Evaluation of Soil Water Content Measurements with Capacitance Probes to Support Irrigation Scheduling in a “Red Beaut” Japanese Plum Orchard
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Plot Description and Climate
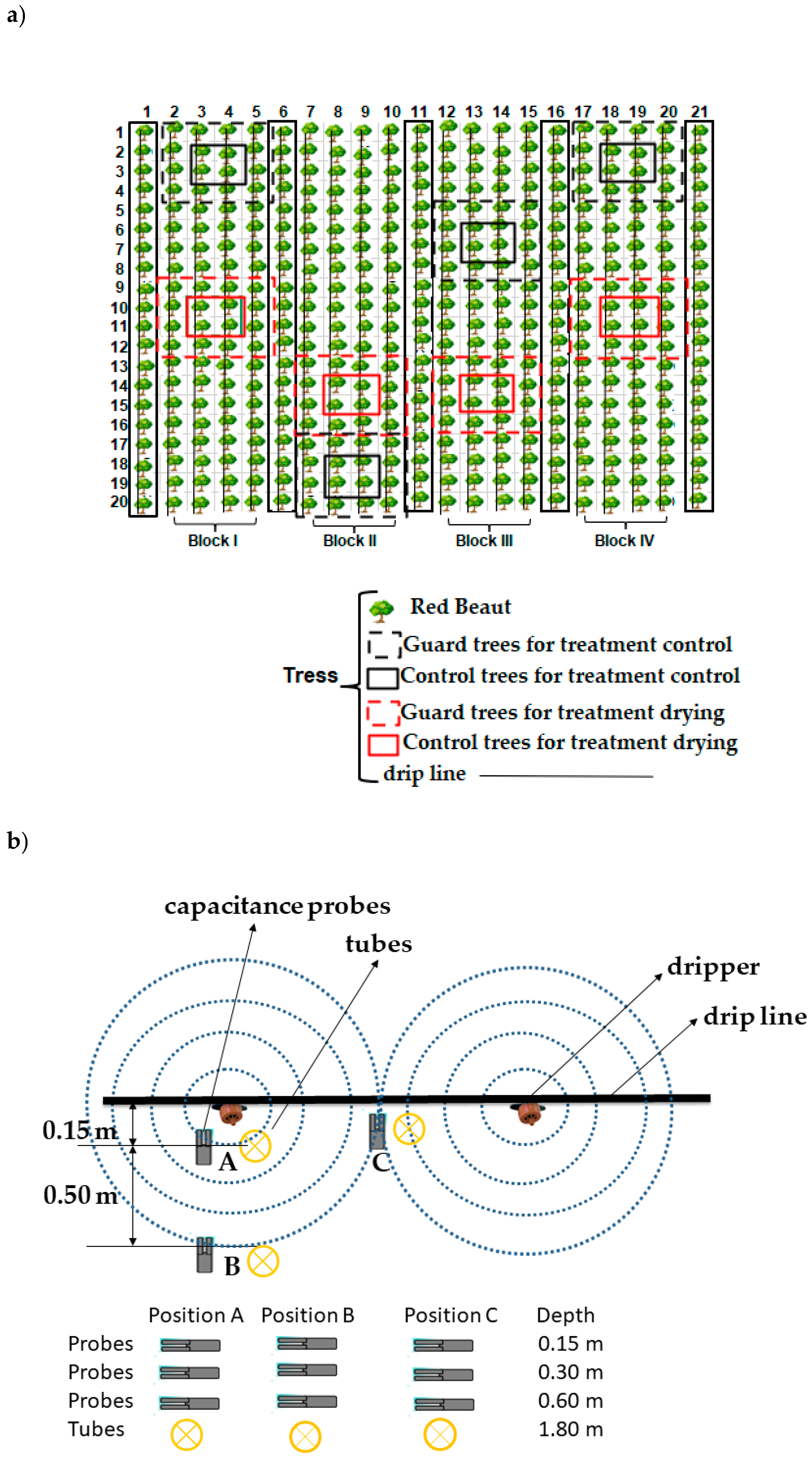
2.2. Irrigation System, Irrigation Treatments and Experimental Design
2.3. Soil Water Content Probes
2.3.1. FDR Probes
2.3.2. Neutron Probes
2.4. Plant Measurements
2.4.1. Plant Water Status
2.4.2. Photosynthesis and Leaf Stomatal Conductance
2.4.3. Sap Flow
2.4.4. The Canopy Photosynthetically Active Radiation Interception
2.5. Signal Intensity, Noise and Sensitivity
- Traditional method (S), as proposed by Goldhamer et al. [50]: S is always higher than 0, and the higher its value the greater the sensitivity.S = SI/CV
- Corrected sensitivity (S*), as proposed by De la Rosa et al. [6]: The interpretation of the values obtained with this algorithm is as follows:S* = SI−1/CV
- (a).
- S* > 1: indicates sensitivity to water deficit.
- (b).
- 1 > S* > 0: The noise is greater than the increase in signal intensity. Therefore, there are no differences between treatments.
- (c).
- S* = 0: no differences between treatments, not sensitive to water deficit.
- (d).
- S* < 0: anomalous behavior.
2.6. Statistical Analysis
3. Results
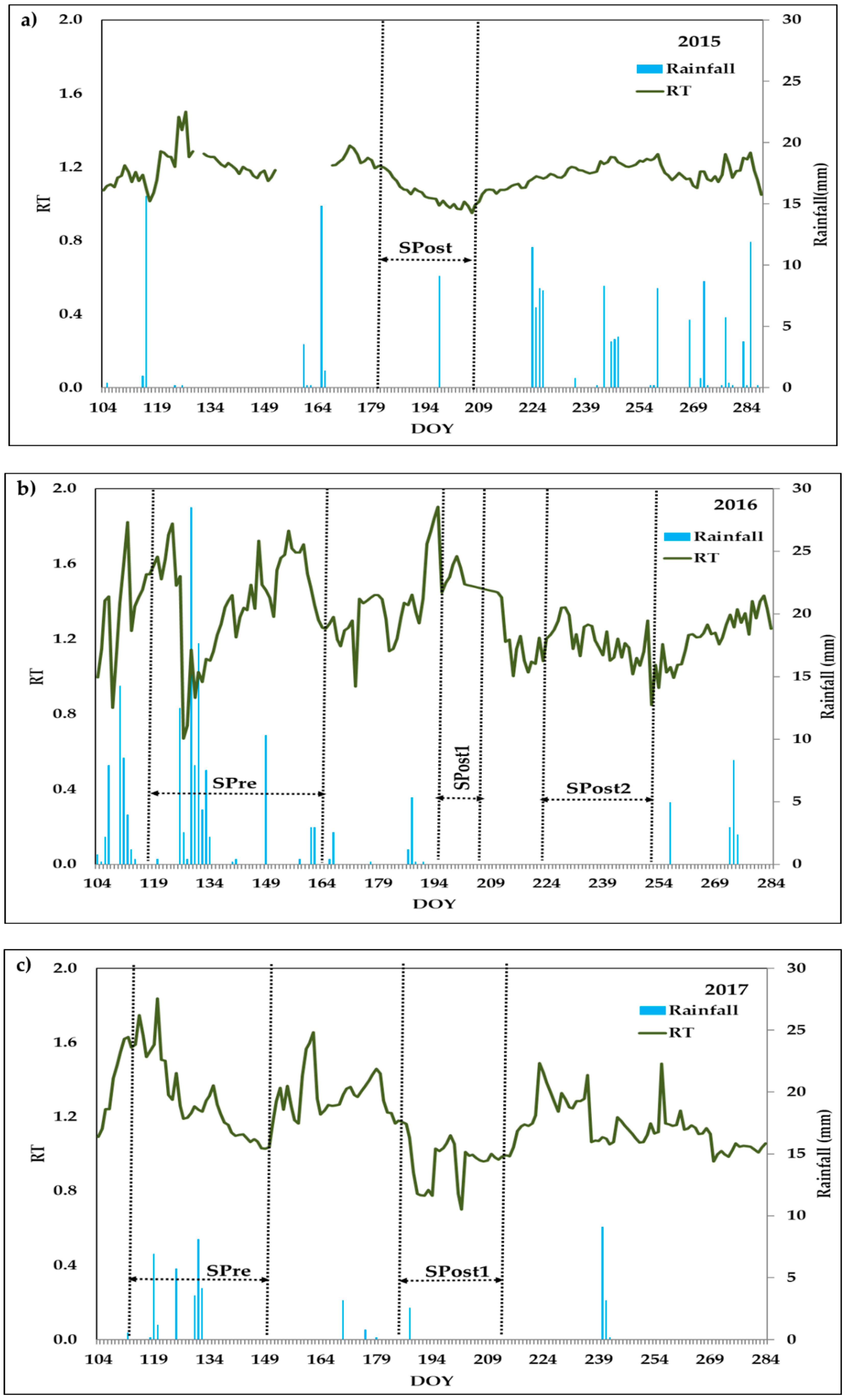
3.1. Climatic Conditions and Water Applied
3.2. Signal Intensity, Noise and Sensitivity
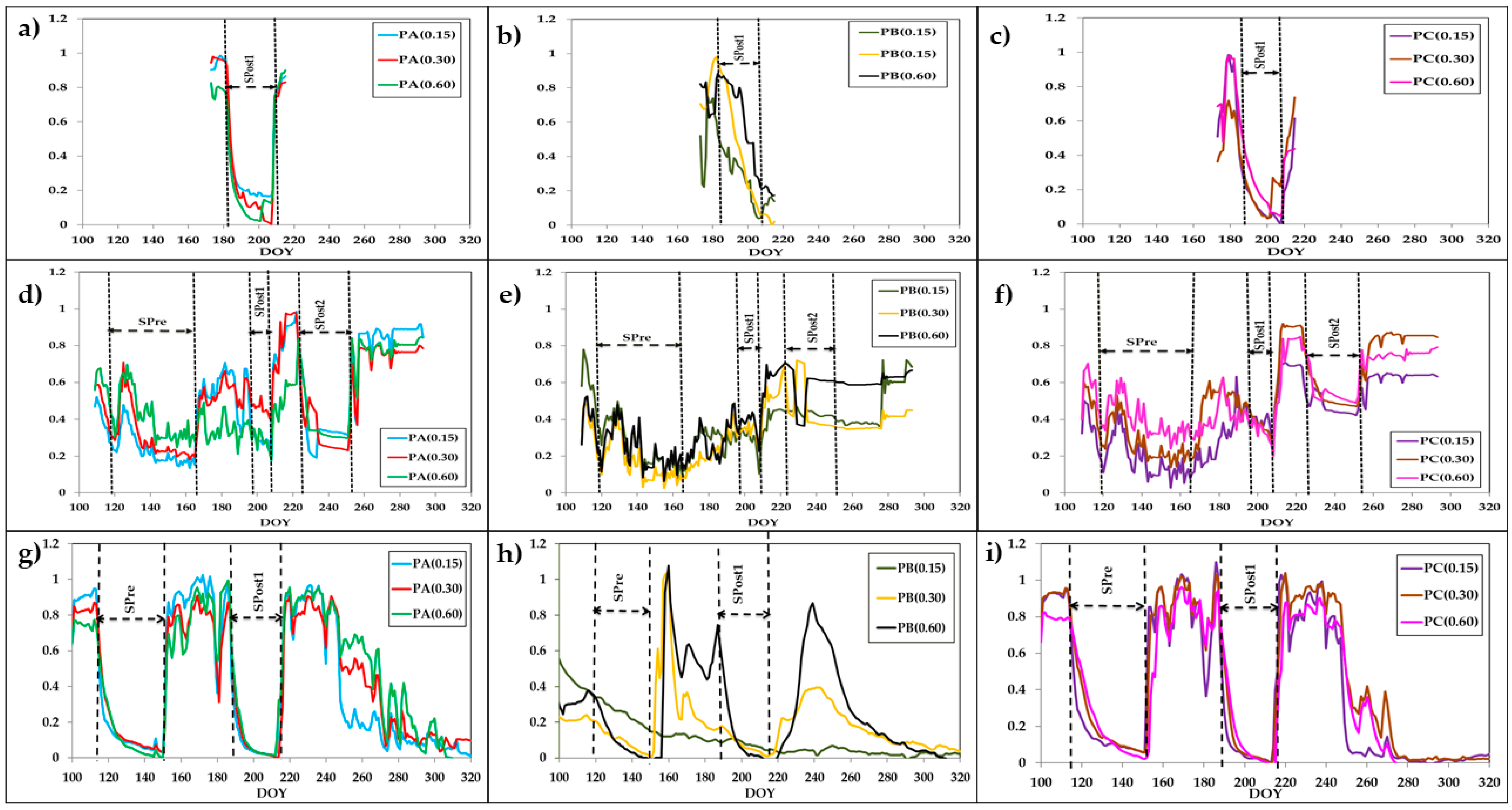
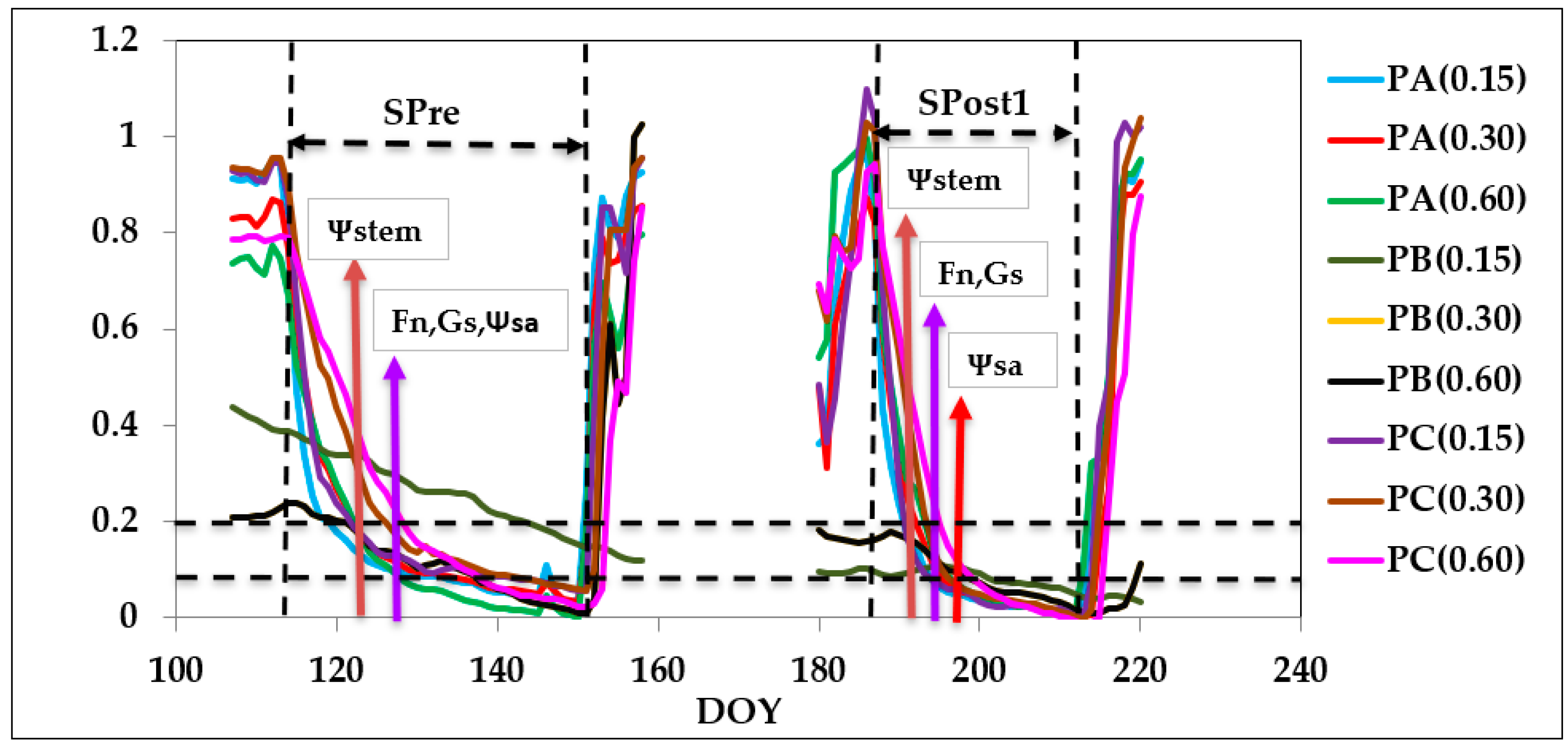
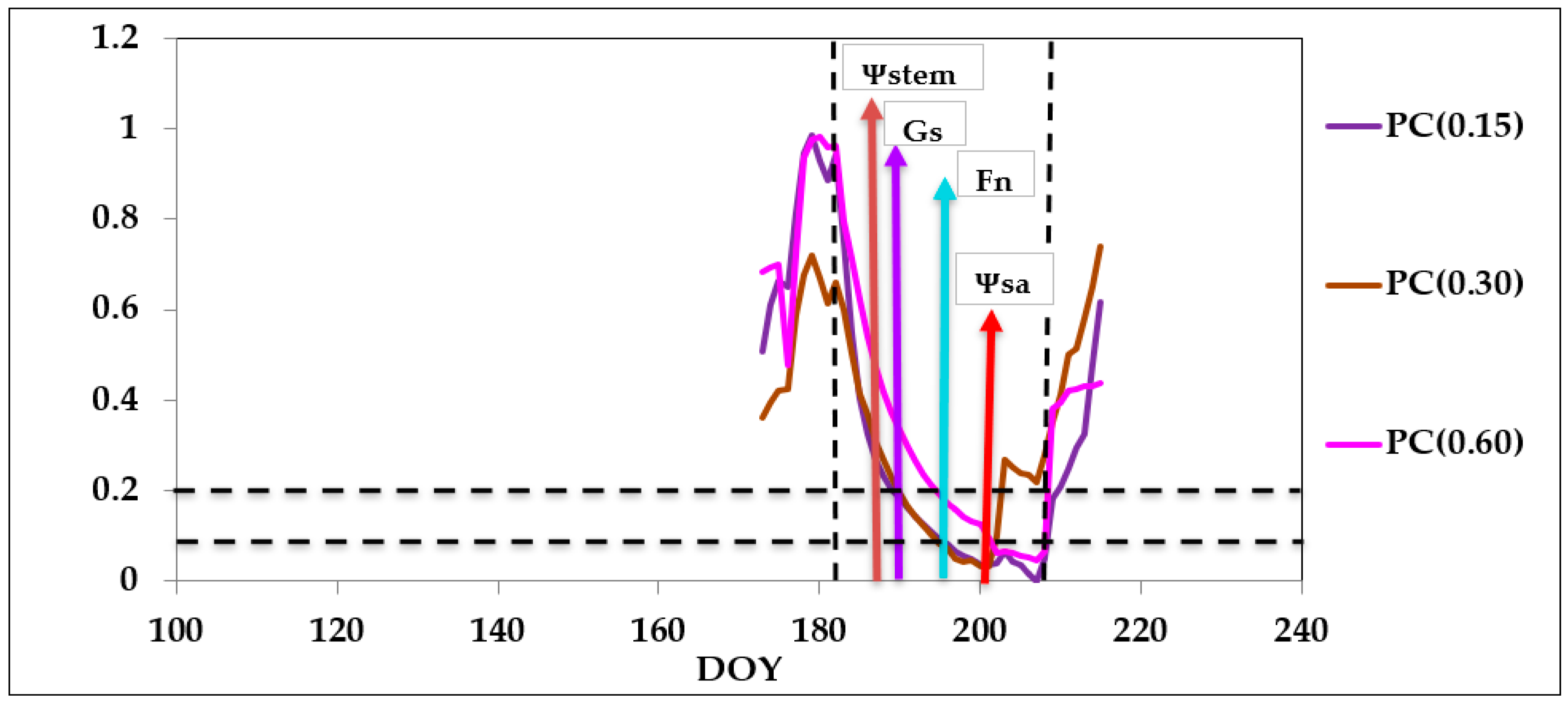
3.3. Seasonal Dynamics
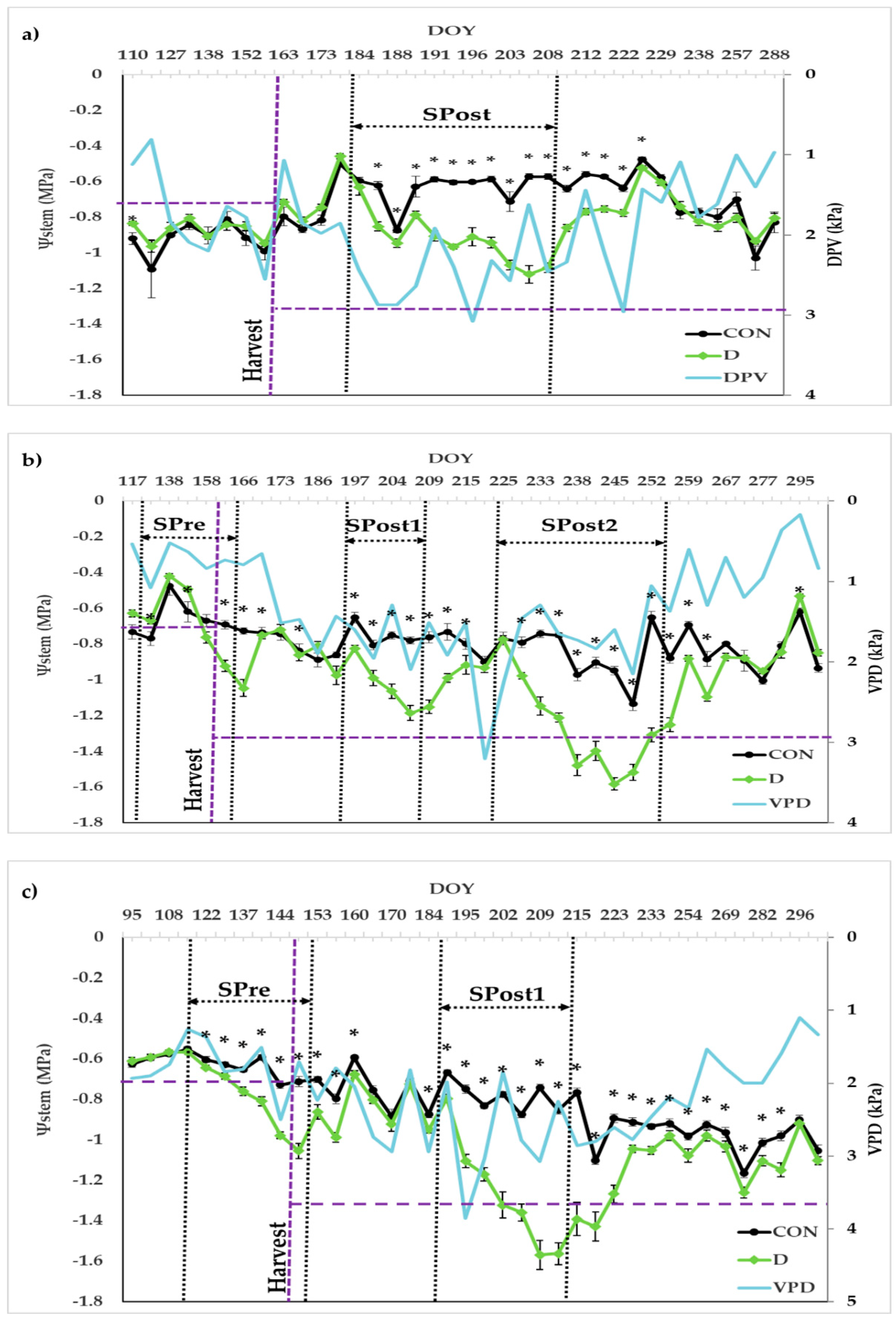
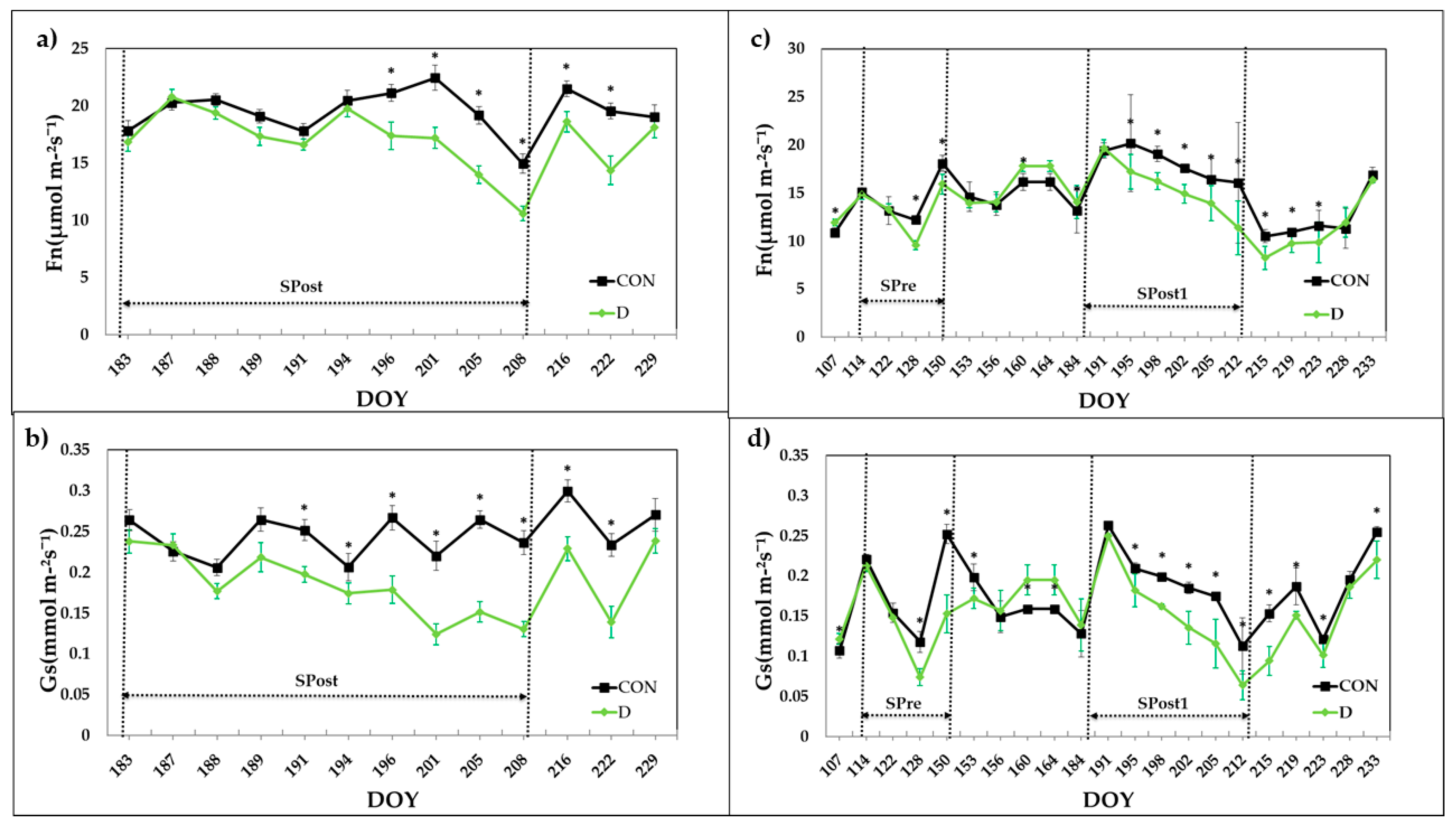
3.3.1. Stem Water Potential and Gas Exchange
3.3.2. Sap Flow
3.3.3. Soil Water Content (FDR Probes)
4. Discussion
4.1. Signal Intensity, Noise and Sensitivity
4.2. Plant Response to Soil Water Content Deprivation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Romero, R.; Muriel, J.; García, I.; de la Peña, D.M. Research on Automatic Irrigation Control: State of the Art and Recent Results. Agric. Water Manag. 2012, 114, 59–66. [Google Scholar] [CrossRef]
- Morales, F.; Ancín, M.; Fakhet, D.; González-Torralba, J.; Gámez, A.L.; Seminario, A.; Soba, D.; Ben Mariem, S.; Garriga, M.; Aranjuelo, I. Photosynthetic Metabolism Under Stressful Growth Conditions as a Bases for Crop Breeding and Yield Improvement. Plants 2020, 9, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houghton, J.T. The Scientific Basis. In Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Edenhofer, O. Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Turral, H.; Burke, J.; Faurès, J. Climate Change, Water and Food Security; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2011. [Google Scholar]
- De la Rosa, J.; Conesa, M.R.; Domingo, R.; Pérez-Pastor, A. A New Approach to Ascertain the Sensitivity to Water Stress of Different Plant Water Indicators in Extra-Early Nectarine Trees. Sci. Hortic. 2014, 169, 147–153. [Google Scholar] [CrossRef]
- Osroosh, Y.; Peters, R.T.; Campbell, C.S.; Zhang, Q. Comparison of Irrigation Automation Algorithms for Drip-Irrigated Apple Trees. Comput. Electron. Agric. 2016, 128, 87–99. [Google Scholar] [CrossRef] [Green Version]
- Casadesús, J.; Mata, M.; Marsal, J.; Girona, J. A General Algorithm for Automated Scheduling of Drip Irrigation in Tree Crops. Comput. Electron. Agric. 2012, 83, 11–20. [Google Scholar] [CrossRef]
- Millán, S.; Casadesús, J.; Campillo, C.; Moñino, M.J.; Prieto, M.H. Using Soil Moisture Sensors for Automated Irrigation Scheduling in a Plum Crop. Water 2019, 11, 2061. [Google Scholar] [CrossRef] [Green Version]
- Millán, S.; Campillo, C.; Casadesús, J.; Pérez-Rodríguez, J.M.; Prieto, M.H. Automatic Irrigation Scheduling on a Hedgerow Olive Orchard using an Algorithm of Water Balance Readjusted with Soil Moisture Sensors. Sensors 2020, 20, 2526. [Google Scholar] [CrossRef]
- Zazueta, F.S.; Xin, J. Soil Moisture Sensors. Soil Sci. 1994, 73, 391–401. [Google Scholar]
- Dobriyal, P.; Qureshi, A.; Badola, R.; Hussain, S.A. A Review of the Methods Available for Estimating Soil Moisture and its Implications for Water Resource Management. J. Hydrol. 2012, 458, 110–117. [Google Scholar] [CrossRef]
- Robock, A.; Vinnikov, K.Y.; Srinivasan, G.; Entin, J.K.; Hollinger, S.E.; Speranskaya, N.A.; Liu, S.; Namkhai, A. The Global Soil Moisture Data Bank. Bull. Am. Meteorol. Soc. 2000, 81, 1281–1300. [Google Scholar] [CrossRef] [Green Version]
- Evett, S.R. Soil Water Measurement by Time Domain Reflectometry. Encycl. Water Sci. 2003, 894–898. [Google Scholar]
- Erlingsson, S.; Baltzer, S.; Baena, J.; Bjarnason, G. Measurement techniques for water flow. In Water in Road Structures; Anonymous, Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 45–67. [Google Scholar]
- Wang, Y.-N.; Fan, J.; Li, S.-Q.; Zeng, C.; Wang, Q.-J. Effects of Sensor’s Laying Depth for Precision Irrigation on Growth Characteristics of Maturate Grapes. Yingyong Shengtai Xuebao 2012, 23, 2062–2068. [Google Scholar] [PubMed]
- Dabach, S.; Shani, U.; Lazarovitch, N. The Influence of Water Uptake on Matric Head Variability in a Drip-Irrigated Root Zone. Soil Tillage Res. 2016, 155, 216–224. [Google Scholar] [CrossRef]
- Soulis, K.X.; Elmaloglou, S. Optimum Soil Water Content Sensors Placement for Surface Drip Irrigation Scheduling in Layered Soils. Comput. Electron. Agric. 2018, 152, 1–8. [Google Scholar] [CrossRef]
- Elmaloglou, S.; Soulis, K.X.; Dercas, N. Simulation of Soil Water Dynamics under Surface Drip Irrigation from Equidistant Line Sources. Water Resour. Manag. 2013, 27, 4131–4148. [Google Scholar] [CrossRef]
- Nolz, R. A Review on the Quantification of Soil Water Balance Components as a Basis for Agricultural Water Management with a Focus on Weighing Lysimeters and Soil Water sensors/Ein Überblick Über Die Ermittlung Von Wasserhaushaltsgrößen Als Basis Für Die Landeskulturelle Wasserwirtschaft Mit Fokus Auf Lysimeter Und Bodenwassersensoren. Die Bodenkult. J. Land Manag. Food Environ. 2016, 67, 133–144. [Google Scholar]
- Bradford, K.; Hsiao, T. Physiological responses to moderate water stress. In Physiological Plant Ecology II; Anonymous, Ed.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 263–324. [Google Scholar]
- Jiménez, S.; Dridi, J.; Gutiérrez, D.; Moret, D.; Irigoyen, J.J.; Moreno, M.A.; Gogorcena, Y. Physiological, Biochemical and Molecular Responses in Four Prunus Rootstocks Submitted to Drought Stress. Tree Physiol. 2013, 33, 1061–1075. [Google Scholar] [CrossRef]
- Shackel, K.A.; Ahmadi, H.; Biasi, W.; Buchner, R.; Goldhamer, D.; Gurusinghe, S.; Hasey, J.; Kester, D.; Krueger, B.; Lampinen, B. Plant Water Status as an Index of Irrigation Need in Deciduous Fruit Trees. HortTechnology 1997, 7, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Samperio, A.; Prieto, M.H.; Blanco-Cipollone, F.; Vivas, A.; Moñino, M.J. Effects of Post-Harvest Deficit Irrigation in `Red Beaut´ Japanese Plum: Tree Water Status, Vegetative Growth, Fruit Yield, Quality and Economic Return. Agric. Water Manag. 2015, 150, 92–102. [Google Scholar] [CrossRef]
- Sperry, J.S.; Wang, Y.; Wolfe, B.T.; Mackay, D.S.; Anderegg, W.R.; McDowell, N.G.; Pockman, W.T. Pragmatic Hydraulic Theory Predicts Stomatal Responses to Climatic Water Deficits. New Phytol. 2016, 212, 577–589. [Google Scholar] [CrossRef] [Green Version]
- Martin-StPaul, N.; Delzon, S.; Cochard, H. Plant Resistance to Drought Depends on Timely Stomatal Closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sanchez, M.C.; Pérez-Pastor, A.; Torrecillas, A.; Domingo, R. Regulated Deficit Irrigation in Apricot Trees. Acta Hortic. 2000, 2, 759–766. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.; Yoon, T. Impact of Drought Stress on Photosynthetic Response, Leaf Water Potential, and Stem Sap Flow in Two Cultivars of Bi-Leader Apple Trees (Malus× Domestica Borkh.). Scientia Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Blanco-Cipollone, F.; Lourenço, S.; Silvestre, J.; Conceição, N.; Moñino, M.J.; Vivas, A.; Ferreira, M.I. Plant Water Status Indicators for Irrigation Scheduling Associated with Iso-and Anisohydric Behavior: Vine and Plum Trees. Horticulturae 2017, 3, 47. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of Photosynthesis of C3 Plants in Response to Progressive Drought: Stomatal Conductance as a Reference Parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Huang, J.; Peng, S.; Xiong, D. Diffusional Conductance to CO2 is the Key Limitation to Photosynthesis in Salt-stressed Leaves of Rice (Oryza Sativa). Physiol. Plant. 2018, 163, 45–58. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Harris, P.J. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Ferree, D. Canopy Development and Yield Efficiency of’ Golden Delicious’ Apple Trees in Four Orchard Management Systems. J. Am. Soc. Hort. Sci. 1980, 105, 376–380. [Google Scholar]
- Casadesus, J.; Mata, M.; Marsal, J.; Girona, J. Automated Irrigation of Apple Trees Based on Measurements of Light Interception by the Canopy. Biosyst. Eng. 2011, 108, 220–226. [Google Scholar] [CrossRef]
- Deutscher, J.; Kupec, P.; Dundek, P.; Holík, L.; Machala, M.; Urban, J. Diurnal Dynamics of Streamflow in an Upland Forested Micro-Watershed during Short Precipitation-Free Periods is Altered by Tree Sap Flow. Hydrol. Process. 2016, 30, 2042–2049. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Sun, Y.; Li, K.; Zhang, C. Sap Flow Characteristics of Three Afforestation Species during the Wet and Dry Seasons in a Dry-Hot Valley in Southwest China. J. For. Res. 2017, 28, 51–62. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.; McMillan, A.M. Warming and Elevated CO2 have Opposing Influences on Transpiration. Which is More Important? Curr. For. Rep. 2018, 4, 51–71. [Google Scholar] [CrossRef] [Green Version]
- Tie, Q.; Hu, H.; Tian, F.; Guan, H.; Lin, H. Environmental and Physiological Controls on Sap Flow in a Subhumid Mountainous Catchment in North China. Agric. For. Meteorol. 2017, 240, 46–57. [Google Scholar] [CrossRef]
- Domingo, F.; Villagarcía, L.; Brenner, A.; Puigdefábregas, J. Evapotranspiration Model for Semi-Arid Shrub-Lands Tested Against Data from SE Spain. Agric. For. Meteorol. 1999, 95, 67–84. [Google Scholar] [CrossRef]
- Goldhamer, D.; Fereres, E. Irrigation Scheduling of Almond Trees with Trunk Diameter Sensors. Irrig. Sci. 2004, 23, 11–19. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Fereres, E. Irrigation Scheduling Protocols using Continuously Recorded Trunk Diameter Measurements. Irrig. Sci. 2001, 20, 115–125. [Google Scholar] [CrossRef]
- Jones, H.G. Irrigation Scheduling: Advantages and Pitfalls of Plant-Based Methods. J. Exp. Bot. 2004, 55, 2427–2436. [Google Scholar] [CrossRef] [Green Version]
- Soil Survey Division Staff. Keys to Soil Taxonomy, 8th ed.; U.S.D.A.-NRCS: Lincoln, NE, USA, 1998. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements-FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998; Volume 300, p. D05109. [Google Scholar]
- Moñino, M.; Samperio, A.; Vivas, A.; Blanco-Cipollone, F.; Prieto, M. Manual Práctico De Riego Ciruelo Japonés; CICYTEX, Gobierno de Extremadura: Badajoz, Spain, 2014. [Google Scholar]
- Swanson, R.; Whitfield, D. A Numerical Analysis of Heat Pulse Velocity Theory and Practice. J. Exp. Bot. 1981, 32, 221–239. [Google Scholar] [CrossRef]
- Testi, L.; Villalobos, F.J. New Approach for Measuring Low Sap Velocities in Trees. Agric. For. Meteorol. 2009, 149, 730–734. [Google Scholar] [CrossRef]
- López-Bernal, Á.; Alcántara, E.; Testi, L.; Villalobos, F.J. Spatial Sap Flow and Xylem Anatomical Characteristics in Olive Trees Under Different Irrigation Regimes. Tree Physiol. 2010, 30, 1536–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samperio, A.; Moñino, M.J.; Marsal, J.; Prieto, M.H.; Stöckle, C. Use of CropSyst as a Tool to Predict Water use and Crop Coefficient in Japanese Plum Trees. Agric. Water Manag. 2014, 146, 57–68. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Viveros, M. Effects of Preharvest Irrigation Cutoff Durations and Postharvest Water Deprivation on Almond Tree Performance. Irrig. Sci. 2000, 19, 125–131. [Google Scholar] [CrossRef]
- Molz, F.J.; Klepper, B.; Browning, V.D. Radial Diffusion of Free Energy in Stem Phloem: An Experimental Study 1. Agron. J. 1973, 65, 219–222. [Google Scholar] [CrossRef]
- Goldhamer, D.; Fereres, E.; Salinas, M. Can Almond Trees Directly Dictate their Irrigation Needs? Calif. Agric. 2003, 57, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Naor, A.; Cohen, S. Sensitivity and Variability of Maximum Trunk Shrinkage, Midday Stem Water Potential, and Transpiration Rate in Response to Withholding Irrigation from Field-Grown Apple Trees. HortScience 2003, 38, 547–551. [Google Scholar] [CrossRef] [Green Version]
- Intrigliolo, D.S.; Castel, J.R. Continuous Measurement of Plant and Soil Water Status for Irrigation Scheduling in Plum. Irrig. Sci. 2004, 23, 93–102. [Google Scholar] [CrossRef]
- Badal, E.; Buesa, I.; Guerra, D.; Bonet, L.; Ferrer, P.; Intrigliolo, D.S. Maximum Diurnal Trunk Shrinkage is a Sensitive Indicator of Plant Water, Stress in Diospyros Kaki (Persimmon) Trees. Agric. Water Manag. 2010, 98, 143–147. [Google Scholar] [CrossRef]
- Robinson, T.L.; Lakso, A.N. Bases of Yield and Production Efficiency in Apple Orchard Systems. J. Am. Soc. Hort. Sci. 1991, 116, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, T.C. Plant Responses to Water Stress. Annu. Rev. Plant Physiol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Marsal, J.; Girona, J.; Basile, B.; Dejong, T. Heterogeneity in Fruit Distribution and Stem Water Potential Variations in Peach Trees under Different Irrigation Conditions. J. Hortic. Sci. Biotechnol. 2005, 80, 82–86. [Google Scholar] [CrossRef] [Green Version]
- Palmer, J. Effects of Varying Crop Load on Photosynthesis, Dry Matter Production and Partitioning of Crispin/M. 27 Apple Trees. Tree Physiol. 1992, 11, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Goldhamer, D.A.; Fereres, E.; Mata, M.; Girona, J.; Cohen, M. Sensitivity of Continuous and Discrete Plant and Soil Water Status Monitoring in Peach Trees Subjected to Deficit Irrigation. J. Am. Soc. Hort. Sci. 1999, 124, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Intrigliolo, D.S.; Puerto, H.; Bonet, L.; Alarcón, J.; Nicolas, E.; Bartual, J. Usefulness of Trunk Diameter Variations as Continuous Water Stress Indicators of Pomegranate (Punica Granatum) Trees. Agric. Water Manag. 2011, 98, 1462–1468. [Google Scholar] [CrossRef]
- Tuccio, L.; Piccolo, E.L.; Battelli, R.; Matteoli, S.; Massai, R.; Scalabrelli, G.; Remorini, D. Physiological Indicators to Assess Water Status in Potted Grapevine (Vitis Vinifera L.). Sci. Hortic. 2019, 255, 8–13. [Google Scholar] [CrossRef]
- Atkinson, D. The Distribution and Effectiveness of the Roots of Tree Crops. Hortic. Rev. 1981. [Google Scholar]
- Nnyamah, J.U.; Black, T. Rates and Patterns of Water Uptake in a Douglas-Fir Forest. Soil Sci. Soc. Am. J. 1977, 41, 972–979. [Google Scholar] [CrossRef]
- Clothier, B.E.; Green, S.R. Rootzone Processes and the Efficient Use of Irrigation Water. Agric. Water Manag. 1994, 25, 1–12. [Google Scholar] [CrossRef]
- Thaler, P.; Pagès, L. Periodicity in the Development of the Root System of Young Rubber Trees (Hevea Brasiliensis Müell. Arg.): Relationship with Shoot Development. Plant Cell Environ. 1996, 19, 56–64. [Google Scholar] [CrossRef]
- Lecompte, F.; Pagès, L.; Ozier-Lafontaine, H. Patterns of Variability in the Diameter of Lateral Roots in the Banana Root System. New Phytol. 2005, 167, 841–850. [Google Scholar] [CrossRef]
- Steudle, E.; Frensch, J. Water Transport in Plants: Role of the Apoplast. Plant Soil 1996, 187, 67–79. [Google Scholar] [CrossRef]
- Barrowclough, D.E.; Peterson, C.A.; Steudle, E. Radial Hydraulic Conductivity along Developing Onion Roots. J. Exp. Bot. 2000, 51, 547–557. [Google Scholar] [PubMed] [Green Version]
- Watt, M.; Magee, L.J.; McCully, M.E. Types, Structure and Potential for Axial Water Flow in the Deepest Roots of Field-Grown Cereals. New Phytol. 2008, 178, 135–146. [Google Scholar] [PubMed]
- Draye, X.; Kim, Y.; Lobet, G.; Javaux, M. Model-Assisted Integration of Physiological and Environmental Constraints Affecting the Dynamic and Spatial Patterns of Root Water Uptake from Soils. J. Exp. Bot. 2010, 61, 2145–2155. [Google Scholar] [CrossRef] [Green Version]
- Soulis, K.X.; Elmaloglou, S. Optimum Soil Water Content Sensors Placement in Drip Irrigation Scheduling Systems: Concept of Time Stable Representative Positions. J. Irrig. Drain. Eng. 2016, 142, 04016054. [Google Scholar] [CrossRef]
- Da Silva, A.J.P.; Coelho, E.F.; Filho, M.A.C.; De Souza, J.L. Water Extraction and Implications on Soil Moisture Sensor Placement in the Root Zone of Banana. Sci. Agric. 2018, 75, 95–101. [Google Scholar] [CrossRef] [Green Version]
| 2015 | 2016 | 2017 | |
|---|---|---|---|
| ISD 1 | 14 April 2015 (104) | 19 April 2016 (109) | 05 April 2017 (95) |
| IED 2 | 15 October 2015 (288) | 20 October 2016 (293) | 08 November 2017 (312) |
| Drying-Pre | 29 April 2016–14 June 2016 | 24 April 2017–31 May 2017 | |
| (119–165) | (114–151) | ||
| First Drying-Post | 02 July 2015–27 July 2015 | 15 July 2016–27 July 2016 | 06 July 2017–01 August 2017 |
| (183–208) | (196–208) | (187–213) | |
| Second Drying-Post | 12 August 2016–10 September 2016 | ||
| (224–253) |
| Year | Phases | Tmean | RHmean | ETo-PM | Irrigation (mm) | Rainfall (mm) | ||
|---|---|---|---|---|---|---|---|---|
| (°C) | (%) | (mm) | CON | D | LF-S 4 | S-LF 3 | ||
| Pre 1 | 19.6 | 56 | 276 | 168 | 172 | 158 | 272 | |
| 2015 | Post 2 | 23.1 | 57 | 720 | 534 | 489 | ||
| Annual | 16.5 | 68 | 1310 | 702 | 661 | 370 | ||
| Pre 1 | 17.8 | 68 | 186 | 33 | 4 | 438 | 291 | |
| 2016 | Post 2 | 23.7 | 54 | 730 | 570 | 327 | ||
| Annual | 16.4 | 71 | 1247 | 603 | 331 | 519.5 | ||
| Pre 1 | 19.7 | 56 | 346 | 245 | 66 | 214 | 108 | |
| 2017 | Post 2 | 23.1 | 52 | 805 | 569 | 452 | ||
| Annual | 17.0 | 64 | 1383 | 814 | 519 | 284 | ||
| Parameters 2015 | A (182DOY) | SPost1 (208DOY) | RPost1 (288DOY) | Average | |
|---|---|---|---|---|---|
| SI | 1.38 | 1.53 | 1.19 | 1.37 | |
| Ψstem | CV | 0.18 | 0.19 | 0.15 | 0.17 |
| S | 7.80 | 9.99 | 8.22 | 8.67 | |
| S* | 4.10 | 4.63 | 5.91 | 4.88 | |
| SI | 0.83 | 0.96 | 0.91 | 0.90 | |
| SF | CV | 0.31 | 0.29 | 0.28 | 0.29 |
| S | 2.67 | 3.34 | 3.24 | 3.08 | |
| S* | 3.86 | 3.61 | 3.89 | 3.79 | |
| SI | 0.95 | 1.24 | 1.36 | 1.18 | |
| Fn | CV | 0.25 | 0.29 | 0.39 | 0.31 |
| S | 3.77 | 4.82 | 3.4 | 4.00 | |
| S* | 15.67 | 17.99 | 6.64 | 13.43 | |
| SI | 0.91 | 1.67 | 1.28 | 1.29 | |
| Gs | CV | 0.36 | 0.54 | 0.63 | 0.53 |
| S | 2.50 | 3.31 | 2.72 | 2.84 | |
| S* | 1.10 | 0.63 | 0.62 | 0.78 | |
| SI | 1.04 | 1.02 | 1.00 | 1.02 | |
| Fipard | CV | 1.21 | 1.25 | 1.09 | 1.18 |
| S | 0.86 | 0.81 | 0.94 | 0.87 | |
| S* | 0.96 | 0.98 | 0.99 | 0.98 |
| Parameters 2016 | A (118) | SPre (165) | RPre (195) | SPost1 (208) | RPost1 (210) | SPost2 (253) | RPost2 (293) | Average | |
|---|---|---|---|---|---|---|---|---|---|
| SI | 0.83 | 1.07 | 0.99 | 1.34 | 1.03 | 1.38 | 1.09 | 1.10 | |
| Ψstem | CV | 0.25 | 0.23 | 0.19 | 0.17 | 0.46 | 0.16 | 0.19 | 0.24 |
| S | 3.37 | 5.72 | 5.04 | 7.96 | 2.91 | 14.71 | 9.03 | 6.96 | |
| S* | 4.89 | 4.65 | 5.25 | 4.47 | 2.35 | 8.08 | 7.6 | 5.33 | |
| SI | 0.82 | 0.74 | 0.75 | 0.64 | 0.87 | 0.86 | 0.8 | 0.78 | |
| SF | CV | 0.50 | 0.44 | 0.6 | 0.62 | 0.55 | 0.47 | 0.5 | 0.53 |
| S | 1.64 | 1.69 | 1.24 | 1.03 | 1.59 | 1.88 | 1.61 | 1.53 | |
| S* | 1.28 | 3.29 | 2.23 | 2.49 | 2.15 | 2.56 | 2.53 | 2.36 | |
| SI | 0.98 | 1.06 | 1.04 | 1.03 | 1.07 | 1,00 | 0.94 | 1.02 | |
| Fipard | CV | 0.88 | 0.87 | 0.85 | 0.87 | 0.89 | 0.96 | 1.25 | 0.94 |
| S | 1.11 | 1.21 | 1.22 | 1.19 | 1.19 | 1.05 | 0.79 | 1.11 | |
| S* | 1.02 | 0.95 | 0.96 | 0.97 | 0.94 | 0.99 | 1.07 | 0.99 |
| Parameters 2017 | A (113) | SPre (151) | RPre (186) | SPost1 (213) | RPost1 (312) | Average | |
|---|---|---|---|---|---|---|---|
| SI | 0.98 | 1.24 | 1.12 | 1.68 | 1.13 | 1.23 | |
| Ψstem | CV | 0.23 | 0.17 | 0.16 | 0.18 | 0.16 | 0.18 |
| S | 4.61 | 7.69 | 7.68 | 9.23 | 7.44 | 7.33 | |
| S* | 4.69 | 5.18 | 6.24 | 3.78 | 5.62 | 5.10 | |
| SI | 0.65 | 0.79 | 0.76 | 1.07 | 0.87 | 0.828 | |
| SF | CV | 0.47 | 0.35 | 0.37 | 0.39 | 0.36 | 0.388 |
| S | 1.45 | 2.29 | 2.1 | 2.74 | 2.49 | 2.21 | |
| S* | 3.51 | 3.66 | 3.54 | 2.5 | 3.23 | 3.29 | |
| SI | 0.96 | 1.16 | 0.95 | 1.22 | 1.18 | 1.09 | |
| Fn | CV | 0.41 | 0.32 | 0.26 | 0.23 | 0.32 | 0.31 |
| S | 2.34 | 3.95 | 3.76 | 5.24 | 3.74 | 3.81 | |
| S* | 2.54 | 2.93 | 4.13 | 3.59 | 2.75 | 3.19 | |
| SI | 0.94 | 1.5 | 1.01 | 1.7 | 1.53 | 1.34 | |
| Gs | CV | 0.46 | 0.46 | 0.76 | 0.63 | 0.57 | 0.58 |
| S | 2.06 | 3.34 | 1.45 | 2.88 | 2.69 | 2.48 | |
| S* | 2.29 | 1.8 | 1.42 | 1.49 | 1.35 | 1.67 | |
| SI | 0.99 | 0.97 | 0.97 | 0.91 | 0.96 | ||
| Fipard | CV | 1.07 | 0.9 | 0.8 | 0.83 | 0.90 | |
| S | 0.92 | 1.08 | 1.21 | 1.1 | 1.08 | ||
| S* | 1.01 | 1.04 | 1.03 | 1.09 | 1.04 |
| Parameters | Year | T_SPre (days) | T_RPre (days) | T_SPost1 (days) | T_RPost1 (days) | T_SPost2 (days) | T_RPost2 (days) |
|---|---|---|---|---|---|---|---|
| 2015 | 4 | 21 | |||||
| Ψstem | 2016 | 5 | 8 | 1 | 14 | 5 | 14 |
| 2017 | 8 | 13 | 4 | 83 | |||
| Fn | 2015 | 13 | 21 | ||||
| 2017 | 14 | 2 | 8 | 15 | |||
| Gs | 2015 | 8 | 21 | ||||
| 2017 | 14 | 5 | 8 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millán, S.; Campillo, C.; Vivas, A.; Moñino, M.J.; Prieto, M.H. Evaluation of Soil Water Content Measurements with Capacitance Probes to Support Irrigation Scheduling in a “Red Beaut” Japanese Plum Orchard. Agronomy 2020, 10, 1757. https://doi.org/10.3390/agronomy10111757
Millán S, Campillo C, Vivas A, Moñino MJ, Prieto MH. Evaluation of Soil Water Content Measurements with Capacitance Probes to Support Irrigation Scheduling in a “Red Beaut” Japanese Plum Orchard. Agronomy. 2020; 10(11):1757. https://doi.org/10.3390/agronomy10111757
Chicago/Turabian StyleMillán, Sandra, Carlos Campillo, Antonio Vivas, María José Moñino, and Maria Henar Prieto. 2020. "Evaluation of Soil Water Content Measurements with Capacitance Probes to Support Irrigation Scheduling in a “Red Beaut” Japanese Plum Orchard" Agronomy 10, no. 11: 1757. https://doi.org/10.3390/agronomy10111757
APA StyleMillán, S., Campillo, C., Vivas, A., Moñino, M. J., & Prieto, M. H. (2020). Evaluation of Soil Water Content Measurements with Capacitance Probes to Support Irrigation Scheduling in a “Red Beaut” Japanese Plum Orchard. Agronomy, 10(11), 1757. https://doi.org/10.3390/agronomy10111757







