Harvested Microalgal Biomass from Different Water Treatment Facilities—Its Characteristics and Potential Use as Renewable Sources of Plant Biostimulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Algae Harvesting Methods
2.2. Analysis of Water Quality
2.3. Analysis of the MB Samples
2.4. Application of the MB Samples on Plant Growth
3. Results and Discussion
3.1. Water Quality and Algal Composition
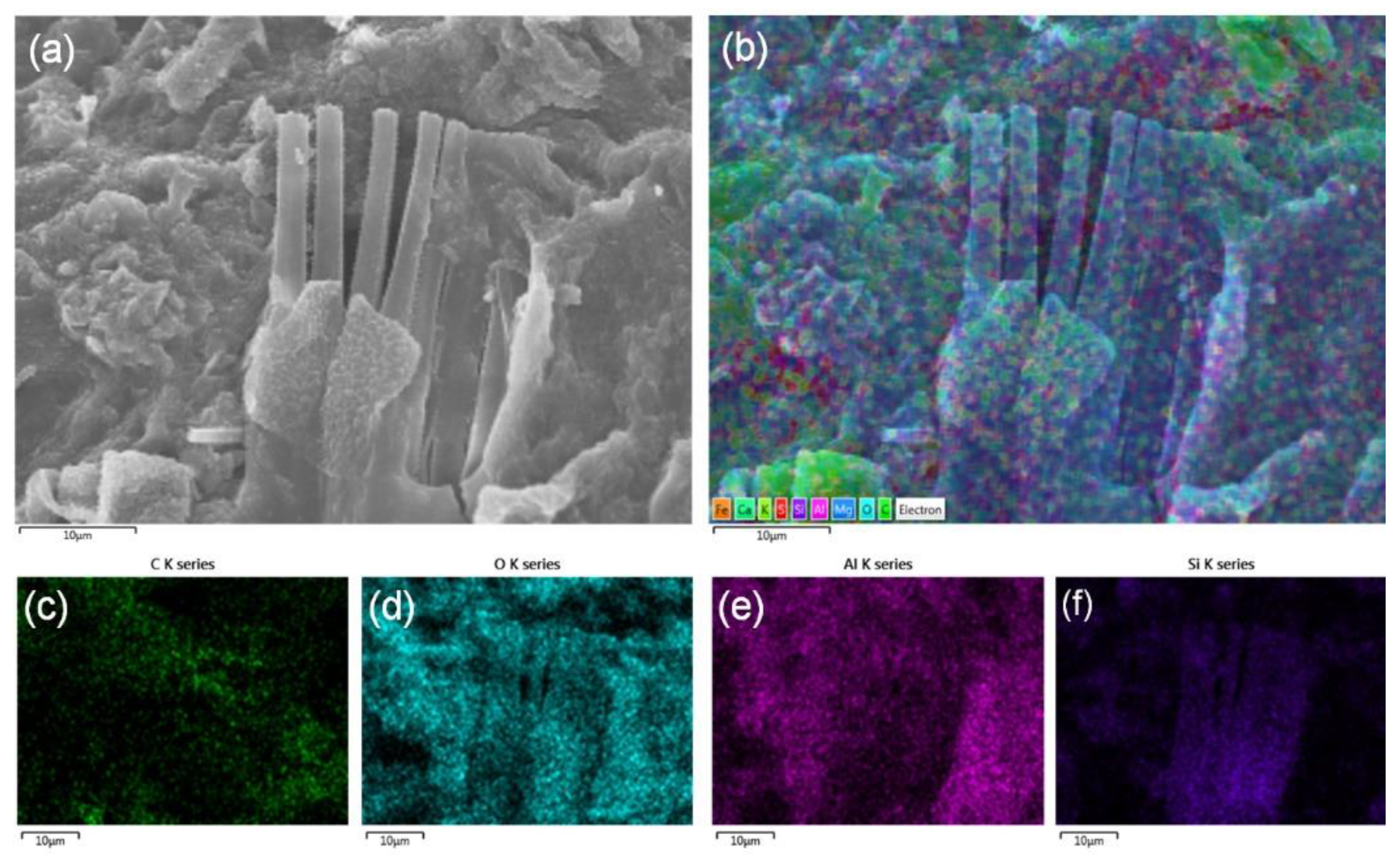
3.2. Morphology and Structure of Dried MB
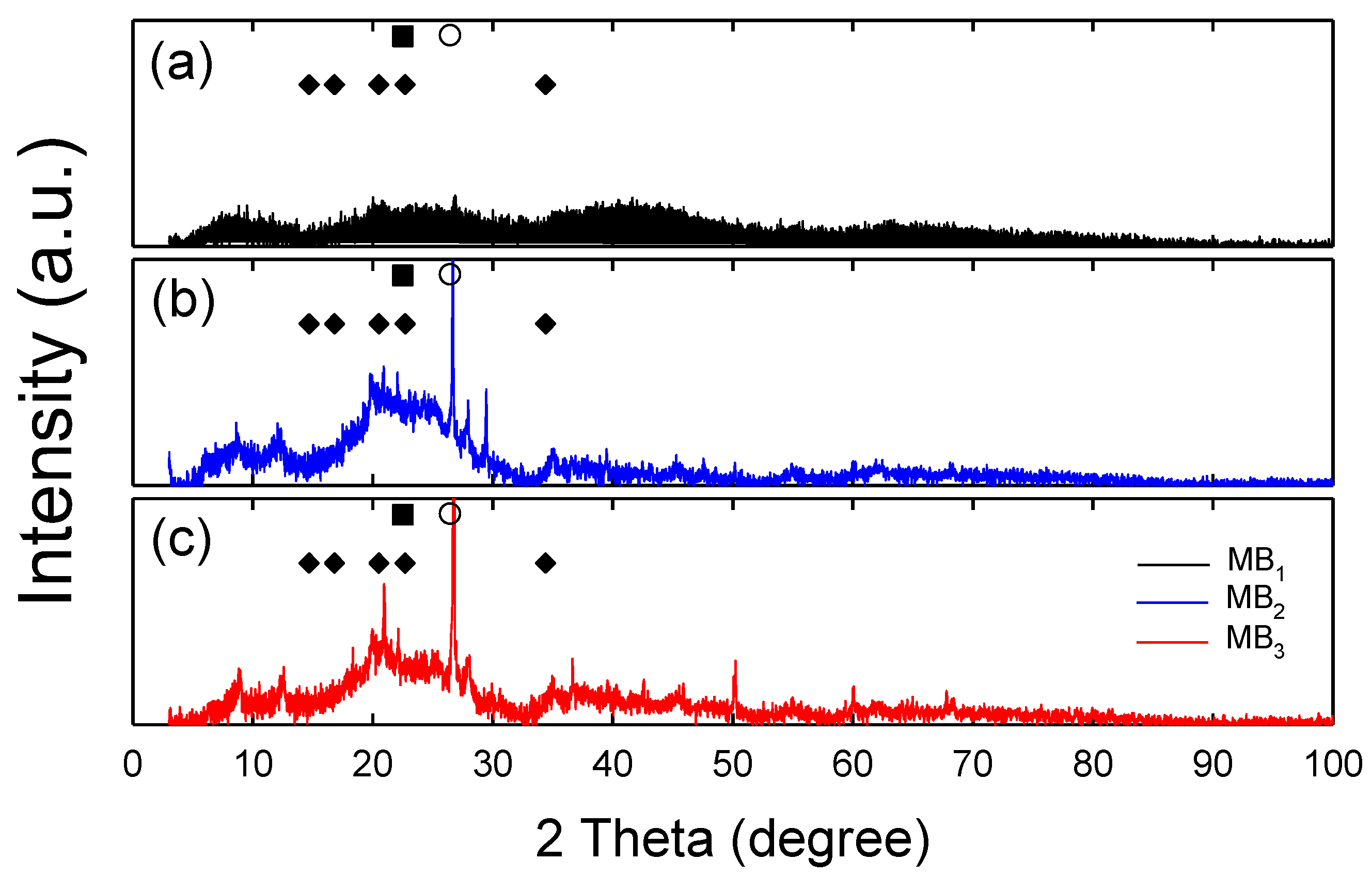
3.3. Mineral Composition and Structure of Dried MB
3.4. Functional Groups of Dried MB
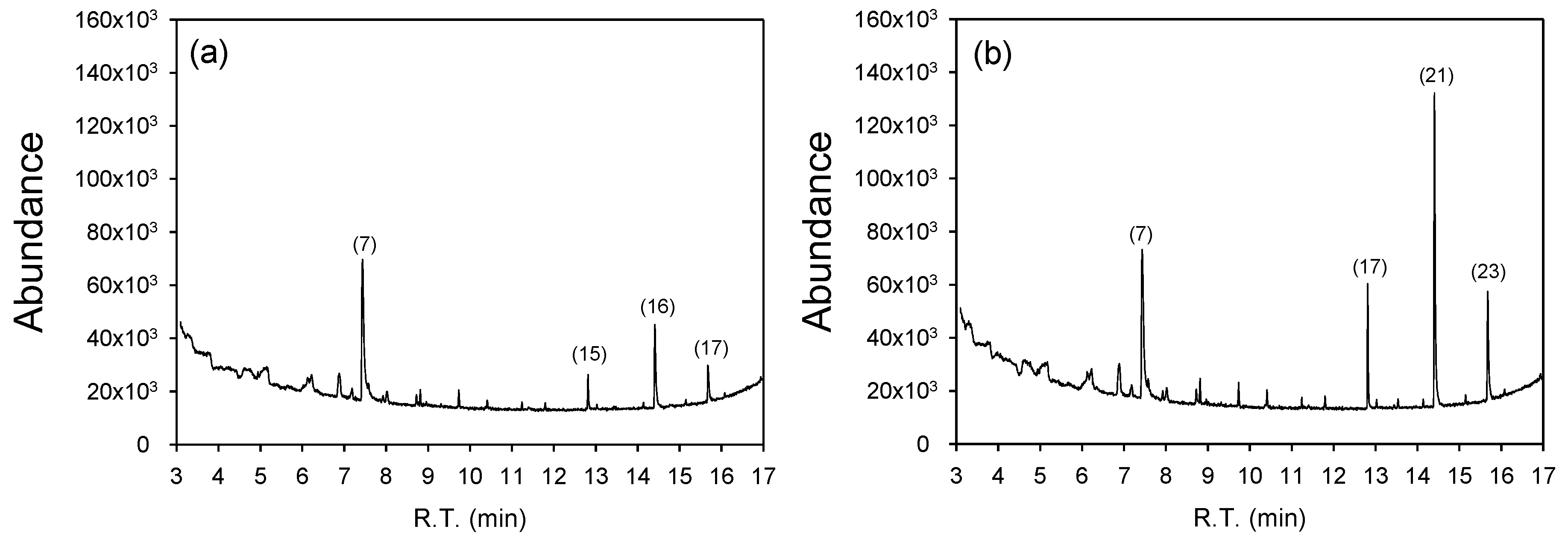
3.5. Bioactive Compounds of Dried MB
3.6. Potential Application of Dried MB as Biostimulant
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Clark, J.H. Green chemistry for the second generation biorefinery–sustainable chemical manufacturing based on biomass. J. Chem. Technol. Biotechnol. 2007, 82, 603–609. [Google Scholar] [CrossRef]
- Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from Algae: Challenges and Potential Importance & Challenges of Algal Biofuels. Biofuels 2010, 1, 763–784. [Google Scholar] [PubMed]
- Anto, S.; Mukherjee, S.S.; Muthappa, R.; Mathimani, T.; Deviram, G.; Kumar, S.S.; Verma, T.N.; Pugazhendhi, A. Algae as green energy reserve: Technological outlook on biofuel production. Chemosphere 2020, 242, 125079. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. 2007, 146, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Microalgal carbohydrates: An overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl. Microbiol. Biotechnol. 2012, 96, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Sharama, N.K.; Tiwari, S.P.; Tripathi, K.; Rai, A.K. Sustainability and cyanobacteria (blue-green algae): Facts and challenges. J. Appl. Phycol. 2011, 23, 1059–1081. [Google Scholar] [CrossRef]
- Christenson, L.; Sims, R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef]
- de Corato, U.; de Bari, I.; Viola, E.; Pugliese, M. Assessing the main opportunities of integrated biorefining from agro-bioenergy co/by-products and agroindustrial residues into high-value added products associated to some emerging markets: A review. Renew. Sustain. Energy Rev. 2018, 88, 326–346. [Google Scholar] [CrossRef]
- Tripathi, R.D.; Dwivedi, S.; Shukla, M.K.; Mishra, S.; Srivastava, S.; Singh, R.; Rai, U.N.; Gupta, D.K. Role of blue green algae biofertilizer in ameliorating the nitrogen demand and fly-ash stress to the growth and yield of rice (Oryza sativa L.) plants. Chemosphere 2008, 70, 1919–1929. [Google Scholar] [CrossRef]
- Wuang, S.C.; Khin, M.C.; Chua, P.Q.D.; Luo, Y.D. Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Res. 2016, 15, 59–64. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Howard-Williams, C.; Turnbull, M.H.; Broady, P.A.; Craggs, R.J. Enhancing microalgal photosynthesis and productivity in wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 2015, 184, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, A.; Craggs, R.; Farid, M.M. Wastewater treatment high rate algal ponds (WWT HRAP) for low-cost biofuel production. Bioresour. Technol. 2015, 184, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Zhao, Z.; Li, D.; Lei, Z.; Zhang, Z.; Lee, D. Algae granulation of nutrients uptake and algae harvesting during wastewater treatment. Chemosphere 2019, 214, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H. Effect of dietary algae on improvement of lipid metabolism in fish. Biomed. Pharmacother. 1997, 51, 345–348. [Google Scholar] [CrossRef]
- Roy, S.S.; Pal, R. Microalgae in aquaculture: A review with special references to nutritional value and fish dietetics. Proc. Zool. Soc. 2015, 68, 1–8. [Google Scholar] [CrossRef]
- Hasan, M.R.; Chakrabarti, R. Use of Algae and Aquatic Macrophytes as Feed in Small-Scale Aquaculture: A Review. FAO Fish. Aquac. Tech. Pap. 2009, 531, vii+123. Available online: https://www.cabdirect.org/cabdirect/abstract/20103235810 (accessed on 27 September 2020).
- Norambuena, F.; Hermon, K.; Skrzypczyk, V.; Emery, J.A.; Sharon, Y.; Beard, A.; Turchini, G.M. Algae in fish feed: Performances and fatty acid metabolism in juvenile Atlantic salmon. PLoS ONE 2015, 10, e0124042. [Google Scholar] [CrossRef]
- Meng, X.; Savage, P.E.; Deng, D. Trash to treasure: From harmful algal blooms to high-performance electrodes for sodium-ion batteries. Environ. Sci. Technol. 2015, 49, 12543–12550. [Google Scholar] [CrossRef]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef]
- Sanseverino, I.; Conduto, D.; Pozzoli, L.; Dobricic, S.; Lettieri, T. Algal Bloom and Its Economic Impact; Institute for Environment and Sustainability: Ispra, Italy, 2016. [Google Scholar]
- Lee, S.; Ahn, C.H.; Kim, E.J.; Park, J.R.; Joo, J.C. Growth inhibition of harmful algae using TiO2-embedded expanded polystyrene balls in the hypereutrophic stream. J. Hazard. Mater. 2020, 398, 123172. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.H.; Schindler, D.W. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, E.; Wiegand, C. Cyanobacterial toxins—Occurrence, biosynthesis and impact on human affairs. Mol. Nutr. Food Res. 2006, 50, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Wang, D.; Ding, S.; Yang, L.; Xu, S.; Yang, C.; Wang, Y.; Zhang, C. Seasonal mobility of antimony in sediment-water systems in algae- and macrophyte-dominated zones of Lake Taihu (China). Chemosphere 2019, 223, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Congestri, R.; di Pippo, F.; de Philippis, R.; Buttino, I.; Paradossi, G.; Albertano, P. Seasonal succession of phototrophic biofilms in an Italian wastewater treatment plant: Biovolume, spatial structure and exopolysaccharides. Aquat. Microb. Ecol. 2006, 45, 301–312. [Google Scholar] [CrossRef]
- Guzzon, A.; di Pippo, F.; Congestri, R. Wastewater Biofilm Photosynthesis in Photobioreactors. Microorganisms 2019, 7, 252. [Google Scholar] [CrossRef]
- di Gregorio, L.; Rossetti, S.; Tandoi, V.; Congestri, R.; di Pippo, F. Unravelling the core microbiome of biofilms in cooling tower systems. Biofouling 2017, 33, 793–806. [Google Scholar] [CrossRef]
- Henderson, R.K.; Parsons, S.A.; Jefferson, B. The impact of differing cell and algogenic organic matter (AOM) characteristics on the coagulation and flotation of algae. Water Res. 2010, 44, 3617–3624. [Google Scholar] [CrossRef]
- Yang, X.; Guo, W.; Shen, Q. Formation of disinfection byproducts from chlor (am) ination of algal organic matter. J. Hazard. Mater. 2011, 197, 378–388. [Google Scholar] [CrossRef]
- Byun, J.; Hwang, S.; Mun, S.; Hwang, S.; Kim, B. Effect of temperature on water quality improvement of natural plant-mineral composites (PMC) in a eutrophic lake, Lake Shingal, Korea. KJEE 2013, 46, 225–233. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2006; pp. 9–72. [Google Scholar]
- Shin, J.; Kang, B.; Hwang, S. Water-blooms (green-tide) dynamics of algae alert system and rainfall-hydrological effects in Daecheong Reservoir, Korea. KJEE 2016, 49, 153–175. [Google Scholar] [CrossRef]
- Kim, M.S.; Chung, Y.R.; Suh, E.H.; Song, W.S. Eutrophication of Nakdong River and statistical analysis of environmental factors. Algae 2002, 17, 105–115. [Google Scholar] [CrossRef]
- Gao, S.; Yang, J.; Tian, J.; Ma, F.; Tu, G.; Du, M. Electro-coagulation–flotation process for algae removal. J. Hazard. Mater. 2010, 177, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Adachi, Y.; Suzuki, M. Flotation and sedimentation of a single microcystis floc collected from surface bloom. Water Res. 1993, 6, 979–983. [Google Scholar] [CrossRef]
- Hou, Q.; Meng, P.; Pei, H.; Hu, W.; Chen, Y. Phosphorus adsorption characteristics of alum sludge: Adsorption capacity and the forms of phosphorus retained in alum sludge. Mater. Lett. 2018, 229, 31–35. [Google Scholar] [CrossRef]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous cellulose-structure and characterization. Cellul. Chem. Technol. 2011, 45, 13–21. [Google Scholar]
- Huo, S.; Wang, Z.; Zhu, S.; Cui, F.; Zou, B.; Wang, F.; Yuan, Z.; Dong, R.; Zhao, P. Process analysis of alkaline flocculation harvesting for Chaetoceros muelleri and Scenedesmus quadricauda. Bioenergy Res. 2016, 9, 682–690. [Google Scholar] [CrossRef]
- Horne, A.J.; Goldman, C.R. Limnology, 2nd ed.; McGraw-Hill Biological Science Series; University of California-Davis: Davis, CA, USA, 1994. [Google Scholar]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 1–10. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef]
- Talbot, J.M.; Treseder, K.K. Interactions among lignin, cellulose, and nitrogen drive litter chemistry-decay relationships. Ecology 2012, 93, 345–354. [Google Scholar] [CrossRef]
- Han, W.; Clarke, W.; Pratt, S. Composting of waste algae: A review. Waste Manag. 2014, 34, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S.; Kumar, A.; Singh, M. Cyanobacteria: A sustainable and commercial bio-resource in production of bio-fertilizer and bio-fuel from waste waters. Environ. Sustain. Indic. 2019, 3–4, 100008. [Google Scholar] [CrossRef]
- Chojnacka, K.; Chojnacki, A.; Górecka, H. Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue–green algae Spirulina sp.: Kinetics, equilibrium and the mechanism of the process. Chemosphere 2005, 59, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Beardall, J.; Young, E.; Roberts, S. Approaches for determining phytoplankton nutrient limitation. Aquat. Sci. 2001, 63, 44–69. [Google Scholar] [CrossRef]
- Kim, H.; Yu, M.; Han, I. Multi-method study of the characteristic chemical nature of aquatic humic substances isolated from the Han River, Korea. Appl. Geochem. 2006, 21, 1226–1239. [Google Scholar] [CrossRef]
- Lee, N.; Amy, G.; Croué, J. Low-pressure membrane (MF/UF) fouling associated with allochthonous versus autochthonous natural organic matter. Water Res. 2006, 40, 2357–2368. [Google Scholar] [CrossRef]
- Kanokkantapong, V.; Marhaba, T.F.; Panyapinyophol, B.; Pavasant, P. FTIR evaluation of functional groups involved in the formation of haloacetic acids during the chlorination of raw water. J. Hazard. Mater. 2006, 136, 188–196. [Google Scholar] [CrossRef]
- Mayers, J.J.; Flynn, K.J.; Shields, R.J. Rapid determination of bulk microalgal biochemical composition by fourier-transform infrared spectroscopy. Bioresour. Technol. 2013, 148, 215–220. [Google Scholar] [CrossRef]
- Kawale, H.D.; Kishore, N. Production of hydrocarbons from a green algae (Oscillatoria) with exploration of its fuel characteristics over different reaction atmospheres. Energy 2019, 178, 344–355. [Google Scholar] [CrossRef]
- Bandekar, J. Amide modes and protein conformation. Biochim. Biophys. Acta 1992, 1120, 123–143. [Google Scholar] [CrossRef]
- Jebsen, C.; Norici, A.; Wagner, H.; Palmucci, M.; Giordano, M.; Wilhelm, C. FTIR spectra of algal species can be used as physiological fingerprints to assess their actual growth potential. Physiol. Plant. 2012, 146, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Dineshkumar, R.; Subramanian, J.; Gopalsamy, J.; Jayasingam, P.; Arumugam, A.; Kannadasan, S.; Sampathkumar, P. The impact of using microalgae as biofertilizer in maize (Zea may L.). Waste Biomass Valorization 2019, 10, 1101–1110. [Google Scholar] [CrossRef]
- Bhalamurugan, G.L.; Valerie, O.; Mark, L. Valuable bioproducts obtained from microalgal biomass and their commercial applications: A review. Environ. Eng. Res. 2018, 23, 229–241. [Google Scholar] [CrossRef]
- Zeroual, W.; Manfait, M.; Choisy, C. FT-IR spectroscopy study of perturbations induced by antibiotic in bacteria (Escherichia coli). Pathol. Biol. 1995, 43, 300–305. [Google Scholar] [PubMed]
- Wiliams, D.H.; Fleming, I. Spectroscopic Methods in Organic Chemistry, 5th ed.; McGraw-Hill International Ltd.: London, UK, 1996. [Google Scholar]
- Ni, L.; Su, L.; Li, S.; Wang, P.; Li, D.; Ye, X.; Li, Y.; Li, Y.; Li, Y.; Wang, C. The characterization of dissolved organic matter extracted from different sources and their influence on cadmium uptake by Microcystis aeruginosa. Environ. Toxicol. Chem. 2017, 36, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Liu, Z.; Langner, U.; Stehfest, K.; Wilhelm, C. The use of FTIR spectroscopy to assess quantitative changes in the biochemical composition of microalgae. J. Biophotonics 2010, 3, 557–566. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Liu, Y.; Rahman, M.M.; Naidu, R.; Dharmarajan, R.; Shon, H.K.; Woo, Y.C. Core−shell interface-oriented synthesis of bowl-structured hollow silica nanospheres using self-assembled ABC triblock copolymeric micelles. Langmuir 2018, 34, 13584–13596. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Kansiz, M.; Heraud, P.; Beardall, J.; Wood, B.; McNaughton, D. Fourier transform infrared spectroscopy as a novel tool to investigate changes in intracellular macromolecular pools in the marine microalga Chaetoceros muellerii (Bacillariophyseae). J. Phycol. 2002, 37, 271–279. [Google Scholar] [CrossRef]
- Faheed, F.; Fattah, Z.A. Effect of Chlorella vulgaris as bio-fertilizer on growth parameters and metabolic aspects of lettuce plant. J. Agric. Soc. Sci. 2008, 4, 164–169. [Google Scholar]
- Uysal, O.; Uysal, F.O.; Ekinci, K. Evaluation of microalgae as microbial fertilizer. Eur. J. Sustain. Dev. 2015, 4, 77–82. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Saeid, A. Plant growth biostimulants, dietary feed supplements and cosmetics formulated with supercritical CO2 algal extracts. Molecules 2017, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.H.; Lee, S.; Park, J.R. Nutritional properties by composting process of algae biomass as soil conditioner. J. Environ. Impact Assess. 2019, 28, 569–580. [Google Scholar]
- Kebelmann, K.; Hornung, A.; Karsten, U.; Griffiths, G. Intermediate pyrolysis and product identification by TGA and Py-GC MS of green microalgae and their extracted protein and lipid components. Biomass Bioenergy 2013, 49, 38–48. [Google Scholar] [CrossRef]
- Aysu, T.; Sanna, A. Nannochloropsis algae pyrolysis with ceria-based catalysts for production of high-quality bio-oils. Bioresour. Technol. 2015, 194, 108–116. [Google Scholar] [CrossRef]
- Moshfegh, A.; Salehzadeh, A.; Shandiz, S.A.S.; Shafaghi, M.; Naeemi, A.S.; Salehi, S. Phytochemical analysis, antioxidant, anticancer and antibacterial properties of the Caspian sea red macroalgae, Laurencia capsica. Iran. J. Sci. Technol. Trans. Sci. 2019, 43, 49–56. [Google Scholar] [CrossRef]
- Chen, W.; Lin, B.; Huang, M.; Chang, J. Thermochemical conversion of microalgal biomass into biofuels: A review. Bioresour. Technol. 2015, 184, 314–327. [Google Scholar] [CrossRef]
- Ragunathan, V.; Pandurangan, J.; Ramakrishnan, T. Gas chromatography-mass spectrometry analysis of methanol extracts from marine red seaweed Gracilaria corticate. Pharmacogn. J. 2019, 11, 547–554. [Google Scholar] [CrossRef]
- Swamy, M.K.; Arumugam, G.; Kaur, R.; Ghasemzadeh, A.; Yusoff, M.M.; Sinniah, U.R. GC-MS based metabolite profiling, antioxidant and antimicrobial properties of different solvent extracts of Malaysian Plectranthus amboinicus leaves. Evid. Based Complementary Altern. Med. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as multi-functional options in modern agriculture: Current trends, prospects and challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef]
- El Arroussi, H.; Benhima, R.; El baouchi, A.; Sijilmassi, B.; El Mernissi, N.; Aafsar, A.; Meftah-Kadmiri, I.; Bendaou, N.; Smouni, A. Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J. Appl. Phycol. 2018, 30, 2929–2941. [Google Scholar] [CrossRef]
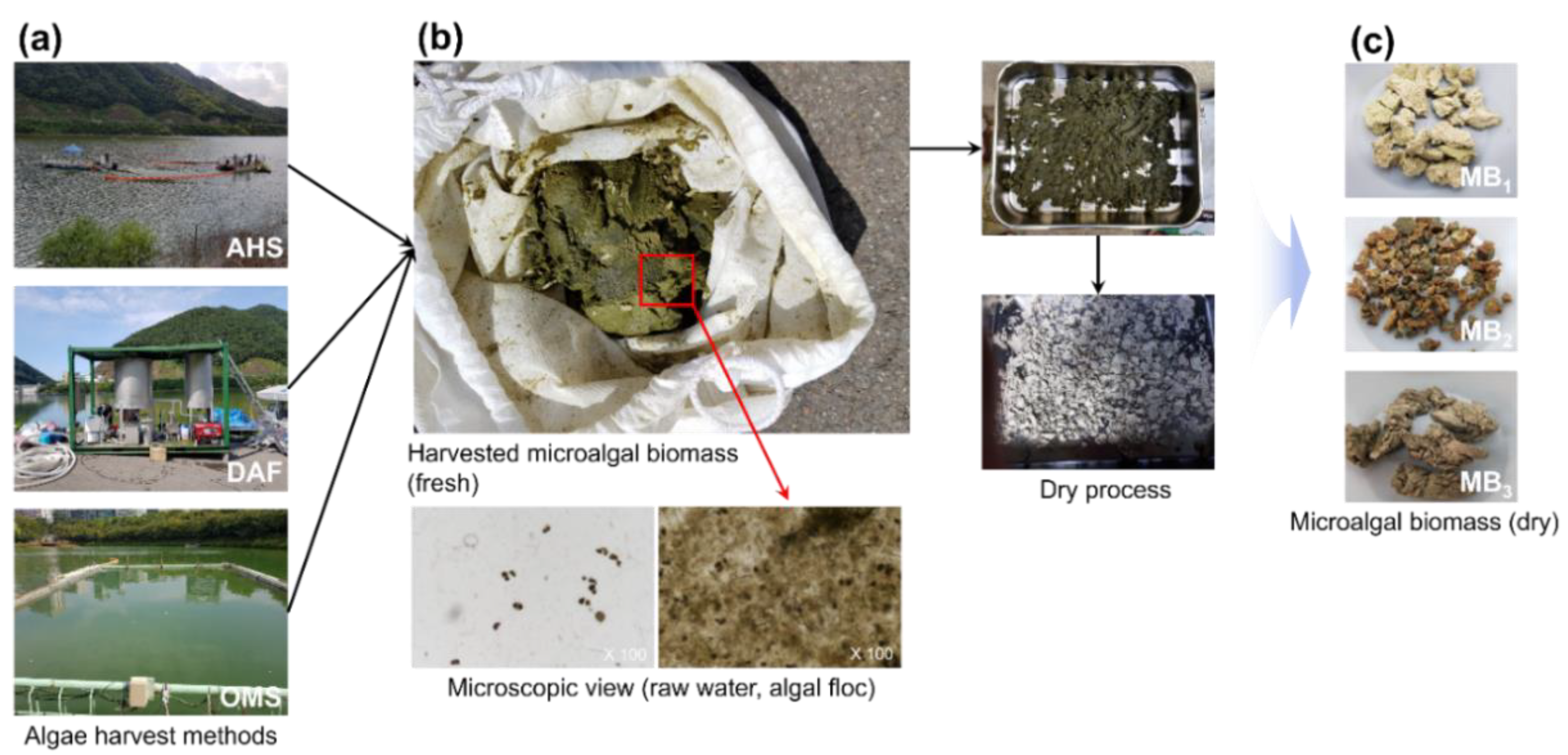



| WTF | Coagulant | Capacity (ton day−1) | Description | Harvested Sample | Site (Date) |
|---|---|---|---|---|---|
| Algae harvest ship (AHS) | Plant mineral composite (PME) | 30,000 | Recovery of surfaced algal sludge from the water after the coagulant is applied to the algal bloom area | MB1 | OD in site B (August 2018) |
| Dissolved air flotation (DAF) | Poly aluminum (PAC) | 240 | Water by algal bloom at the waterbody edge is transferred to the pilot plant to separate the algal solid from the water using the cyclonic-DAF method | MB2 | ED in site B (August 2018) |
| On-site microbubble ship (OMS) | Al2(SO4)3 (Alum) | 30,000 | Algae sludge is collected on the water surface via the coagulation-floating process using microbubbles in a compact facility on-site | MB3 | AL in site C (Sepember 2018) |
| Description | OD | ED | AL |
|---|---|---|---|
| Temperature (°C) | 27.6 | 27.7 | 26.0 |
| pH | 9.6 | 9.5 | 8.9 |
| DO (mg L−1) | 12.0 | 11.6 | 9.2 |
| DO saturation (%) | 149 | 148 | 115 |
| EC (μS cm−1) | 114 | 124 | 152 |
| Turbidity (NTU) | 17.8 | 17.3 | 6.7 |
| SS (mg L−1) | 6.4 | 6.6 | 3.5 |
| TOC (mg L−1) | 5.8 | 5.3 | 2.6 |
| BOD (mg L−1) | 4.0 | 3.9 | 1.5 |
| CODMn (mg L−1) | 12.0 | 9.6 | 3.2 |
| TN (mg L−1) | 0.97 | 1.16 | 0.79 |
| NH3+-N (μg L−1) | 0.07 | 0.46 | 0.14 |
| NO3−-N (μg L−1) | ND a | ND a | 0.43 |
| TP (μg L−1) | 0.047 | 0.052 | 0.062 |
| PO43−-P (μg L−1) | ND a | ND a | 0.035 |
| Chl-a (μg L−1) | 91.7 | 46.4 | 19.5 |
| Element | MB1 | MB2 | MB3 | |||
|---|---|---|---|---|---|---|
| Weight (%) | Atomic Level (%) | Weight (%) | Atomic Level (%) | Weight (%) | Atomic Level (%) | |
| C | 38.78 | 48.57 | 23.44 | 33.52 | 41.32 | 51.50 |
| O | 46.23 | 43.47 | 44.14 | 47.39 | 43.17 | 40.40 |
| Al | 11.17 | 6.23 | 9.52 | 6.06 | 7.95 | 4.41 |
| Si | 1.89 | 1.01 | 18.35 | 11.22 | 5.48 | 2.92 |
| Fe | 0.59 | 0.16 | 2.06 | 0.63 | 0.78 | 0.21 |
| Ca | 0.29 | 0.11 | 0.88 | 0.38 | 0.52 | 0.19 |
| S | 0.56 | 0.26 | 0.60 | 0.32 | 0.40 | 0.19 |
| K | 0.18 | 0.07 | 0.86 | 0.38 | 0.20 | 0.08 |
| Mg | 0.07 | 0.05 | 0.15 | 0.11 | 0.18 | 0.11 |
| Mn | 0.25 | 0.07 | ND a | ND a | ND a | ND a |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Compound | Formulate | Peak Number | R.T. a (min) | MW b (g moL−1) | Class/Subclass |
|---|---|---|---|---|---|
| Undecane | C11H24 | (7), (7) | 7.444 | 156 | Saturated hydrocarbons/Alkanes |
| Heptadecane | C17H36 | (15), (17) | 12.823 | 240 | Saturated hydrocarbons/Alkanes |
| Hxadecanoic acid, methyl ester | C17H34O2 | (16), (21) | 14.420 | 270 | Fatty Acyls/Fatty acid esters |
| Methyl stearate | C19H38O2 | (17), (23) | 15.689 | 298 | Fatty Acyls/Fatty acid esters |
| Experimental Groups | Fresh Weight (g) | Leaves (num.) | Harmful Effects |
|---|---|---|---|
| Control | 7.7 ± 0.33d | 9.1 ± 0.59d | None |
| Composted sawdust | 7.8 ± 0.40d | 9.3 ± 0.51d | None |
| Composted MB sample (11.7% w/w) | 8.2 ± 0.21d | 10.0 ± 0.22d | None |
| Composted MB sample (21.6% w/w) | 9.1 ± 0.35c | 13.6 ± 0.68b | None |
| Composted MB sample (37.6% w/w) | 11.3 ± 0.53a | 15.9 ± 0.55a | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, C.H.; Lee, S.; Park, J.R.; Hwang, T.-M.; Joo, J.C. Harvested Microalgal Biomass from Different Water Treatment Facilities—Its Characteristics and Potential Use as Renewable Sources of Plant Biostimulation. Agronomy 2020, 10, 1882. https://doi.org/10.3390/agronomy10121882
Ahn CH, Lee S, Park JR, Hwang T-M, Joo JC. Harvested Microalgal Biomass from Different Water Treatment Facilities—Its Characteristics and Potential Use as Renewable Sources of Plant Biostimulation. Agronomy. 2020; 10(12):1882. https://doi.org/10.3390/agronomy10121882
Chicago/Turabian StyleAhn, Chang Hyuk, Saeromi Lee, Jae Roh Park, Tae-Mun Hwang, and Jin Chul Joo. 2020. "Harvested Microalgal Biomass from Different Water Treatment Facilities—Its Characteristics and Potential Use as Renewable Sources of Plant Biostimulation" Agronomy 10, no. 12: 1882. https://doi.org/10.3390/agronomy10121882
APA StyleAhn, C. H., Lee, S., Park, J. R., Hwang, T.-M., & Joo, J. C. (2020). Harvested Microalgal Biomass from Different Water Treatment Facilities—Its Characteristics and Potential Use as Renewable Sources of Plant Biostimulation. Agronomy, 10(12), 1882. https://doi.org/10.3390/agronomy10121882





