Extending the CSM-CERES-Beet Model to Simulate Impact of Observed Leaf Disease Damage on Sugar Beet Yield
Abstract
1. Introduction
2. Materials and Methods
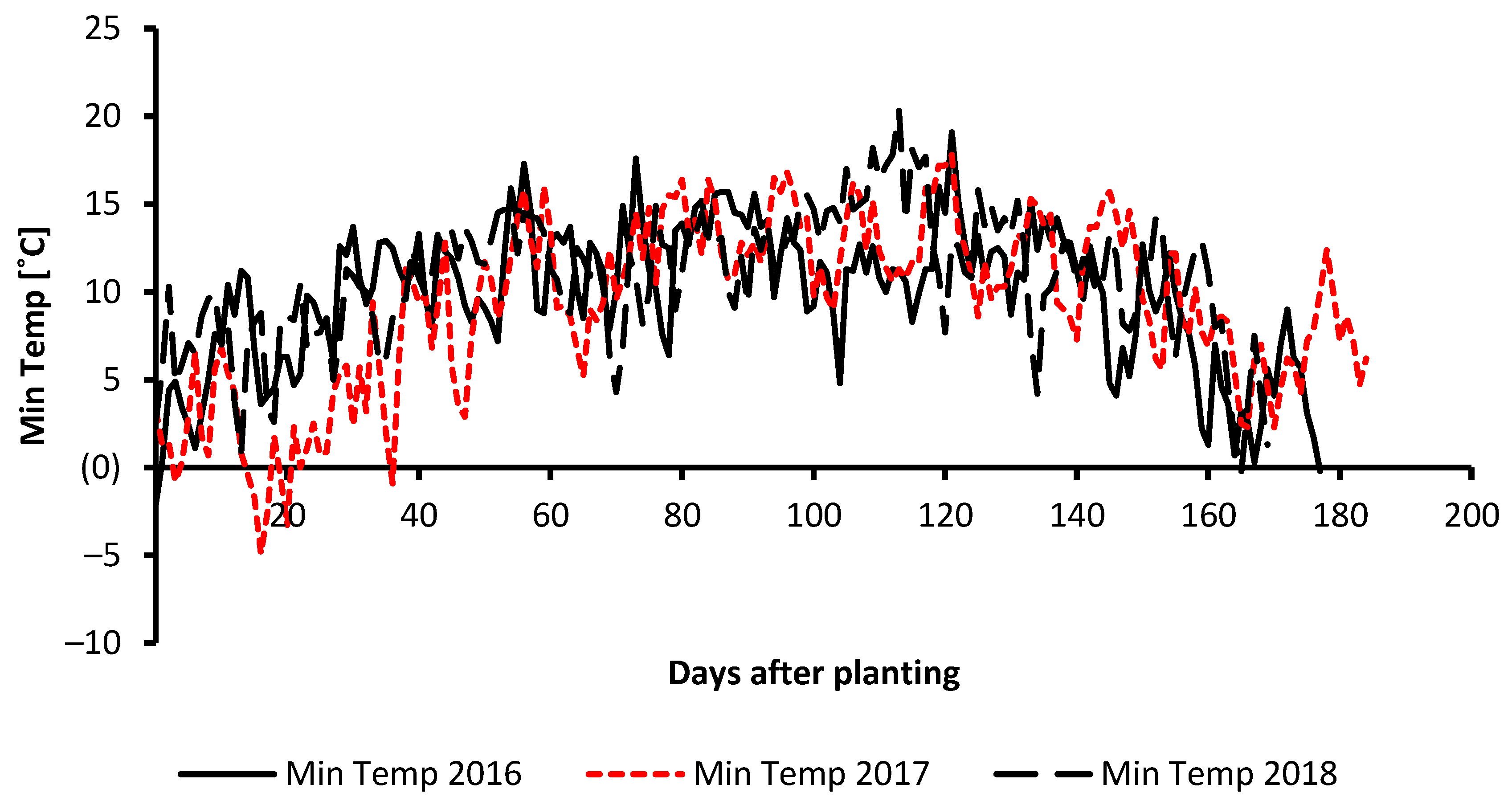
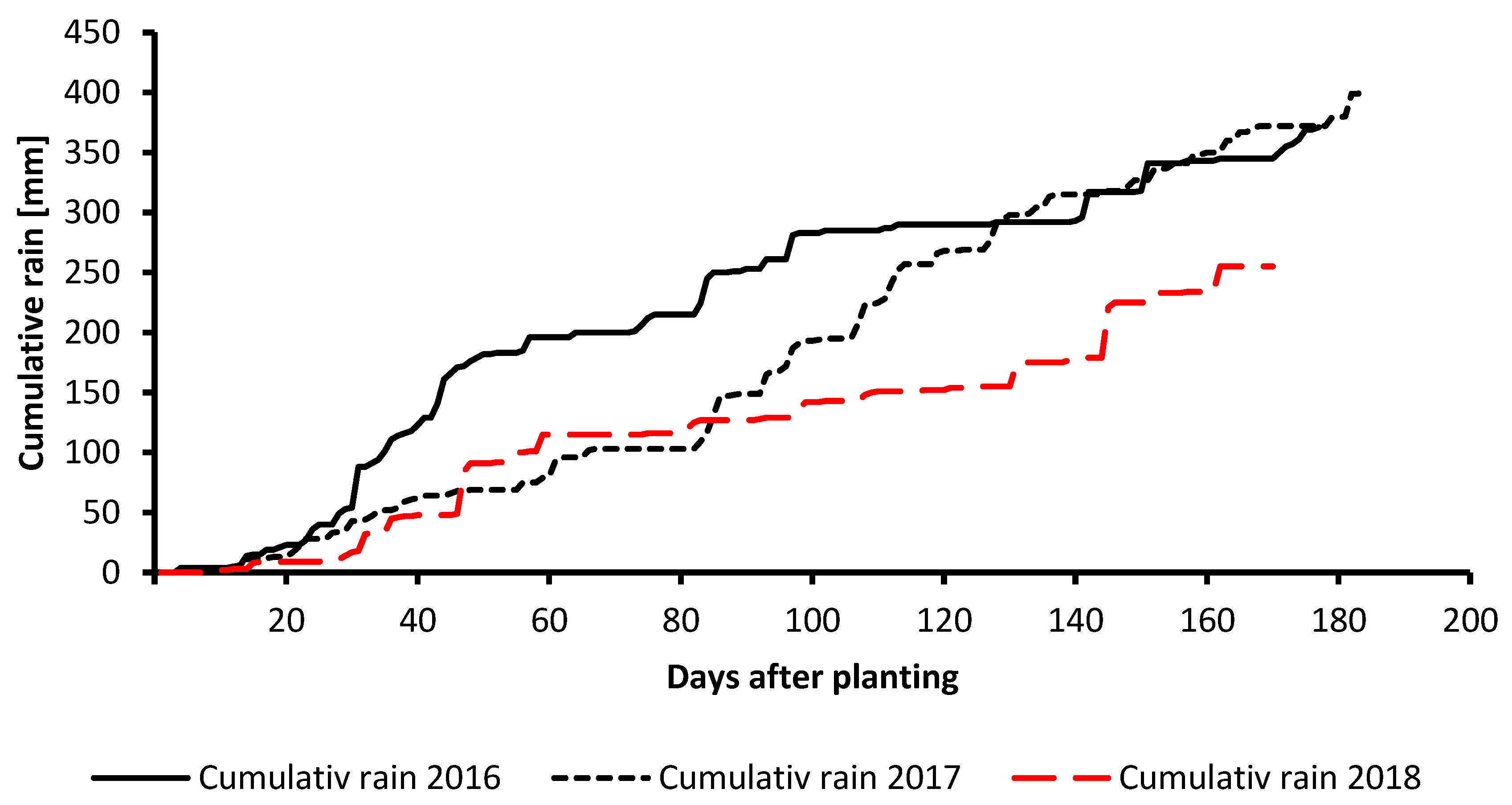
2.1. Field Experiment Description and Data Collection
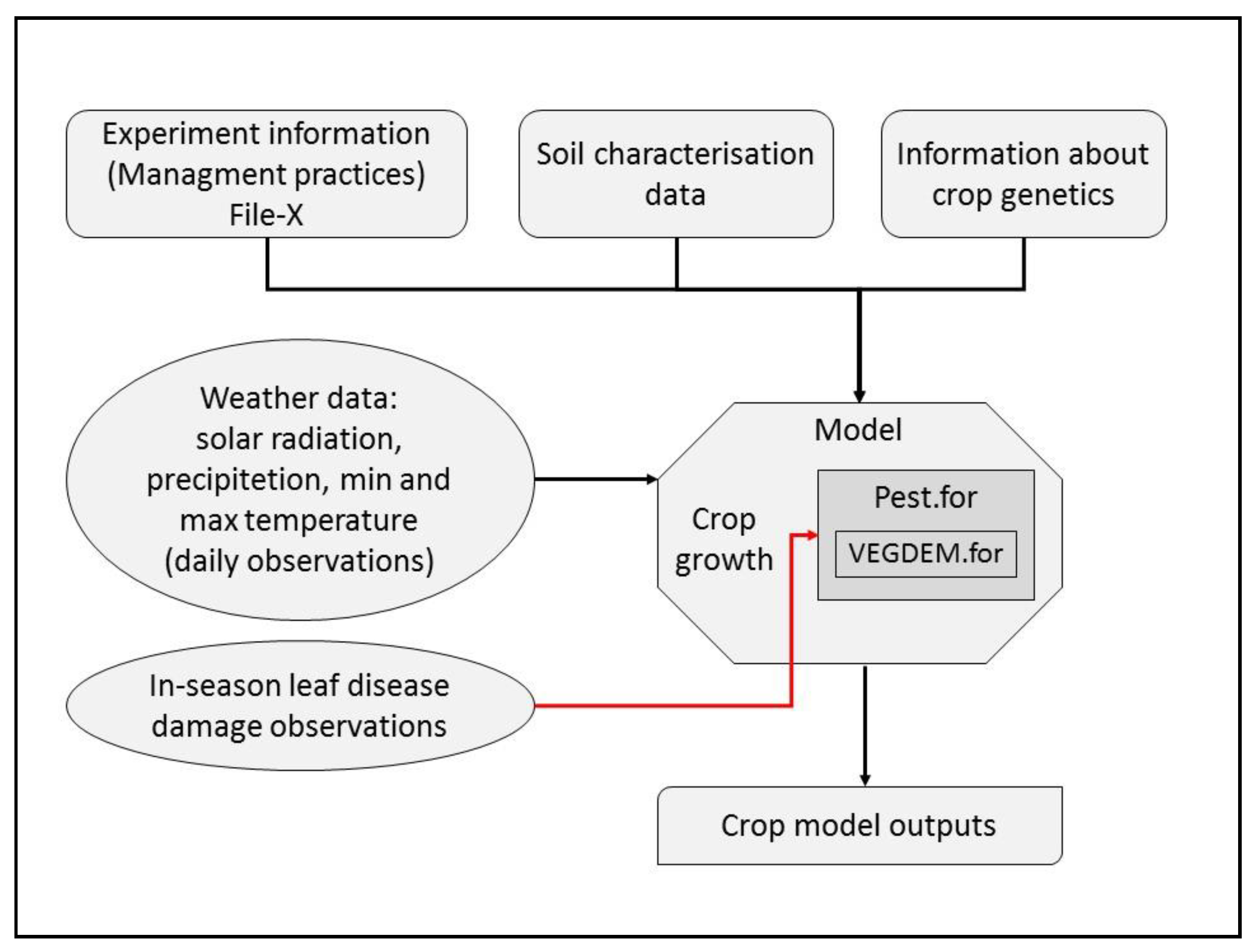
2.2. Leaf Disease Damage Coupling Points
3. Results
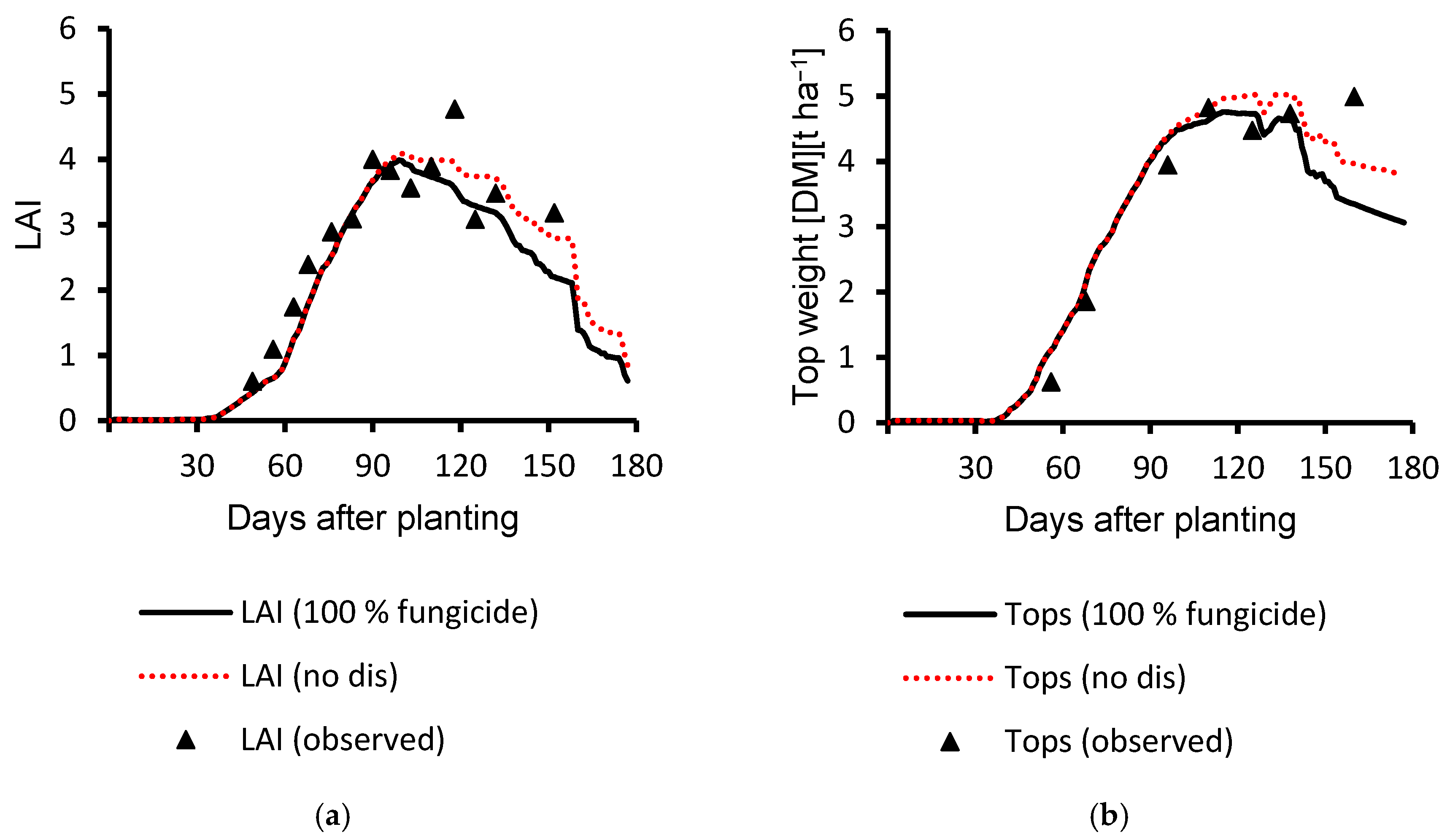
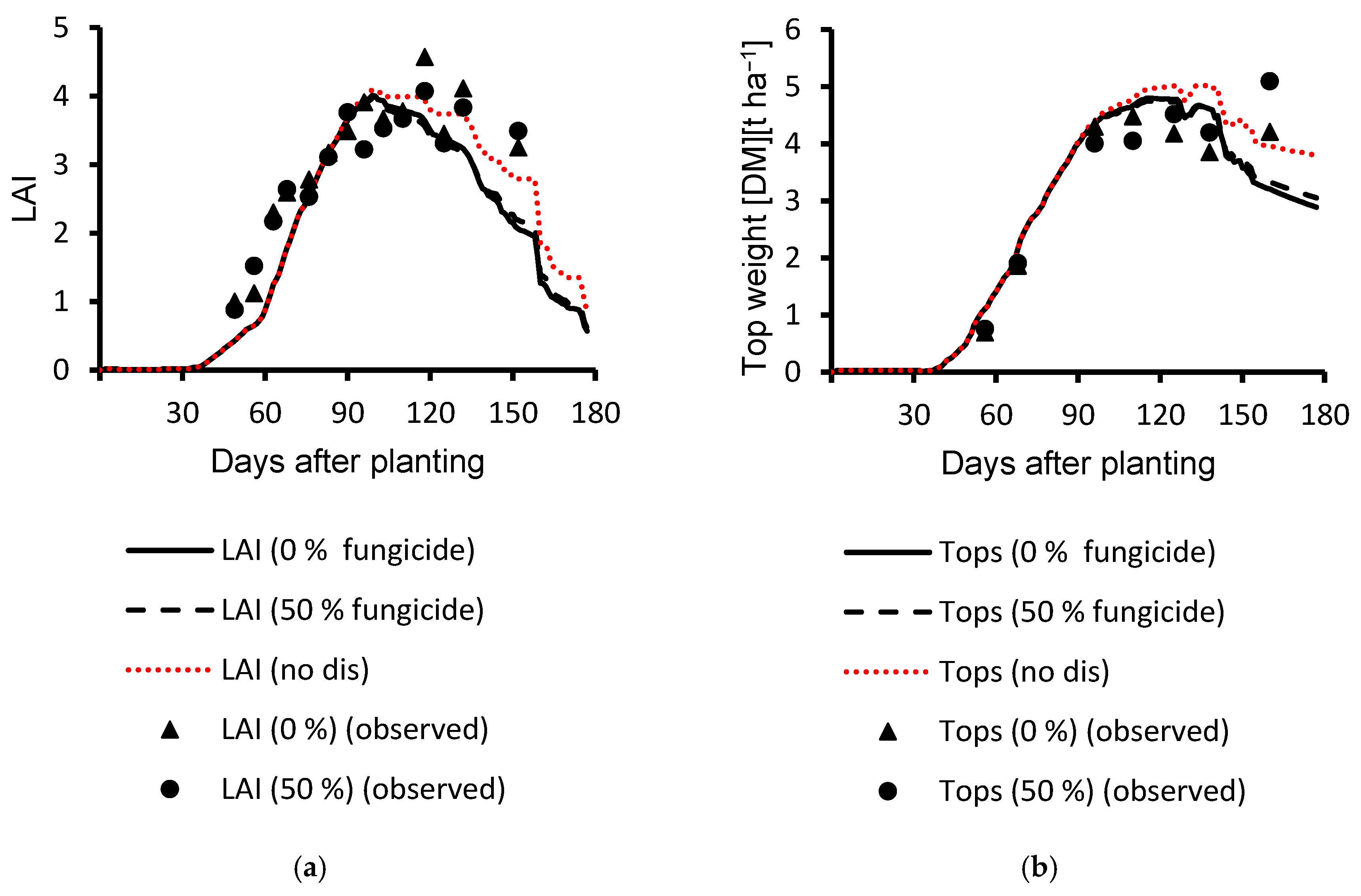
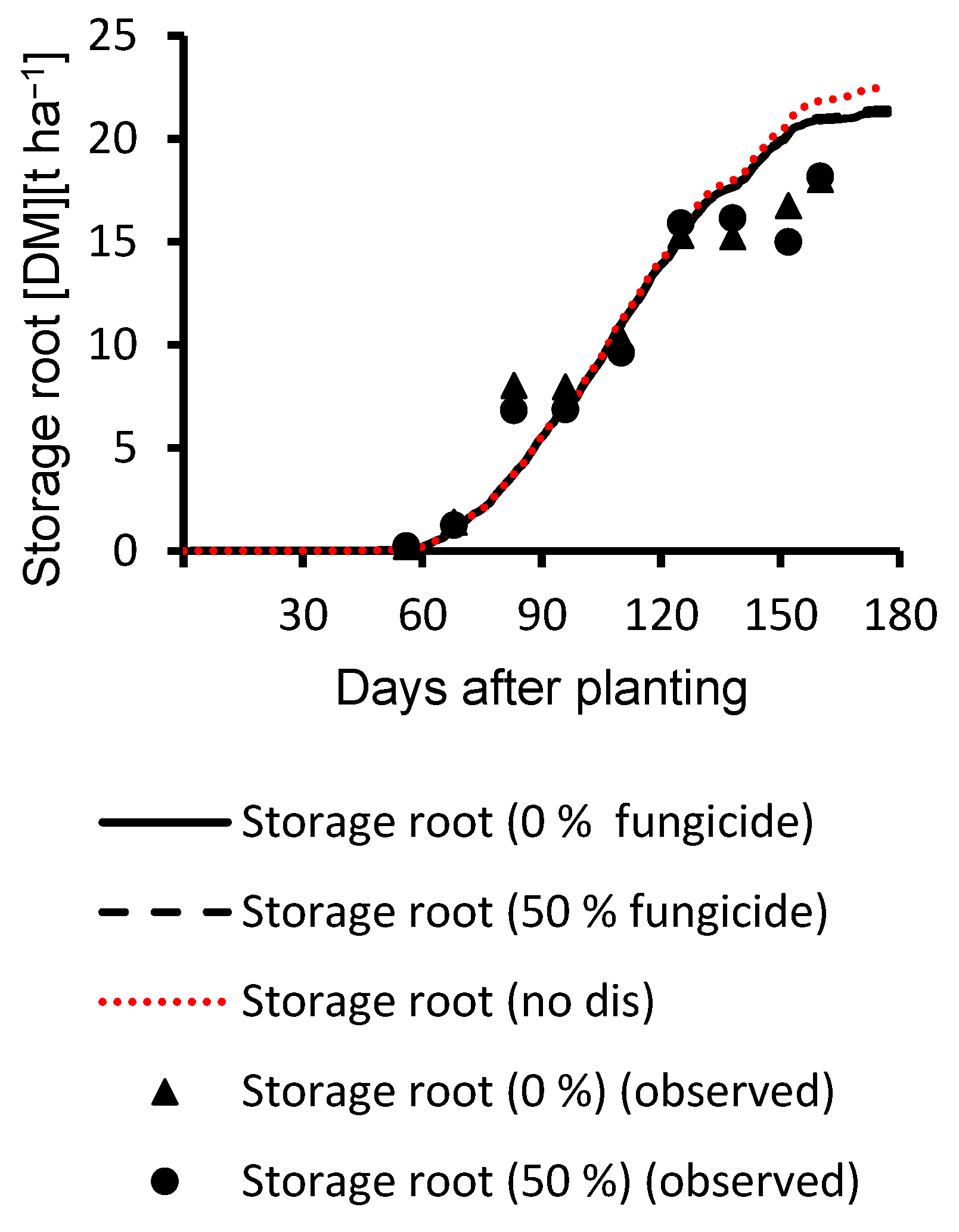
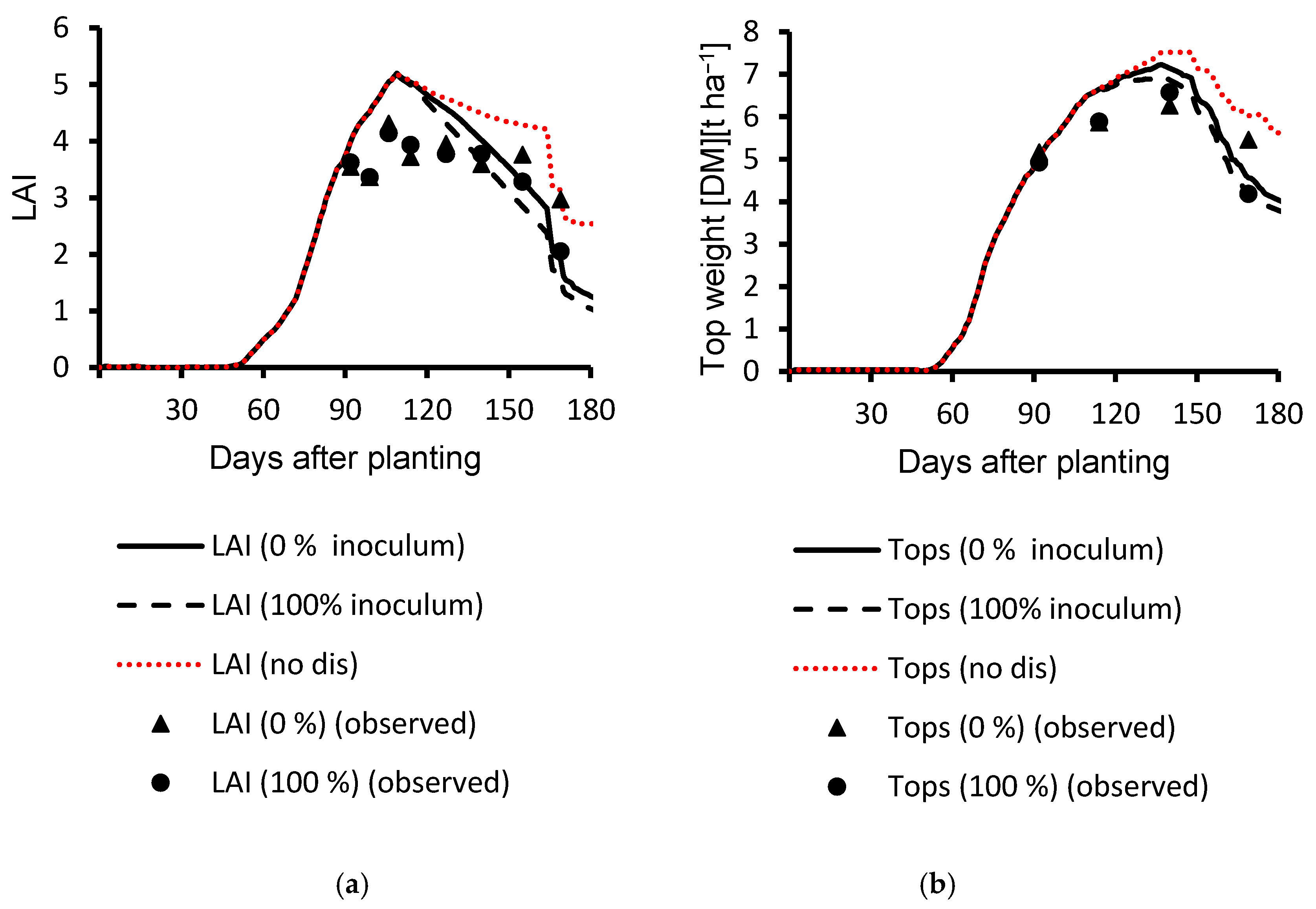
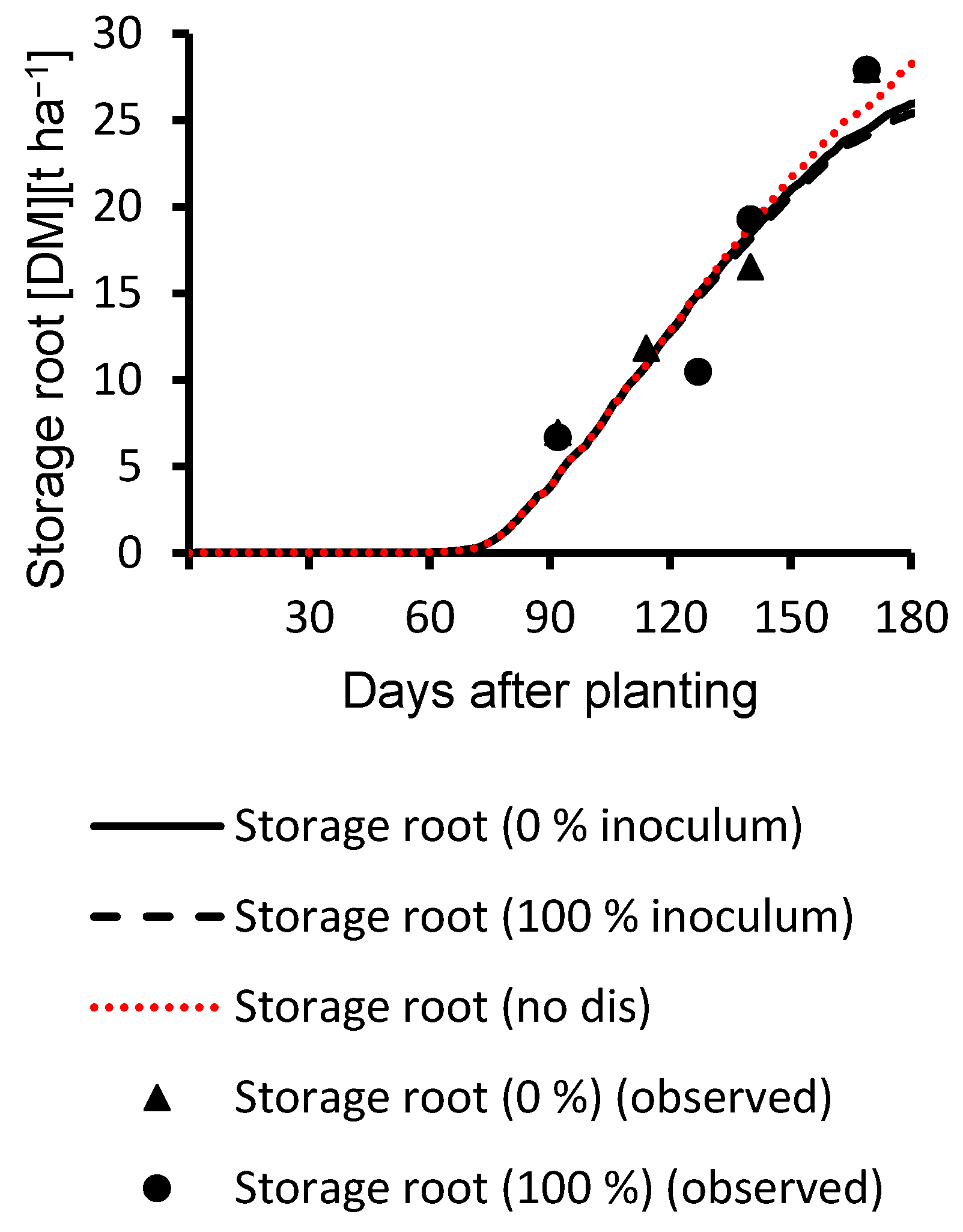
3.1. Calibration Results
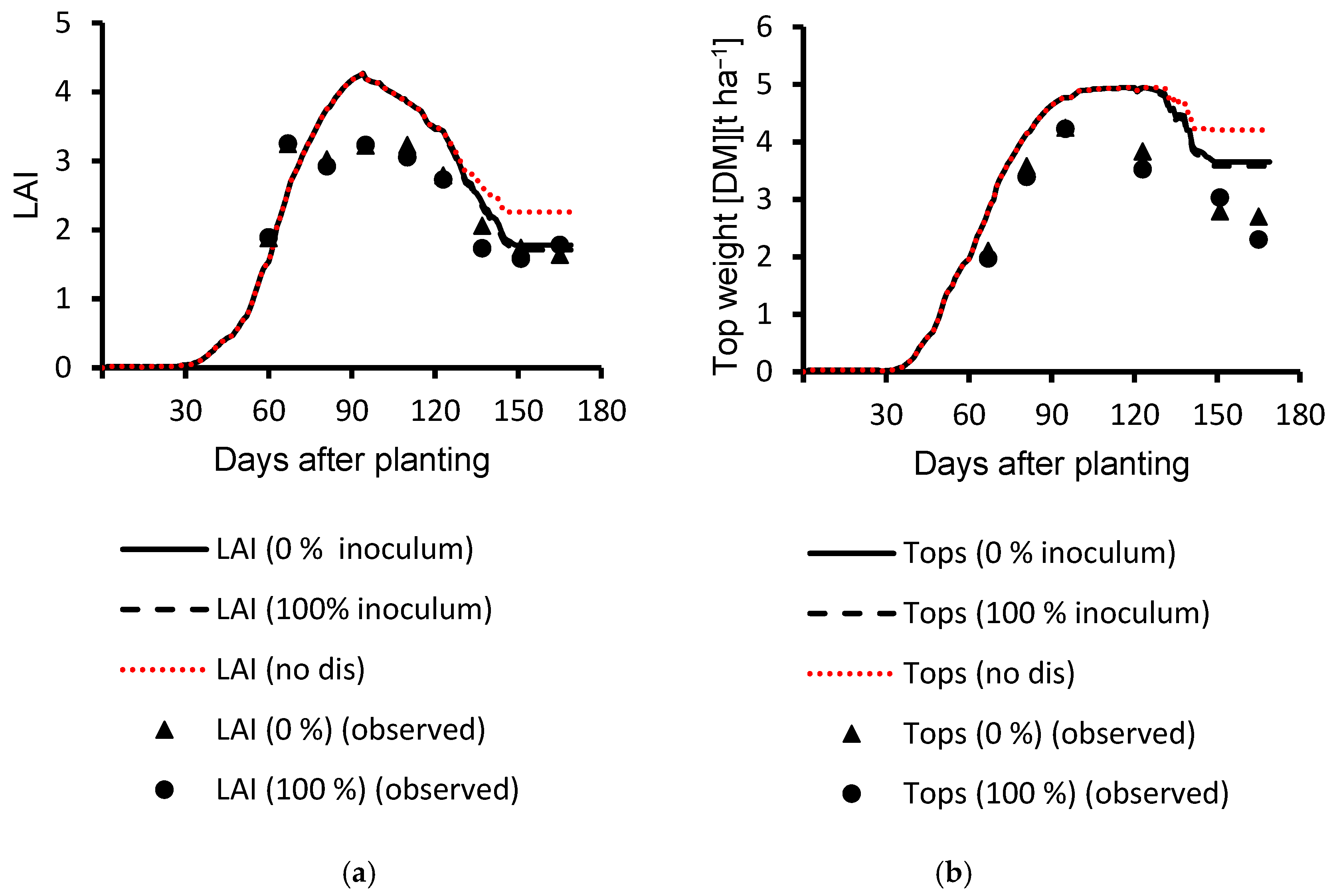
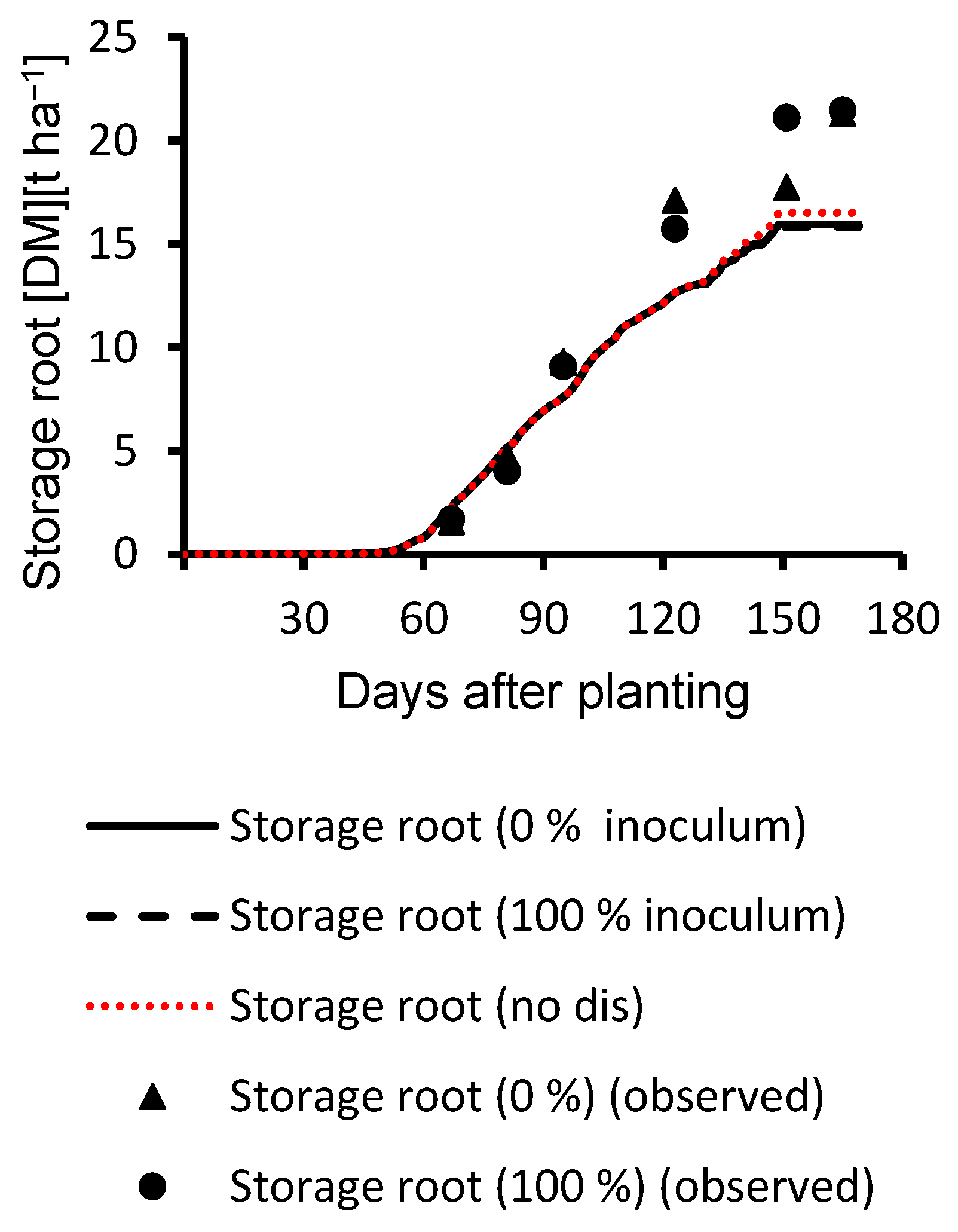
3.2. Evaluation Results
3.3. Model-Based Yield Losses Evaluation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eurostat, Agricultural Production—Crops. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Agricultural_production_-_crops#cite_note-3 (accessed on 5 October 2020).
- Wolf, P.F.J.; Verreet, J.A. An integrated pest management system in Germany for the control of fungal leaf diseases in sugar beet. The IPM sugar beet model. Plant Dis. 2002, 86, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Shane, W.; Teng, P. Impact of Cercospora leaf spot on root weight, sugar yield, and purity of Beta vulgaris. Plant Dis. 1992, 76, 812–820. [Google Scholar] [CrossRef]
- Jones, J.W.; Hoogenboom, G.; Porter, C.; Boote, K.J.; Batchelor, W.D.; Hunt, L.A.; Wilkens, P.; Singh, U.; Gijsman, A.; Ritchie, J.T. DSSAT cropping system model. Eur. J. Agron. 2003, 18, 235–265. [Google Scholar] [CrossRef]
- Olesen, J.E.; Trnka, M.; Kersebaum, K.C.; Skjelvåg, A.O.; Seguin, B.; Peltonen-Sainio, P.; Rossi, F.; Kozyra, J.; Micale, F. Impacts and adaptation of European crop production systems to climate change. Eur. J. Agron. 2011, 34, 96–112. [Google Scholar] [CrossRef]
- Röll, G.; Batchelor, W.D.; Castro, A.C.; Simón, M.R.; Graeff-Hönninger, S. Development and Evaluation of a Leaf Disease Damage Extension in Cropsim-CERES Wheat. Agronomy 2019, 9, 120. [Google Scholar] [CrossRef]
- Batchelor, W.D.; Suresh, L.M.; Zhen, X.; Beyene, Y.; Wilson, M.; Kruseman, G.; Prasanna, B. Simulation of Maize Lethal Necrosis (MLN) Damage Using the CERES-Maize Model. Agronomy 2020, 10, 710. [Google Scholar] [CrossRef]
- Bourgeois, G. Interrelationships between Late Leafspot Disease and Florunner Peanut: A Modelling Approach. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 1989. [Google Scholar]
- Boote, K.J.; Jones, J.W.; Mishoe, J.W.; Wilkerson, G.G. Modelling growth and yield of groundnut. In Agrometeorology of Groundnut Proceedings of the International Symposium, ICRISAT Sahelian Centre, Niamey, Niger, 21–26 August 1985; Sivakumar, M.V.K., Virmani, S.M., Eds.; ICRISAT: Patancheru, India, 1986; pp. 243–254. [Google Scholar]
- Nokes, S.E.; Young, J.H. Simulation of the temporal spread of leafspot and the effect on peanut growth. Trans. ASAE 1991, 34, 653–662. [Google Scholar] [CrossRef]
- Batchelor, W.D.; Jones, J.W.; Boote, K.J.; Pinnschmidt, H.O. Extending the use of crop models to study pest damage. Trans. ASAE 1993, 36, 551–558. [Google Scholar] [CrossRef]
- Leviel, B. Evaluation of Risks and Monitoring of Nitrogen Fluxes at the Crop Level on the Romanian and Bulgarian Plain. Application to Maize, Wheat, Rapeseed and Sugar-Beet. Ph.D. Thesis, Institut National Polytechnique de Toulouse, Toulouse, France, 2000. [Google Scholar]
- Leviel, B.; Crivineanu, C.; Gabrielle, B. CERES-Beet, a model for the production and environmental impact of sugar beet. In Proceedings of the Joint Colloquium on Sugar Beet Growing and Modelling, Lille, France, 12 September 2003; pp. 143–152. [Google Scholar]
- Anar, M.J.; Lin, Z.; Teboh, J.; Ostlie, M. Modeling energy beets using the Decision Support System for Agrotechnology Transfer. Trans. ASABE 2015, RRV15-048. [Google Scholar] [CrossRef]
- Baey, C.; Didier, A.; Lemaire, S.; Maupas, F.; Cournède, P.H. Parameterization of five classical plant growth models applied to sugar beet and comparison of their predictive capacity on root yield and total biomass. Ecol. Modell. 2014, 290, 11–20. [Google Scholar] [CrossRef]
- Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Shelia, V.; Wilkens, P.W.; Singh, U.; White, J.W.; Asseng, S.; Lizaso, J.I.; Moreno, L.P.; et al. The DSSAT crop modeling ecosystem. In Advances in Crop Modeling for a Sustainable Agriculture; Boote, K.J., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 173–216. [Google Scholar] [CrossRef]
- Hoogenboom, G.; Porter, C.H.; Shelia, V.; Boote, K.J.; Singh, U.; White, J.W.; Hunt, L.A.; Ogoshi, R.; Lizaso, J.I.; Koo, J.; et al. Decision Support System for Agrotechnology Transfer (DSSAT) Version 4.7 (www.DSSAT.net); DSSAT Foundation: Gainesville, FL, USA, 2019. [Google Scholar]
- Anar, M.J.; Lin, Z.; Hoogenboom, G.; Shelia, V.; Batchelor, W.D.; Teboh, J.M.; Ostlie, M.; Schatz, B.G.; Khan, M. Modeling Growth, Development and Yield of Sugarbeet using DSSAT. Agric. Syst. 2019, 169, 58–70. [Google Scholar] [CrossRef]
- Jones, C.A.; Kiniry, J.R. CERES-Maize: A Simulation Model of Maize Growth and Development; Texas A&M University Press: College Station, TX, USA, 1986; p. 194. [Google Scholar]
- López-Cedrón, X.F.; Boote, J.K.; Piñeiro, J.; Sau, F. Improving the CERES-Maize Model Ability to Simulate Water Deficit Impact on Maize Production and Yield Components. Agron. J. 2008, 100, 296–307. [Google Scholar] [CrossRef]
- Memic, E.; Graeff, S.; Batchelor, W. Extending the CERES-Beet model to simulate leaf disease in sugar beet. In Precision Agriculture ’19 Proceedings of the European Conference on Precision Agriculture, Montpellier, France, 8 July 2019; John, V.S., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources. World Soil Resources Reports No. 103; FAO: Rome, Italy, 2006. [Google Scholar]
- ICUMSA: Methods Book. Method GS6-3: The determination of the polarisation of sugar beet by the macerator or cold aqueous digestion method using aluminium sulphate as clarifying agent-official. In Internation-al Commission for Uniform Methods of Sugar Analysis; Elsevier: Berlin, Germany, 2003. [Google Scholar]
- Jones, F.G.W.; Dunning, R.A.; Hamphries, K.P. The effect of defoliation and loss of stand upon yield of sugar beets. Ann. Appl. Biol. 1955, 43, 63–70. [Google Scholar] [CrossRef]
- Yang, M.; Yang, J.Y.; Liu, S.; Hoogenboom, G. An evaluation of the statistical methods for testing the performance of crop models with observed data. Agric. Syst. 2014, 127, 81–89. [Google Scholar] [CrossRef]
- Rossi, V.; Battilani, P. CERCOPRI: A forecasting model for primary infections of Cercospora leaf spot of sugar beet. Bull. OEPP 1991, 21, 527–531. [Google Scholar] [CrossRef]
- Rossberg, D.; Racca, P.; Joerg, E.; Kleinhenz, B. Erste Erfahrung mit dem Modell CERCBET 1 (First application of CERCBEET 1 model). Nachr Dtsch Pflanzenschutzd 2000, 52, 153–159. [Google Scholar]
- Racca, P.; Jörg, E.; Mittler, S.; Petersen, J. CERCBET 3—A forecaster for epidemic development of Cercospora beticola. In Proceedings of the 67th IIRB Congress, Brüssel, Belgium, 11–12 February 2004. [Google Scholar]
- Windels, C.E.; Lamey, H.A.; Hilde, D.; Widner, J.; Knudsen, T.A. Cercospora leaf spot model for sugar beet: In practice by an industry. Plant Dis. 1998, 82, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Mittler, S.; Petersen, J.; Jörg, E.; Racca, P. Integrierte Bekämpfung von Blattkrankheiten bei Zuckerrüben (Integrated treatment of leaf disease in sugar beet). In Proceedings of the 67th IIRB Congress, Brüssel, Belgium, 11–12 February 2004. [Google Scholar]
- Bock, C.H.; Barbedo, J.G.A.; Del Ponte, E.M.; Chiang, K.S.; Bohnenkamp, D.; Mahlein, A.K. From visual estimates to fully automated sensor-based measurements of plant disease severity: Status and challenges for improving accuracy. Phytopathol. Res. 2020, 2. [Google Scholar] [CrossRef]
- Leucker, M.; Wahabzada, M.; Kersting, K.; Peter, M.; Beyer, W.; Steiner, U.; Mahlein, A.K.; Oerke, E.C. Hyperspectral imaging reveals the effect of sugar beet quantitative trait loci on Cercospora leaf spot resistance. Funct. Plant Biol. 2017, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]


| Year | DAP | Cercospora Leaf Spot Leaf Area Disease Progress (%) | ||
|---|---|---|---|---|
| 2016 | 0% fungicide | 50% fungicide | 100% fungicide | |
| 63 | 0 | 0 | 0 | |
| 83 | 3 | 3 | 1 | |
| 103 | 13 | 20 | 20 | |
| 125 | 22 | 21 | 24 | |
| 138 | 47 | 25 | 22 | |
| 152 | 48 | 33 | 33 | |
| 177 | 48 | 33 | 33 | |
| 2017 | 0% inoculum | 100% inoculum | ||
| 106 | 0 | 0 | ||
| 127 | 18 | 46 | ||
| 140 | 56 | 74 | ||
| 169 | 92 | 100 | ||
| 184 | 92 | 100 | ||
| 2018 | 0% inoculum | 100% inoculum | ||
| 106 | 0 | 0 | ||
| 119 | 1 | 7 | ||
| 126 | 46 | 52 | ||
| 133 | 49 | 57 | ||
| 147 | 75 | 79 | ||
| 154 | 100 | 100 | ||
| 169 | 100 | 100 | ||
| No. | PID 1 | Method Name | DM 2 | CP 3 | Coeff. 4 |
|---|---|---|---|---|---|
| 1 | PCLA 5 | Observed % defoliation | 3 | LAD | 2.0 |
| Definition | Units | BTS940 | |
|---|---|---|---|
| P1 | Growing Degree Days from the seedling emergence to the end of juvenile phase (juvenile group of leaves, depending on the cultivar up to 15–20 leaves) | °C-d | 760.0 |
| P2 | Photo period sensitivity | hr−1 | 0.0 |
| P5 | Thermal time from leaf growth to physiological maturity | °C-d | 700.0 |
| G2 | Leaf expansion rate during leaf growth stage | cm2 cm−2 d−1 | 420.0 |
| G3 | Maximum root growth rate | gm−2 d−1 | 27.5 |
| PHINT | Phyllochron interval, the interval in thermal time between successive leaf tip appearances | °C-d | 43.0 |
| Year | Variable | Treatment | R2 | RMSE | d Stat. | Total Obs. 1 |
|---|---|---|---|---|---|---|
| 2016 | LAI | 100% fungicide | 0.87 | 0.52 | 0.95 | 14 |
| Top weight | 100% fungicide | 0.82 | 686 | 0.94 | 7 | |
| Storage root | 100% fungicide | 0.95 | 1696 | 0.99 | 9 |
| Year | Variable | Treatment | R2 | RMSE (kg ha−1) | d Stat. | Total Obs. 1 |
|---|---|---|---|---|---|---|
| 2016 | LAI | 0% fungicide | 0.85 | 0.63 | 0.92 | 14 |
| LAI | 50% fungicide | 0.80 | 0.63 | 0.91 | 14 | |
| Top weight | 0% fungicide | 0.85 | 565 | 0.95 | 7 | |
| Top weight | 50% fungicide | 0.74 | 751 | 0.92 | 7 | |
| Storage root | 0% fungicide | 0.94 | 2270 | 0.97 | 9 | |
| Storage root | 50% fungicide | 0.94 | 2362 | 0.97 | 9 |
| Year | Variable | Treatment | R2 | RMSE (kg ha−1) | d Stat. | Total Obs. 1 |
|---|---|---|---|---|---|---|
| 2017 | LAI | 0% inoculum | 0.54 | 0.85 | 0.63 | 8 |
| LAI | 100% inoculum | 0.81 | 0.72 | 0.83 | 8 | |
| Top weight | 0% inoculum | 0.80 | 747 | 0.74 | 4 | |
| Top weight | 100% inoculum | 0.96 | 402 | 0.96 | 4 | |
| Storage root | 0% inoculum | 0.93 | 2399 | 0.98 | 4 | |
| Storage root | 100% inoculum | 0.87 | 3124 | 0.96 | 4 |
| Year | Variable | Treatment | R2 | RMSE (kg ha−1) | d Stat. | Total Obs. 1 |
|---|---|---|---|---|---|---|
| 2018 | LAI | 0% inoculum | 0.76 | 0.57 | 0.87 | 9 |
| LAI | 100% inoculum | 0.70 | 0.64 | 0.84 | 9 | |
| Top weight | 0% inoculum | 0.92 | 812 | 0.78 | 6 | |
| Top weight | 100% inoculum | 0.81 | 953 | 0.71 | 6 | |
| Storage root | 0% inoculum | 0.97 | 3045 | 0.94 | 6 | |
| Storage root | 100% inoculum | 0.99 | 3486 | 0.93 | 6 |
| DAY | SRW kg ha−1 | SY kg ha−1 | Sc (%) | SRW kg ha−1 | SY kg ha−1 | Sc (%) | SRW kg ha−1 | SY kg ha−1 | Sc (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | 0% fungicide | 50% fungicide | 100% fungicide | |||||||
| 138 | 15,241 | 10,171 | 67 | 16,153 | 10,625 | 66 | 18,319 | 12,438 | 68 | |
| 152 | 16,768 | 11,343 | 68 | 15,005 | 9906 | 66 | 18,091 | 12,366 | 68 | |
| 160 | 18,024 | 12,072 | 67 | 18,180 | 12,231 | 67 | 22,184 | 14,911 | 67 | |
| Avg. | 67 | 66 | 68 | |||||||
| 2017 | 0% inoculum | 100% inoculum | ||||||||
| 114 | 11,820 | 8233 | 70 | 10,461 | 7109 | 68 | ||||
| 140 | 16,538 | 10,970 | 66 | 19,270 | 13,384 | 69 | ||||
| 169 | 27,956 | 20,166 | 72 | 27,915 | 19,944 | 71 | ||||
| Avg. | 69 | 69 | ||||||||
| 2018 | 0% inoculum | 100% inoculum | ||||||||
| 119 | 17,115 | 11,839 | 69 | 15,736 | 10,937 | 70 | ||||
| 147 | 17,748 | 12,842 | 72 | 21,111 | 13,941 | 66 | ||||
| 161 | 21,308 | 14,890 | 70 | 21,462 | 15,533 | 72 | ||||
| Avg. | 70 | 69 | ||||||||
| Simulated Storage Root (DM) (kg ha−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| DAY | ‘No Dis’ | ‘Dis’ | SRW Loss | ‘Dis’ | SRW Loss | ‘Dis’ | SRW Loss | |
| 2016 | 0% fungicide | 50% fungicide | 100% fungicide | |||||
| 63 | 478 | 478 | 0 | 478 | 0 | 478 | 0 | |
| 83 | 3738 | 3728 | 10 | 3728 | 10 | 3735 | 3 | |
| 103 | 8867 | 8776 | 91 | 8741 | 126 | 8759 | 108 | |
| 125 | 15,565 | 15,269 | 296 | 15,202 | 363 | 15,207 | 358 | |
| 138 | 17,983 | 17,636 | 347 | 17,645 | 338 | 17,655 | 328 | |
| 152 | 20,802 | 20,221 | 581 | 20,291 | 511 | 20,307 | 495 | |
| 177 | 22,506 | 21,232 | 1274 | 21,424 | 1082 | 21,440 | 1066 | |
| 2017 | 0% inoculum | 100% inoculum | ||||||
| 106 | 2742 | 2742 | 0 | 2742 | 0 | |||
| 127 | 9792 | 9787 | 5 | 9779 | 13 | |||
| 140 | 12,577 | 12,529 | 48 | 12,456 | 121 | |||
| 169 | 21,108 | 20,481 | 627 | 20,145 | 963 | |||
| 184 | 28,518 | 26,068 | 2450 | 25,489 | 3029 | |||
| 2018 | 0% inoculum | 100% inoculum | ||||||
| 106 | 10,971 | 10,971 | 0 | 10,971 | 0 | |||
| 119 | 12,654 | 12,651 | 3 | 12,632 | 22 | |||
| 126 | 13,171 | 13,082 | 89 | 13,047 | 124 | |||
| 133 | 14,418 | 14,187 | 231 | 14,133 | 285 | |||
| 147 | 16,506 | 15,939 | 567 | 15,841 | 665 | |||
| 154 | 16,506 | 15,939 | 567 | 15,841 | 665 | |||
| 169 | 16,506 | 15,939 | 567 | 15,841 | 665 | |||
| Year | DAY | Treatments | SRW Loss (DM) (kg ha−1) | SY Loss (kg ha−1) |
|---|---|---|---|---|
| 2016 | 177 | 0% fungicide | 1274 | 866 |
| 50% fungicide | 1082 | 735 | ||
| 100% fungicide | 1066 | 725 | ||
| 2017 | 184 | 0% inoculum | 2450 | 1666 |
| 100% inoculum | 3029 | 2060 | ||
| 2018 | 169 | 0% inoculum | 567 | 386 |
| 100% inoculum | 665 | 452 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memic, E.; Graeff-Hönninger, S.; Hensel, O.; Batchelor, W.D. Extending the CSM-CERES-Beet Model to Simulate Impact of Observed Leaf Disease Damage on Sugar Beet Yield. Agronomy 2020, 10, 1930. https://doi.org/10.3390/agronomy10121930
Memic E, Graeff-Hönninger S, Hensel O, Batchelor WD. Extending the CSM-CERES-Beet Model to Simulate Impact of Observed Leaf Disease Damage on Sugar Beet Yield. Agronomy. 2020; 10(12):1930. https://doi.org/10.3390/agronomy10121930
Chicago/Turabian StyleMemic, Emir, Simone Graeff-Hönninger, Oliver Hensel, and William D. Batchelor. 2020. "Extending the CSM-CERES-Beet Model to Simulate Impact of Observed Leaf Disease Damage on Sugar Beet Yield" Agronomy 10, no. 12: 1930. https://doi.org/10.3390/agronomy10121930
APA StyleMemic, E., Graeff-Hönninger, S., Hensel, O., & Batchelor, W. D. (2020). Extending the CSM-CERES-Beet Model to Simulate Impact of Observed Leaf Disease Damage on Sugar Beet Yield. Agronomy, 10(12), 1930. https://doi.org/10.3390/agronomy10121930







