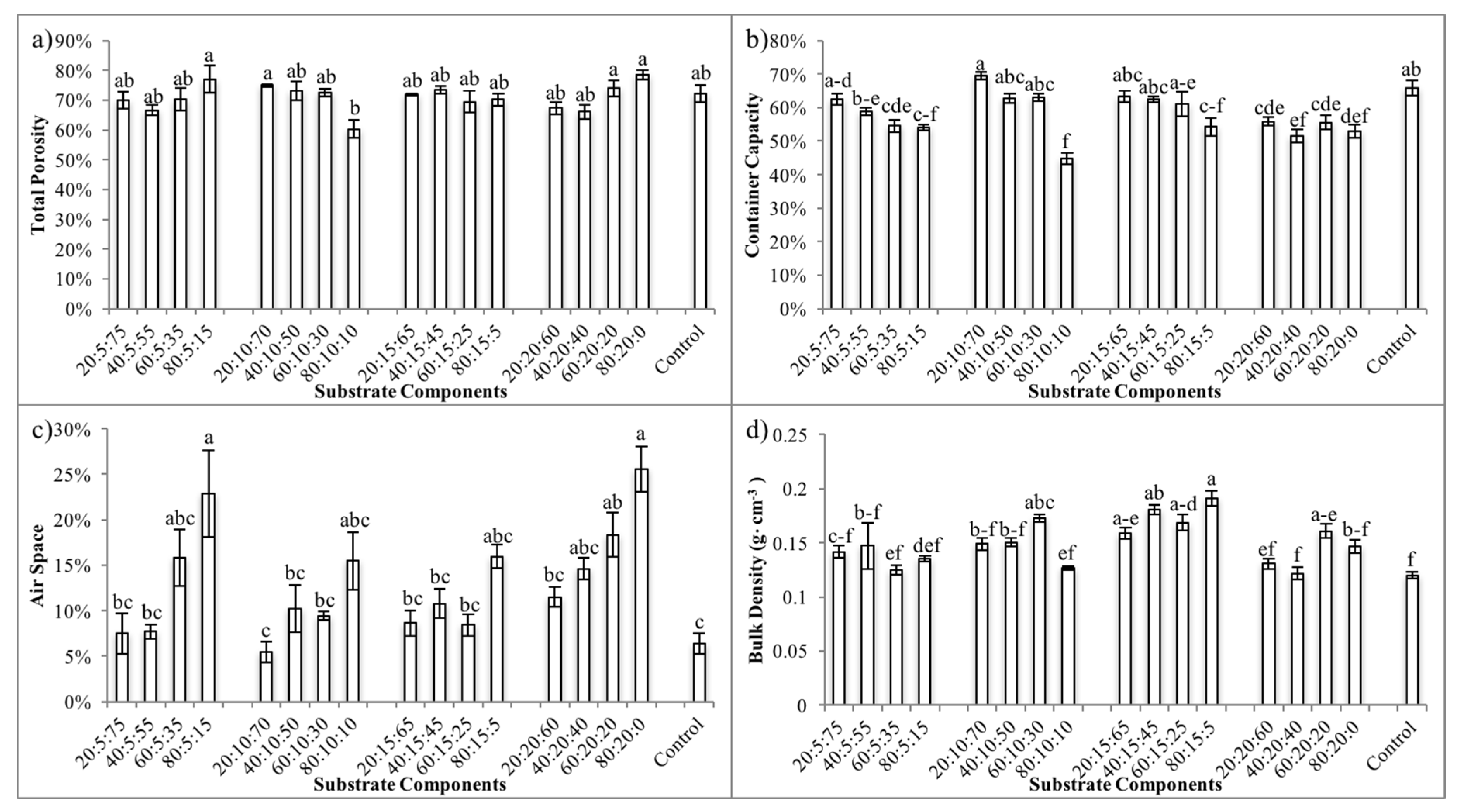
3.1. Physical Properties of the Container Substrates
3.2. Substrate Leachate pH
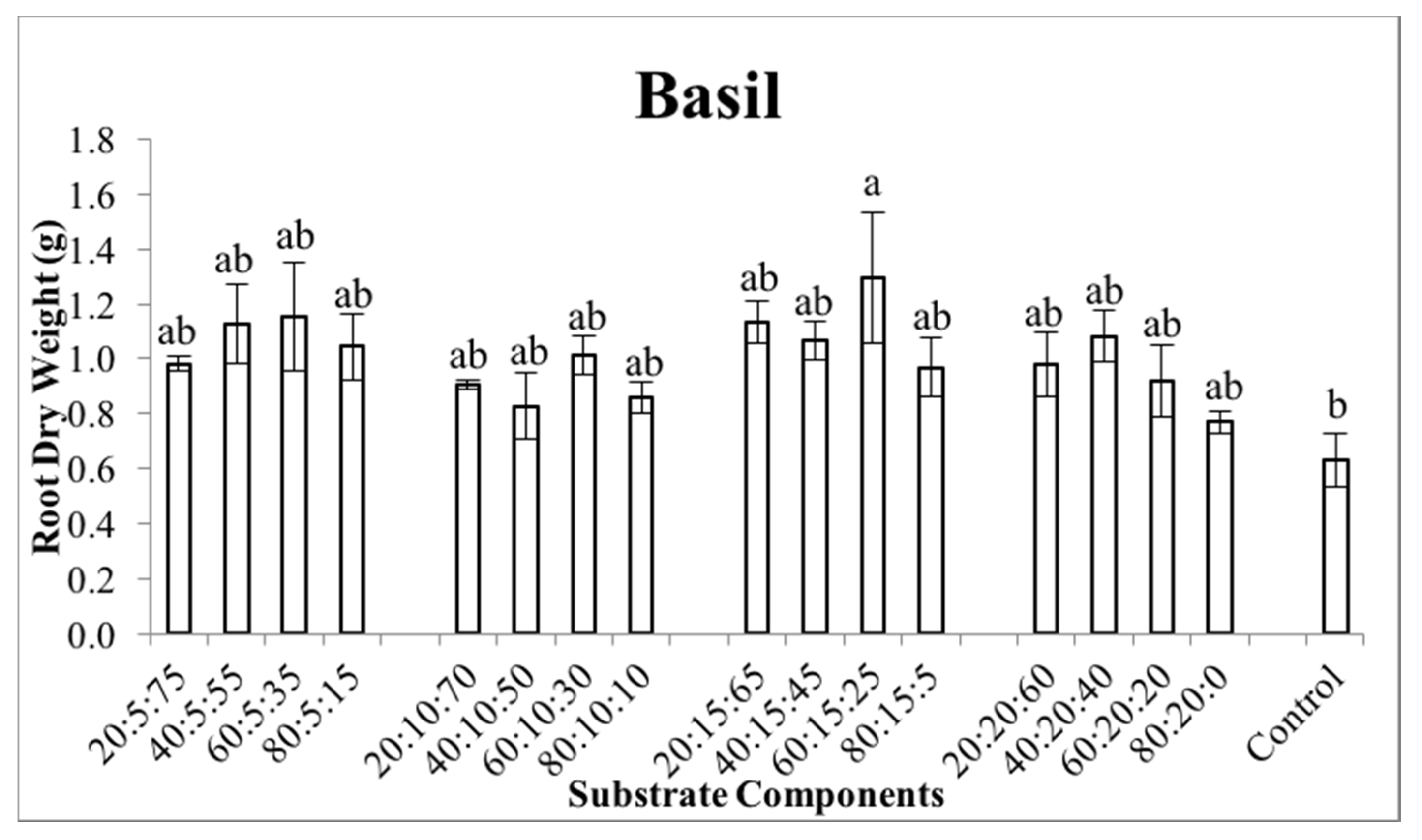
3.3. Plant Growth and Development
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Webber, C.L., III; White, P.M., Jr.; Gu, M.; Spaunhorst, D.J.; Lima, I.M.; Petrie, E.C. Sugarcane and pine biochar as amendments for greenhouse growing media for the production of bean (Phaseolus vulgaris L.) seedlings. J. Agric. Sci. 2018, 10, 58–68. [Google Scholar] [CrossRef]
- Choi, H.-S.; Zhao, Y.; Dou, H.; Cai, X.; Gu, M.; Yu, F. Effects of biochar mixtures with pine-bark based substrates on growth and development of horticultural crops. Horti. Environ. Biotechnol. 2018, 59, 345–354. [Google Scholar] [CrossRef]
- Peng, D.; Gu, M.; Zhao, Y.; Yu, F.; Choi, H.-S. Effects of biochar mixes with peat-moss based substrates on growth and development of horticultural crops. Hortic. Sci. Technol. 2018, 36, 501–512. [Google Scholar]
- Gvero, P.M.; Papuga, S.; Mujanic, I.; Vaskovic, S. Pyrolysis as a key process in biomass combustion and thermochemical conversion. Therm. Sci. 2016, 20, 154. [Google Scholar] [CrossRef]
- Rahman, A.A.; Sulaiman, F.; Abdullah, N. Influence of washing medium pre-treatment on pyrolysis yields and product characteristics of palm kernel shell. J. Phys. Sci. 2016, 27, 53. [Google Scholar]
- Hansen, V.; Müller-Stöver, D.; Ahrenfeldt, J.; Holm, J.K.; Henriksen, U.B.; Hauggaard-Nielsen, H. Gasification biochar as a valuable by-product for carbon sequestration and soil amendment. Biomass Bioenergy 2015, 72, 300–308. [Google Scholar] [CrossRef]
- Altland, J.E.; Locke, J.C. Gasified rice hull biochar is a source of phosphorus and potassium for container-grown plants. J. Environ. Hortic. 2013, 31, 138–144. [Google Scholar]
- Laird, D.A. The charcoal vision: A win–win–win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agron. J. 2008, 100, 178–181. [Google Scholar]
- Zheng, W.; Sharma, B.; Rajagopalan, N. Using Biochar as a Soil Amendment for Sustainable Agriculture; Illinois Sustainable Technology Center: Champaign, IL, USA, 2010. [Google Scholar]
- Tian, Y.; Sun, X.; Li, S.; Wang, H.; Wang, L.; Cao, J.; Zhang, L. Biochar made from green waste as peat substitute in growth media for Calathea rotundifola cv. Fasciata. Sci. Hortic. 2012, 143, 15–18. [Google Scholar] [CrossRef]
- Buss, W.; Graham, M.C.; Shepherd, J.G.; Mašek, O. Risks and benefits of marginal biomass-derived biochars for plant growth. Sci. Total Environ. 2016, 569, 496–506. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Sun, J.; Shao, H. Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci. Total Environ. 2016, 568, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, S.F.; Kenar, J.A.; Thompson, A.R.; Peterson, S.C. Comparison of biochars derived from wood pellets and pelletized wheat straw as replacements for peat in potting substrates. Ind. Crops Prod. 2013, 51, 437–443. [Google Scholar] [CrossRef]
- Hansen, V.; Hauggaard-Nielsen, H.; Petersen, C.T.; Mikkelsen, T.N.; Müller-Stöver, D. Effects of gasification biochar on plant-available water capacity and plant growth in two contrasting soil types. Soil Till. Res. 2016, 161, 1–9. [Google Scholar] [CrossRef]
- Locke, J.C.; Altland, J.E.; Ford, C.W. Gasified rice hull biochar affects nutrition and growth of horticultural crops in container substrates. J. Environ. Hortic. 2013, 31, 195–202. [Google Scholar]
- Yu, F.; Steele, P.H.; Gu, M.; Zhao, Y. Using Biochar as Container Substrate for Plant Growth. U.S. Patent 9,359,267 B2, 7 June 2016. [Google Scholar]
- Huang, L.; Gu, M. Effects of biochar on container substrate properties and growth of plants—A review. Horticulturae 2019, 5, 14. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Prasad, M.; Kavanagh, A.; Tzortzakis, N. Biochar type and ratio as a peat additive/partial peat replacement in growing media for cabbage seedling production. Agronomy 2019, 9, 693. [Google Scholar] [CrossRef]
- Prasad, M.; Chrysargyris, A.; McDaniel, N.; Kavanagh, A.; Gruda, N.S.; Tzortzakis, N. Plant nutrient availability and pH of biochars and their fractions, with the possible use as a component in a growing media. Agronomy 2020, 10, 10. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Hatala, J.A.; Detto, M.; Sonnentag, O.; Deverel, S.J.; Verfaillie, J.; Baldocchi, D.D. Greenhouse gas (CO2, CH4, H2O) fluxes from drained and flooded agricultural peatlands in the Sacramento-San Joaquin Delta. Agric. Ecosyst. Environ. 2012, 150, 1–18. [Google Scholar] [CrossRef]
- Leifeld, J.; Menichetti, L. The underappreciated potential of peatlands in global climate change mitigation strategies. Nat. Commun. 2018, 9, 1071. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Heiskanen, J.; Englund, K.; Tervahauta, A. Pelleted biochar: Chemical and physical properties show potential use as a substrate in container nurseries. Biomass Bioenergy 2011, 35, 2018–2027. [Google Scholar] [CrossRef]
- Crutchfield, E.F.; McGiffen, M.E., Jr.; Merhaut, D.J. Effects of biochar on nutrient leaching and begonia plant growth. J. Environ. Hortic. 2018, 36, 126–132. [Google Scholar]
- Zulfiqar, F.; Younis, A.; Chen, J. Biochar or biochar-compost amendment to a peat-based substrate improves growth of Syngonium podophyllum. Agronomy 2019, 9, 460. [Google Scholar] [CrossRef]
- Dispenza, V.; De Pasquale, C.; Fascella, G.; Mammano, M.M.; Alonzo, G. Use of biochar as peat substitute for growing substrates of Euphorbia × lomi potted plants. Span. J. Agric. Res. 2016, 14, e0908. [Google Scholar] [CrossRef]
- Nieto, A.; Gascó, G.; Paz-Ferreiro, J.; Fernández, J.; Plaza, C.; Méndez, A. The effect of pruning waste and biochar addition on brown peat based growing media properties. Sci. Hortic. 2016, 199, 142–148. [Google Scholar] [CrossRef]
- Kadota, M.; Niimi, Y. Effects of charcoal with pyroligneous acid and barnyard manure on bedding plants. Sci. Hortic. 2004, 101, 327–332. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Eller, F.J.; Evangelista, R.L.; Moser, B.R.; Lee, E.; Wagner, R.E.; Peterson, S.C. Evaluation of biochar-anaerobic potato digestate mixtures as renewable components of horticultural potting media. Ind. Crops Prod. 2015, 65, 467–471. [Google Scholar] [CrossRef]
- Yu, P.; Huang, L.; Li, Q.; Lima, I.M.; White, P.M.; Gu, M. Effects of mixed hardwood and sugarcane biochar as bark-based substrate substitutes on container plants production and nutrient leaching. Agronomy 2020, 10, 156. [Google Scholar] [CrossRef]
- Gu, M.; Li, Q.; Steele, P.H.; Niu, G.; Yu, F. Growth of ‘fireworks’ gomphrena grown in substrates amended with biochar. J. Food Agric. Environ. 2013, 11, 819–821. [Google Scholar]
- Housley, C.; Kachenko, A.; Singh, B. Effects of eucalyptus saligna biochar-amended media on the growth of Acmena smithii, Viola var. hybrida, and Viola × wittrockiana. J. Hortic. Sci. Biotechnol. 2015, 90, 187–194. [Google Scholar] [CrossRef]
- Webber, C.L., III; White, P.M., Jr.; Spaunhorst, D.J.; Lima, I.M.; Petrie, E.C. Sugarcane biochar as an amendment for greenhouse growing media for the production of cucurbit seedlings. J. Agric. Sci. 2018, 10, 104. [Google Scholar] [CrossRef]
- Mitchell, M.; Hornor, S.; Abrams, B. Decomposition of sewage sludge in drying beds and the potential role of the earthworm, Eisenia foetida. J. Environ. Qual. 1980, 9, 373–378. [Google Scholar] [CrossRef]
- Edwards, C. Production of feed protein from animal waste by earthworms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985, 310, 299–307. [Google Scholar]
- Chan, P.L.; Griffiths, D. The vermicomposting of pre-treated pig manure. Biol. Wastes 1988, 24, 57–69. [Google Scholar] [CrossRef]
- Hartenstein, R.; Bisesi, M.S. Use of earthworm biotechnology for the management of effluents from intensively housed livestock. Outlook Agric. 1989, 18, 72–76. [Google Scholar] [CrossRef]
- Manna, M.; Jha, S.; Ghosh, P.; Ganguly, T.; Singh, K.; Takkar, P. Capacity of various food materials to support growth and reproduction of epigeic earthworms on vermicompost. J. Sustain. For. 2005, 20, 1–15. [Google Scholar] [CrossRef]
- Sinha, R.K.; Agarwal, S.; Chauhan, K.; Valani, D. The wonders of earthworms & its vermicompost in farm production: Charles darwin’s ‘friends of farmers’, with potential to replace destructive chemical fertilizers. Agric. Sci. 2010, 1, 76. [Google Scholar]
- Atiyeh, R.; Subler, S.; Edwards, C.; Bachman, G.; Metzger, J.; Shuster, W. Effects of vermicomposts and composts on plant growth in horticultural container media and soil. Pedobiologia 2000, 44, 579–590. [Google Scholar] [CrossRef]
- Edwards, C.A.; Burrows, I. Potential of earthworm composts as plant growth media. In Earthworms in Waste and Environmental Management; Edwards, C.A., Neuhauser, E.F., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 1988. [Google Scholar]
- McGinnis, M.; Bilderback, T.; Warren, S. Vermicompost amended pine bark provides most plant nutrients for Hibiscus moscheutos ‘Luna Blush’. Acta Hortic. 2011, 891, 249–256. [Google Scholar] [CrossRef]
- Abbey, L.; Young, C.; Teitel-Payne, R.; Howe, K. Evaluation of proportions of vermicompost and coir in a medium for container-grown Swiss chard. Int. J. Veg. Sci. 2012, 18, 109–120. [Google Scholar] [CrossRef]
- Alvarez, J.; Pasian, C.; Lal, R.; López, R.; Fernández, M. Vermicompost and biochar as substitutes of growing media in ornamental-plant production. J. Appl. Hortic. 2017, 19, 205–214. [Google Scholar]
- Álvarez, J.M.; Pasian, C.; Lal, R.; López, R.; Díaz, M.J.; Fernández, M. Morpho-physiological plant quality when biochar and vermicompost are used as growing media replacement in urban horticulture. Urban For. Urban Green. 2018, 34, 175–180. [Google Scholar] [CrossRef]
- Szczech, M.; Smolińska, U. Comparison of suppressiveness of vermicomposts produced from animal manures and sewage sludge against Phytophthora nicotianae Breda de Haan var. nicotianae. J. Phytopathol. 2001, 149, 77–82. [Google Scholar] [CrossRef]
- Liu, R.; Gu, M.; Huang, L.; Yu, F.; Jung, S.-K.; Choi, H.-S. Effect of pine wood biochar mixed with two types of compost on growth of bell pepper (Capsicum annuum L.). Hortic. Environ. Biotechnol. 2019, 60, 313–319. [Google Scholar] [CrossRef]
- Abushita, A.A.; Hebshi, E.A.; Daood, H.G.; Biacs, P.A. Determination of antioxidant vitamins in tomatoes. Food Chem. 1997, 60, 207–212. [Google Scholar] [CrossRef]
- Jones, J.B., Jr. Tomato plant culture. In The Field, Greenhouse, and Home Garden; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Makri, O.; Kintzios, S. Ocimum sp. (basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs Spices Med. Plants 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Bernstein, N.; Sela, S.; Dudai, N.; Gorbatsevich, E. Salinity stress does not affect root uptake, dissemination and persistence of salmonella in sweet-basil (Ocimum basilicum). Front. Plant Sci. 2017, 8, 675. [Google Scholar] [CrossRef]
- Grayer, R.J.; Kite, G.C.; Goldstone, F.J.; Bryan, S.E.; Paton, A.; Putievsky, E. Infraspecific taxonomy and essential oil chemotypes in sweet basil, Ocimum basilicum. Phytochemistry 1996, 43, 1033–1039. [Google Scholar] [CrossRef]
- Sharafzadeh, S.; Alizadeh, O. Nutrient supply and fertilization of basil. Adv. Environ. Biol. 2011, 5, 956–960. [Google Scholar]
- Tesi, R.; Chisci, G.; Nencini, A.; Tallarico, R. Growth response to fertilisation of sweet basil (Ocimum basilicum L.). Acta Hortic. 1995, 390, 93–96. [Google Scholar] [CrossRef]
- BWI Companies, Inc. Available online: https://www.bwicompanies.com/ (accessed on 2 February 2020).
- LeBude, A.; Bilderback, T. The Pour-Through Extraction Procedure: A Nutrient Management Tool for Nursery Crops; AG-717-W:2009; North Carolina Cooperative Extension: Raleigh, NC, USA, 2009. [Google Scholar]
- Fonteno, W.; Hardin, C.; Brewster, J. Procedures for Determining Physical Properties of Horticultural Substrates Using the NCSU Porometer; Horticultural Substrates Laboratory, North Carolina State University: Raleigh, NC, USA, 1995. [Google Scholar]
- Guo, Y.; Niu, G.; Starman, T.; Volder, A.; Gu, M. Poinsettia growth and development response to container root substrate with biochar. Horticulturae 2018, 4, 1. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.-Y.; Tian, Y.; Gong, X.-Q. Biochar and humic acid amendments improve the quality of composted green waste as a growth medium for the ornamental plant Calathea insignis. Sci. Hortic. 2014, 176, 70–78. [Google Scholar] [CrossRef]
- Fan, R.; Luo, J.; Yan, S.; Zhou, Y.; Zhang, Z. Effects of biochar and super absorbent polymer on substrate properties and water spinach growth. Pedosphere 2015, 25, 737–748. [Google Scholar] [CrossRef]
- Méndez, A.; Paz-Ferreiro, J.; Gil, E.; Gascó, G. The effect of paper sludge and biochar addition on brown peat and coir based growing media properties. Sci. Hortic. 2015, 193, 225–230. [Google Scholar] [CrossRef]
- Guo, Y.; Niu, G.; Starman, T.; Gu, M. Growth and development of Easter lily in response to container substrate with biochar. J. Hortic. Sci. Biotechnol. 2018, 94, 80–86. [Google Scholar] [CrossRef]
- Yu, P.; Li, Q.; Huang, L.; Niu, G.; Gu, M. Mixed hardwood and sugarcane bagasse biochar as potting mix components for container tomato and basil seedling production. Appl. Sci. 2019, 9, 4713. [Google Scholar] [CrossRef]
- Méndez, A.; Cárdenas-Aguiar, E.; Paz-Ferreiro, J.; Plaza, C.; Gascó, G. The effect of sewage sludge biochar on peat-based growing media. Biol. Agric. Hortic. 2017, 33, 40–51. [Google Scholar] [CrossRef]
- Landis, T.D. Growing media. Contain. Grow. Med. 1990, 2, 41–85. [Google Scholar]
- Ducey, T.F.; Novak, J.M.; Johnson, M.G. Effects of biochar blends on microbial community composition in two coastal plain soils. Agriculture 2015, 5, 1060–1075. [Google Scholar] [CrossRef]
- Streubel, J.; Collins, H.; Garcia-Perez, M.; Tarara, J.; Granatstein, D.; Kruger, C. Influence of contrasting biochar types on five soils at increasing rates of application. Soil Sci. Soc. Am. J. 2011, 75, 1402–1413. [Google Scholar] [CrossRef]
- Chan, K.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Soil Res. 2008, 45, 629–634. [Google Scholar] [CrossRef]
- Li, Y.; Alva, A.; Calvert, D.; Zhang, M. A rapid nondestructive technique to predict leaf nitrogen status of grapefruit tree with various nitrogen fertilization practices. HortTechnology 1998, 8, 81–86. [Google Scholar] [CrossRef]
- Nair, A.; Carpenter, B. Biochar rate and transplant tray cell number have implications on pepper growth during transplant production. HortTechnology 2016, 26, 713–719. [Google Scholar] [CrossRef]
- Dickson, R.W.; Fisher, P.R.; Padhye, S.R.; Argo, W.R. Evaluating calibrachoa (Calibrachoa× hybrida cerv.) genotype sensitivity to iron deficiency at high substrate ph. HortScience 2016, 51, 1452–1457. [Google Scholar] [CrossRef]
- Radhamani, R.; Kannan, R.; Rakkiyappan, P. Leaf chlorophyll meter readings as an indicator for sugarcane yield under iron deficient typic haplustert. Sugar Tech. 2016, 18, 61–66. [Google Scholar] [CrossRef]
- Wallihan, E.F. Relation of chlorosis to concentration of iron in citrus leaves. Am. J. Bot. 1955, 42, 101–104. [Google Scholar] [CrossRef]
- Huang, L.; Niu, G.; Feagley, S.E.; Gu, M. Evaluation of a hardwood biochar and two composts mixes as replacements for a peat-based commercial substrate. Ind. Crops Prod. 2019, 129, 549–560. [Google Scholar] [CrossRef]

| Substrate | N | P | K | Ca | Mg | S | Fe | B | Cu | Mn | Na | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (mg kg−1) | |||||||||||
| CS | 0.70 b z | 540 b | 1265 c | 25108 a | 4237 a | 1744 b | 1508 b | 11 b | 17 b | 98 c | 953 a | 46 b |
| BC | 0.23 c | 456 b | 6362 a | 27507 a | 1299 b | 231 b | 2039 b | 15 b | 9 b | 905 a | 107 c | 13 b |
| VC | 2.43 a | 4901 a | 3714 b | 25841 a | 3819 a | 5996 a | 4835 a | 42 a | 165 a | 374 b | 351 b | 385 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Gu, M.; Yu, P.; Zhou, C.; Liu, X. Biochar and Vermicompost Amendments Affect Substrate Properties and Plant Growth of Basil and Tomato. Agronomy 2020, 10, 224. https://doi.org/10.3390/agronomy10020224
Huang L, Gu M, Yu P, Zhou C, Liu X. Biochar and Vermicompost Amendments Affect Substrate Properties and Plant Growth of Basil and Tomato. Agronomy. 2020; 10(2):224. https://doi.org/10.3390/agronomy10020224
Chicago/Turabian StyleHuang, Lan, Mengmeng Gu, Ping Yu, Chunling Zhou, and Xiuli Liu. 2020. "Biochar and Vermicompost Amendments Affect Substrate Properties and Plant Growth of Basil and Tomato" Agronomy 10, no. 2: 224. https://doi.org/10.3390/agronomy10020224
APA StyleHuang, L., Gu, M., Yu, P., Zhou, C., & Liu, X. (2020). Biochar and Vermicompost Amendments Affect Substrate Properties and Plant Growth of Basil and Tomato. Agronomy, 10(2), 224. https://doi.org/10.3390/agronomy10020224






