Performance and Potentiality of Camelina (Camelina sativa L. Crantz) Genotypes in Response to Sowing Date under Mediterranean Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Characteristics
2.2. Experimental Setup
2.3. Sampling and Measurements
2.4. Statistical Analyses
3. Results
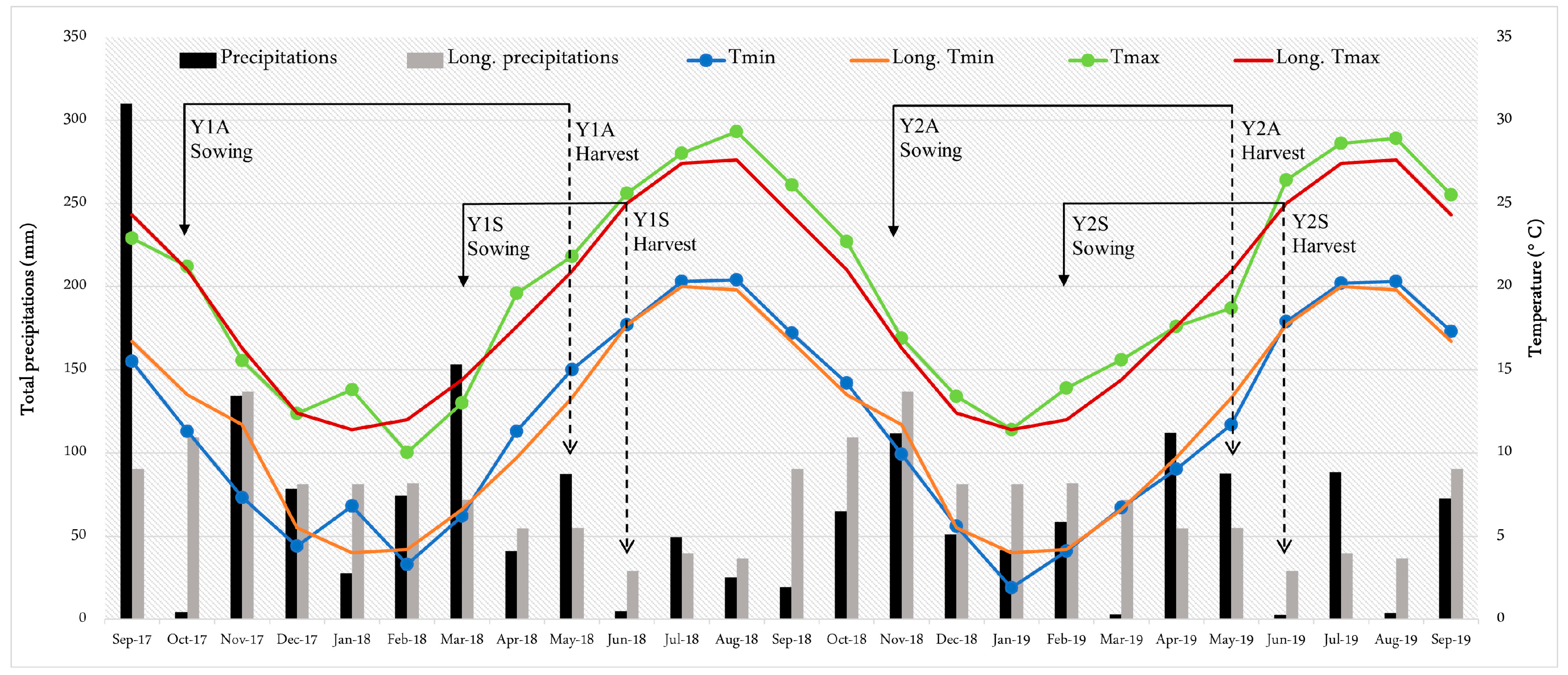
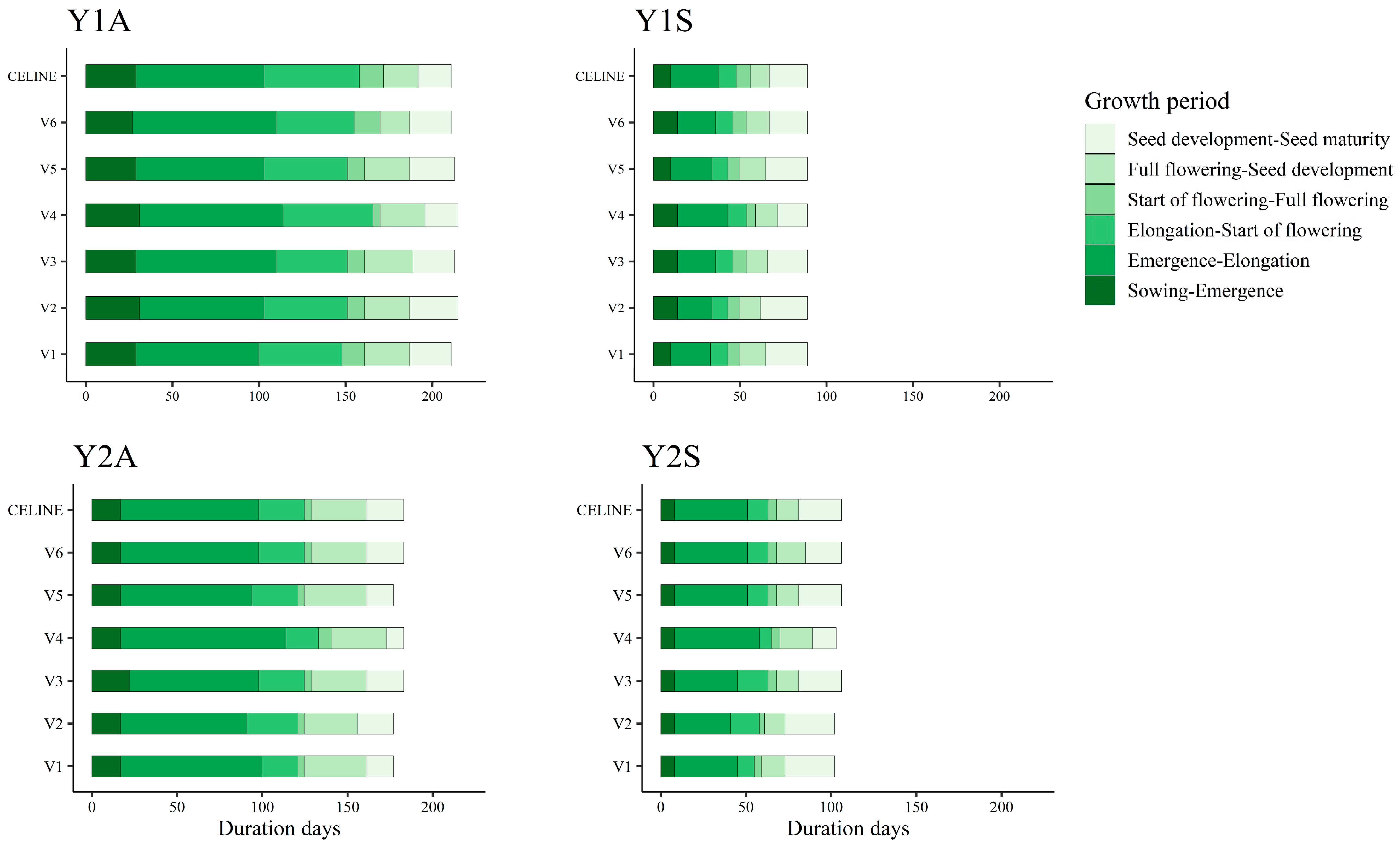
3.1. Weather and Phenological Stages
3.2. Seed Yield and Yield Components
3.3. Oil Yield and Seed Quality
3.4. Relationships between Environmental Factors, Seed Yield, Yield Components and Oil Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berti, M.; Gesch, R.; Eynck, C.; Anderson, J.; Cermak, S. Camelina uses, genetics, genomics, production, and management. Ind. Crops Prod. 2016, 94, 690–710. [Google Scholar] [CrossRef]
- Zubr, J. Oil-seed crop: Camelina sativa. Ind. Crops Prod. 1997, 6, 113–119. [Google Scholar] [CrossRef]
- Rode, J. Study of autochthon Camelina sativa (L.) Crantz in Slovenia. J. Herbs Spices Med. Plants 2002, 9, 313–318. [Google Scholar] [CrossRef]
- Li, X.; Mupondwa, E. Life cycle assessment of camelina oil derived biodiesel and jet fuel in the Canadian Prairies. Sci. Total Environ. 2014, 481, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Shonnard, D.R.; Williams, L.; Kalnes, T.N. Camelina-derived jet fuel and diesel: Sustainable advanced biofuels. Environ. Prog. Sustain. Energy 2010, 29, 382–392. [Google Scholar] [CrossRef]
- Matteo, R.; D’Avino, L.; Ramirez-Cando, L.J.; Pagnotta, E.; Angelini, L.G.; Spugnoli, P.; Tavarini, S.; Ugolini, L.; Foschi, L.; Lazzeri, L. Camelina (Camelina sativa L. Crantz) under low-input management systems in northern Italy: Yields, chemical characterization and environmental sustainability. Ital. J. Agron. 2020, 15, 132–143. [Google Scholar] [CrossRef]
- Gesch, R.W.; Cermak, S.C. Sowing date and tillage effects on fall-seeded camelina in the northern corn belt. Agron. J. 2011, 103, 980–987. [Google Scholar] [CrossRef]
- Wysocki, D.J.; Chastain, T.G.; Schillinger, W.F.; Guy, S.O.; Karow, R.S. Camelina: Seed yield response to applied nitrogen and sulfur. Field Crop Res. 2013, 145, 60–66. [Google Scholar] [CrossRef]
- Séguin-Swartz, G.; Eynck, C.; Gugel, R.K.; Strelkov, S.E.; Olivier, C.Y.; Li, J.L.; Klein-Gebbinck, H.; Borhan, H.; Caldwell, C.D.; Falk, K.C. Diseases of Camelina sativa (false flax). Can. J. Plant Pathol. 2010, 31, 375–386. [Google Scholar] [CrossRef]
- Von Cossel, M.; Lewandowski, I.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; Iqbal, Y.; Mantel, S.; Scordia, D.; Testa, G.; Cosentino, S.L.; et al. Marginal agricultural land low-input systems for biomass production. Energies 2019, 12, 3123. [Google Scholar] [CrossRef]
- Chen, C.; Bekkerman, A.; Afshar, R.K.; Neill, K. Intensification of dryland cropping systems for bio-feedstock production: Evaluation of agronomic and economic benefits of Camelina sativa. Ind. Crops Prod. 2015, 71, 114–121. [Google Scholar] [CrossRef]
- Berti, M.; Gesch, R.; Johnson, B.; Ji, Y.; Seames, W.; Aponte, A. Double-and relay-cropping of energy crops in the northern Great Plains, USA. Ind. Crops Prod. 2015, 75, 26–34. [Google Scholar] [CrossRef]
- Keshavarz-Afshar, R.; Chen, C. Intensification of dryland cropping systems for bio-feedstock production: Energy analysis of camelina. BioEnergy Res. 2015, 8, 1877–1884. [Google Scholar] [CrossRef]
- Berti, M.; Johnson, B.; Ripplinger, D.; Gesch, R.; Aponte, A. Environmental impact assessment of double-and relay-cropping with winter camelina in the northern Great Plains, USA. Agric. Syst. 2017, 156, 1–12. [Google Scholar] [CrossRef]
- Royo-Esnal, A.; Valencia-Gredilla, F. Camelina as a rotation crop for weed control in organic farming in a semiarid mediterranean climate. Agriculture 2018, 8, 156. [Google Scholar] [CrossRef]
- Eberle, C.A.; Thom, M.D.; Nemec, K.T.; Forcella, F.; Lundgren, J.G.; Gesch, R.W.; Riedell, W.E.; Papiernik, S.K.; Wagner, A.; Peterson, D.H.; et al. Using pennycress, camelina, and canola cash cover crops to provision pollinators. Ind. Crops Prod. 2015, 75, 20–25. [Google Scholar] [CrossRef]
- Szterk, A.; Roszko, M.; Sosińska, E.; Derewiaka, D.; Lewicki, P.P. Chemical composition and oxidative stability of selected plant oils. J. Am. Oil Chem. Soc. 2010, 87, 637–645. [Google Scholar] [CrossRef]
- Zubr, J.; Matthäus, B. Effects of growth conditions on fatty acids and tocopherols in Camelina sativa oil. Ind. Crops Prod. 2002, 15, 155–162. [Google Scholar] [CrossRef]
- Lunn, J.; Theobald, H.E. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Li, Y.H.; Sun, X.S. Camelina oil derivatives and adhesion properties. Ind. Crops Prod. 2015, 73, 73–80. [Google Scholar] [CrossRef]
- Righini, D.; Zanetti, F.; Monti, A. The bio-based economy can serve as the springboard for camelina and crambe to quit the limbo. OCL 2016, 23, D504. [Google Scholar] [CrossRef]
- Obour, A.K.; Sintim, H.Y.; Obeng, E.; Zheljazkov, V.D.J. Oilseed camelina Camelina sativa L Crantz production systems prospects and challenges in the USA great plains. Adv. Plants Agric. Res. 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Belayneh, H.D.; Wehling, R.L.; Cahoon, E.; Ciftci, O.N. Lipid composition and emulsifying properties of Camelina sativa seed lecithin. Food Chem. 2018, 242, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Meher, L.C.; Mittal, M.; Dwivedi, S.K. Hydroxy fatty acid from camelina sativa seed oil for industrial application. In Advances in Plant & Microbial Biotechnology; Kundu, R., Narula, R., Eds.; Springer: Singapore, 2019; pp. 69–75. [Google Scholar]
- Matthäus, B.; Angelini, L.G. Anti-nutritive constituents in oilseed crops from Italy. Ind. Crops Prod. 2005, 21, 89–99. [Google Scholar] [CrossRef]
- Orczewska-Dudek, S.; Pietras, M. The Effect of Dietary Camelina sativa Oil or Cake in the Diets of Broiler Chickens on Growth Performance, Fatty Acid Profile, and Sensory Quality of Meat. Animals 2019, 9, 734. [Google Scholar] [CrossRef]
- Szumacher-Strabel, M.; Cieślak, A.; Zmora, P.; Pers-Kamczyc, E.; Bielińska, S.; Stanisz, M.; Wójtowski, J. Camelina sativa cake improved unsaturated fatty acids in ewe’s milk. J. Sci. Food Agric. 2011, 91, 2031–2037. [Google Scholar] [CrossRef]
- Vollmann, J.; Moritz, T.; Kargl, C.; Baumgartner, S.; Wagentristl, H. Agronomic evaluation of camelina genotypes selected for seed quality characteristics. Ind. Crops Prod. 2007, 26, 270–277. [Google Scholar] [CrossRef]
- Pavlista, A.D.; Isbell, T.A.; Baltensperger, D.D.; Hergert, G.W. Planting date and development of spring-seeded irrigated canola, brown mustard and camelina. Ind. Crops Prod. 2011, 33, 451–456. [Google Scholar] [CrossRef]
- Berti, M.; Wilckens, R.; Fischer, S.; Solis, A.; Johnson, B. Seeding date influence on camelina seed yield, yield components, and oil content in Chile. Ind. Crops Prod. 2011, 34, 1358–1365. [Google Scholar] [CrossRef]
- Masella, P.; Martinelli, T.; Galasso, I. Agronomic evaluation and phenotypic plasticity of Camelina sativa growing in Lombardia. Italy. Crop Pasture Sci. 2014, 65, 453–460. [Google Scholar] [CrossRef]
- Zanetti, F.; Eynck, C.; Christou, M.; Krzyżaniak, M.; Righini, D.; Alexopoulou, E.; Stolarski, M.; Van Loo, E.; Puttick, D.; Monti, A. Agronomic performance and seed quality attributes of Camelina (Camelina sativa L. crantz) in multi-environment trials across Europe and Canada. Ind. Crops Prod. 2017, 107, 602–608. [Google Scholar] [CrossRef]
- Righini, D.; Zanetti, F.; Martínez-Force, E.; Mandrioli, M.; Toschi, T.G.; Monti, A. Shifting sowing of camelina from spring to autumn enhances the oil quality for bio-based applications in response to temperature and seed carbon stock. Ind. Crops Prod. 2019, 137, 66–73. [Google Scholar] [CrossRef]
- Gesch, R. Influence of genotype and sowing date on camelina growth and yield in the north central U.S. Ind. Crops Prod. 2014, 54, 209–215. [Google Scholar] [CrossRef]
- Obeng, E.; Obour, A.K.; Nelson, N.O.; Moreno, J.A.; Ciampitti, I.A.; Wang, D.; Durrett, T.P. Seed yield and oil quality as affected by Camelina genotype and planting date. J. Crop Improv. 2019, 33, 202–222. [Google Scholar] [CrossRef]
- Soil Survey Staff USA. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys; USDA-SCS Agricultural Handbook, 436; United States Government Publishing Office: Washington, DC, USA, 1975.
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen total. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph 9; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Mehlich, A. Determination of cation- and anion-exchange properties of soils. Soil Sci. 1948, 66, 429–446. [Google Scholar] [CrossRef]
- Thomas, G.W. Exchangeable cations. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 159–165. [Google Scholar]
- Izza, C.; Mangione, D.; Indiati, R.; Figliolia, A. Heavy metal pollution: Role of the soil organic matter in the dynamic of Cd, Pb, Cu and Zn. In Proceedings of the XXIV ESNA Annual Meeting, Varna, Bulgaria, 12–16 September 1994; p. 24. [Google Scholar]
- Nelson, P.W.; Sommers, C.E. Total Carbon, organic Carbon and organic matter. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Dreimanis, A. Quantitative determination of calcite and dolomite by using Chittick apparatus. J. Sediment. Petrol. 1962, 32, 520–529. [Google Scholar]
- Soil Survey Laboratory Methods Manual; Inv. Rep. No. 42. USDA-NRCS; Soil Survey Laboratory: Washington, DC, USA, 1996.
- Martinelli, T.; Galasso, I. Phenological growth stages of Camelina sativa according to the extended BBCH scale. Ann. Appl. Biol. 2011, 158, 87–94. [Google Scholar] [CrossRef]
- International Rules for Seed Testing, 2005 ed.; The International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 2005.
- Christie, W.W. Preparation of lipid extracts from tissues. In Advances in Lipid Methodology—Two; Christie, W.W., Ed.; Oily Press: Dundee, UK, 1993; pp. 195–213. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2019; Available online: http://www.rstudio.com/ (accessed on 1 October 2020).
- Hergert, G.W.; Margheim, J.F.; Pavlista, A.D.; Martin, D.L.; Isbell, T.A.; Supalla, R.J. Irrigation response and water productivity of deficit to fully irrigated spring camelina. Agric. Water Manag. 2016, 177, 46–53. [Google Scholar] [CrossRef]
- Blackshaw, R.; Johnson, E.; Gan, Y.; May, W.; McAndrew, D.; Barthet, V.; McDonald, T.; Wispinski, D. Alternative oilseed crops for biodiesel feedstock on the Canadian prairies. Can. J. Plant Sci. 2011, 91, 889–896. [Google Scholar] [CrossRef]
- Guy, S.O.; Wysocki, D.J.; Schillinger, W.F.; Chastain, T.G.; Karow, R.S.; Garland-Campbell, K.; Burke, I.C. Camelina: Adaptation and performance of genotypes. Field Crops Res. 2014, 155, 224–232. [Google Scholar] [CrossRef]
- Angelini, L.G.; Moscheni, E.; Colonna, G.; Belloni, P.; Bonari, E. Variation in agronomic characteristics and seed oil composition of new oilseed crops in central Italy. Ind. Crops Prod. 1997, 6, 313–323. [Google Scholar] [CrossRef]
- Hossain, Z.; Johnson, E.N.; Wang, L.; Blackshaw, R.E.; Cutforth, H.; Gan, Y. Plant establishment, yield and yield components of Brassicaceae oilseeds as potential biofuel feedstock. Ind. Crops Prod. 2019, 141, 111800. [Google Scholar] [CrossRef]
- Gesch, R.W.; Matthees, H.L.; Alvarez, A.L.; Gardner, R.D. Winter camelina: Crop growth, seed yield, and quality response to cultivar and seeding rate. Crop Sci. 2018, 58, 2089–2098. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Zheljazkov, V.D.; Obour, A.K.; Garcia y Garcia, A.; Foulke, T.K. Evaluating agronomic responses of camelina to seeding date under rain-fed conditions. Agron. J. 2016, 108, 349–357. [Google Scholar] [CrossRef]
- Pecchia, P.; Russo, R.; Brambilla, I.; Reggiani, R.; Mapelli, S. Biochemical seed traits of Camelina sativa—An emerging oilseed crop for biofuel: Environmental and genetic influences. J. Crop Improv. 2014, 28, 465–483. [Google Scholar] [CrossRef]
- Manca, A.; Pecchia, P.; Mapelli, S.; Masella, P.; Galasso, I. Evaluation of genetic diversity in a Camelina sativa (L.) Crantz collection using microsatellite markers and biochemical traits. Genet. Resour. Crop Evol. 2013, 60, 1223–1236. [Google Scholar] [CrossRef]
- Champolivier, L.; Merrien, A. Effects of water stress applied at different growth stages to Brassica napus L. var. oleifera on yield, yield components and seed quality. Eur. J. Agron. 1996, 5, 153–160. [Google Scholar] [CrossRef]
- Aslam, M.; Nelson, M.; Kailis, S.; Bayliss, K.; Speijers, J.; Cowling, W. Canola oil increases in polyunsaturated fatty acids and decreases in oleic acid in drought-stressed Mediterranean-type environments. Plant Breed. 2009, 128, 348–355. [Google Scholar] [CrossRef]
- Velasco, L.; Fernandez-Martinez, J.M. Breeding oilseed crops for improved oil quality. J. Crop Prod. 2002, 5, 309–344. [Google Scholar] [CrossRef]
- Ratusz, K.; Symoniuk, E.; Wroniak, M.; Rudzińska, M. Bioactive compounds, nutritional quality and oxidative stability of cold-pressed Camelina (Camelina sativa L.) oils. Appl. Sci. 2018, 8, 2606. [Google Scholar] [CrossRef]
- Singer, S.D.; Zou, J.; Weselake, R.J. Abiotic factors influence plant storage lipid accumulation and composition. Plant Sci. 2016, 243, 1–9. [Google Scholar] [CrossRef]
- Schillinger, W.F. Camelina: Long-term cropping systems research in a dry Mediterranean climate. Field Crops Res. 2019, 235, 87–94. [Google Scholar] [CrossRef]
- Rondanini, D.P.; Menendez, Y.C.; Gomez, N.V.; Miralles, D.J.; Botto, J.F. Vegetative plasticity and floral branching compensate low plant density in modern spring rapeseed. Field Crops Res. 2017, 210, 104–113. [Google Scholar] [CrossRef]
- Angadi, S.V.; Cutforth, H.W.; McConkey, B.G.; Gan, Y. Yield adjustment by canola grown at different plant populations under semiarid conditions. Crop Sci. 2003, 43, 1358–1366. [Google Scholar] [CrossRef]
| 2017–2018 | 2018–2019 | |
|---|---|---|
| Sand (%) | 50.97 | 45.86 |
| Silt (%) | 31.39 | 39.00 |
| Clay (%) | 17.64 | 15.14 |
| pH | 7.9 | 8.0 |
| Electric conductivity (mS/cm) | 0.044 | 0.097 |
| Total N (g kg−1) | 1.18 | 1.27 |
| NO3−-N (mg kg−1) | 11.08 | 12.70 |
| Available P Olsen (mg kg−1) | 5.48 | 9.09 |
| Exchangeable K (mg kg−1) | 86.4 | 83.8 |
| Exchangeable Mg (mg kg−1) | 172.5 | 169.7 |
| Total S (g kg−1) | 0.3 | 0.3 |
| Organic matter (%) | 1.91 | 1.98 |
| Total CaCO3 (%) | 6.57 | 6.34 |
| Active CaCO3 (%) | 2.87 | 3.03 |
| C/N | 9.41 | 9.04 |
| Cation-exchange capacity (meq/100 g) | 9.22 | 9.04 |
| Bulk density (g cm−3) | 1.38 | 1.35 |
| Sowing Time | Cycle Length (days) | GDD (°C d) | Cumulative Rainfall (mm) | ||||
|---|---|---|---|---|---|---|---|
| Vegetative † | Reproductive †† | Vegetative | Reproductive | Vegetative | Reproductive | ||
| Y1A | 18 October 2017 | 154 | 58 | 762 | 580 | 442.8 | 128.5 |
| Y1S | 28 March 2018 | 46 | 43 | 491 | 663 | 93.4 | 52.6 |
| Y2A | 20 November 2018 | 124 | 56 | 522 | 479 | 181.7 | 170.0 |
| Y2S | 26 February 2019 | 61 | 46 | 442 | 563 | 109.0 | 94.0 |
| Term | Seed Yield | No. Siliques Plant−1 † | No. Seeds Silique−1 † | 1000-Seed Weight | Plant Height | Plant Density † | Above-Ground Biomass †† | HI ††† | Oil Content | Oil Yield | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (Mg ha−1) | (g) | (cm) | (No. m−2 ) | (Mg ha−1) | (% dry Weight) | (Mg ha−1) | |||||
| Year | 2017–2018 | 1.57 ± 0.06 b | 137.5 ± 1.6 a | 8.4 ± 0.4 a | 0.94 ± 0.01 b | 90.4 ± 0.9 b | 221.0 ± 3.7 b | 4.74 ± 0.10 b | 0.25 ± 0.003 a | 39.3 ± 0.4 a | 0.65 ± 0.02 b |
| 2018–2019 | 1.95 ± 0.06 a | 139.2 ±1.6 a | 7.4 ± 0.4 a | 1.03 ± 0.01 a | 96.1 ± 0.9 a | 288.9 ± 4.6 a | 7.19 ± 0.10 a | 0.21 ± 0.003 b | 39.2 ± 0.4 a | 0.76 ± 0.02 a | |
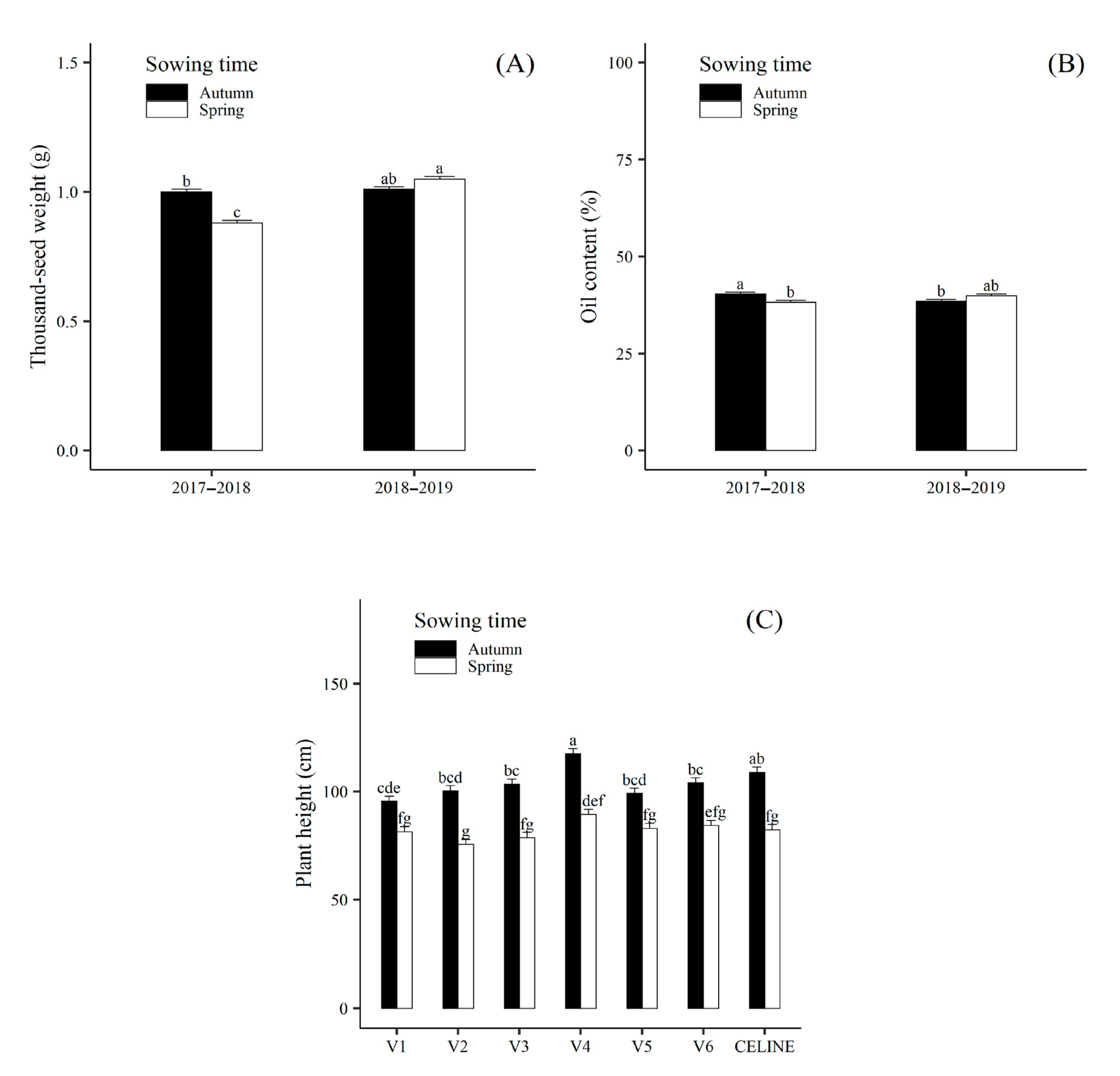
| Sowing time | Autumn | 1.90 ± 0.06 a | 167.8 ± 1.7 a | 8.1 ± 0.4 a | 1.01 ± 0.01 a | 104.2 ± 0.9 a | 220.7 ± 3.7 b | 6.88 ± 0.10 a | 0.22 ± 0.003 b | 39.4 ± 0.4 a | 0.77 ± 0.02 a |
| Spring | 1.61 ± 0.06 b | 114.0 ± 1.4 b | 7.6 ± 0.4 a | 0.97 ± 0.01 b | 82.2 ± 0.9 b | 289.4 ± 4.6 a | 5.05 ± 0.10 b | 0.24 ± 0.003 a | 39.1 ± 0.4 a | 0.65 ± 0.02 b | |
| Genotype | V1 | 1.86 ± 0.09 ab | 122.9 ± 2.8 de | 7.7 ± 0.7 ab | 1.13 ± 0.02 ab | 88.6 ± 1.7 c | 290.9 ±5.9 a | 5.72 ± 0.20 ab | 0.25 ± 0.01 a | 40.3 ± 0.6 a | 0.77 ± 0.03 ab |
| V2 | 1.62 ± 0.09 bc | 150.6 ± 3.1b | 6.9 ± 0.7 b | 0.90 ± 0.02 cd | 88.0 ± 1.7 c | 248.6 ± 5.3 bc | 5.22 ± 0.20 b | 0.24 ± 0.01 ab | 39.7 ± 0.6 a | 0.66 ± 0.03 bc | |
| V3 | 2.08 ± 0.09 a | 140.2 ± 3.1 bc | 7.7 ± 0.7 b | 1.17 ± 0.02 a | 91.2 ± 1.7 bc | 224.5 ± 4.9 d | 5.95 ± 0.20 ab | 0.26 ± 0.01 a | 38.7 ± 0.6 a | 0.83 ± 0.03 a | |
| V4 | 1.44 ± 0.09 c | 113.9 ± 2.7 e | 10.2 ± 0.8 a | 0.86 ± 0.02 de | 103.5 ± 1.7 a | 261.6 ± 5.5 b | 6.06 ± 0.20 ab | 0.20 ± 0.01 c | 38.9 ± 0.6 a | 0.58 ± 0.03 c | |
| V5 | 1.75 ± 0.09 b | 128.8 ± 2.8 cd | 8.4 ± 0.7 ab | 1.10 ± 0.02 b | 91.1 ± 1.7 bc | 235.2 ± 5.1 cd | 6.35± 0.20 a | 0.22 ± 0.01 b | 38.6 ± 0.6 a | 0.69 ± 0.03 bc | |
| V6 | 1.70 ± 0.09 bc | 189.6 ± 3.5 a | 7.6 ± 0.7 ab | 0.82 ± 0.02 e | 94.3 ± 1.7 bc | 221.5 ± 4.9 d | 6.02 ± 0.20 ab | 0.22 ± 0.01 b | 39.1 ± 0.6 a | 0.69 ± 0.03 bc | |
| CELINE | 1.84 ± 0.09 ab | 134.5 ± 2.9 cd | 7.0 ± 0.7 b | 0.95 ± 0.02 c | 95.8 ± 1.7 b | 297.7 ± 6.1a | 6.43 ± 0.20 a | 0.22 ± 0.01 b | 39.5 ± 0.6 a | 0.74 ± 0.03 ab | |
| G | <0.001 | <0.0001 | 0.02 | <0.0001 | <0.001 | <0.0001 | <0.001 | <0.001 | 0.35 | <0.001 | |
| ST | <0.001 | <0.0001 | 0.33 | 0.001 | <0.001 | <0.0001 | <0.001 | 0.002 | 0.43 | <0.001 | |
| Y | <0.001 | 0.95 | 0.08 | <0.0001 | <0.001 | <0.0001 | <0.001 | 0.002 | 0.80 | <0.001 | |
| G × ST | <0.001 | <0.0001 | 0.98 | 0.34 | 0.02 | <0.0001 | <0.001 | <0.001 | 0.48 | <0.001 | |
| G × Y | 0.17 | <0.0001 | 0.54 | 0.28 | 0.44 | <0.0001 | 0.02 | 0.06 | 0.06 | 0.10 | |
| ST × Y | <0.001 | <0.0001 | 0.82 | <0.0001 | 0.09 | 0.002 | <0.001 | 0.90 | <0.001 | <0.001 | |
| G × ST × Y | <0.001 | <0.0001 | 0.97 | 0.06 | 0.29 | <0.0001 | 0.001 | <0.001 | 0.06 | <0.001 |
| Genotype | Sowing Time | Seed Yield (Mg ha−1) | No. Siliques Plant−1 | Plant Density (No. m−2) | Above-Ground Biomass (Mg ha−1) | HI | Oil Yield (Mg ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | ||
| V1 | Autumn | 1.6 ± 0.2 ab | 2.0 ± 0.2 bc | 170.7 ± 6.5 bc | 150.7 ± 6.5 c | 198.1 ± 7.5 ef | 283.6 ± 9.3 def | 4.8 ± 0.4 ab | 7.2 ± 0.4 bc | 0.25 ± 0.01a | 0.22 ± 9.3 abc | 0.64 ± 0.06 ab | 0.81 ± 0.06 bc |
| Spring | 2.0 ± 0.2 a | 1.8 ± 0.2 bc | 110.7 ± 5.3 fgh | 80.0 ± 4.5 g | 271.6 ± 9.1 bc | 468.8 ± 12.6 a | 5.0 ± 0.4 ab | 5.9 ± 0.4 cd | 0.29 ± 0.01a | 0.23 ± 0.01 abc | 0.79 ± 0.06 a | 0.76 ± 0.06 bc | |
| V2 | Autumn | 1.4 ± 0.2 ab | 2.0 ± 0.2 bc | 192.7 ± 6.9 ab | 138.7 ± 5.9 cd | 158.4 ± 6.7 g | 254.9 ± 8.7 fg | 4.5 ± 0.4 ab | 7.2 ± 0.4 bc | 0.25 ± 0.01a | 0.21 ± 0.01 abcd | 0.59 ± 0.06 ab | 0.74 ± 0.06 bc |
| Spring | 1.5 ± 0.2 ab | 1.6 ± 0.2 c | 121.7 ± 5.5 efg | 158.0 ± 6.3 c | 288.4 ± 9.4 b | 327.8 ± 10.1 bc | 3.7 ± 0.4 b | 5.5 ± 0.4 cd | 0.28 ± 0.01a | 0.23 ± 0.01 abc | 0.59 ± 0.06 ab | 0.64 ± 0.06 c | |
| V3 | Autumn | 1.9 ± 0.2 a | 3.4 ± 0.2 a | 188.0 ± 6.8 ab | 224.5 ± 7.5 b | 173.2 ± 7.0 fg | 217.9 ± 8.0 g | 4.8 ± 0.4 ab | 10.2 ± 0.4 a | 0.28 ± 0.01a | 0.25 ± 0.01 a | 0.74 ± 0.06 a | 1.35 ± 0.06 a |
| Spring | 1.5 ± 0.2 ab | 1.4 ± 0.2 c | 98.0 ± 4.9 gh | 93.5 ± 4.8 fg | 251.4 ± 8.7 bc | 267.9 ± 9.0 ef | 3.9 ± 0.4 b | 4.8 ± 0.4 d | 0.28 ± 0.01a | 0.23 ± 0.01 abc | 0.57 ± 0.06 ab | 0.56 ± 0.06 c | |
| V4 | Autumn | 1.4 ± 0.2 ab | 1.5 ± 0.2 c | 156.0 ± 6.2 cd | 110.7 ± 5.3 ef | 194.9 ± 7.5 ef | 298.6 ± 9.6 bcde | 5.4 ± 0.4 ab | 8.6 ± 0.4 ab | 0.21 ± 0.01a | 0.15 ± 0.01 e | 0.58 ± 0.06 ab | 0.59 ± 0.06 c |
| Spring | 1.1 ± 0.2 b | 1.7 ± 0.2 bc | 73.7 ± 4.3 i | 132.0 ± 5.7 cde | 243.4 ± 8.5 cd | 330.6 ± 10.2 bc | 3.7 ± 0.4 b | 6.5 ± 0.4 cd | 0.22 ± 0.01a | 0.21 ± 0.01 bcd | 0.38 ± 0.06 b | 0.70 ± 0.06 bc | |
| V5 | Autumn | 1.5 ± 0.2 ab | 2.1 ± 0.2 bc | 134.0 ± 5.8 def | 130.7 ± 5.7 cde | 241.4 ± 8.5 cd | 304.5 ± 9.7 bcde | 5.5 ± 0.4 ab | 9.9 ± 0.4 a | 0.22 ± 0.01a | 0.18 ± 0.01 de | 0.65 ± 0.06 ab | 0.75 ± 0.06 bc |
| Spring | 1.6 ± 0.2 ab | 1.8 ± 0.2 bc | 132.0 ± 5.7 def | 119.0 ± 5.4 de | 183.2 ± 7.2 efg | 227.2 ± 8.2 gh | 4.4 ± 0.4 ab | 5.1 ± 0.4 cd | 0.26 ± 0.01a | 0.24 ± 0.01 ab | 0.60 ± 0.06 ab | 0.68 ± 0.06 c | |
| V6 | Autumn | 1.6 ± 0.2 ab | 2.4 ± 0.2 b | 206.5 ± 7.2a | 283.5 ± 8.4 a | 168.4 ± 6.9 fg | 199.9 ± 7.6 h | 5.3 ± 0.4 ab | 8.8 ± 0.4 ab | 0.23 ± 0.01a | 0.21 ± 0.01 abcd | 0.63 ± 0.06 ab | 0.97 ± 0.06 b |
| Spring | 1.4 ± 0.2 ab | 1.4 ± 0.2 c | 141.0 ± 5.9 de | 156.5 ± 6.3 c | 209.9 ± 7.8 de | 340.6 ± 10.4 b | 3.9 ± 0.4 b | 6.0 ± 0.4 cd | 0.26 ± 0.01a | 0.19 ± 0.01 cde | 0.53 ± 0.06 ab | 0.54 ± 0.06 c | |
| CELINE | Autumn | 1.5 ± 0.2 ab | 2.1 ± 0.2 bc | 193.0 ± 6.9 ab | 141.7 ± 5.9 cd | 180.2 ± 7.2 efg | 292.4 ± 9.5 cdef | 5.4 ± 0.4 ab | 8.7 ± 0.4 ab | 0.22 ± 0.01a | 0.19 ± 0.01 cd | 0.61 ± 0.06 ab | 0.81 ± 0.06 bc |
| Spring | 1.9 ± 0.2 a | 1.9 ± 0.2 bc | 92.2 ± 4.8 hi | 129.7 ± 5.7 cde | 464.8 ± 12.6 a | 320.6 ± 10.0 bcd | 6.0 ± 0.4 a | 5.7 ± 0.4 cd | 0.24 ± 0.01a | 0.25 ± 0.01 ab | 0.72 ± 0.06 a | 0.77 ± 0.06 bc | |
| Term | C18:3 | C18:2 | C20:1 | C18:1 | C16:0 | C22:1 | C18:0 | C20:2 | C20:0 | |
|---|---|---|---|---|---|---|---|---|---|---|
| % | ||||||||||
| Year | 2017–2018 | 34.62 ± 0.35 b | 18.37 ± 0.07 a | 14.84 ± 0.12 b | 14.33 ± 0.21 a | 5.13 ± 0.02 a | 3.90 ± 0.11 a | 2.07 ± 0.02 a | 2.02 ± 0.01 b | 1.33 ± 0.02 a |
| 2018–2019 | 35.82 ± 0.35 a | 17.16 ± 0.07 b | 16.54 ± 0.12 a | 12.02 ± 0.21 b | 4.86 ± 0.02 b | 2.72 ± 0.11 b | 2.06 ± 0.02 a | 2.12 ± 0.01 a | 1.29 ± 0.02 b | |
| Sowing time | Autumn | 36.68 ± 0.35 a | 16.92 ± 0.07 b | 16.25 ± 0.12 a | 11.76 ± 0.21 b | 4.85 ± 0.02 b | 3.58 ± 0.11 a | 2.06 ± 0.02 a | 2.22 ± 0.01 a | 1.29 ± 0.02 b |
| Spring | 33.76 ± 0.35 b | 18.60 ± 0.07 a | 15.13 ± 0.12 b | 14.59 ± 0.21 a | 5.14 ± 0.02 a | 3.05 ± 0.11 b | 2.07 ± 0.02 a | 1.93 ± 0.01 b | 1.32 ± 0.02 a | |
| Genotype | V1 | 35.17 ± 0.42 ab | 17.10 ± 0.13 cd | 16.54 ± 0.21a | 12.45 ± 0.26 c | 5.04 ± 0.03 ab | 3.54 ± 0.16 a | 1.99 ± 0.03 cd | 2.16 ± 0.02 b | 1.41 ± 0.03 a |
| V2 | 36.02 ± 0.42 a | 16.26 ± 0.13 e | 15.69 ± 0.21 abc | 14.16 ± 0.26 a | 4.83 ± 0.03 d | 3.16 ± 0.16 a | 2.16 ± 0.03 ab | 1.83 ± 0.02 d | 1.30 ± 0.03 bc | |
| V3 | 35.44 ± 0.42 a | 18.46 ± 0.13 b | 15.79 ± 0.21 abc | 11.59 ± 0.26 d | 5.17 ± 0.03 a | 3.52 ± 0.16 a | 1.95 ± 0.03 d | 2.30 ± 0.02 a | 1.38 ± 0.03 ab | |
| V4 | 33.97 ± 0.42 b | 19.89 ± 0.13 a | 15.00 ± 0.21c | 12.86 ± 0.26 bc | 4.89 ± 0.03 cd | 3.27 ± 0.16 a | 1.93 ± 0.03 d | 2.32 ± 0.02 a | 1.25 ± 0.03 cd | |
| V5 | 35.20 ± 0.42 ab | 16.59 ± 0.13 de | 16.01 ± 0.21 ab | 13.60 ± 0.26 ab | 4.99 ± 0.03 bc | 3.34 ± 0.16 a | 2.13 ± 0.03 ab | 1.86 ± 0.02 d | 1.42 ± 0.03 a | |
| V6 | 35.37 ± 0.42 a | 17.63 ± 0.13 c | 15.60 ± 0.21 abc | 14.01 ± 0.26 a | 4.98 ± 0.03 bc | 3.27 ± 0.16 a | 2.21± 0.03 a | 1.98 ± 0.02 c | 1.25 ± 0.03 cd | |
| CELINE | 35.37 ± 0.42 a | 18.41 ± 0.13 b | 15.19 ± 0.21 bc | 13.50 ± 0.26 ab | 5.10 ± 0.03 ab | 3.10 ± 0.16 a | 2.09 ± 0.03 bc | 2.05 ± 0.02 c | 1.15 ± 0.03 d | |
| G | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.28 | <0.001 | <0.001 | <0.001 | |
| ST | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.56 | <0.001 | 0.05 | |
| Y | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.42 | <0.001 | 0.04 | |
| G × ST | 0.49 | <0.001 | 0.81 | 0.10 | 0.06 | 0.61 | 0.05 | 0.72 | 0.75 | |
| G × Y | 0.07 | 0.02 | 0.04 | 0.62 | 0.67 | 0.48 | 0.01 | 0.56 | 0.20 | |
| ST × Y | 0.34 | 0.01 | 0.77 | <0.001 | <0.001 | 0.009 | 0.45 | <0.001 | 0.003 | |
| G × ST × Y | 0.23 | 0.003 | 0.49 | 0.19 | 0.36 | 0.82 | 0.45 | 0.15 | 0.38 | |
| Term | SFA † | MUFA †† | PUFA ††† | PUFA/SFA | ω-3 | ω-6/ω-3 | Crude protein | |
|---|---|---|---|---|---|---|---|---|
| % | ||||||||
| Year | 2017–2018 | 9.07 ± 0.04 a | 30.1 ± 0.1 b | 60.1 ± 0.3 a | 6.64 ± 0.04 b | 39.5 ± 0.3 a | 0.53 ± 0.01 a | 20.7 ± 0.4 b |
| 2018–2019 | 8.51 ± 0.04 b | 33.1 ± 0.1 a | 57.6 ± 0.3 b | 6.77 ± 0.04 a | 38.2 ± 0.3 b | 0.51 ± 0.01 b | 22.1 ± 0.4 a | |
| Sowing time | Autumn | 8.62 ± 0.04 b | 30.7 ± 0.1 b | 59.9 ± 0.3 a | 6.96 ± 0.04 a | 40.6 ± 0.3 a | 0.47 ± 0.01 b | 21.0 ± 0.4 b |
| Spring | 8.97 ± 0.04 a | 32.5 ± 0.1 a | 57.7 ± 0.3 b | 6.45 ± 0.04 b | 37.1 ± 0.3 b | 0.56 ± 0.01 a | 21.8 ± 0.4 a | |
| Genotype | V1 | 8.83 ± 0.07 ab | 31.8 ± 0.3 ab | 58.5 ± 0.5 abc | 6.64 ± 0.07 bc | 39.1 ± 0.5 ab | 0.50 ± 0.01 d | 20.7 ± 0.5 a |
| V2 | 8.66 ± 0.07 bc | 32.6 ± 0.3 a | 57.8 ± 0.5 bc | 6.69 ± 0.07 bc | 39.5 ± 0.5 a | 0.46 ± 0.01 e | 21.9 ± 0.5 a | |
| V3 | 8.96 ± 0.07 ab | 30.1 ± 0.3 c | 59.9 ± 0.5 a | 6.69 ± 0.07 bc | 38.9 ± 0.5 ab | 0.54 ± 0.01 b | 21.3 ± 0.5 a | |
| V4 | 8.50 ± 0.07 c | 30.7 ± 0.3 bc | 59.9 ± 0.5 a | 7.05 ± 0.07 a | 37.5 ± 0.5 b | 0.60 ± 0.01 a | 21.1 ± 0.5 a | |
| V5 | 8.99 ± 0.07 a | 32.4 ± 0.3 a | 57.4 ± 0.5 c | 6.40 ± 0.07 c | 38.8 ± 0.5 ab | 0.48 ± 0.01 de | 21.4 ± 0.5 a | |
| V6 | 8.85 ± 0.07 ab | 32.3 ± 0.3 a | 58.8 ± 0.5 abc | 6.65 ± 0.07 bc | 39.0 ± 0.5 ab | 0.51 ± 0.01 cd | 21.6 ± 0.5 a | |
| CELINE | 8.76 ± 0.07 abc | 31.5 ± 0.3 ab | 59.6 ± 0.5 ab | 6.80 ± 0.07 ab | 39.0 ± 0.5 ab | 0.53 ± 0.01 bc | 22.0 ± 0.5 a | |
| G | <0.001 | <0.001 | <0.001 | <0.001 | 0.03 | <0.001 | 0.25 | |
| ST | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.01 | |
| Y | <0.001 | <0.001 | <0.001 | 0.01 | <0.001 | 0.02 | <0.001 | |
| G × ST | 0.07 | 0.52 | 0.46 | 0.38 | 0.82 | 0.02 | 0.67 | |
| G × Y | 0.13 | 0.05 | 0.50 | 0.23 | 0.15 | 0.02 | 0.70 | |
| ST × Y | <0.001 | 0.001 | 0.35 | 0.07 | 0.89 | 0.08 | 0.01 | |
| G × ST × Y | 0.37 | 0.28 | 0.76 | 0.90 | 0.39 | 0.03 | 0.38 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelini, L.G.; Abou Chehade, L.; Foschi, L.; Tavarini, S. Performance and Potentiality of Camelina (Camelina sativa L. Crantz) Genotypes in Response to Sowing Date under Mediterranean Environment. Agronomy 2020, 10, 1929. https://doi.org/10.3390/agronomy10121929
Angelini LG, Abou Chehade L, Foschi L, Tavarini S. Performance and Potentiality of Camelina (Camelina sativa L. Crantz) Genotypes in Response to Sowing Date under Mediterranean Environment. Agronomy. 2020; 10(12):1929. https://doi.org/10.3390/agronomy10121929
Chicago/Turabian StyleAngelini, Luciana G., Lara Abou Chehade, Lara Foschi, and Silvia Tavarini. 2020. "Performance and Potentiality of Camelina (Camelina sativa L. Crantz) Genotypes in Response to Sowing Date under Mediterranean Environment" Agronomy 10, no. 12: 1929. https://doi.org/10.3390/agronomy10121929
APA StyleAngelini, L. G., Abou Chehade, L., Foschi, L., & Tavarini, S. (2020). Performance and Potentiality of Camelina (Camelina sativa L. Crantz) Genotypes in Response to Sowing Date under Mediterranean Environment. Agronomy, 10(12), 1929. https://doi.org/10.3390/agronomy10121929





