Biofertilizers Enhance Quality of Onion
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Analyses
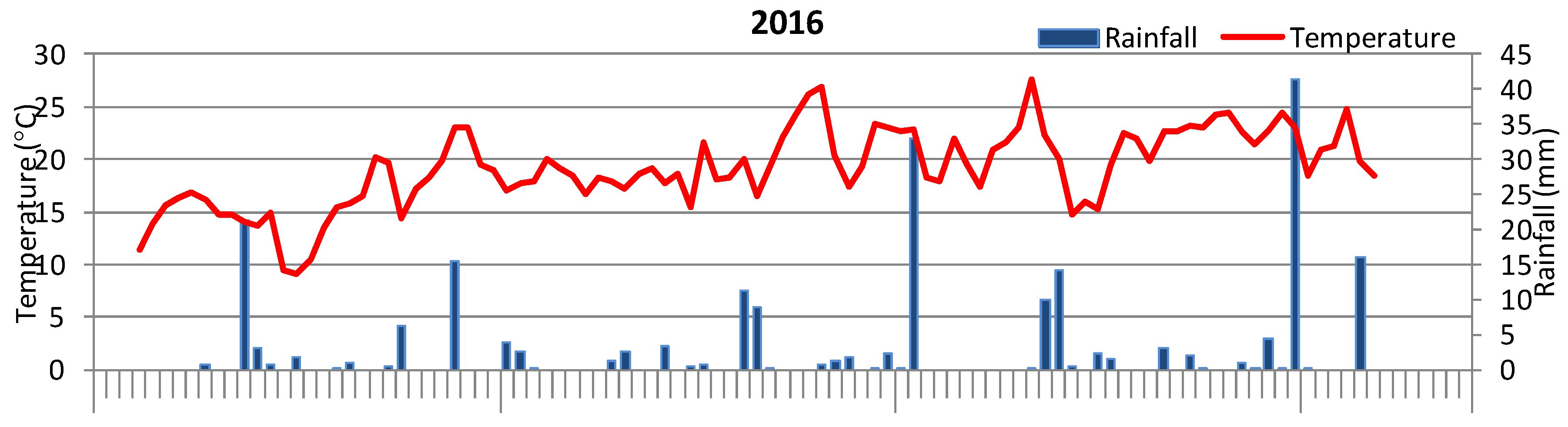
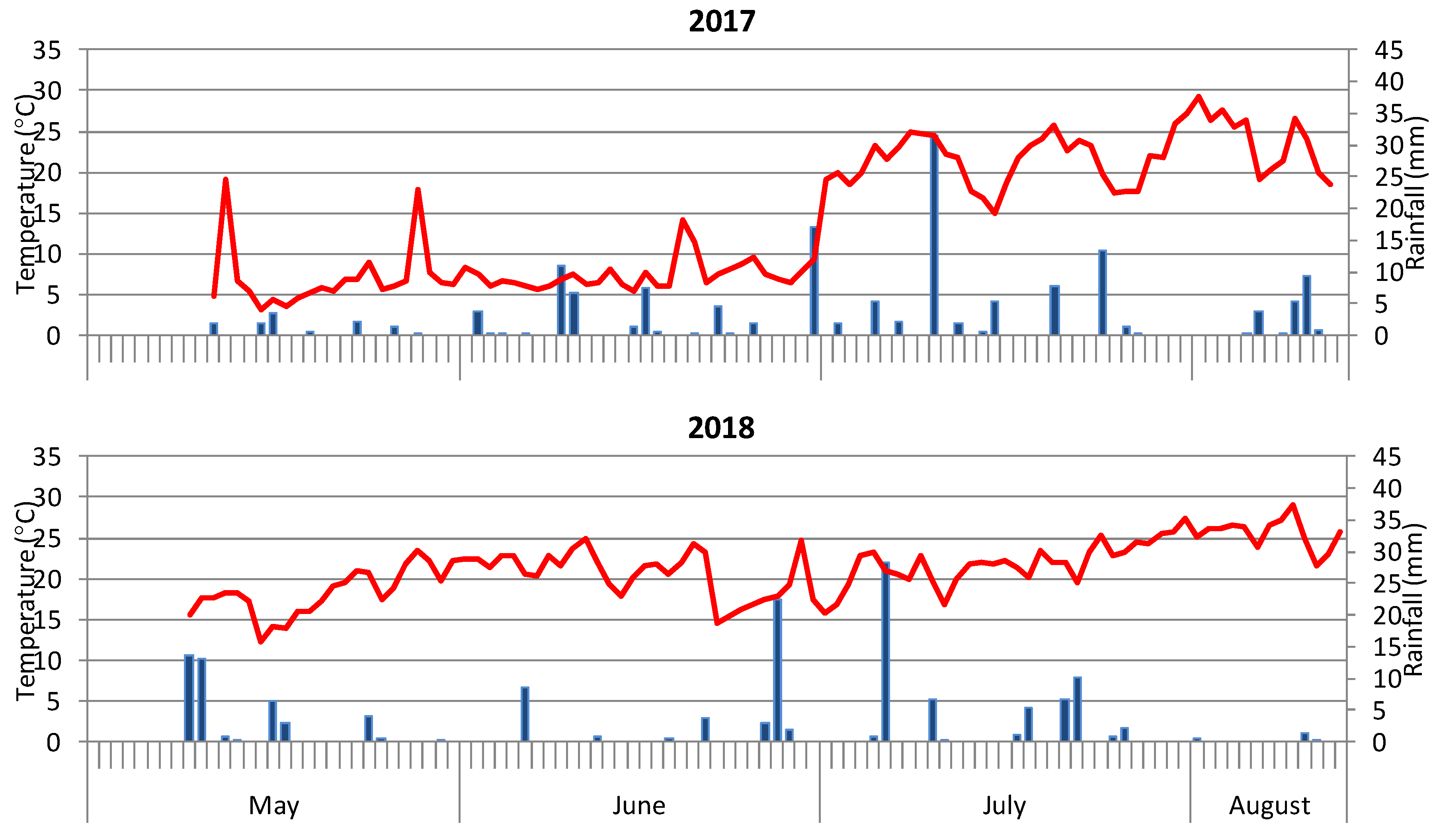
2.2. Weather Conditions in Onion Growing Season
2.3. Soil Analyses
2.4. Statistical Analyses
3. Results
3.1. Chlorophyll a and Chlorophyll b Content
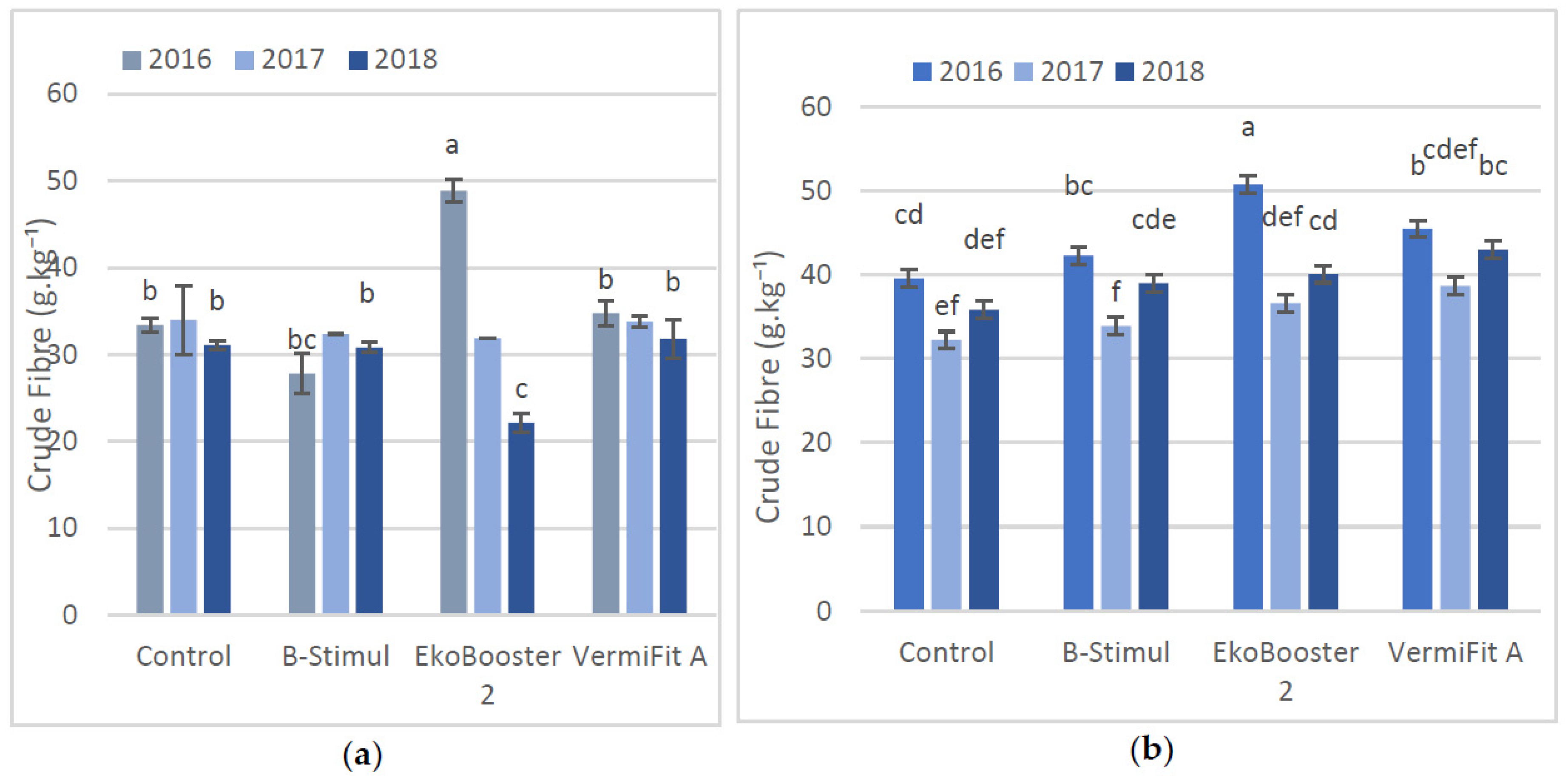
3.2. Crude Fibre
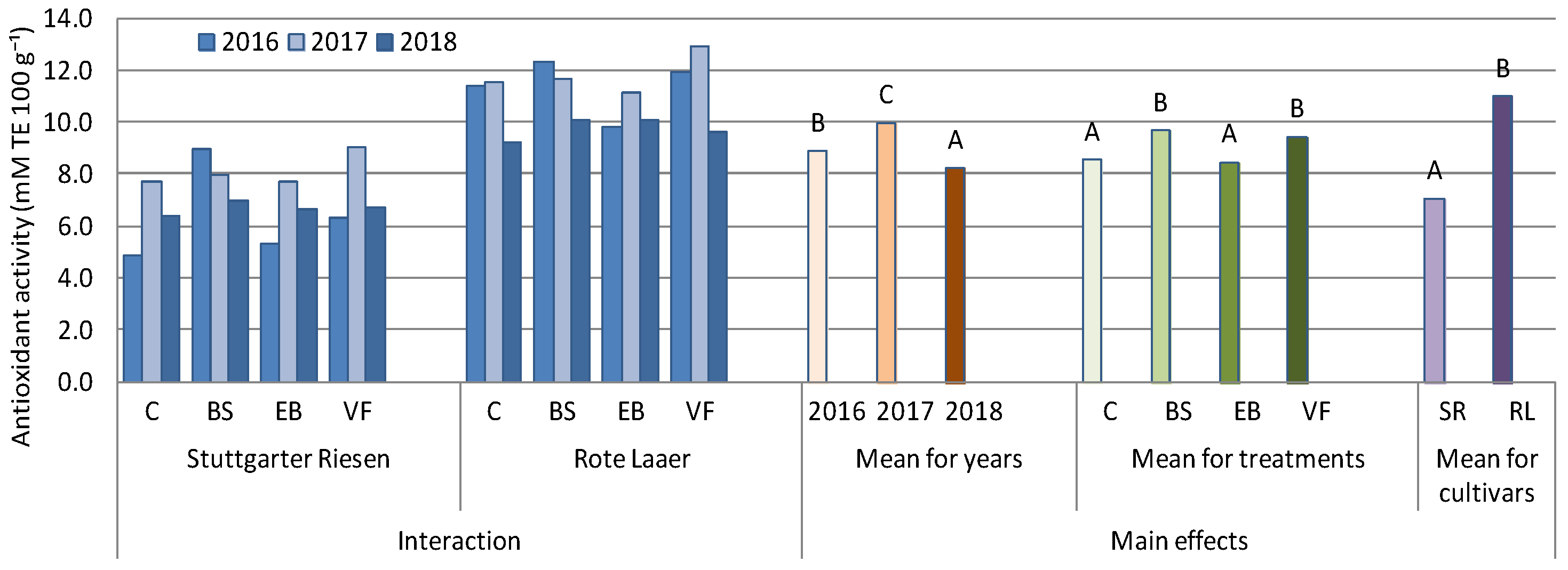
3.3. Antioxidant Activity
3.4. Zinc Content
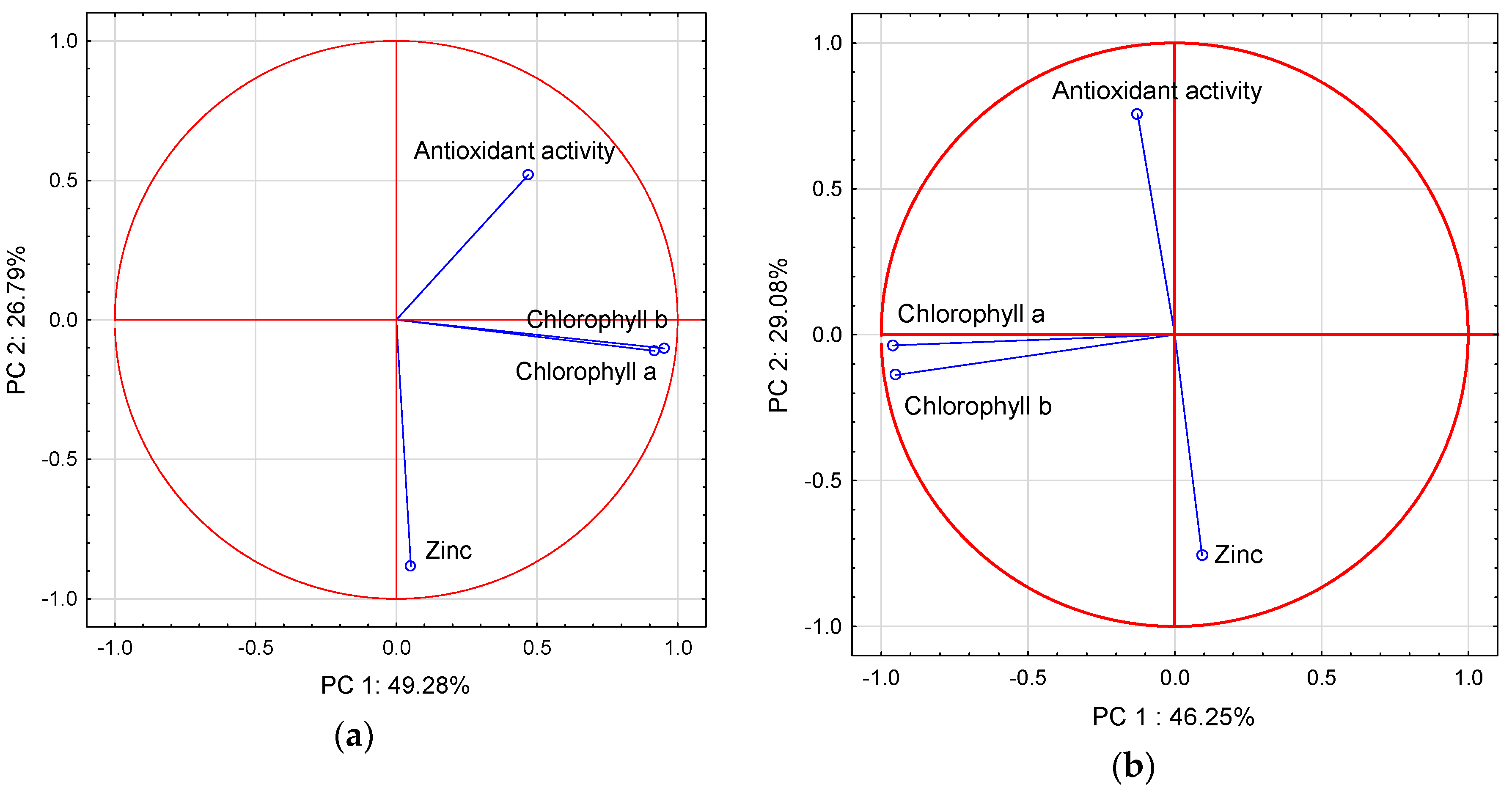
3.5. Cluster and Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roldan, E.; Sanchez-Moreno, C.; de Ancos, B.; Cano, M.P. Characterization of onion (Allium cepa L.) by-products as food ingredients with antioxidant and antibrowning properties. Food Chem. 2008, 108, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Sękara, A.; Pokluda, R.; Del Vacchio, L.; Somma, S.; Caruso, G. Interactions among genotype, environment and agronomic practices on production and quality of storage onion (Allium cepa L.)—A review. Hort. Sci. (Prague) 2017, 44, 201–212. [Google Scholar] [CrossRef]
- Gadelrabh, H.M.; Elamin, S.M. Effect of different organic fertilizers on growth, yield and total soluble solid of the onion (Allium cepa L.) variety Baftaim-s. J. Agric. Vet. Sci. 2013, 14, 61–67. [Google Scholar]
- Ibrahim, A. The effect of inorganic fertilizer on onion production. Int. J. Biol. Sci. 2014, 1, 21–29. [Google Scholar]
- Enping, Z.; Yu, D.; Fulei, T.; Shuhong, Z. Effects of long-term nitrogen and organic fertilization on antioxidants content of tomato fruits. Hort. J. 2016, 3, 8–12. [Google Scholar]
- Shedeed, I.S.; Sayed-el, S.A.A.; Doaa, M.A.B. Effectiveness of bio-fertilizers with organic matter on the growth, yield and nutrient content of onion (Allium cepa L.) plants. Eur. Inter. J. Sci.Tech. 2014, 3, 115–122. [Google Scholar]
- Moldovan, C.; Ianculov, I.; Nicoleta, G.H.; Delia, D.; Crăiniceanuc, E.; Drugă, M.; Liana, A.; Moldovan, G.Z. Influence of chlorophyll content from onion (Allium cepa) after selenium and zinc adding. J. Agroaliment. Proc. Technol. 2009, 15, 437–440. [Google Scholar]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; Esteban, R.M. Physicochemical properties and in vitro antidiabetic potential of fibre concentrates from onion by-products. J. Funct. Foods 2017, 36, 34–42. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Abida, S.; Muhammad, H.S. Analysis of the proximate composition and energy values of two varieties of onion (Allium cepa L.) bulbs of different origin: A comparative study. Int. J. Food Sci. 2013, 2, 246–253. [Google Scholar] [CrossRef]
- Modu, S.; Chamba, G.; Falmata, A.; Laminu, H.; Babagana, M.; Hauwa, H. Studies on the effect of drying and varietal differences on chemical composition of some selected onion cultivars. Nutr. Res. 2015, 2, 376–385. [Google Scholar]
- Krishnamurthy, V.M.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fibre intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Inter. 2012, 81, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Manas, D. The determination of vitamin C, total phenol and antioxidant activity of some commonly cooking spices crops used in west Bengal. Int. J. Plant Physiol. Biochem. 2014, 6, 66–70. [Google Scholar] [CrossRef]
- Lu, X.; Jun, W.; Al-Qadiri, H.M.; Carolyn, F.R.; Joseph, R.P.; Juming, T.; Barbara, A.R. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 2011, 129, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Kandoliya, U.K.; Bodar, N.P.; Bajaniya, V.K.; Bhadja, N.V.; Golakiya, B.A. Determination of nutritional value and antioxidant from bulbs of different onion (Allium cepa) variety. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 635–641. [Google Scholar]
- Feiyue, R.; Kim, R.; Joseph, P.K.; Gaffney, M.; Hossain, M.; Rai, D.K. Higher antioxidant activity, total flavonols, and specific quercetin glucosides in two different onion (Allium cepa L.) varieties grown under organic production: Results from a 6-year field study. J. Agric. Food Chem. 2017, 65, 5122–5132. [Google Scholar]
- Sriram, K.; Lonchyna, V.A. Micronutrient supplementation in adult nutrition therapy: Practical considerations. J. Parenter. Enteral Nutr. 2009, 33, 548–562. [Google Scholar] [CrossRef]
- Kosesakal, T.; Unal, M. Role of Zn deficiency in photosynthetic pigments and peroxidase activity of tomato seedlings IUFS. J. Biol. 2009, 68, 113–120. [Google Scholar]
- Holm, G. Chlorophyll mutations in barley. Acta Agric. Scand. 1954, 4, 457–471. [Google Scholar] [CrossRef]
- Zloch, Z.; Čelakovský, J.; Aujezdska, A. Determination of Polyphenol Content and Total Antioxidant Capacity in Plant Food; Final Report Implementation of the DANONE 2004 Research Project; Plzeň, Czech Republic, 2004; Available online: https://docplayer.cz/14474044-Stanoveni-obsahu-polyfenolu-a-celkove-antioxidacni-kapacity-v-potravinach-rostlinneho-puvodu-z-zloch-j-celakovsky-a.html (accessed on 30 December 2004).
- AOCS. Crude Fibre Analysis in Feeds Filter Bag Technique. 2006. Available online: http://www.ssco.com.tw/Ankom/PDF_file/Crude%20Fiber%20Method%20A200.pdf (accessed on 21 October 2005).
- Zbíral, J.; Malý, S.; Váňa, M. Soil Analysis III. In Central Institute for Supervising and Testing in Agriculture; Soil analysis: Brno, Czech Republic, 2011. (In Czech) [Google Scholar]
- Stajner, D.; Milic, N.; Candanovic-Brunet, J.; Kapor, A.; Stajner, M.; Popovic, B.M. Exploring allium species as a source of potential medicinal agents. Phytother. Res. 2006, 20, 581–584. [Google Scholar] [CrossRef]
- Nassar, R.M.A.; Seleem, E.A.; Caruso, G.; Sekara, A.; Abdelhamid, M.T. The Nitrogen-fixing bacteria—Effctive enhancers of growth and chemical composition of Egyptian henbane under varied mineral N nutrition. Agronomy 2020, 10, 921. [Google Scholar] [CrossRef]
- Petrovic, B.; Kopta, T.; Pokluda, R. Effect of biofertilizers on yield and morphological parameters of onion cultivars. Folia Hort. 2019, 31, 51–59. [Google Scholar] [CrossRef]
- Hanci, F.; Cebeci, E. Investigation of proline, chlorophyll and carotenoids changes under drought stress in some onion (Allium cepa L.) cultivars. Türk Tarım ve Doğa Bilimleri Dergisi 2014, 2, 1499–1504. [Google Scholar]
- Jaime, L.; Mollá, E.; Fernández, A.; Martín-Cabrejas, M.A.; López-Andréu, F.J.; Esteban, R.M. Structural carbohydrate differences and potential source of dietary fiber of onion (Allium cepa L.) tissues. J. Agric. Food Chem. 2002, 50, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Akeem, A.; Azeez, O.Y. Phytochemical screening and proximate analysis of newbouldia laevis and allium sativum. Nigerian J. Anim. Sci. 2016, 1, 242–256. [Google Scholar]
- Ombodi, A.; Lugasi, A.; Daood, H.G.; Berki, M.; Helyes, L. Water supply and temperature effects on some nutritive constituents of direct sown onion. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 245–249. [Google Scholar] [CrossRef][Green Version]
- Available online: https://ec.europa.eu/jrc/en/health-knowledge-gateway/promotion-prevention/nutrition/fibre (accessed on 10 November 2020).
- Sirag, N.; Imd, M.T.; Mohamed, A.; Alaa, M. Determination of antioxidant activity of some varieties of onion (Allium cepa L.) grown in Sudan. Gezira J. Health Sci. 2015, 2, 1–7. [Google Scholar] [CrossRef]
- Cheng, A.; Xiangyan, C.; Qiong, J.; Wenliang, W.; John, S.; Yaobo, L. Comparison of phenolic content and antioxidant capacity of red and yellow onions. Czech J. Food Sci. 2013, 31, 501–508. [Google Scholar] [CrossRef]
- Zhang, S.L.; Peng, D.E.N.G.; Xu, Y.C.; Lü, S.W.; Wang, J.J. Quantification and analysis of anthocyanin and flavonoids compositions, and antioxidant activities in onions with three different colors. J. Integr. Agric. 2016, 15, 2175–2181. [Google Scholar] [CrossRef]
- Prakash, D.; Singh, B.N.; Upadhyay, G. Antioxidant and free radical scavenging activities of phenols from onion (Allium cepa). Food Chem. 2007, 102, 1389–1393. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Ali, M.; Al-Rashdan, A.; Ahmed, N. Onion (Allium cepa L.) is potentially a good source of important antioxidants. J. Food Sci. Technol. 2019, 56, 1811–1819. [Google Scholar] [CrossRef]
- Gökçe, A.F.; Kaya, C.; Serçe, S.; Özgen, M. Effect of scale color on the antioxidant capacity of onions. Sci. Hort. 2010, 123, 431–435. [Google Scholar] [CrossRef]
- Gamelli, H.H. The effect of some foliar fertilizers application on growth, bulb plant biomass, quality and storage ability of Giza 20 onion cultivar (Allium cepa L.). Ann. Agric. Sci. Moshtohor. 2000, 38, 1727–1737. [Google Scholar]
- Machado, R.M.A.; Shahidian, S.; Pivetta, C.R.; Oliveira, M.R.G. Nitrogen fertilization effects on rooting pattern and plant biomass of intermediate-day onions bulb in Alentejo region. Rev. Ciênc. Agrárias. 2009, 32, 113–122. [Google Scholar]
- Almendros, P.; Obrador, A.; Gonzalez, D.; Alvarez, J.M. Biofortification of zinc in onions (Allium cepa L.) and soil Zn status by the application of different organic Zn complexes. Sci. Hortic. 2015, 186, 254–265. [Google Scholar] [CrossRef]
- Mlcek, J.; Valsikova, M.; Druzbikova, H.; Ryant, P.; Jurikova, T.; Sochor, J.; Borkovcova, M. The antioxidant capacity and macroelement content of several onion cultivars. Turk. J. Agric. For. 2015, 39, 999–1004. [Google Scholar] [CrossRef]
- Yogita; Ram, R.B. Effect of chemical and bio-fertilizers on quality of onion. HortFlora Res. Spectrum. 2012, 1, 367–370. [Google Scholar]
- Yatsenko, V.; Ulianych, O.; Yanowskiy, Y. Effect of iron, zinc and boron on the growth, physiological state, productivity and storability of Allium sativum L. Ukr. J. Ecol. 2020, 10, 33–42. [Google Scholar] [CrossRef]
- Cota, J.; Gvozdanovic-Varga, J.; Hadžic, A.; Petrovic, A.; Saraic, E.; Savic, A.; Cota, J. Yield and mineral composition of two new onion varieties from Bosnia and Herzegovina. In Proceedings of the Fourth International Scientific Symposium, Agrosym, Jahorina, Bosnia and Herzegovina, 3–6 October 2013; pp. 251–256. [Google Scholar]
- Akinwande, B.A.; Olatunde, S.J. Comparative evaluation of the mineral profile and other selected components of onion and garlic. Int. Food Res. J. 2015, 22, 332–336. [Google Scholar]
- Gautam, S.; Platel, K.; Srinivasan, K. Higher bioaccessibility of iron and zinc from food grains in the presence of garlic and onion. J. Agric. Food Chem. 2010, 58, 8426–8429. [Google Scholar] [CrossRef]
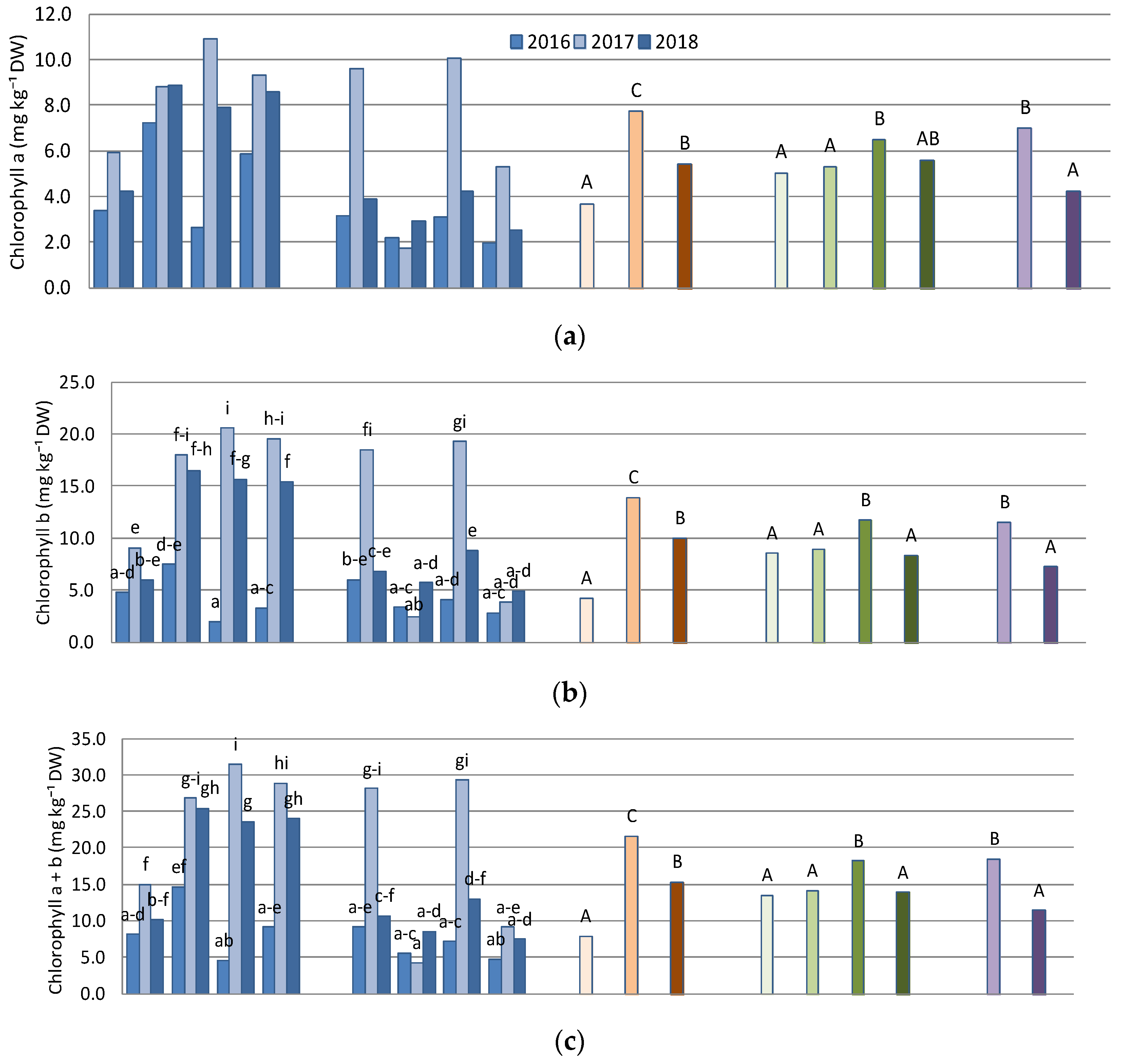
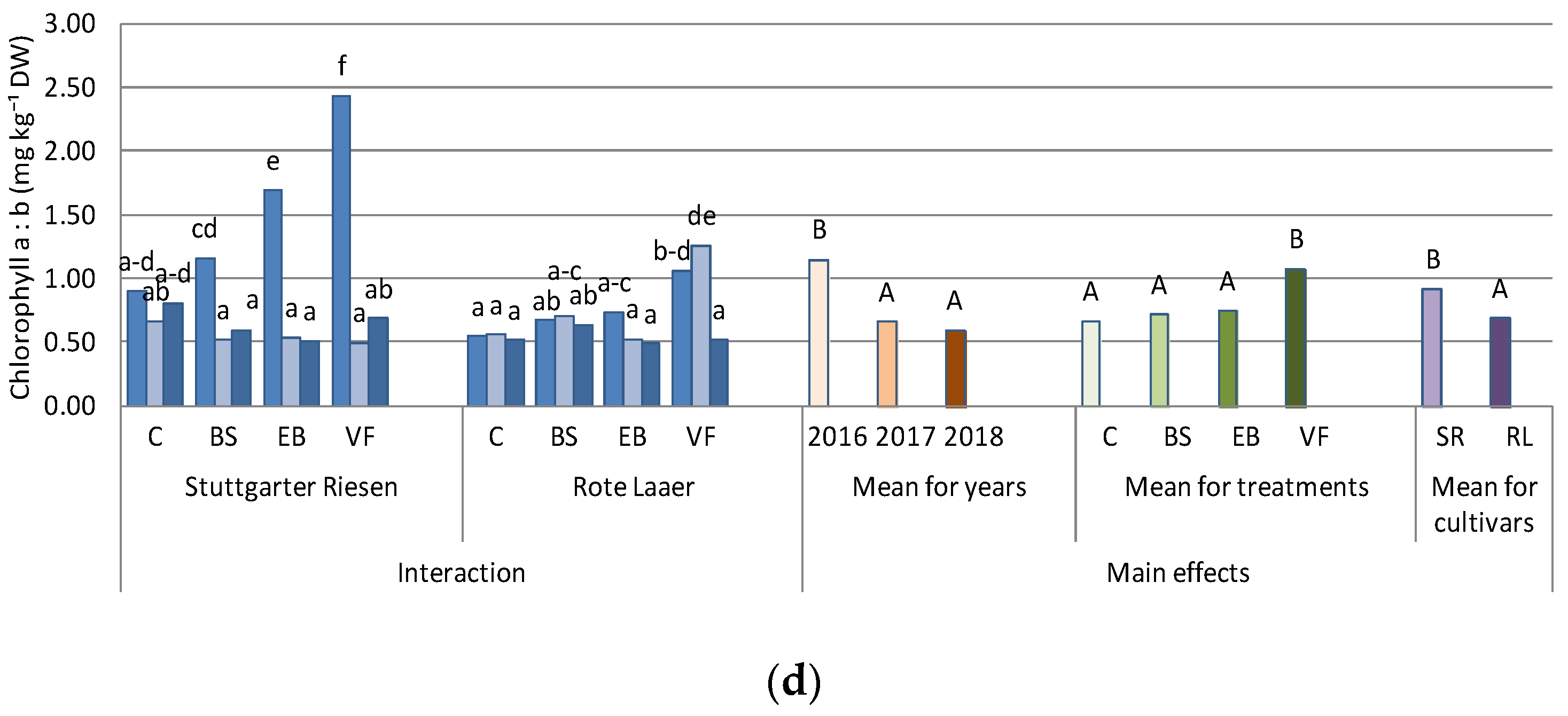


| ANOVA Source of Variation | Chlorophyll a | Chlorophyll b | Chl. a + b | Chl. a:b | Antioxidant Activity | Zn |
|---|---|---|---|---|---|---|
| Treatment (T) | * | *** | *** | *** | *** | n.s. |
| Cultivar (C) | *** | *** | *** | *** | *** | *** |
| Year (Y) | *** | *** | *** | *** | *** | *** |
| T × C | *** | *** | *** | n.s. | n.s. | *** |
| C × Y | * | *** | *** | *** | *** | *** |
| T × Y | *** | *** | *** | *** | *** | *** |
| T × C × Y | n.s. | *** | *** | ** | n.s. | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovic, B.; Sękara, A.; Pokluda, R. Biofertilizers Enhance Quality of Onion. Agronomy 2020, 10, 1937. https://doi.org/10.3390/agronomy10121937
Petrovic B, Sękara A, Pokluda R. Biofertilizers Enhance Quality of Onion. Agronomy. 2020; 10(12):1937. https://doi.org/10.3390/agronomy10121937
Chicago/Turabian StylePetrovic, Bojana, Agnieszka Sękara, and Robert Pokluda. 2020. "Biofertilizers Enhance Quality of Onion" Agronomy 10, no. 12: 1937. https://doi.org/10.3390/agronomy10121937
APA StylePetrovic, B., Sękara, A., & Pokluda, R. (2020). Biofertilizers Enhance Quality of Onion. Agronomy, 10(12), 1937. https://doi.org/10.3390/agronomy10121937







