Is Drought Stress Tolerance Affected by Biotypes and Seed Size in the Emerging Oilseed Crop Camelina?
Abstract
1. Introduction
2. Materials and Methods
2.1. Genetic Material and Its Characterization
2.2. Seed Germination Test
2.3. Statistical Analysis
3. Results
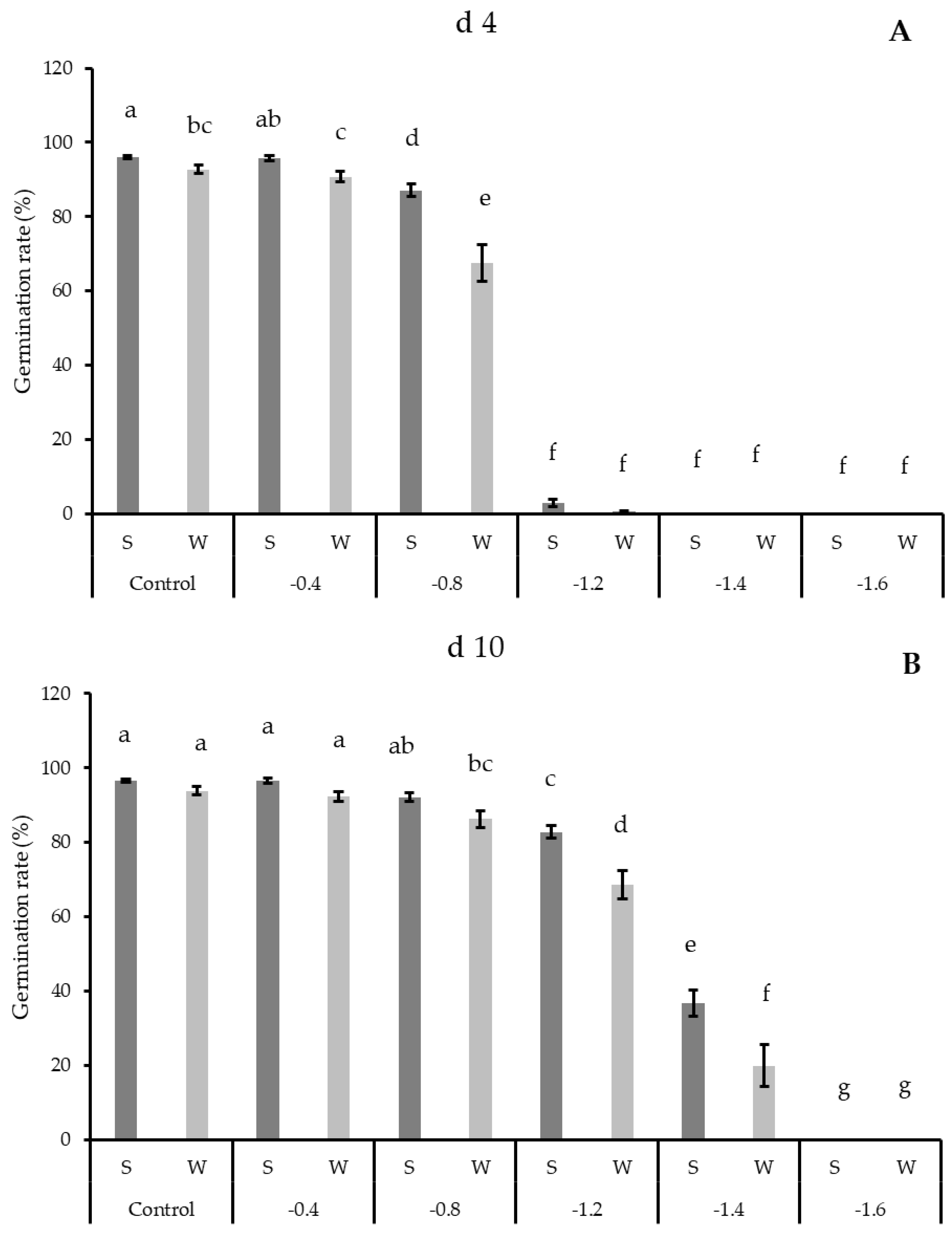
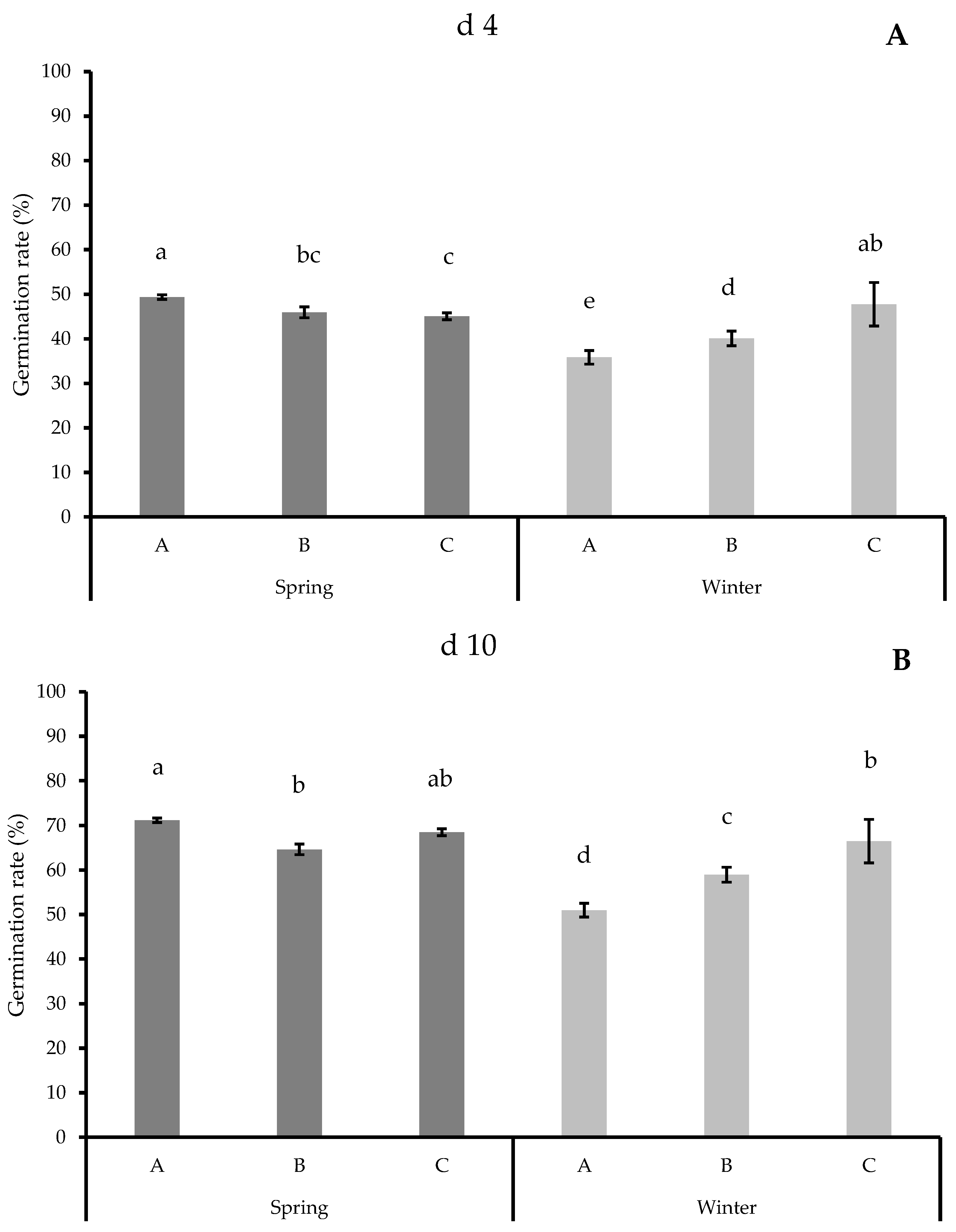
3.1. Germination Results
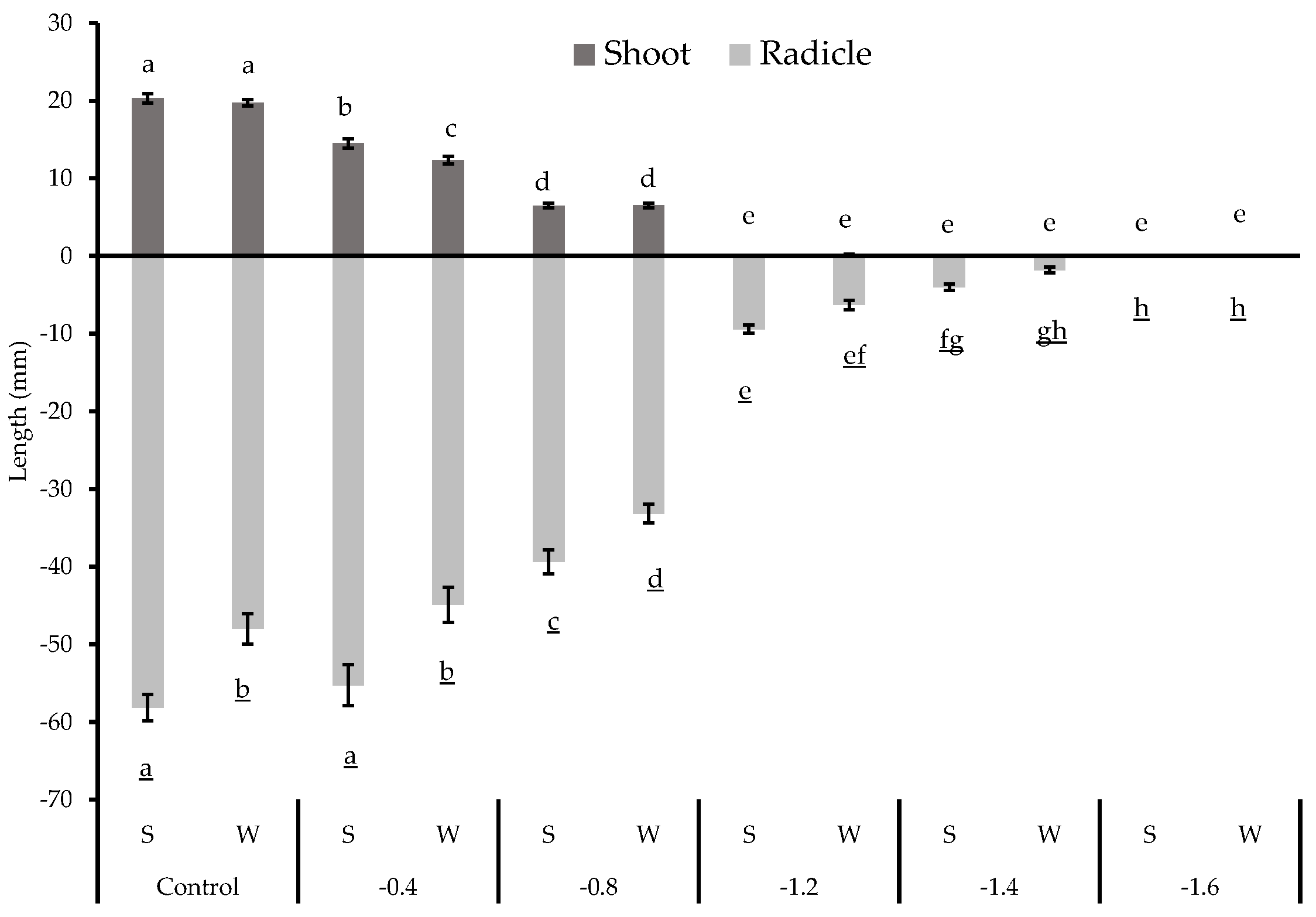
3.2. Seedling Morphology Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Narusaka, Y.; Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Furihata, T.; Abe, H.; Narusaka, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high salinity stresses. Plant J. 2003, 34, 137–149. [Google Scholar] [CrossRef]
- De la Pena, R.; Hughes, J. Improving vegetable productivity in a variable and changing climate. J. SAT Agric. Res. 2007, 4, 1–22. [Google Scholar]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, Q.; Xie, B.; Wang, Z.; Cui, J.; Hu, T. Effects of drought and salt stress on seed germination of three leguminous species. Afr. J. Biotechnol. 2011, 10, 17954–17961. [Google Scholar] [CrossRef]
- Ashraf, M.; Bokhari, M.H.; Cristiti, S.N. Variation in osmotic adjustment of lentil (Lens culinaris, Medik) in response to drought. Acta Bot. Neerl. 1992, 41, 51–62. [Google Scholar] [CrossRef]
- Almansouri, M.; Kinet, J.M.; Lutts, S. Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.). Plant Soil 2001, 231, 243–254. [Google Scholar] [CrossRef]
- Ansari, O.; Azadi, M.S.; Sharif-Zadeh, F.; Younesi, E. Effect of hormone priming on germination characteristics and enzyme activity of mountain rye (Secale montanum) seeds under drought stress conditions. J. Stress Physiol. Biochem. 2013, 9, 61–71. [Google Scholar]
- George, N.; Levers, L.; Thompson, S.; Hollingsworth, J.; Kaffka, S. Modeling identifies optimal fall planting times and irrigation requirements for canola and camelina at locations across California. Calif. Agric. 2017, 71, 214–220. [Google Scholar] [CrossRef]
- Ashraf, M.; Mehmood, S. Response of four Brassica species to drought stress. Environ. Expt. Bot. 1990, 30, 93–100. [Google Scholar] [CrossRef]
- de Figueiredo e Albuquerque, M.C.; de Carvalho, N.M. Effect of type of environmental stress on the emergence of sunflower (Helianthus annuus L.), soybean (Glycine max L. Merril) and maize (Zea mays L.) seeds with different levels of vigor. Seed Sci. Technol. 2003, 31, 465–467. [Google Scholar] [CrossRef]
- Brown, S.C.; Gregory, P.J.; Cooper, P.J.M.; Keatinge, J.D.H. Root and shoot growth and water use of chickpea (Cicer arietinum) grown in dryland conditions: Effects of sowing date and genotype. J. Agric. Sci. 1989, 113, 41–49. [Google Scholar] [CrossRef]
- Khajeh-Hosseini, M.; Powell, A.A.; Bingham, I.J. The interaction between salinity stress and seed vigour during germination of soybean seeds. Seed Sci. Technol. 2003, 31, 715–725. [Google Scholar] [CrossRef]
- Donohue, K.; de Casas, R.R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, postgermination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Zanetti, F.; Eynck, C.; Christou, M.; Krzyżaniak, M.; Righini, D.; Alexopoulou, E.; Stolarski, M.J.; Van Loo, E.N.; Puttick, D.; Monti, A. Agronomic performance and seed quality attributes of Camelina (Camelina sativa L. Crantz) in multi-environment trials across Europe and Canada. Ind. Crop Prod. 2017, 107, 602–608. [Google Scholar] [CrossRef]
- Gesch, R. Influence of genotype and sowing date on camelina growth and yield in the north central US. Ind. Crops Prod. 2014, 54, 209–215. [Google Scholar] [CrossRef]
- Berti, M.; Wilckens, R.; Fischer, S.; Solis, A.; Gonzalez, W.; Johnson, B.L. Seeding date influence on camelina seed yield, yield components, and oil content in Chile. Ind. Crops Prod. 2011, 34, 1358–1365. [Google Scholar] [CrossRef]
- Berti, M.; Gesch, R.; Eynck, C.; Anderson, J.; Cermak, S. Camelina uses, genetics, genomics, production, and management. Ind. Crops Prod. 2016, 94, 690–710; [Google Scholar] [CrossRef]
- Larsson, M. Cultivation and processing of Linum usitatissimum and Camelina sativa in southern Scandinavia during the Roman Iron Age. Veg. Hist. Archaeobot. 2013, 22, 509–520. [Google Scholar] [CrossRef]
- George, N.; Thompson, S.E.; Hollingsworth, J.; Orloff, S.; Kaffka, S. Measurement and simulation of water-use by canola and camelina under cool-season conditions in California. Agric. Water Manag. 2018, 196, 15–23. [Google Scholar] [CrossRef]
- Vollmann, J.; Eynck, C. Camelina as a sustainable oilseed crop: Contributions of plant breeding and genetic engineering. Biotechnol. J. 2015, 10, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, A.; Anderson, J.V.; Berti, M.T. Winter and summer annual biotypes of camelina have different morphology and seed characteristics. Ind. Crops Prod. 2019, 135, 230–237. [Google Scholar] [CrossRef]
- Fabre, J.F.; Lacroux, E.; Gravé, G.; Mouloungui, Z. Extraction of camelina mucilage with ultrasound and high flow rate fluid circulation. Ind. Crops Prod. 2020, 144, 112057. [Google Scholar] [CrossRef]
- Cherian, G. Camelina sativa in poultry diets: Opportunities and challenges. In Biofuel Co-Products as Livestock Feed—Opportunities and Challenges; Makkar, H.P.S., Ed.; FAO: Rome, Italy, 2012; pp. 303–310. [Google Scholar]
- Channaoui, S.; El Idrissi, I.S.; Mazouz, H.; Nabloussi, A. Reaction of some rapeseed (Brassica napus L.) genotypes to different drought stress levels during germination and seedling growth stages. OCL 2019, 26, 23. [Google Scholar] [CrossRef]
- Royo-Esnal, A.; Valencia-Gredilla, F. Camelina as a Rotation Crop for Weed Control in Organic Farming in a Semiarid Mediterranean Climate. Agriculture 2018, 8, 156. [Google Scholar] [CrossRef]
- Metz, J.; Liancourt, P.; Kigel, J.; Harel, D.; Sternberg, M.; Tielbörger, K. Plant survival in relation to seed size along environmental gradients: A long-term study from semi-arid and Mediterranean annual plant communities. J. Ecol. 2010, 98, 697–704. [Google Scholar] [CrossRef]
- Willenborg, C.J.; Wildeman, J.C.; Miller, A.K.; Rossnagel, B.G.; Shirtliffe, S.J. Oat germination characteristics differ among genotypes, seed sizes, and osmotic potentials. Crop Sci. 2005, 45, 2023–2029. [Google Scholar] [CrossRef]
- Moles, A.T.; Ackerly, D.D.; Webb, C.O.; Tweddle, J.C.; Dickie, J.B.; Westoby, M. A Brief History of Seed Size. Science 2005, 307, 576–580. [Google Scholar] [CrossRef]
- Leishman, M.R.; Wright, I.J.; Moles, A.T.; Westoby., M. The evolutionary ecology of seed size. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CAB International: Wallingford, UK, 2000; pp. 31–57. [Google Scholar]
- Li, Y.; Beisson, F.; Pollard, M.; Ohlrogge, J. Oil content of Arabidopsis seeds: The influence of seed anatomy, light and plant-to-plant variation. Phytochemistry 2006, 67, 904–915. [Google Scholar] [CrossRef]
- Razeq, F.M.; Kosma, D.K.; Rowland, O.; Molina, I. Extracellular lipids of Camelina sativa: Characterization of chloroform-extractable waxes from aerial and subterranean surfaces. Phytochemistry 2014, 106, 188–196. [Google Scholar] [CrossRef] [PubMed]
- North, H.M.; Berger, A.; Saez-Aguayo, S.; Ralet, M.C. Understanding polysaccharide production and properties using seed coat mutants: Future perspectives for the exploitation of natural variants. Ann. Bot. 2014, 114, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.L.; Benton, R.A. The behavior of seeds in soil: II. The germination of seeds on the surface of a water supplying substrate. J. Ecol. 1996, 54, 151–166. [Google Scholar] [CrossRef]
- Cui Jiang, J.W.; Qun, S.; Baoqi, S. Drought Resistance of Camelina Sativa (L.) Crantz’s Seeds in Germination. Chin. Agric. Sci. Bull. 2006, 10, 203–205. [Google Scholar]
- Yadav, P.V.; Khatri, D.; Nasim, M. Salt and PEG Induced Osmotic Stress Tolerance at Germination and Seedling Stage in Camelina sativa: A Potential Biofuel Crop. J. Seed Sci. 2017, 10, 27–32. [Google Scholar] [CrossRef][Green Version]
- Righini, D.; Zanetti, F.; Martinez-Force, E.; Toschi, T.G.; Monti, A. Shifting sowing of camelina from spring to autumn enhances the oil quality for bio-based applications in response to temperature and seed carbon stock. Ind. Crops Prod. 2019, 137, 66–73. [Google Scholar] [CrossRef]
- Christou, M.; Alexopoulou, E.; Zanetti, F.; Krzyżaniak, M.; Stolarski, M.; Righini, D.; Monti, A. Sowing dates effect on Camelina growth in different EU climatic zones. In Proceedings of the 26th European Biomass Conference and Exhibition, Copenhagen, Denmark, 14–18 May 2018; pp. 133–135. [Google Scholar]
- European Pharmacopoeia. European Pharmacopoeia 8.0.; Method 2.8.4; Strasbourg Council of Europe: Strasbourg, France, 2013; Volume 1. [Google Scholar]
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Trenberth, K.E. Changes in precipitation with climate change. Clim. Res. 2011, 47, 123–138. [Google Scholar] [CrossRef]
- Enjalbert, J.N.; Zheng, S.; Johnson, J.J.; Mullen, J.L.; Byrne, P.F.; McKay, J.K. Brassicaceae germplasm diversity for agronomic and seed quality traits under drought stress. Ind. Crops Prod. 2013, 47, 176–185. [Google Scholar] [CrossRef]
- Berti, M.; Samarappuli, D.; Burton, L.; Johnson, B.L.; Gesch, R.W. Integrating winter camelina into maize and soybean cropping systems. Ind. Crops Prod. 2017, 107, 595–601. [Google Scholar] [CrossRef]
- Kaya, M.D.; Okcu, G.; Atak, M.; Cikili, Y.; Kolsarici, O. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Eur. J. Agron. 2006, 24, 291–295. [Google Scholar] [CrossRef]
- Singh, B.; Reddy, K.R.; Redona, E.; Walker, T. Developing a screening tool for osmotic stress tolerance classification of rice cultivars based on in vitro seed germination. Crop Sci. 2017, 57, 387–394. [Google Scholar] [CrossRef]
- Wijewardana, C.; Alsajri, F.A.; Reddy, K.R. Soybean Seed Germination Response to In Vitro Osmotic Stress. Seed Technol. 2018, 39, 143–154. [Google Scholar]
- Guy, S.A.; Wysocki, D.J.; Schillinger, W.F.; Chastain, T.G.; Karow, R.S.; Garland-Campbell, K.; Burke, I.C. Camelina: Adaptation and performance of genotypes. Field Crop Res. 2014, 155, 224–232; [Google Scholar] [CrossRef]
- Panikashvili, D.; Shi, J.X.; Schreiber, L. The Arabidopsis DCR Encoding a Soluble BAHD Acyltransferase Is Required for Cutin Polyester Formation and Seed Hydration Properties. Plant Physiol. 2009, 151, 1773–1789. [Google Scholar] [CrossRef]
- Gorai, M.; El Aloui, W.; Yang, X.; Neffati, M. Toward understanding the ecological role of mucilage in seed germination of a desert shrub Henophyton deserti: Interactive effects of temperature, salinity and osmotic stress. Plant Soil 2014, 374, 727–738. [Google Scholar] [CrossRef]
- Easton, L.C.; Kleindorfer, S. Interaction Effects of Seed Mass and Temperature on Germination in Australian Species of Frankenia (Frankeniaceae). Folia Geobot. 2008, 43, 383–396; [Google Scholar] [CrossRef]
- Kaydan, D.; Yagmur, M. Germination, seedling growth and relative water content of shoot in different seed sizes of triticale under osmotic stress of water and NaCl. Afr. J. Biotechnol. 2008, 7, 2862–2868; [Google Scholar] [CrossRef]
- Gholami, A.; Sharafi, S.; Sharafi, A.; Ghasemi, S. Germination of different seed size of pinto bean cultivars as affected by salinity and drought stress. J. Food Agric. Environ. 2009, 7, 555–558. [Google Scholar]
- Mut, Z.; Akay, H. Effect of seed size and drought stress on germination and seedling growth of naked oat (Avena sativa L.). Bulg. J. Agric. Sci. 2010, 16, 459–467. [Google Scholar]
- Bakhshandeh, E.; Jamali, M. Population-based threshold models: A reliable tool for describing aged seeds response of rapeseed under salinity and water stress. Environ. Exp. Bot. 2020, 176, 104077. [Google Scholar] [CrossRef]
- Koirala, P.S.; Neff, M.M. Improving seed size, seed weight and seedling emergence in Camelina sativa by overexpressing the Atsob3-6 gene variant. Transgenic Res. 2020, 29, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Okcu, G.; Kaya, M.D.; Atak, M. Effects of salt and drought stresses on germination and seedling growth of pea (Pisum sativum L.). Turk. J. Agric. For. 2005, 29, 237–242. [Google Scholar]


| Variety Name | Biotype | Provider | TSW (g) | TSW Class | Mucilage (%) |
|---|---|---|---|---|---|
| Midas | S | Smart Earth Camelina (Saskatoon, SK, Canada) | 1.26 | B | 10.63 |
| Cypress | 1.60 | A | 10.80 | ||
| EICA | Landesbetrieb Landwirtschaft Hessen (Alsfeld, Germany) | 1.83 | A | 10.41 | |
| NS Zlatka | IFVCNS (Novi Sad, Serbia) | 1.01 | B | 10.48 | |
| Omega | Poznan University (Poznan, Poland) | 0.96 | C | 12.06 | |
| Calena | Saatbau Linz (Leonding, Austria) | 1.13 | B | 7.64 | |
| Joelle | W | R. Gesch, USDA-ARS (Morris, MN, USA) | 0.88 | C | 10.86 |
| WG1 | C. Rife, High Plains Crop Development (Torrington, WY, USA) | 1.11 | B | 13.78 | |
| WG4 | 1.16 | B | 15.01 | ||
| Bison | 1.31 | A | 9.49 | ||
| Luna | Poznan University (Poznan, Poland) | 0.99 | B | 11.46 | |
| Merlin | J. Vollmann, BOKU University (Vienna, Austria) | 0.93 | C | 10.86 |
| Factors | Germ d 4 | Germ d 10 | Shoot Length | Radicle Length |
|---|---|---|---|---|
| OS | 3335.20 *** | 576.61 *** | 1153.85 *** | 549.26 *** |
| Biotype | 67.19 *** | 47.20 *** | 7.18 ** | 36.73 *** |
| SWC | 11.42 *** | 8.10 ** | 0.89 ns | 0.04 ns |
| OS × Biotype | 20.21 *** | 6.71 *** | 5.10 *** | 3.65 ** |
| OS × SWC | 3.91 *** | 1.39 ns | 1.03 ns | 0.58 ns |
| Biotype × SWC | 20.21 *** | 6.71 *** | 5.03 ** | 1.86 ns |
| OS × Biotype × SWC | 11.47 *** | 2.88 ** | 2.60 ** | 3.16 *** |
| Main Factors | Germination d 4 | Germination d 10 |
|---|---|---|
| % | ||
| Osmotic stress (MPa) | ||
| Control (0.0) | 94.42 ± 0.71 a | 95.19 ± 0.62 a |
| −0.4 | 93.22 ± 0.93 a | 94.42 ± 0.78 a |
| −0.8 | 77.28 ± 3.03 b | 89.22 ± 1.33 b |
| −1.2 | 1.69 ± 0.54 c | 75.72 ± 2.44 c |
| −1.4 | 0.00 ± 0.00 c | 28.31 ± 3.59 d |
| −1.6 | 0.00 ± 0.00 c | 0.00 ± 0.00 e |
| Biotype | ||
| Spring | 46.94 ± 4.47 a | 67.47 ± 3.60 a |
| Winter | 41.34 ± 4.20 b | 60.15 ± 3.76 b |
| Seed weight class | ||
| A | 44.85 ± 6.15 b | 64.46 ± 5.26 b |
| B | 43.00 ± 4.27 b | 61.80 ± 3.67 b |
| C | 46.87 ± 6.39 a | 67.18 ± 5.32 a |
| Osmotic Stress | Biotype | Seed Weight Class | Germination d 4 | Germination d 10 |
|---|---|---|---|---|
| % | ||||
| Control (0.0) | Spring | A | 98.00 ± 0.52 a | 98.17 ± 0.54 a |
| B | 95.22 ± 0.57 ab | 96.11 ± 0.65 a | ||
| C | 94.67 ± 1.45 abc | 94.67 ± 1.45 ab | ||
| Winter | A | 84.00 ± 1.00 def | 86.33 ± 0.88 abc | |
| B | 92.22 ± 0.78 bc | 93.33 ± 0.71 ab | ||
| C | 98.00 ± 0.78 a | 98.33 ± 0.33 a | ||
| −0.4 MPa | Spring | A | 98.33 ± 0.33 a | 98.50 ± 0.34 a |
| B | 94.22 ± 1.04 abc | 95.44 ± 0.73 ab | ||
| C | 94.64 ± 2.03 abc | 96.00 ± 2.00 ab | ||
| Winter | A | 81.00 ± 3.21 ef | 83.33 ± 2.91 bcd | |
| B | 89.56 ± 0.80 cd | 91.78 ± 0.64 ab | ||
| C | 97.50 ± 0.85 a | 97.50 ± 0.85 a | ||
| −0.8 MPa | Spring | A | 93.33 ± 1.02 abc | 95.67 ± 0.61 ab |
| B | 85.56 ± 2.10 de | 90.78 ± 1.79 ab | ||
| C | 79.00 ± 2.52 f | 89.33 ± 1.76 abc | ||
| Winter | A | 50.00 ± 2.00 h | 71.33 ± 1.45 de | |
| B | 58.67 ± 5.91 g | 84.22 ± 1.31 bc | ||
| C | 89.50 ± 2.67 cd | 96.83 ± 0.91 a | ||
| −1.2 MPa | Spring | A | 6.50 ± 2.13 i | 90.83 ± 2.12 ab |
| B | 0.67 ± 0.44 j | 77.56 ± 1.60 cd | ||
| C | 2.00 ± 1.53 ij | 82.67 ± 1.33 bcd | ||
| Winter | A | 0.00 ± 0.00 j | 60.67 ± 0.88 ef | |
| B | 0.00 ± 0.00 j | 60.11 ± 5.40 ef | ||
| C | 1.67 ± 0.80 ij | 85.33 ± 1.41 bc | ||
| −1.4 MPa | Spring | A | 0.00 ± 0.00 j | 44.00 ± 7.30 g |
| B | 0.00 ± 0.00 j | 28.00 ± 3.70 h | ||
| C | 0.00 ± 0.00 j | 48.33 ± 0.33 fg | ||
| Winter | A | 0.00 ± 0.00 j | 4.33 ± 1.33 i | |
| B | 0.00 ± 0.00 j | 24.22 ± 10.49 h | ||
| C | 0.00 ± 0.00 j | 21.17 ± 5.34 h | ||
| −1.6 MPa | Spring | A | 0.00 ± 0.00 j | 0.00 ± 0.00 i |
| B | 0.00 ± 0.00 j | 0.00 ± 0.00 i | ||
| C | 0.00 ± 0.00 j | 0.00 ± 0.00 i | ||
| Winter | A | 0.00 ± 0.00 j | 0.00 ± 0.00 i | |
| B | 0.00 ± 0.00 j | 0.00 ± 0.00 i | ||
| C | 0.00 ± 0.00 j | 0.00 ± 0.00 i | ||
| Osmotic Stress | Biotype | Seed Weight Class | Shoot Length | Radicle Length |
|---|---|---|---|---|
| mm | ||||
| Control (0.0) | Spring | A | 20.80 ± 1.27 a | 56.05 ± 2.96 ab |
| B | 19.74 ± 0.64 a | 58.66 ± 2.80 a | ||
| C | 21.17 ± 2.03 a | 61.07 ± 1.16 a | ||
| Winter | A | 19.07 ± 1.19 a | 55.40 ± 2.24 abc | |
| B | 20.01 ± 0.62 a | 49.04 ± 2.99 cd | ||
| C | 19.65 ± 0.74 a | 42.73 ± 2.48 ef | ||
| −0.4 MPa | Spring | A | 15.33 ± 1.19 b | 51.82 ± 3.33 bcd |
| B | 14.26 ± 0.94 b | 55.69 ± 4.70 ab | ||
| C | 13.60 ± 0.38 bc | 60.87 ± 3.30 a | ||
| Winter | A | 8.83 ± 0.43 d | 54.17 ± 5.09 abc | |
| B | 12.29 ± 0.27 c | 46.38 ± 3.21 de | ||
| C | 14.17 ± 0.76 b | 38.10 ± 1.92 f | ||
| −0.8 MPa | Spring | A | 7.48 ± 0.64 de | 38.93 ± 3.11 f |
| B | 6.10 ± 0.23 e | 40.61 ± 2.26 f | ||
| C | 5.73 ± 0.33 e | 36.47 ± 2.03 fgh | ||
| Winter | A | 6.77 ± 0.03 de | 27.80 ± 1.51 h | |
| B | 6.06 ± 0.44 e | 32.12 ± 1.58 gh | ||
| C | 7.17 ± 0.52 de | 37.42 ± 1.24 fg | ||
| −1.2 MPa | Spring | A | 0.00 ± 0.00 f | 9.98 ± 1.31 i |
| B | 0.00 ± 0.00 f | 9.69 ± 0.52 i | ||
| C | 0.00 ± 0.00 f | 7.47 ± 0.72 ijk | ||
| Winter | A | 0.00 ± 0.00 f | 4.93 ± 0.42 ijk | |
| B | 0.00 ± 0.00 f | 5.86 ± 0.82 ijk | ||
| C | 0.47 ± 0.23 f | 7.65 ± 1.23 ij | ||
| −1.4 MPa | Spring | A | 0.00 ± 0.00 f | 3.43 ± 0.71 jk |
| B | 0.00 ± 0.00 f | 3.72 ± 0.42 jk | ||
| C | 0.00 ± 0.00 f | 6.00 ± 1.46 ijk | ||
| Winter | A | 0.00 ± 0.00 f | 1.07 ± 0.22 jk | |
| B | 0.00 ± 0.00 f | 1.53 ± 0.62 jk | ||
| C | 0.00 ± 0.00 f | 2.60 ± 0.66 jk | ||
| −1.6 MPa | Spring | A | 0.00 ± 0.00 f | 0.00 ± 0.00 k |
| B | 0.00 ± 0.00 f | 0.00 ± 0.00 k | ||
| C | 0.00 ± 0.00 f | 0.00 ± 0.00 k | ||
| Winter | A | 0.00 ± 0.00 f | 0.00 ± 0.00 k | |
| B | 0.00 ± 0.00 f | 0.00 ± 0.00 k | ||
| C | 0.00 ± 0.00 f | 0.00 ± 0.00 k | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čanak, P.; Jeromela, A.M.; Vujošević, B.; Kiprovski, B.; Mitrović, B.; Alberghini, B.; Facciolla, E.; Monti, A.; Zanetti, F. Is Drought Stress Tolerance Affected by Biotypes and Seed Size in the Emerging Oilseed Crop Camelina? Agronomy 2020, 10, 1856. https://doi.org/10.3390/agronomy10121856
Čanak P, Jeromela AM, Vujošević B, Kiprovski B, Mitrović B, Alberghini B, Facciolla E, Monti A, Zanetti F. Is Drought Stress Tolerance Affected by Biotypes and Seed Size in the Emerging Oilseed Crop Camelina? Agronomy. 2020; 10(12):1856. https://doi.org/10.3390/agronomy10121856
Chicago/Turabian StyleČanak, Petar, Ana Marjanović Jeromela, Bojana Vujošević, Biljana Kiprovski, Bojan Mitrović, Barbara Alberghini, Erika Facciolla, Andrea Monti, and Federica Zanetti. 2020. "Is Drought Stress Tolerance Affected by Biotypes and Seed Size in the Emerging Oilseed Crop Camelina?" Agronomy 10, no. 12: 1856. https://doi.org/10.3390/agronomy10121856
APA StyleČanak, P., Jeromela, A. M., Vujošević, B., Kiprovski, B., Mitrović, B., Alberghini, B., Facciolla, E., Monti, A., & Zanetti, F. (2020). Is Drought Stress Tolerance Affected by Biotypes and Seed Size in the Emerging Oilseed Crop Camelina? Agronomy, 10(12), 1856. https://doi.org/10.3390/agronomy10121856







