15N-Fertilizer Recovery in Maize as an Additional Strategy for Understanding Nitrogen Fertilization Management with Blends of Controlled-Release and Conventional Urea
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Site Description
2.2. Experimental Setup and Treatment Description
2.3. Quantification of Ammonia Volatilization
2.4. Analyses of Plant and Soil Samples
2.5. 15N-Fertilizer Recovery Analyses
2.6. Statistical Analyses
3. Results
3.1. Weather Conditions
3.2. Ammonia Volatilization
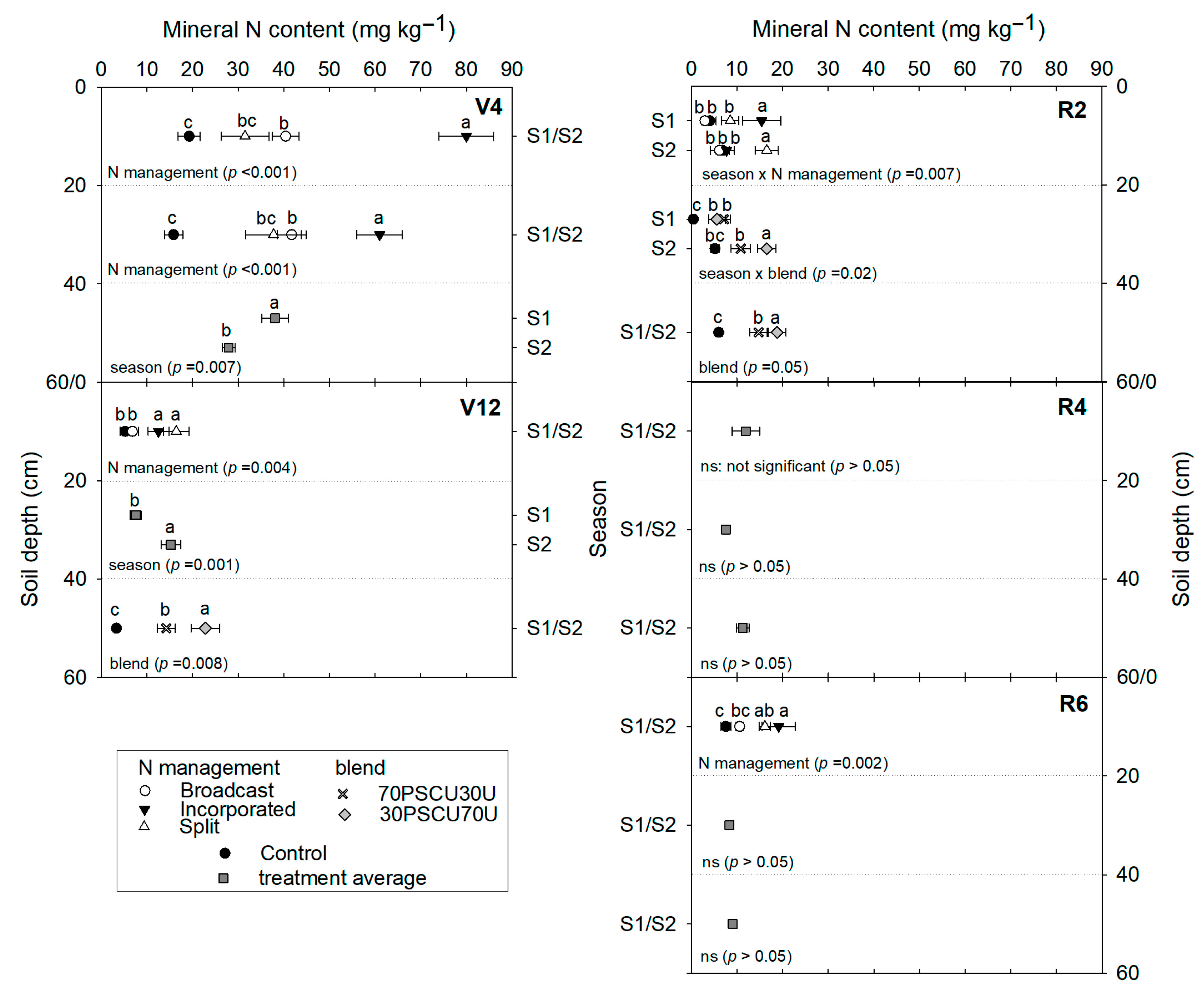
3.3. Mineral N Content in the Soil
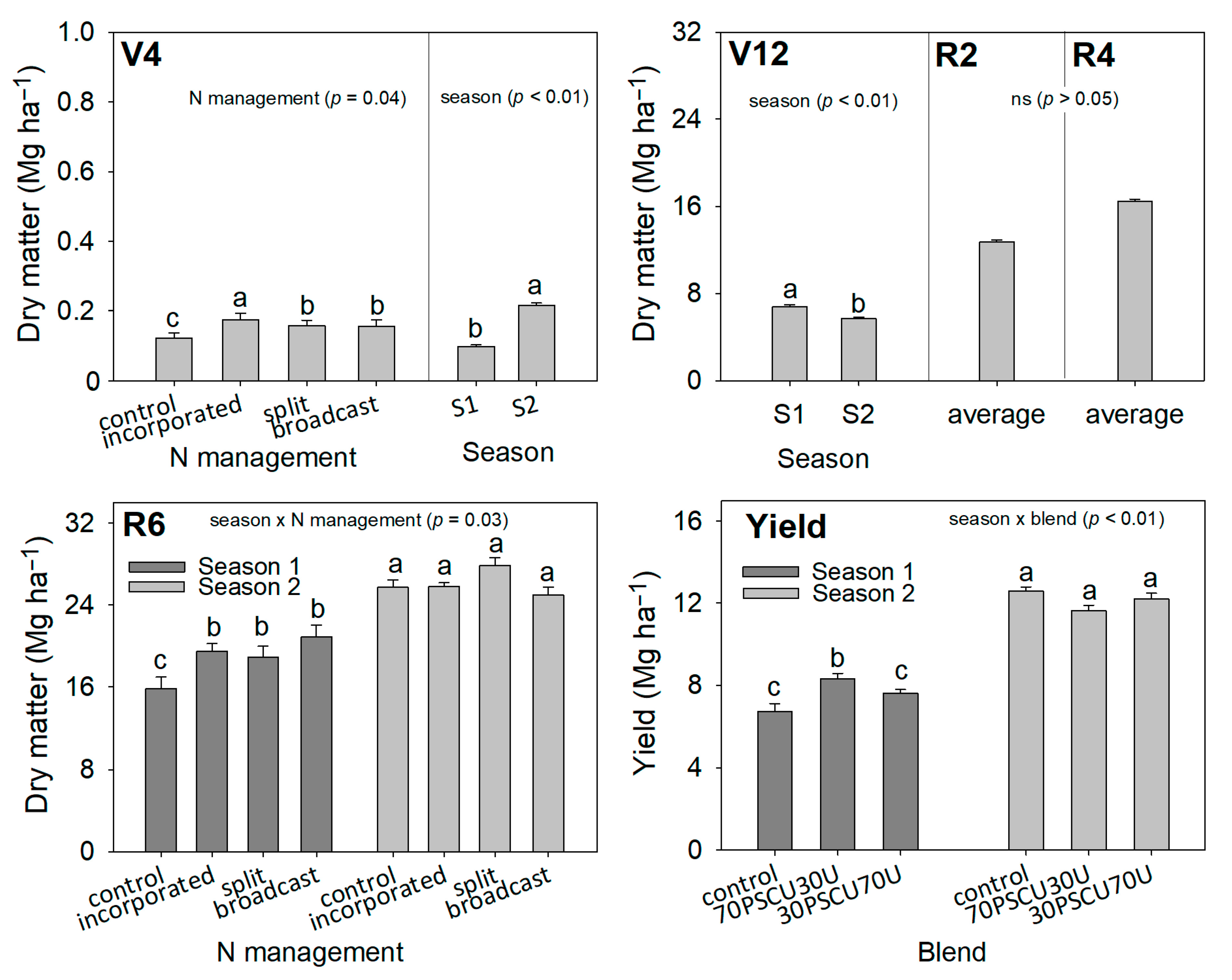
3.4. Biomass (Dry Matter) Accumulation in Plants and Maize Yield
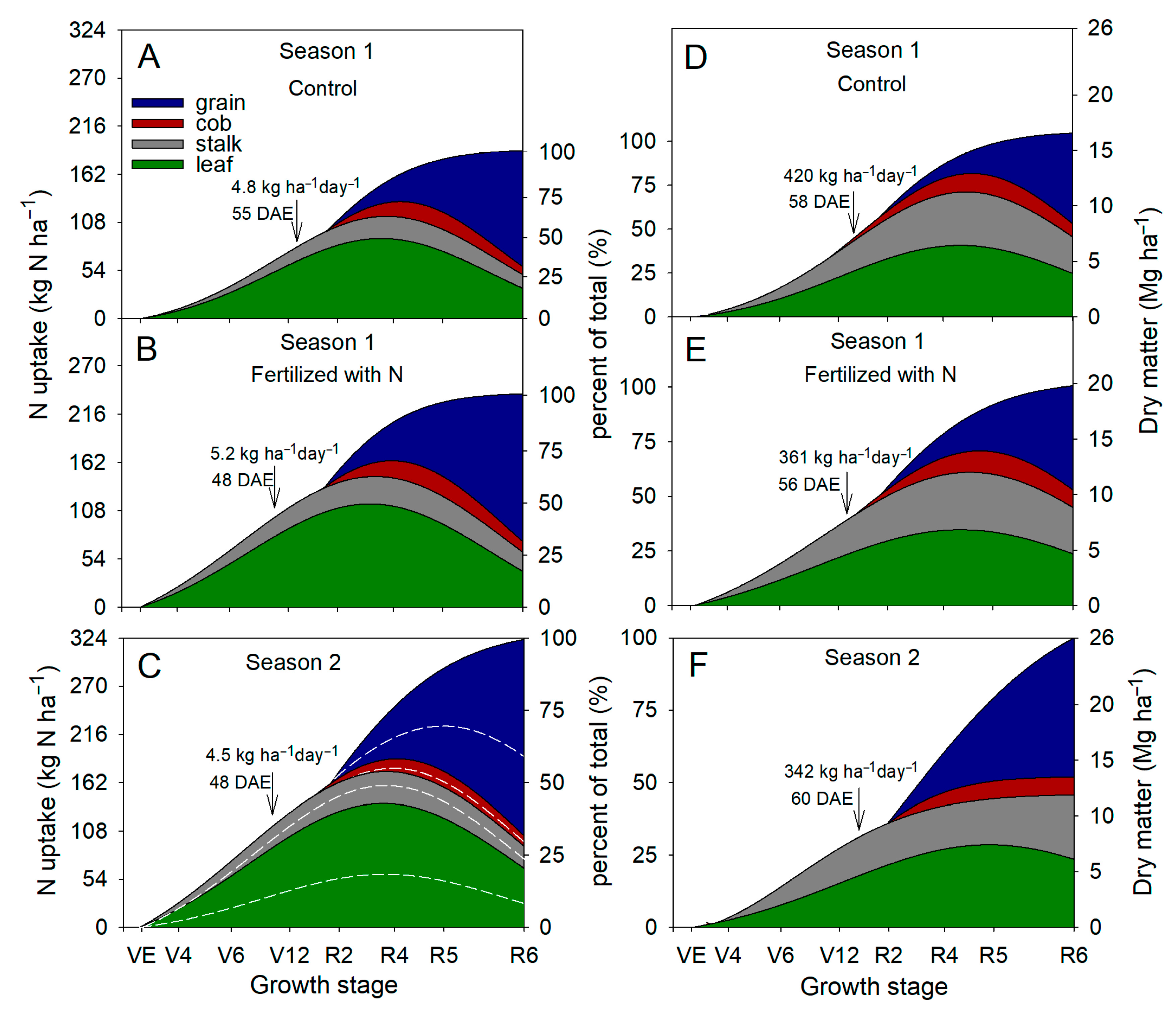
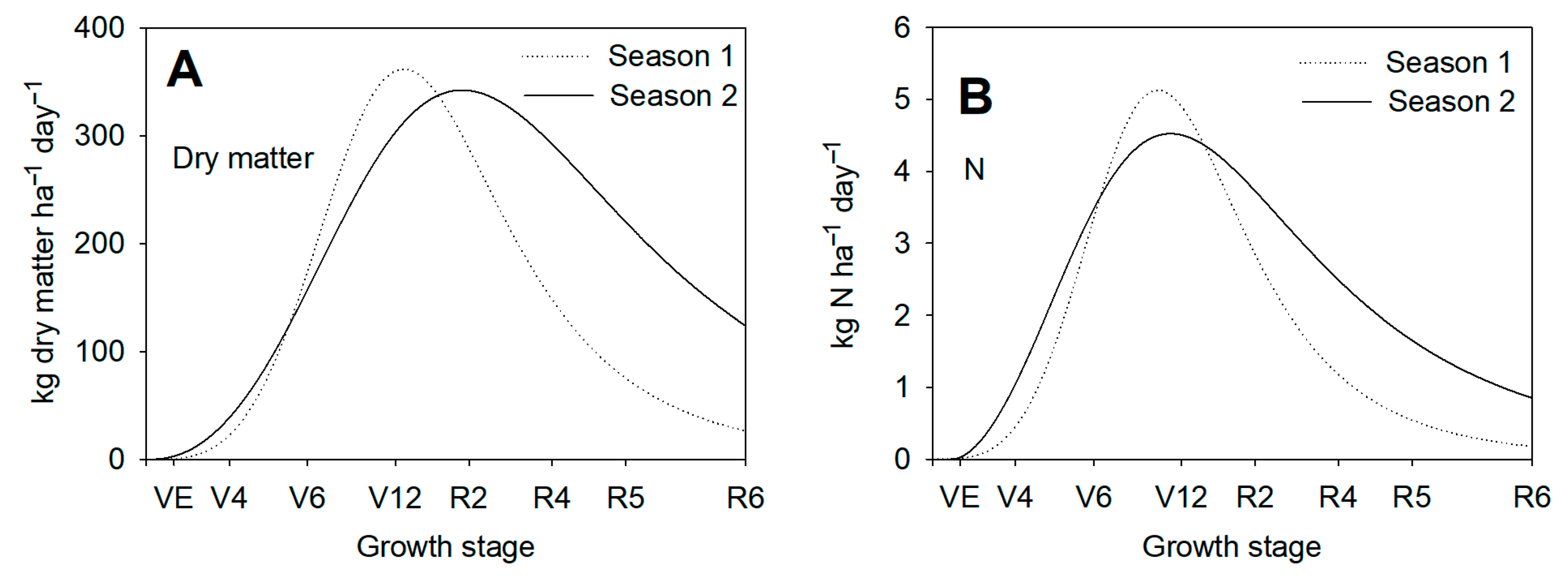
3.5. Nitrogen Uptake in Maize Plants
3.6. 15N-Fertilizer Recovery in Maize Plants and Nitrogen Balance
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ke, J.; Xing, X.; Li, G.; Ding, Y.; Dou, F.; Wang, S.; Liu, Z.; Tang, S.; Ding, C.; Chen, L. Effects of different controlled-release nitrogen fertilisers on ammonia volatilisation, nitrogen use efficiency and yield on blanket-seeding machine-transplanted rice. Field Crop. Res. 2017, 205, 147–156. [Google Scholar] [CrossRef]
- [USDA] United States Department of Agriculture. World Corn Supply and Use. 2020. Available online: https://www.usda.gov/oce/commodity/wasde/wasde0720.pdf (accessed on 24 November 2020).
- Rinaldi, L.F.; Garcia, P.L.; Sermarini, R.A.; Trivelin, P.C.O. 15N-Urea efficiency in maize as influenced by humic substances and urease inhibitors treatments. Commun. Soil Sci. Plant Anal. 2019, 50, 198–208. [Google Scholar] [CrossRef]
- Cantarella, H. Nitrogen. In Soil Fertility; Novais, R.F., Ed.; Brazilian Society of Soil Science: Viçosa, Brazil, 2007; pp. 271–276. [Google Scholar]
- Graming, B.M.; Massey, R.; Yun, S.D. Nitrogen application decision-making under climate risk in the U.S. Corn Belt. Clim. Risk Manag. 2017, 15, 82–89. [Google Scholar] [CrossRef]
- Apostolopoulou, E. The Global Market for Slow-Release, Controlled-Release and Stabilized Fertilizers; International Fertilizer Association—IFA: Beijing, China, 2016; 19p. [Google Scholar]
- IPNI. Slow or Controlled Release and Stabilized Nitrogen Fertilizers 2017. Available online: http://www.ipni.net/PUBLICATION/IA-BRASIL.NSF/0/90DE38570A7216CB832580FB0066E3B4/$FILE/Jornal-157.pdf (accessed on 24 November 2020).
- Trenkel, M.E. Slow and Controlled Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture; International Fertilizer Industry Association: Paris, France, 2010; 160p. [Google Scholar]
- Shapiro, C.; Attia, A.; Ulloa, S.; Mainz, M. Use of five nitrogen source and placement systems for improved nitrogen management of irrigated corn. Soil Sci. Soc. Am. J. 2016, 80, 1663–1674. [Google Scholar] [CrossRef]
- Guo, L.; Ning, T.; Nie, L.; Li, Z.; Lal, R. Interaction of deep placed controlled-release urea and water retention agent on nitrogen and water use and maize yield. Eur. J. Agron. 2016, 75, 118–129. [Google Scholar] [CrossRef]
- Cancellier, E.L.; Silva, G.R.D.; Faquin, V.; Gonçalvez, A.B.; Cancellier, L.L.; Spehar, R.C. Ammonia volatilization from enhanced-efficiency urea on no-till maize in Brazilian cerrado with improved soil fertility. Ciência Agrotecnol. 2016, 40, 133–144. [Google Scholar] [CrossRef]
- Wilson, M.L.; Rosen, C.J.; Moncrief, J.F. Potato response to polymer coated-urea on an irrigated coarsed-texture soil. Agron. J. 2009, 101, 897–905. [Google Scholar] [CrossRef]
- Grant, C.A.; Wu, R.; Selles, F.; Harker, K.N.; Clayton, G.W.; Bittman, S.; Zebarth, B.J.; Lupwayi, N.Z. Crop yield and nitrogen concentration with controlled release urea and split applications of nitrogen as compared to non-coated urea applied at seeding. Field Crop. Res. 2012, 127, 170–180. [Google Scholar] [CrossRef]
- Garcia, P.L.; Sermarini, R.A.; Trivelin, P.C.O. Effects of nitrogen rates applying controlled-release and conventional urea blend in maize. J. Plant Nutr. 2019, 42, 2199–2208. [Google Scholar] [CrossRef]
- Hong, N.; Scharf, P.C.; Davis, J.G.; Kitchen, N.R.; Sudduth, K.A. Economically optimal nitrogen rate reduces soil residual nitrate. J. Environ. Qual. 2007, 36, 354–362. [Google Scholar] [CrossRef]
- Garcia, P.L.; González-Villalba, H.A.; Sermarini, R.A.; Trivelin, P.C.O. Nitrogen use efficiency and nutrient partitioning in maize as affected by blends of controlled-release and conventional urea. Arch. Agron. Soil Sci. 2018, 64, 1944–1962. [Google Scholar] [CrossRef]
- Villalba, H.A.G. Blending Polymer-Sulfur Coated and NBPT-Treated Urea to Improve Nitrogen Use Efficiency and Grain Yield in Corn Production Systems. Ph.D. Dissertation, University of São Paulo, Piracicaba, Brazil, 2018. Available online: https://www.teses.usp.br/teses/disponiveis/11/11140/tde-14082018-100857/pt-br.php (accessed on 24 November 2020).
- Nkebiwe, P.M.; Weinmann, M.; Bar-Tal, A.; Müller, T. Fertilizer placement to improve crop nutrient acquisition and yield: A review and meta-analysis. Field Crop. Res. 2016, 196, 389–401. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Li, V.; Zhu, L.; Cao, Y.; Zhao, X.; Feng, G.; Gao, Q. Mixture of controlled-release and normal urea to improve nitrogen management for maize across contrasting soil types. Agron. J. 2020, 112, 3101–3313. [Google Scholar] [CrossRef]
- Li, C.L.; Cao, Y.Q.; Wang, Y.; Li, X.Y.; Li, Y.X.; Zhu, L.; Zhao, X.H.; Gao, Q. Effects of mixed controlled release and normal urea on maize (Zea mays L.) growth, grain yield and nitrogen balance and use efficiency in northeast China. Appl. Ecol. Environ. Res. 2020, 18, 5367–5382. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Blaylock, A.D.; Chen, X. Mixture of controlled release and normal urea to optimize nitrogen management for high-yielding (>15 Mg ha−1) maize. Field Crop. Res. 2017, 204, 23–30. [Google Scholar] [CrossRef]
- Garcia, P.L.; Sermarini, R.A.; Trivelin, P.C.O. Placement effect of controlled-release and conventional urea blend in maize. Commun. Soil Sci. Plant Anal. 2019, 50, 2321–2329. [Google Scholar] [CrossRef]
- Kong, L. Maize residues, soil quality, and wheat growth in China: A review. Agron. Sustain. Dev. 2014, 34, 405–416. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-NRCS.: Whashington, DC, USA, 2014. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analyzis. In Methods of Soil Analysis: Physical and Mineralogical Methods; Klaute, A., Ed.; ASA and SSSA: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Bataglia, O.C.; Furlani, A.M.C.; Teixeira, J.P.F.; Furlani, P.R.; Gallo, J.R. Methods of Chemical Analysis of Plants; Instituto Agronômico: Campinas, Brazil, 1983; pp. 1–48. [Google Scholar]
- van Raij, B.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Chemical Analysis for Fertility Evaluation of Tropical Soils; Instituto Agronômico: Campinas, Brazil, 2001. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, organic carbon and organic matter. In Methods of Soil Analysis: Chemical Methods; Sparks, D.L., Ed.; ASA and SSSA: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Barrie, A.; Prosser, S.J. Automated analyses of light-element stable isotopes by isotope ratio mass spectrometry. In Mass Spectrometry of Soils; Boutton, T.W., Yamasaki, S., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 1–46. [Google Scholar]
- van Raij, B.; Cantarella, H. Corn for grain and silage. In Recommendation of Fertilization and Liming for the State of São Paulo; Cantarella, H., Quaggio, J.A., Furlani, A.M.C., Eds.; Instituto Agronômico: Campinas, Brazil, 1997; pp. 56–59. [Google Scholar]
- Mariano, E.; Filho, C.R.S.A.; Bortoletto-Santos, R.; Bendassoli, J.A.; Trivelin, P.C.O. Ammonia losses following surface application of enhanced-efficiency nitrogen fertilizers and urea. Atmos. Environ. 2019, 203, 242–251. [Google Scholar] [CrossRef]
- Fritsche, L.S. Urea-15N. Master’s Thesis, University of São Paulo, Piracicaba, Brazil, 2019. Available online: https://www.teses.usp.br/teses/disponiveis/64/64135/tde-09102019-091454/publico/Leticia_Sbrana_Fritsche_Revisada.pdf (accessed on 24 November 2020).
- Landis, E.N.; Keane, D.T. X-ray microtomography. Mater. Charact. 2010, 61, 1305–1316. [Google Scholar] [CrossRef]
- Reis, B.F.; Vieira, J.A.; Krug, F.J.; Giné, M.F. Development of a flow injections system two analytical paths for ammonium determination in soil extracts by conductometry. J. Braz. Chem. Soc. 1997, 8, 524–528. [Google Scholar] [CrossRef]
- Gonzaga, M.M.; Trivelin, P.C.O. Open Collector Efficiency to Capture NH3 Volatilized from the Soil. SIICUSP. 2018. Available online: https://uspdigital.usp.br/siicusp/siicPublicacao.jsp?codmnu=7210 (accessed on 24 November 2020).
- Ritchie, S.W.; Hanway, J.J.; Benson, G.O. How a Corn Plant Develops; Special Report 48; Iowa State University Cooperative Extension Service: Ames, IA, USA, 1997. [Google Scholar]
- Laviola, B.G.; Martinez, H.E.P.; Souza, R.B.; Salomão, L.C.C.; Cruz, C.D. Macronutrient accumulation in coffee fruits at Brazilian Zona da Mata conditions. J. Plant Nutr. 2009, 32, 980–995. [Google Scholar] [CrossRef]
- Trivelin, P.C.O.; Lara Cabezas, W.A.R.; Victoria, R.L.; Reichardt, K. Evaluation of a 15N plot design for estimating plant recovery of fertilizer nitrogen applied to sugar cane. Sci. Agric. 1994, 51, 226–234. [Google Scholar] [CrossRef]
- Hauck, R.D.; Bremmer, J.M. Use of tracers for soil and fertilizer nitrogen research. Adv. Agron. 1976, 28, 219–260. [Google Scholar]
- R development Core Team. R: A Language and Environment for Statistical Computing, R. Foundation for Statistical Computing 2015. Available online: http://www.R-project.org/ (accessed on 24 November 2020).
- Karlen, D.L.; Flannery, R.L.; Sadler, E.J. 1988. Aerial accumulation and partitioning of nutrients by corn. Agron. J. 1988, 80, 232–242. [Google Scholar] [CrossRef]
- Ghagas, W.F.T.; Guelfi, D.R.; Caputo, A.L.C.; Souza, T.L.; Andrade, A.B.; Faquim, V. Ammonia volatilization from blends with stabilized and controlled-release urea in the coffee system. Ciênc. Agrotecnol. 2016, 40, 497–509. [Google Scholar]
- Miyazawa, M.; Gil, L.G.; Costa, A.; Anjos Reis Júnior, R.; Tiski, I. Volatilization of ammonia in tropical soil with different moisture after application of polymer-coated urea. Aust. J. Crop Sci. 2020, 14, 712–720. [Google Scholar] [CrossRef]
- Loss, A.; Pereira, M.G.; Costa, E.M.; Beutler, S.J. Carbon, nitrogen and the natural abundance of 13C and 15N in macro and microaggregates. Idesia 2014, 32, 15–21. [Google Scholar]
- Magalhães, P.C.; Durães, F.O.M. Physiology of Maize Production; Embrapa: Sete Lagoas, Brazil, 2006; Volume 76, pp. 1–10. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/490408/fisiologia-da-producao-de-milho (accessed on 24 November 2020).
- Feldman, L. The maize root. In The Maize Handbook; Freeling, M., Walbot, V., Eds.; Springer Lab Manuals: New York, NY, USA, 1994; pp. 29–37. [Google Scholar]
- Vicensi, M.; Lopes, C.; Koszalka, V.; Umburanas, R.C.; Kawakami, J.; Pott, C.A.; Müller, M.M.L. Gypsum Rates and Splitting Under No-Till: Soil Fertility, Corn Performance, Accumulated Yield and Profits. J. Soil Sci. Plant Nutr. 2020, 20, 690–702. [Google Scholar] [CrossRef]
- Schoninger, E.L.; Villalba, H.A.G.; Bendassolli, A.; Trivelin, P.C.O. Corn grain yield and 15N-fertilizer recovery as a function of urea sidedress timing. Ann. Braz. Acad. Sci. 2018, 90, 3299–3312. [Google Scholar] [CrossRef] [PubMed]
- Boschiero, B.N.; Mariano, E.; Torres-Dorante, L.O.; Sattolo, T.M.S.; Otto, R.; Garcia, P.L.; Dias, C.T.S.D.; Trivelin, P.C.O. Nitrogen fertilizer effects on sugarcane growth, nutritional status, and productivity in tropical acid soils. Nutr. Cycl. Agroecosyst. 2020, 117, 367–382. [Google Scholar] [CrossRef]
- Sangoi, L. Understanding plant density effects on maize growth and development: An important issue to maximize grain yield. Ciência Rural 2001, 31, 159–168. [Google Scholar] [CrossRef]
- Roberts, T.L.; Slaton, N.A.; Kelley, J.P.; Greub, C.E.; Fulford, A.M. Fertilizer nitrogen recovery efficiency of furrow-irrigated corn. Agron. J. 2016, 108, 2123–2128. [Google Scholar] [CrossRef]
- Walsh, O.; Raun, W.; Klatt, A.; Solie, J. Effect of delayed nitrogen fertilization on maize (Zea mays L.) grain yields and nitrogen use efficiency. J. Plant Nutr. 2012, 35, 538–555. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Qin, S.; Yang, G.; Frang, K.; Zhu, B.; Kuzyakov, Y.; Chen, P.; Xu, Y.; Yang, Y. Regulation of priming effect by soil organic matter stability over a broad geography scale. Nat. Commun. 2019, 10, 5112. [Google Scholar] [CrossRef]
- Moschini, B.P. Management of Nitrogen Fertilization with Mixtures of NBPT-Treated Urea and Polymer Sulfur Coated Urea in Corn Production Systems. Ph.D. Dissertation, University of São Paulo, Piracicaba, Brazil, 2019. Available online: https://www.teses.usp.br/teses/disponiveis/11/11140/tde-07052019-180546/pt-br.php (accessed on 24 November 2020).
- Ladha, J.K.; Pathak, H.; Krupinic, T.J.; Six, J.; van Kessel, C. Efficiency of fertilizer nitrogen in cereal production: Retrospects and prospects. Adv. Agron. 2005, 87, 85–156. [Google Scholar]
- Gava, G.J.C.; Oliveira, M.W.; Silva, M.A.; Jerônimo, E.M.; Cruz, J.C.S.; Trivelin, P.C.O. Phytomass production and nitrogen accumulation in maize cultivated with different doses of 15N-urea. Semina 2010, 31, 851–862. [Google Scholar]
- Lara Cabezas, W.A.R.; Couto, P.A. Nitrogen immobilization of urea and ammonium sulphate applied to maize before planting and as top-dressing in a no-till system. Rev. Bras. Cienc. Solo 2005, 29, 215–226. [Google Scholar]
- Wang, S.; Luo, S.; Yue, S.; Shen, Y.; Li, S. Fate of 15N fertilizer under different nitrogen split applications to plastic mulched maize in semiarid farmland. Nutr. Cycl. Agroecosyst. 2016, 105, 129–140. [Google Scholar] [CrossRef]
- Hauck, R.D. Nitrogen tracers in nitrogen cycle studies-past use and future needs. J. Environ. Qual. 1973, 2, 317–327. [Google Scholar] [CrossRef]
- Torbert, H.A.; Mulvaney, R.M.; Heuvel, V.; Hoeft, R.G. Soil type and moisture regime effects on fertilizer efficiency calculation methods in a N-15 tracer study. Agron. J. 1992, 84, 66–70. [Google Scholar] [CrossRef]
- Lange, A.; Lara Cabezas, W.A.R.; Trivelin, P.C.O. Timing and sources of sidedress nitrogen on no-tillage corn two years after soy crop. Rev. Ceres 2010, 57, 817–824. [Google Scholar] [CrossRef]
- Gava, G.J.C.; Trivelin, P.C.O.; Oliveira, M.W.; Heinrichs, R.; Silva, M.A. Balance of nitrogen from urea (15N) in the soil-plant system at the establishment of no-till in maize. Bragantia 2006, 65, 477–486. [Google Scholar] [CrossRef]
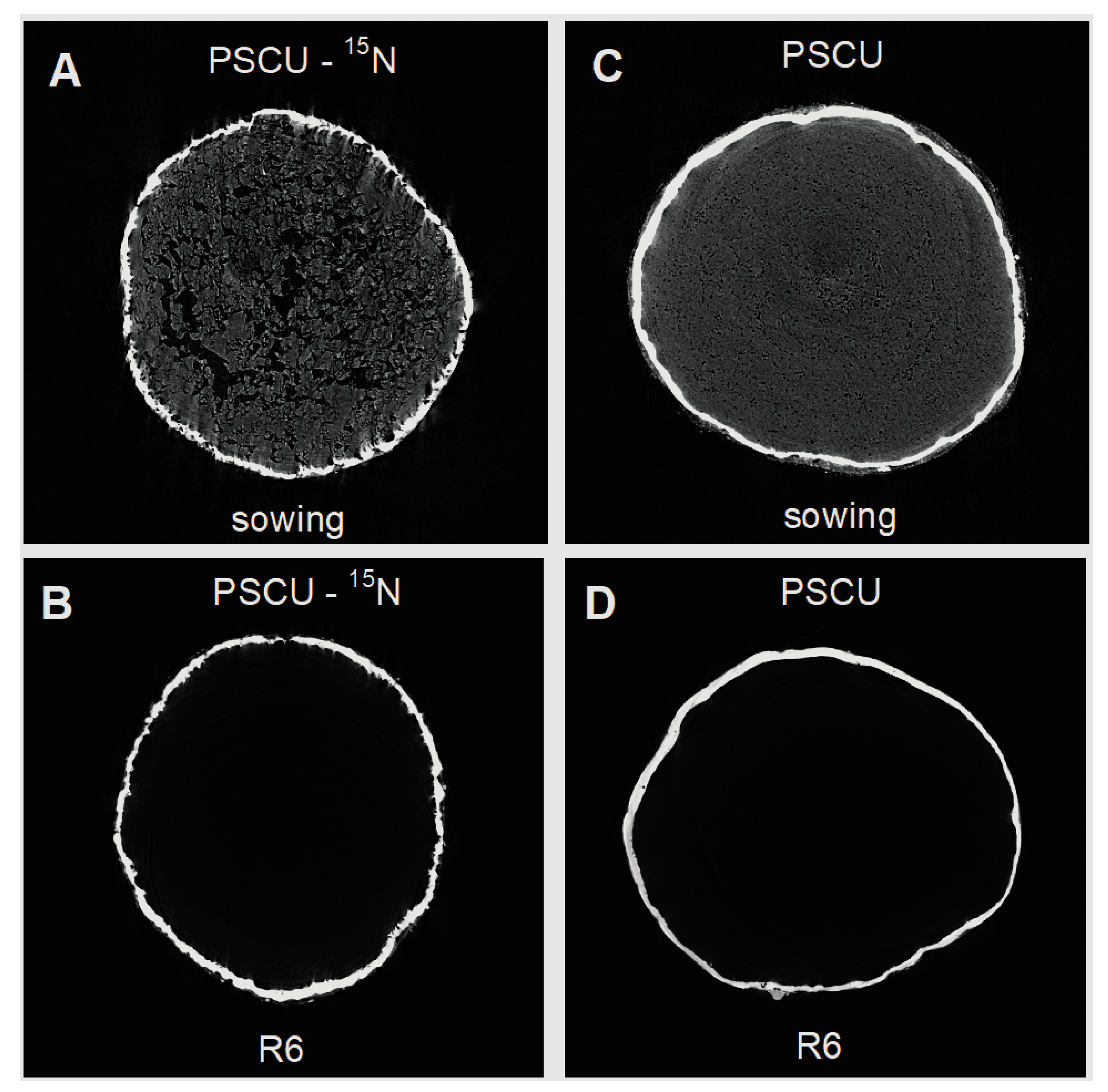
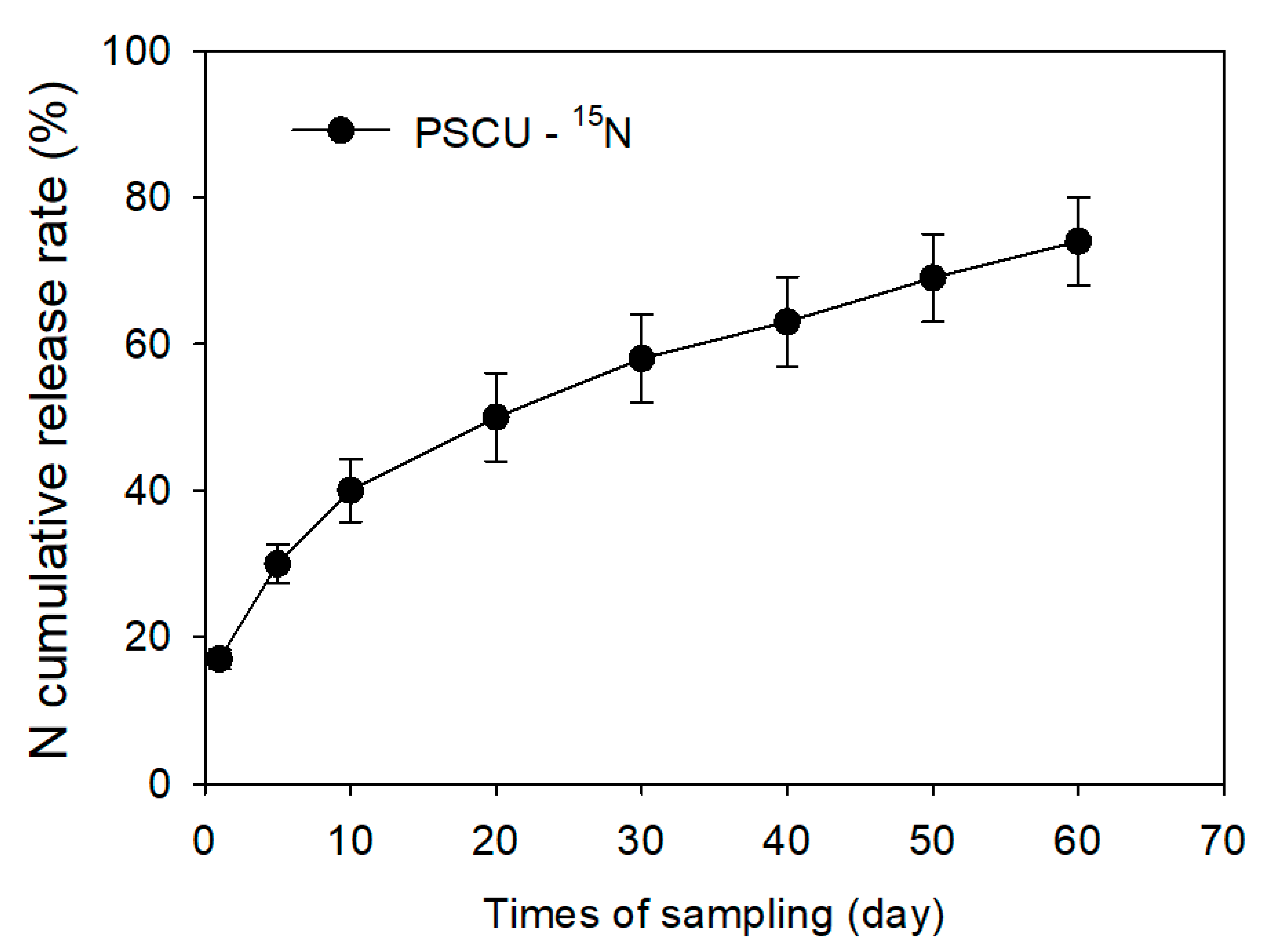

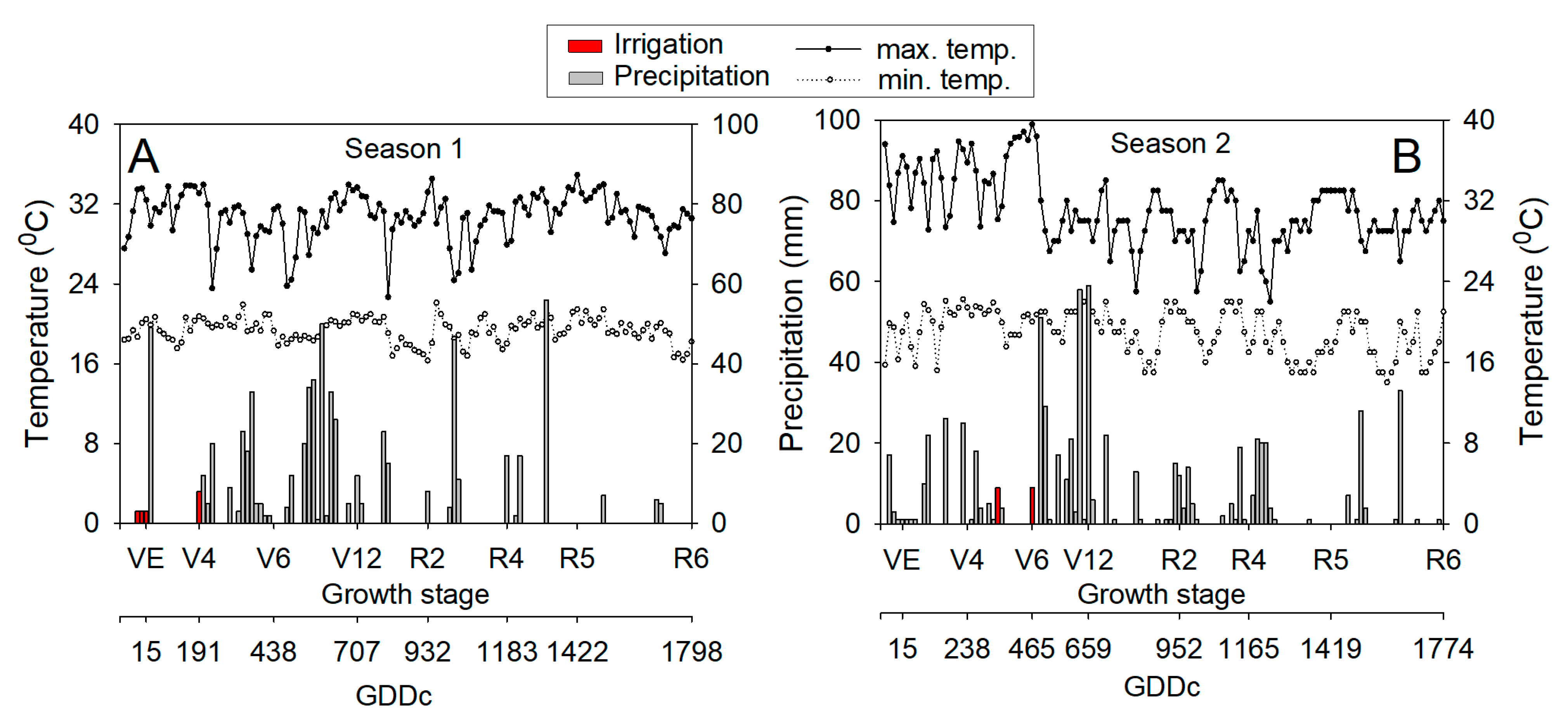
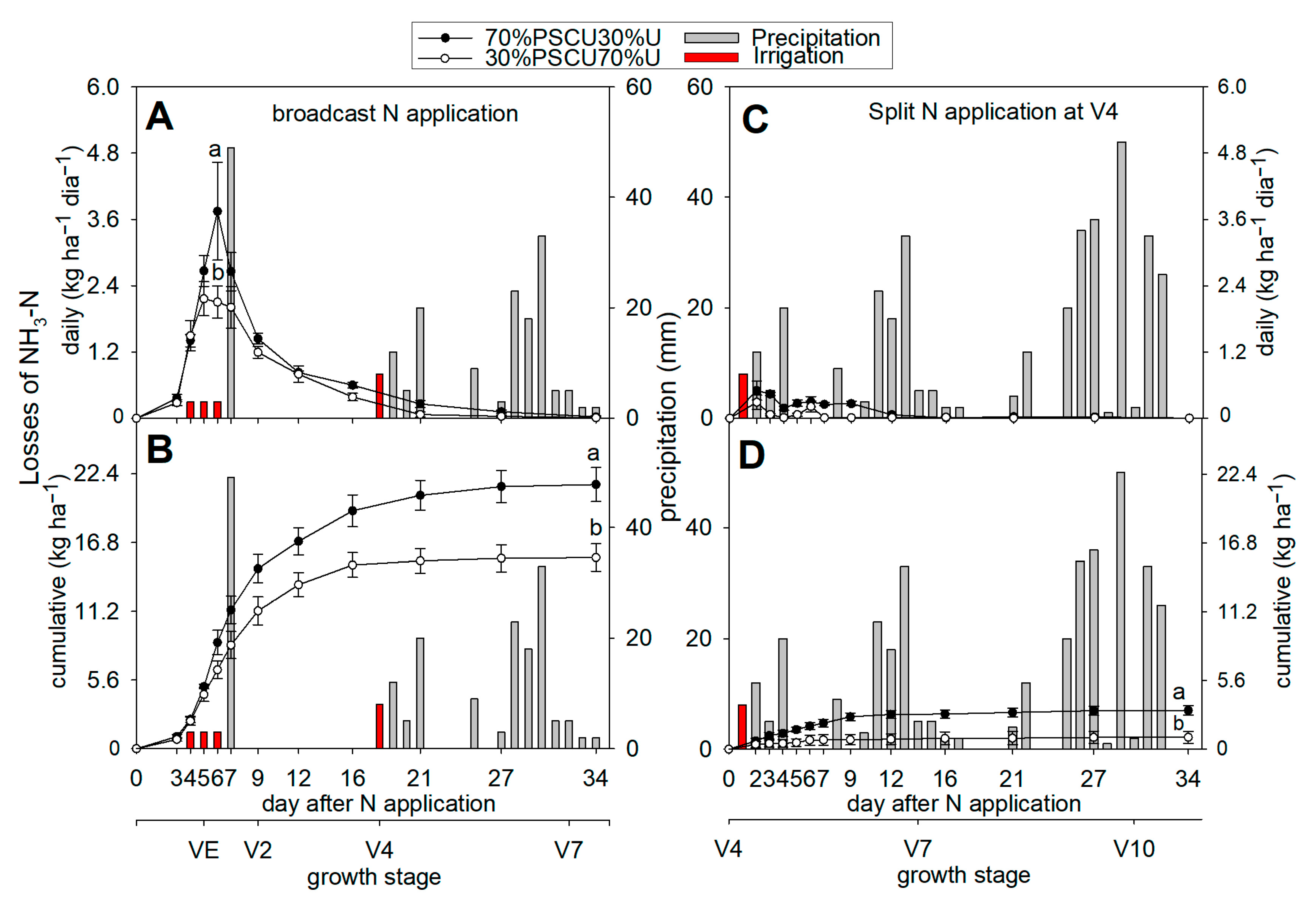
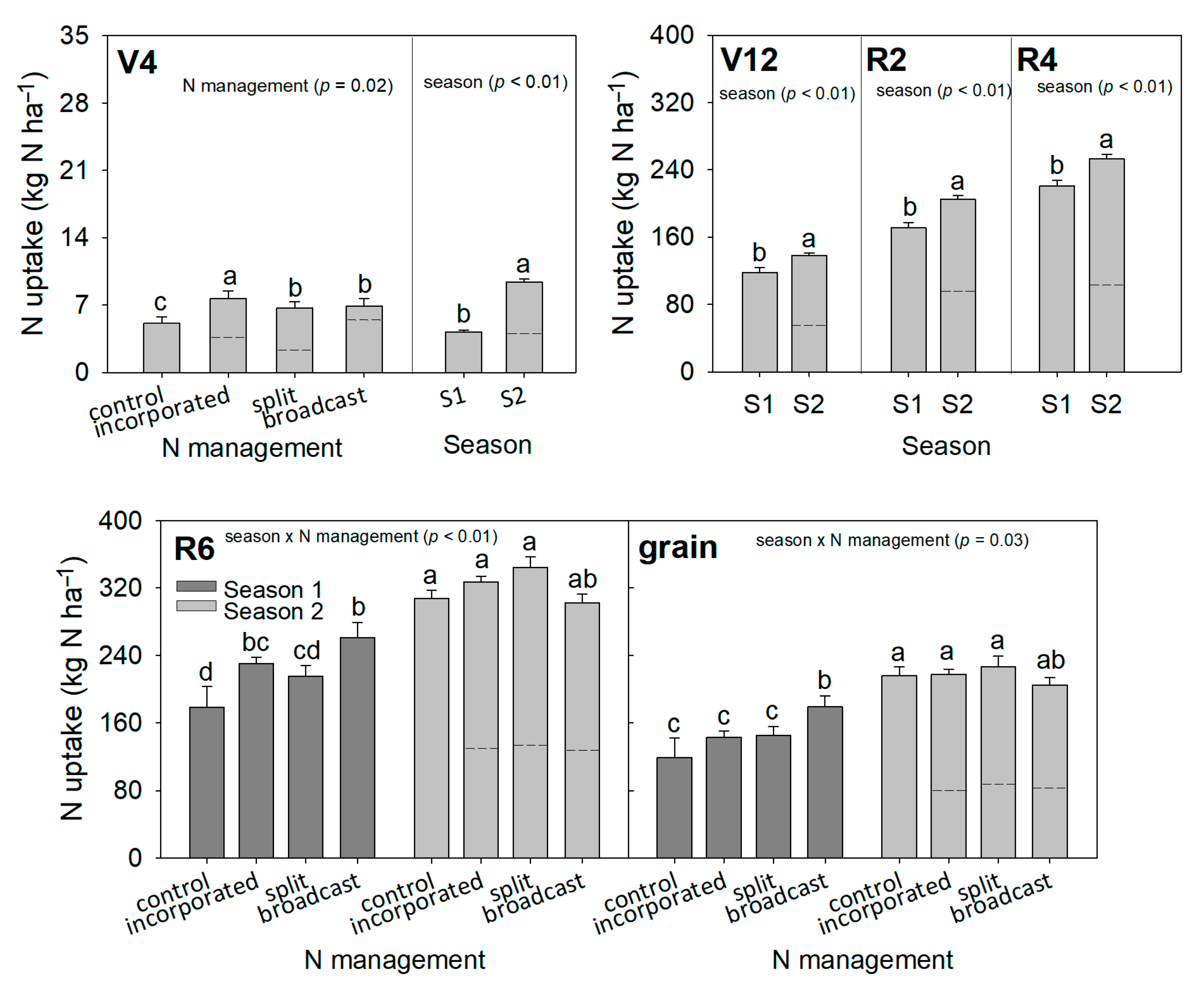

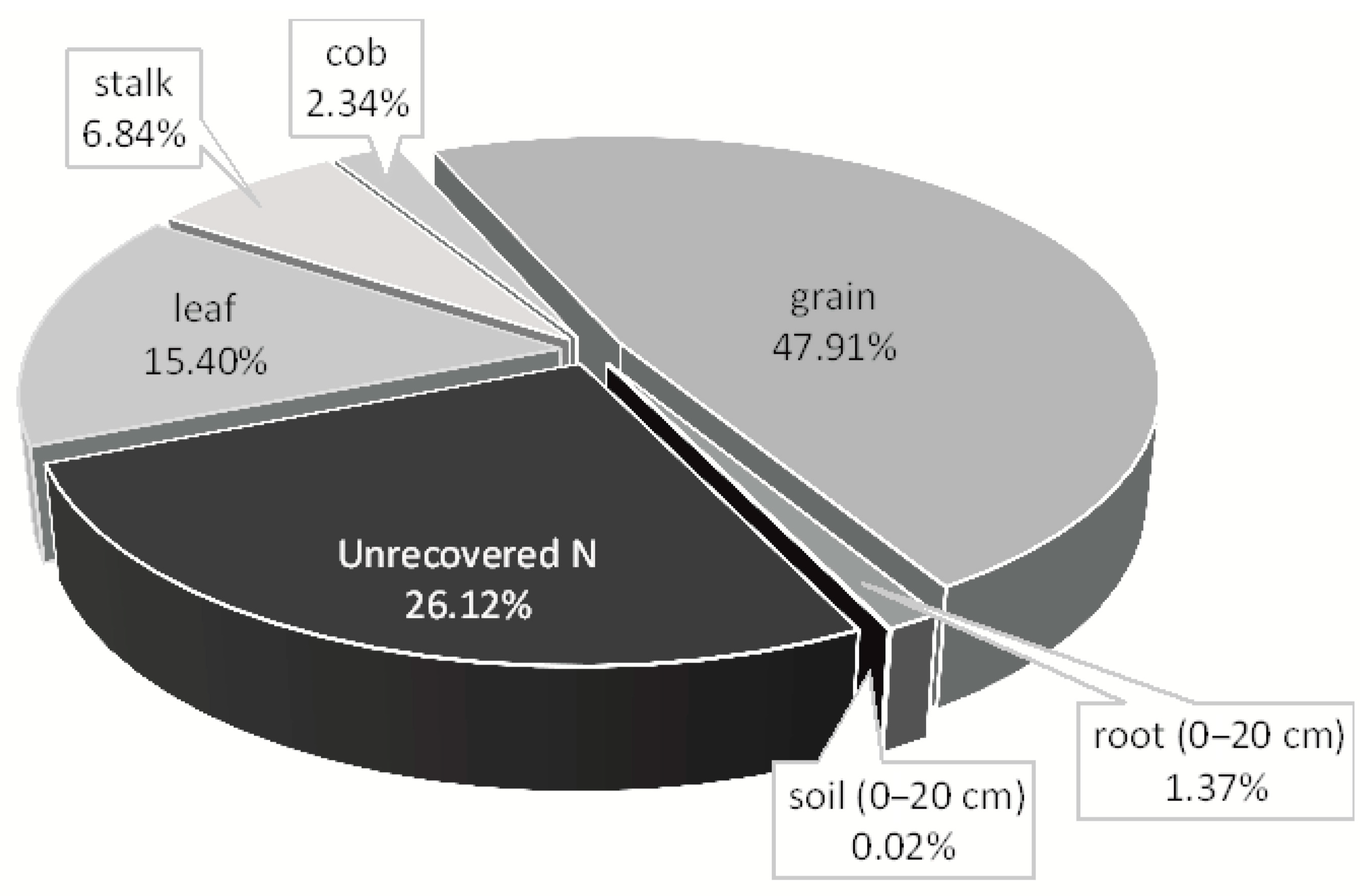
| Depth | pH | SOM 1 | TSN 2 | NH4+ | NO3− | S | P | K | Ca | Mg | Al | CEC 3 | AlS 4 | BS 5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cm | g dm−3 | mg kg−1 | mg dm−3 | mmolc dm−3 | % | |||||||||
| Season 1: 2017–2018 | ||||||||||||||
| 0–20 | 5.0 | 24 | 1100 | 3.7 | 15 | 125 | 10 | 2.5 | 28 | 12 | 1 | 68 | 2 | 63 |
| 20–40 | 4.3 | 18 | 800 | 1.7 | 4.8 | 269 | <3 | 1.3 | 6 | 5 | 11 | 43 | 47 | 28 |
| 40–60 | 4.2 | 15 | 600 | 3.0 | 4.3 | 318 | <3 | 1.0 | 2 | 3 | 11 | 34 | 65 | 18 |
| Season 2: 2019–2020 | ||||||||||||||
| 0–20 | 5.5 | 25 | 1100 | 1.5 | 22 | 14 | 30 | 5.4 | 39 | 26 | 0 | 95 | 0 | 74 |
| 20–40 | 5.1 | 23 | 900 | 1.4 | 12 | 38 | 14 | 4.7 | 25 | 16 | 3 | 90 | 5 | 62 |
| 40–60 | 5.0 | 23 | 700 | 0.7 | 9 | 46 | 12 | 4.9 | 27 | 16 | 2 | 82 | 4 | 58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, P.L.; Sermarini, R.A.; Filho, C.R.d.S.A.; Bendassolli, J.A.; Boschiero, B.N.; Trivelin, P.C.O. 15N-Fertilizer Recovery in Maize as an Additional Strategy for Understanding Nitrogen Fertilization Management with Blends of Controlled-Release and Conventional Urea. Agronomy 2020, 10, 1932. https://doi.org/10.3390/agronomy10121932
Garcia PL, Sermarini RA, Filho CRdSA, Bendassolli JA, Boschiero BN, Trivelin PCO. 15N-Fertilizer Recovery in Maize as an Additional Strategy for Understanding Nitrogen Fertilization Management with Blends of Controlled-Release and Conventional Urea. Agronomy. 2020; 10(12):1932. https://doi.org/10.3390/agronomy10121932
Chicago/Turabian StyleGarcia, Pedro Lopes, Renata Alcarde Sermarini, Carlos Roberto de Sant Ana Filho, José Albertino Bendassolli, Beatriz Nastaro Boschiero, and Paulo Cesar Ocheuze Trivelin. 2020. "15N-Fertilizer Recovery in Maize as an Additional Strategy for Understanding Nitrogen Fertilization Management with Blends of Controlled-Release and Conventional Urea" Agronomy 10, no. 12: 1932. https://doi.org/10.3390/agronomy10121932
APA StyleGarcia, P. L., Sermarini, R. A., Filho, C. R. d. S. A., Bendassolli, J. A., Boschiero, B. N., & Trivelin, P. C. O. (2020). 15N-Fertilizer Recovery in Maize as an Additional Strategy for Understanding Nitrogen Fertilization Management with Blends of Controlled-Release and Conventional Urea. Agronomy, 10(12), 1932. https://doi.org/10.3390/agronomy10121932






