Morphological, Physiological, and Biochemical Impacts of Different Levels of Long-Term Water Deficit Stress on Linum album Ky. ex Boiss. Accessions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Soil Materials
2.2. Water Stress Treatment
2.3. Determination of Phenological Stages
2.4. Relative Water Content
2.5. Chlorophyll and Carotenoid Contents
2.6. Determination of Proline and Glycine Betaine
2.7. Measurement of Electrolyte Leakage and Malondialdehyde Contents
2.8. Enzymatic Antioxidant Activity
2.9. Abscisic Acid (ABA) Extraction and Quantification
2.10. Statistical Analysis
3. Results
3.1. Morphological Properties
3.2. Phenological Stages
3.3. RWC, Chlorophyll, and Carotenoid Contents
3.4. Proline and Glycine Betaine
3.5. Electrolyte Leakage and Malondialdehyde
3.6. Abscisic Acid
3.7. Enzymatic Antioxidant Activity
3.8. Correlations between Traits
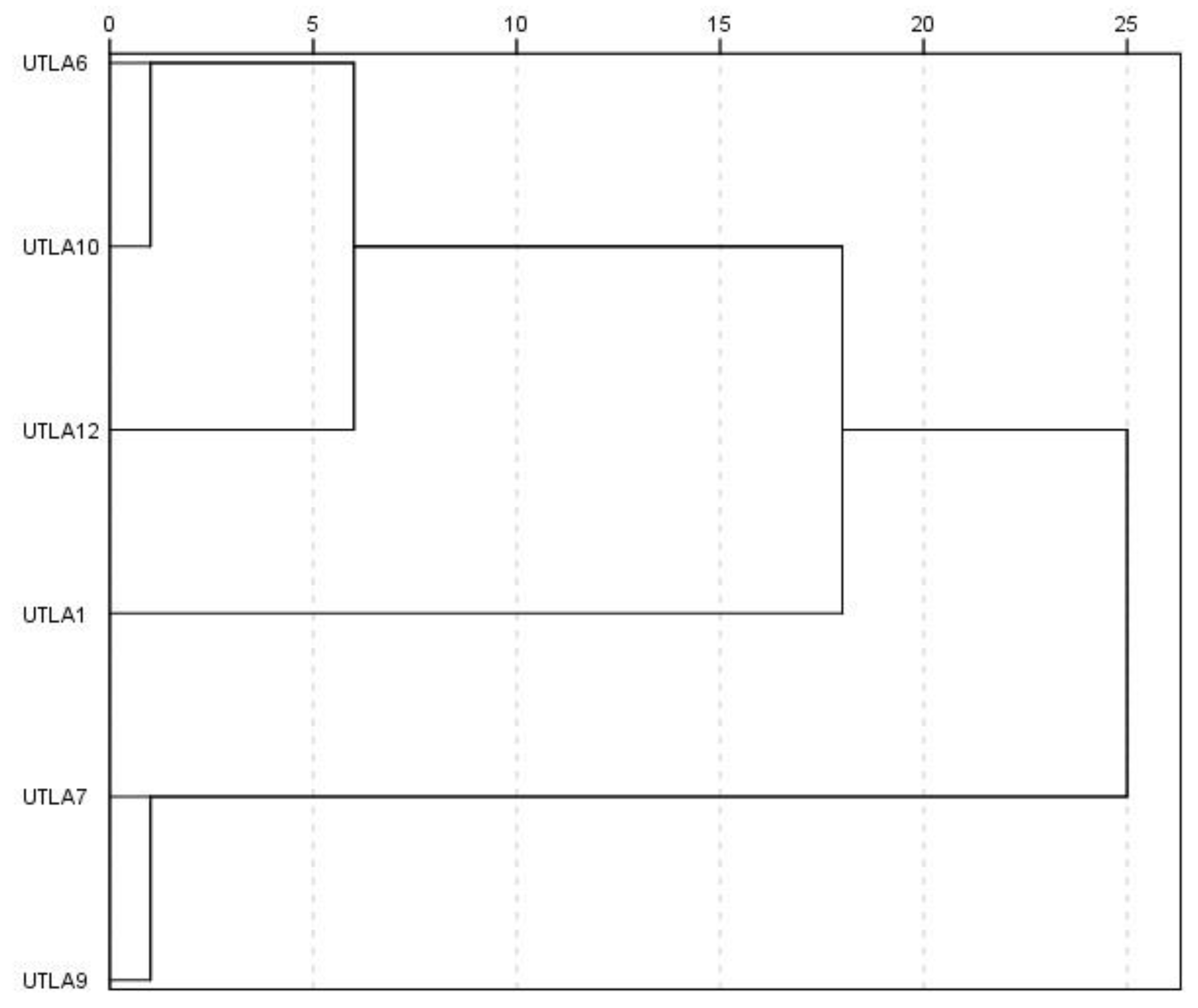
3.9. Cluster Analysis and Factor Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rechinger, K.H. Linaceae. In Flora Iranica; Akademische Druck-u. Verlagsanstalt: Graz, Austria, 1974; Volume 106, pp. 1–20. [Google Scholar]
- Sharifnia, F.; Asadi, M. A phenetic study of the genus Linum L. (Linaceae) in Iran. Iran. J. Bot 2002, 9, 135–139. [Google Scholar]
- Vardapetyan, H.R.; Kirakosyan, A.B.; Oganesyan, A.A.; Penesyan, A.R.; Alfermann, W.A. Effect of various elicitors on lignan biosynthesis in callus cultures of Linum austriacum. Russ. J. Plant Physiol. 2003, 50, 297–300. [Google Scholar] [CrossRef]
- Koulman, A.; Quax, W.J.; Pras, N. Podophyllotoxin and related lignans produced by plants. In Biotechnology of Medicinal Plants: Vitalizer and Therapeutic; Ramawat, K.G., Ed.; Science Publishers: New York, NY, USA, 2004; pp. 225–266. [Google Scholar]
- Mikac, S.; Markulin, L.; Drouet, S.; Corbin, C.; Tungmunnithum, D.; Kiani, R.; Kabra, A.; Abassi, B.H.; Renouard, S.; Fuss, E.; et al. Bioproduction of Anticancer Podophyllotoxin and Related Aryltretralin-Lignans in Hairy Root Cultures of Linum Flavum L. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Ramawat, K., Ekiert, H., Goyal, S., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–38. [Google Scholar]
- Bedir, E.; Khan, I.; Moraes, R.M. Bioprospecting for Podophyllotoxin. In Trends New Crop and New Uses; ASHS Press: Alexandria, VA, USA, 2002; pp. 545–549. [Google Scholar]
- Huynh Cong, L.; Dauwe, R.; Lequart, M.; Vinchon, S.; Renouard, S.; Fliniaux, O.; Colas, C.; Corbin, C.; Doussot, J.; Hano, C.; et al. Kinetics of glucosylated and non-glucosylated aryltetralin lignans in Linum hairy root cultures. Phytochemistry 2015, 115, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Doussot, J.; Mathieu, V.; Colas, C.; Molinie, R.; Corbin, C.; Montguillon, J.; Moreno, Y.; Banuls, L.; Renouard, S.; Lamblin, F.; et al. Investigation of the lignan content in extracts from Linum, Callitris and Juniperus species in relation to their in vitro antiproliferative activities. Planta Med. 2017, 83, 574–581. [Google Scholar] [CrossRef]
- Renouard, S.; Corbin, C.; Drouet, S.; Medvedec, B.; Doussot, J.; Colas, C.; Maunit, B.; Bhambra, A.S.; Gontier, E.; Jullian, N.; et al. Investigation of Linum flavum (L.) hairy root cultures for the production of anticancer aryltetralin lignans. Int. J. Mol. Sci. 2018, 19, 990. [Google Scholar] [CrossRef]
- Malik, S.; Bilba, O.; Gruz, J.; Arroo, R.R.J.; Strnad, M. Biotechnological approaches for producing aryltetralin lignans from Linum species. Phytochem. Rev. 2014, 13, 893–913. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Klaes, M.; Sendker, J. Lignans in seeds of Linum species. Phytochemistry 2012, 82, 89–99. [Google Scholar] [CrossRef]
- Farkya, S.; Bisaria, V.S.; Srivastava, A.K. Biotechnological aspects of the production of the anticancer drug podophyllotoxin. Appl. Microbiol. Biotechnol. 2004, 65, 504–519. [Google Scholar] [CrossRef]
- Nazeri, V.; Kiani, R.; Rezaei, K.; Kalvandi, R. Diversity study of some ecological, morphological and fatty acid profile of Linum album Ky. ex Boiss. Iran. J. Med. Aromat. Plants 2017, 33, 168–183. [Google Scholar]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal; Artmed Editora: Porto Alegre, Brazil, 2017; pp. 731–759. [Google Scholar]
- Tabari, H.; Talaee, P.H. Temporal variability of precipitation over Iran: 1966–2005. J. Hydrol. 2011, 396, 313–320. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, J. Effects of water stress on photosystem II photochemistry and its thermostability in wheat plants. J. Exp. Bot. 1999, 50, 1199–1206. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Guo, K.; Fan, D.; Li, G.; Zheng, Y.; Yu, L.; Yang, R. Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environ. Exp. Bot. 2011, 71, 174–183. [Google Scholar] [CrossRef]
- Tabari, H.; Abghari, H.; Hosseinzadeh Talaee, P. Temporal trends and spatial characteristics of drought and rainfall in arid and semiarid regions of Iran. Hydrol. Process. 2012, 26, 3351–3361. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.B.S.M.A.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Talbi, S.; Romero-Puertas, M.C.; Hernández, A.; Terrón, L.; Ferchichi, A.; Sandalio, L.M. Drought tolerance in a Saharian plant Oudneya africana: Role of antioxidant defences. Environ. Exp. Bot. 2015, 111, 114–126. [Google Scholar] [CrossRef]
- Dietz, K.J.; Mittler, R.; Noctor, G. Recent progress in understanding the role of reactive oxygen species in plant cell signaling. Plant Physiol. 2016, 171, 1535–1539. [Google Scholar] [CrossRef]
- Hall, A.E. Crop Responses to Environment; CRC Press: Boca Raton, FL, USA, 2000; pp. 137–150. [Google Scholar]
- Sikder, S. Accumulated heat unit and phenology of wheat cultivars as influenced by late sowing heat stress condition. J. Agric. Rural Dev. 2009, 7, 59–64. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Fischer, R.A. Crop Temperature Modification and Yield Potential in a Dwarf Spring Wheat 1. Crop Sci. 1976, 16, 855–859. [Google Scholar] [CrossRef]
- Nemeskéri, E.; Neményi, A.; Bőcs, A.; Pék, Z.; Helyes, L. Physiological factors and their relationship with the productivity of processing tomato under different water supplies. Water 2019, 11, 586. [Google Scholar] [CrossRef]
- Bishnoi, O.P.; Singh, S.; Niwas, R. Effect of temperature on phenological development of wheat (Triticum aestivum) crop in different orientation. Indian J. Agric. Sci. 1995, 65, 211–214. [Google Scholar]
- Powell, W. Abiotic Stresses: Plant Resistance through Breeding and Molecular Approaches; Ashraf, M., Harris, P., Eds.; The Haworth Press: Binghamton, NH, USA, 2005; pp. 367–373. [Google Scholar]
- Wollenweber, B.; Porter, J.R.; Schellberg, J. Lack of interaction between extreme high-temperature events at vegetative and reproductive growth stages in wheat. J. Agron. Crop Sci. 2003, 189, 142–150. [Google Scholar] [CrossRef]
- Finkelstein, R. Abscisic acid synthesis and response. Arab. Book Am. Soc. Plant Biol. 2013, 11, e0166. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Kouna, T.; Antonopoulou, C.I.; Therios, I. Exogenous proline induces soluble sugar accumulation and alleviates drought stress effects on photosystem II functioning of Arabidopsis thaliana leaves. Plant Growth Regul. 2011, 65, 315. [Google Scholar] [CrossRef]
- Zaher-Ara, T.; Boroomand, N.; Sadat-Hosseini, M. Physiological and morphological response to drought stress in seedlings of ten citrus. Trees 2016, 30, 985–993. [Google Scholar] [CrossRef]
- Hong, Z.; Lakkineni, K.; Zhang, Z.; Verma, D.P.S. Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 2000, 122, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Deuschle, K.; Funck, D.; Hellmann, H.; Däschner, K.; Binder, S.; Frommer, W.B. A nuclear gene encoding mitochondrial Δ1-pyrroline-5-carboxylate dehydrogenase and its potential role in protection from proline toxicity. Plant J. 2001, 27, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian Chashmi, N.; Sharifi, M.; Behmanesh, M. Lignan enhancement in hairy root cultures of Linum album using coniferaldehyde and methylenedioxycinnamic acid. Prep. Biochem. Biotechnol. 2016, 46, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, A.; Chellappan, K.P. Accumulation of proline and glycine betaine in Ipomoea pes-caprae induced by NaCl. Biol. Plant. 1998, 41, 271–276. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Nitrogen containing compounds and adaptation of plants to salinity stress. Biol. Plant. 2000, 43, 491–500. [Google Scholar] [CrossRef]
- Yang, W.J.; Rich, P.J.; Axtell, J.D.; Wood, K.V.; Bonham, C.C.; Ejeta, G.; Rhodes, D. Genotypic variation for glycinebetaine in sorghum. Crop Sci. 2003, 43, 162–169. [Google Scholar] [CrossRef]
- Quan, R.; Shang, M.; Zhang, H.; Zhao, Y.; Zhang, J. Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebetaine synthesis in maize. Plant Sci. 2004, 166, 141–149. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Wheeler, R.M.; Levine, L.H.; Stutte, G.W. Glycine betaine accumulation, ionic and water relations of red-beet at contrasting levels of sodium supply. J. Plant Physiol. 2001, 158, 767–776. [Google Scholar] [CrossRef]
- Javadian, N.; Karimzadeh, G.; Sharifi, M.; Moieni, A.; Behmanesh, M. In vitro polyploidy induction: Changes in morphology, podophyllotoxin biosynthesis, and expression of the related genes in Linum album (Linaceae). Planta 2017, 245, 1165–1178. [Google Scholar] [CrossRef]
- Tashackori, H.; Sharifi, M.; Chashmi, N.A.; Safaie, N.; Behmanesh, M. Induced-differential changes on lignan and phenolic acid compounds in Linum album hairy roots by fungal extract of Piriformospora indica. Plant Cell Tissue Organ Cult. 2016, 127, 187–194. [Google Scholar] [CrossRef]
- Kiani, R. Evaluation of Morphological and Phytochemical Diversity of Linum album Ky. ex Bioss., an Endemic Medicinal Plant in Iran. Master’s Thesis, Department of Horticultural Sciences College of Agriculture and Natural Resources, University of Tehran, Tehran, Iran, 2016. [Google Scholar]
- Miller, P.; Lanier, W.; Brandt, S. Using Growing Degree Days to Predict Plant Stages; Ag/Extension Communications Coordinator, Communications Services, Montana State University-Bozeman: Bozeman, MO, USA, 2001; Volume 59717, pp. 994–2721. [Google Scholar]
- Turner, N.C. Crop water deficits: A decade of progress. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 1986; Volume 39, pp. 1–51. [Google Scholar]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 2231, 606X. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Nayyar, H.; Walia, D.P. Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Biol. Plant. 2003, 46, 275–279. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hadwan, M.H. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem. 2018, 19, 7. [Google Scholar] [CrossRef]
- Plewa, M.J.; Smith, S.R.; Wagner, E.D. Diethyldithiocarbamate suppresses the plant activation of aromatic amines into mutagens by inhibiting tobacco cell peroxidase. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 1991, 247, 57–64. [Google Scholar] [CrossRef]
- Ranieri, A.; D’urso, G.; Nali, C.; Lorenzini, G.; Soldatini, G.F. Ozone stimulates apoplastic antioxidant systems in pumpkin leaves. Physiol. Plant. 1996, 97, 381–387. [Google Scholar] [CrossRef]
- Kiani, R.; Nazeri, V.; Kalvandi, R. Investigation of genetic diversity in some populations of flax (Linum album Ky. ex Bioss.) using morphological traits. In Proceedings of the 4th National Congress on Medicinal Plants, Tehran, Iran, 12–13 May 2015. [Google Scholar]
- Kiani, R.; Nazeri, V.; Rezaei, K.; Kalvandi, R. Evaluation of morphological diversity in Linum album Ky. ex Boiss. populations in Karaj conditions. In Proceedings of the 11th Iranian Horticultural Science, Urmia, Iran, 26 August 2019. [Google Scholar]
- Plaut, Z. Plant exposure to water stress during specific growth stages. In Encyclopedia of Water Science; Stewart, B.A., Howell, T., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 673–685. [Google Scholar]
- Schuppler, U.; He, P.H.; John, P.C.; Munns, R. Effect of water stress on cell division and Cdc2-like cell cycle kinase activity in wheat leaves. Plant Physiol. 1998, 117, 667–678. [Google Scholar] [CrossRef]
- Kavar, T.; Maras, M.; Kidrič, M.; Šuštar-Vozlič, J.; Meglič, V. Identification of genes involved in the response of leaves of Phaseolus vulgaris to drought stress. Mol. Breed. 2008, 21, 159–172. [Google Scholar] [CrossRef]
- Ahmadi, A.; Baker, D.A. The effect of water stress on the activities of key regulatory enzymes of the sucrose to starch pathway in wheat. Plant Growth Regul. 2001, 35, 81–91. [Google Scholar] [CrossRef]
- Morgan, P.W. Effects of abiotic stresses on plant hormone systems. In Stress Responses in Plants: Adaptation and Acclimation Mechanisms; Alscher, R.G., Cumming, J.R., Eds.; Wiley-Liss, Inc.: New York, NY, USA, 1991; pp. 113–146. [Google Scholar]
- Ober, E.S.; Setter, T.L.; Madison, J.T.; Thompson, J.F.; Shapiro, P.S. Influence of water deficit on maize endosperm development: Enzyme activities and RNA transcripts of starch and zein synthesis, abscisic acid, and cell division. Plant Physiol. 1991, 97, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Sionit, N.; Kramer, P.J. Effect of water stress during different stages of growth of soybean. Agron. J. 1977, 69, 274–278. [Google Scholar] [CrossRef]
- Estrada-Campuzano, G.; Miralles, D.J.; Slafer, G.A. Genotypic variability and response to water stress of pre-and post-anthesis phases in triticale. Eur. J. Agron. 2008, 28, 171–177. [Google Scholar] [CrossRef]
- Uzilday, B.; Turkan, I.; Sekmen, A.H.; Ozgur, R.E.N.G.İ.N.; Karakaya, H.C. Comparison of ROS formation and antioxidant enzymes in Cleome gynandra (C4) and Cleome spinosa (C3) under drought stress. Plant Sci. 2012, 182, 59–70. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Arjenaki, F.G.; Jabbari, R.; Morshedi, A. Evaluation of drought stress on relative water content, chlorophyll content and mineral elements of wheat (Triticum aestivum L.) varieties. Int. J. Agric. Crop Sci. 2012, 4, 726–729. [Google Scholar]
- Guo, R.; Hao, W.; Gong, D. Effects of water stress on germination and growth of linseed seedlings (Linum usitatissimum L), photosynthetic efficiency and accumulation of metabolites. J. Agric. Sci. 2012, 4, 253. [Google Scholar] [CrossRef]
- Talebi, R.; Ensafi, M.H.; Baghebani, N.; Karami, E.; Mohammadi, K. Physiological responses of chickpea (Cicer arietinum) genotypes to drought stress. Environ. Exp. Biol. 2013, 11, 9–15. [Google Scholar]
- Pirzad, A.; Shakiba, M.R.; Zehtab-Salmasi, S.; Mohammadi, S.A.; Darvishzadeh, R.; Samadi, A. Effect of water stress on leaf relative water content, chlorophyll, proline and soluble carbohydrates in Matricaria chamomilla L. J. Med. Plants Res. 2011, 5, 2483–2488. [Google Scholar]
- Anjum, F.; Yaseen, M.; Rasul, E.; Wahid, A.; Anjum, S. Water stress in barley (Hordeum vulgare L.). II. Effect on chemical composition and chlorophyll contents. Pak. J. Agric. Sci. 2003, 40, 45–49. [Google Scholar]
- Monakhova, O.F.; Chernyad’ev, I.I. Protective role of kartolin-4 in wheat plants exposed to soil draught. Appl. Biochem. Microbiol. 2002, 38, 373–380. [Google Scholar] [CrossRef]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Wahid, A.; Close, T.J. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol. Plant. 2007, 51, 104–109. [Google Scholar] [CrossRef]
- Askari, E.; Ehsanzadeh, P. Osmoregulation-mediated differential responses of field-grown fennel genotypes to drought. Ind. Crop. Prod. 2015, 76, 494–508. [Google Scholar] [CrossRef]
- Dhar, P.; Ojha, D.; Kar, C.S.; Mitra, J. Differential response of tossa jute (Corchorus olitorius) submitted to water deficit stress. Ind. Crop. Prod. 2018, 112, 141–150. [Google Scholar] [CrossRef]
- Zarrinabadi, I.G.; Razmjoo, J.; Mashhadi, A.A.; Boroomand, A. Physiological response and productivity of pot marigold (Calendula officinalis) genotypes under water deficit. Ind. Crop. Prod. 2019, 139, 111488. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B.J.B.P. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Fallon, K.M.; Phillips, R. Responses to water stress in adapted and unadapted carrot cell suspension cultures. J. Exp. Bot. 1989, 40, 681–687. [Google Scholar] [CrossRef]
- McCue, K.F.; Hanson, A.D. Drought and salt tolerance: Towards understanding and application. Trends Biotechnol. 1990, 8, 358–362. [Google Scholar] [CrossRef]
- Rhodes, D.; Hanson, A.D. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant Biol. 1993, 44, 357–384. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar] [PubMed]
- Masia, A. Physiological effects of oxidative stress in relation to ethylene in postharvest produce. In Postharvest Oxidative Stress in Horticultural Crops; Food Products Press: New York, NY, USA, 2003; pp. 165–197. [Google Scholar]
- Xu, Z.Z.; Zhou, G.S.; Wang, Y.L.; Han, G.X.; Li, Y.J. Changes in chlorophyll fluorescence in maize plants with imposed rapid dehydration at different leaf ages. J. Plant Growth Regul. 2008, 27, 83–92. [Google Scholar] [CrossRef]
- Khoshro, H.H.; Taleei, A.; Bihamta, M.R.; Shahbazi, M.; Abbasi, A. Expression analysis of the genes involved in osmotic adjustment in bread wheat (Triticum aestivum L.) cultivars under terminal drought stress conditions. J. Crop Sci. Biotechnol. 2013, 16, 173–181. [Google Scholar] [CrossRef]
- Shanjani, P.S.; Izadpanah, M.; Mohamadpour, M.R. Effects of water stress on germination of yarrow populations (Achillea spp.) from different bioclimatic zones in Iran. Plant Breed. Seed Sci. 2014, 68, 39. [Google Scholar] [CrossRef]
- Dhanda, S.S.; Sethi, G.S.; Behl, R.K. Indices of drought tolerance in wheat genotypes at early stages of plant growth. J. Agron. Crop Sci. 2004, 190, 6–12. [Google Scholar] [CrossRef]
- Yousfi, N.; Slama, I.; Ghnaya, T.; Savoure, A.; Abdelly, C. Effects of water deficit stress on growth, water relations and osmolyte accumulation in Medicago truncatula and M. laciniata populations. Comptes Rendus Biol. 2010, 333, 205–213. [Google Scholar] [CrossRef]
- Davies, W.J.; Jones, H.G. Physiology and Biochemistry of Abscisic Acid. J. Exp. Bot. 1991, 42, 7–17. [Google Scholar] [CrossRef]
- Sharp, R.E.; Wu, Y.; Voetberg, G.S.; Saab, I.N.; LeNoble, M.E. Confirmation that abscisic acid accumulation is required for maize primary root elongation at low water potentials. J. Exp. Bot. 1994, 45, 1743–1751. [Google Scholar] [CrossRef]
- Turner, N.C.; Wright, G.C.; Siddique, K.H.M. Adaptation of grain legumes (pulses) to water-limited environments. Adv. Agron. 2001, 71, 194–233. [Google Scholar]
- Abedi, T.; Pakniyat, H. Antioxidant enzymes changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.). Czech J. Genet. Plant Breed. 2010, 46, 27–34. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016, 39. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.A.D.; Suzuki, N.; Ciftci-Yilmaz, S.U.L.T.A.N.; Mittler, R.O.N. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Tunc-Ozdemir, M.; Miller, G.; Song, L.; Kim, J.; Sodek, A.; Koussevitzky, S.; Shintani, D. Thiamin confers enhanced tolerance to oxidative stress in Arabidopsis. Plant Physiol. 2009, 151, 421–432. [Google Scholar] [CrossRef]
- Orvar, B.L.; Ellis, B.E. Transgenic tobacco plants expressing antisense RNA for cytosolic ascorbate peroxidase show increased susceptibility to ozone injury. Plant J. 1997, 11, 1297–1305. [Google Scholar] [CrossRef]
- Shigeoka, S.; Ishikawa, T.; Tamoi, M.; Miyagawa, Y.; Takeda, T.; Yabuta, Y.; Yoshimura, K. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 2002, 53, 1305–1319. [Google Scholar] [CrossRef]
- Rahimi, Y.; Taleei, A.; Ranjbar, M. Long-term water deficit modulates antioxidant capacity of peppermint (Mentha piperita L.). Sci. Hortic. 2018, 237, 36–43. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Salami, S.A.; Nazeri, V.; Craker, L.E. Prolonged Water Stress on Growth and Constituency of Iranian of Oregano (Origanum vulgare L.). J. Med. Act. Plants 2017, 5, 7–19. [Google Scholar]



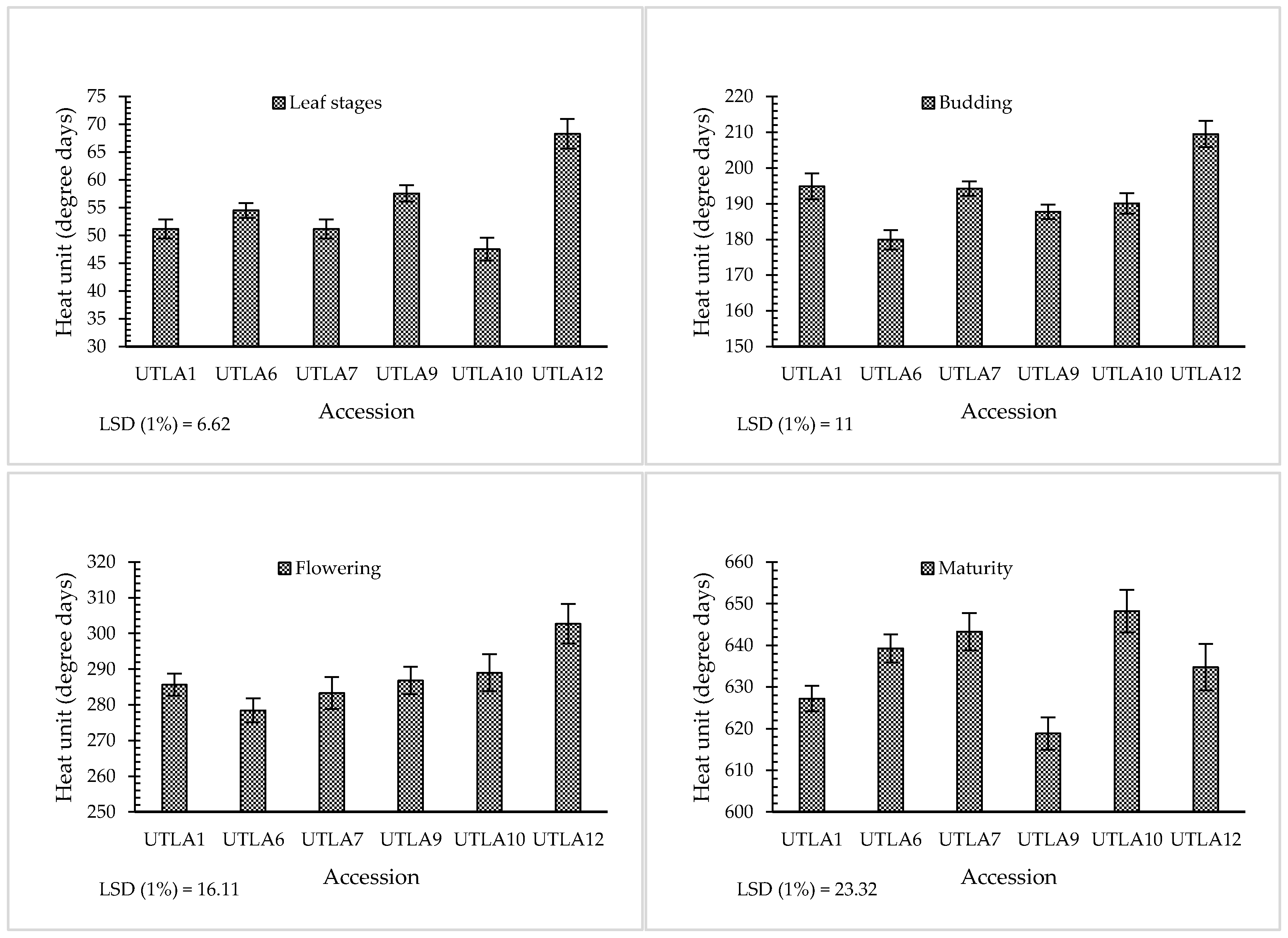
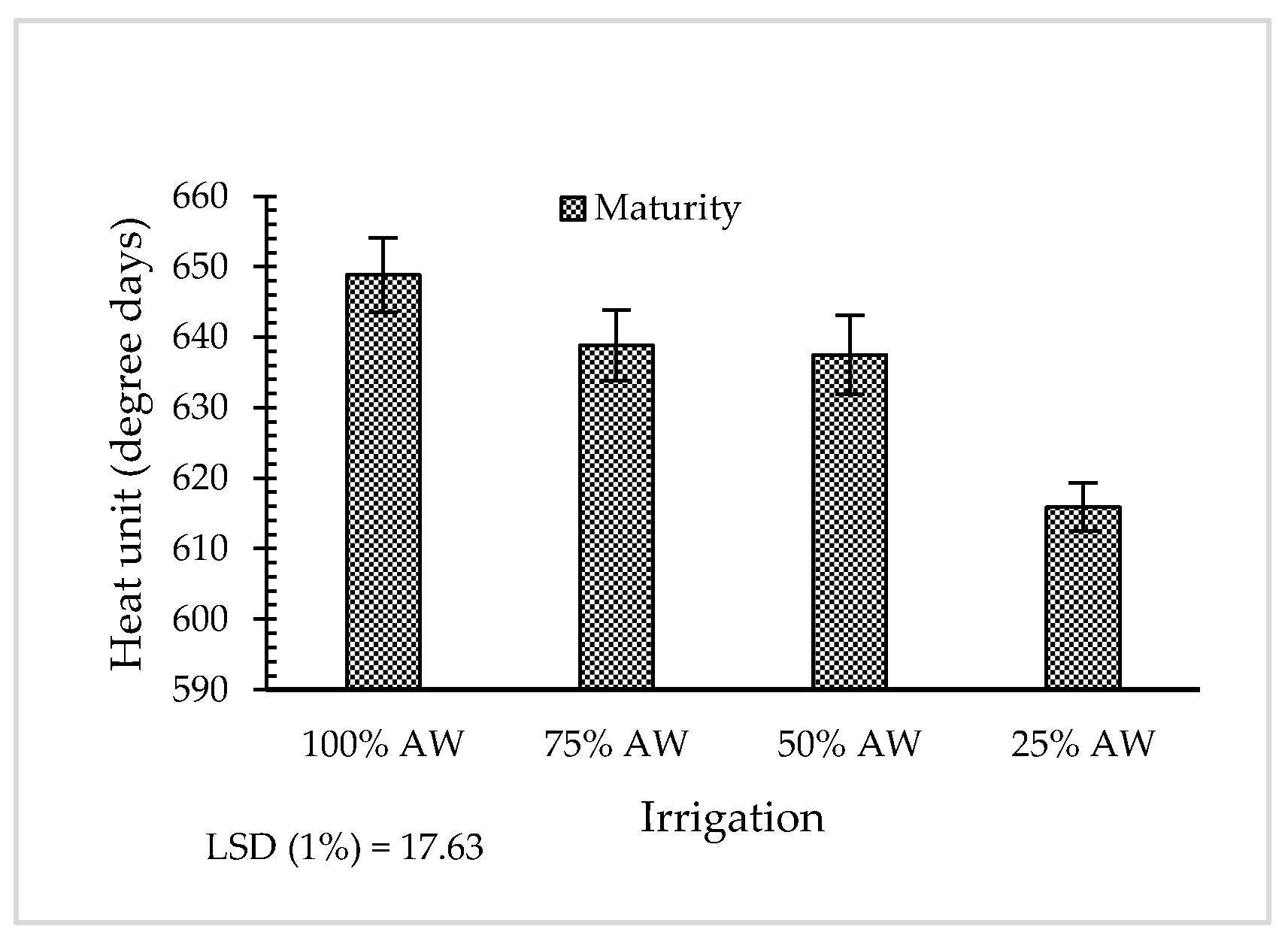
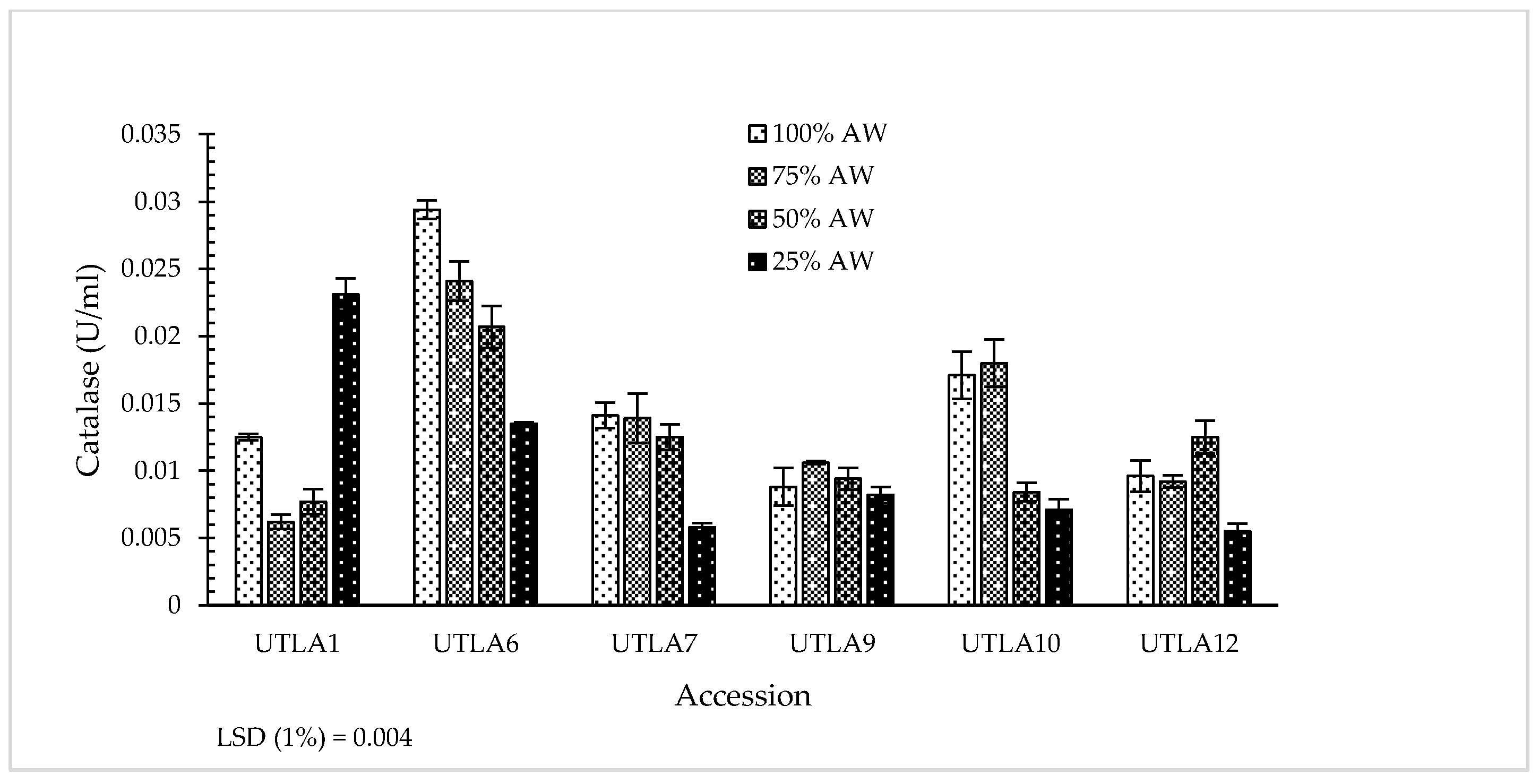
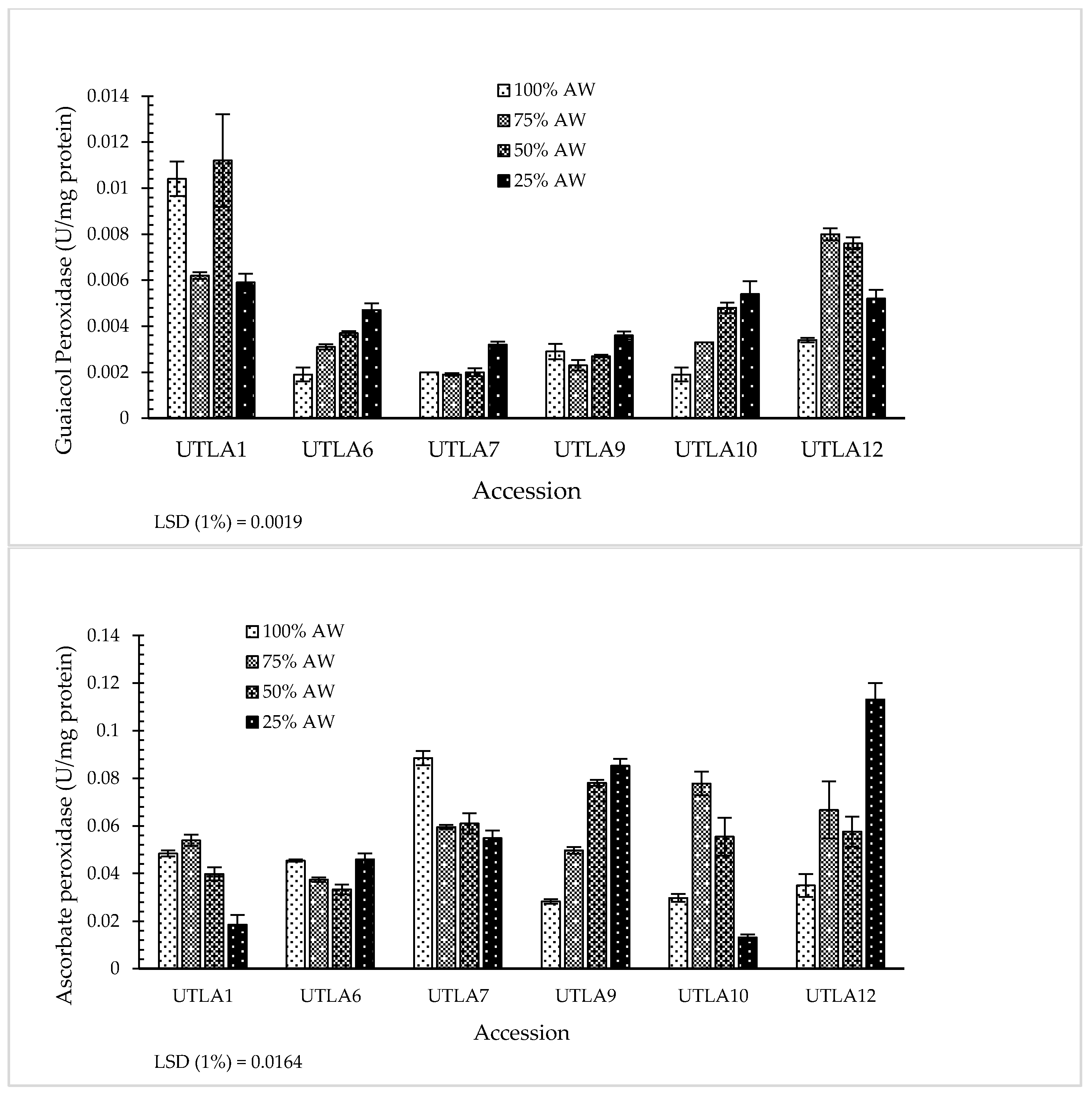

| Accession Code | Latitude (N) | Longitude (E) | Altitude (m) | Voucher Number a |
|---|---|---|---|---|
| UTLA1 | 34°13′56″ | 48°57′25″ | 1904 | 6426 |
| UTLA6 | 34°55′50″ | 48°11′34″ | 2176 | 6425 |
| UTLA7 | 34°41′12″ | 48°38′02″ | 2124 | 6430 |
| UTLA9 | 34°46′11″ | 48°43′17″ | 1955 | 6427 |
| UTLA10 | 34°22′45″ | 48°40′02″ | 1721 | 6428 |
| UTLA12 | 32°54′11″ | 50°4′39″ | 2630 | - |
| pH | EC (Ds/m) | Silt (%) | Clay (%) | Sand (%) | Soil CLASS | FC (%) | PWP (%) | OC (%) | Total N (%) | Usable K (mg/kg) | Usable P (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 8.1 | 2.8 | 21 | 11 | 68 | Sandy loam | 24.98 | 12.52 | 3.18 | 0.25 | 397 | 47.6 |
| Accession/Irrigation | Number of Seeds per Capsule | Seed Yield per Plant (g) | Seed Width (mm) | Weight of 1000 Seeds (g) | Number of Flowers in Inflorescence | Number of Mature Capsules | Inflorescence Length (cm) | Length of the Main Branch (cm) | Plant Height (cm) |
|---|---|---|---|---|---|---|---|---|---|
| UTLA1 | |||||||||
| 100% AW | 8.7 ± 0.15 | 1.86 ± 0.03 | 2.35 ± 0.03 | 3.12 ± 0.1 | 7.33 ± 0.51 | 6.76 ± 0.57 | 13.24 ± 0.38 | 14.83 ± 0.44 | 27.5 ± 0.76 |
| 75% AW | 8.31 ± 0.18 | 0.61 ± 0.04 | 2.29 ± 0.06 | 3.03 ± 0.14 | 11.67 ± 0.67 | 11 ± 1 | 13.33 ± 1.2 | 13.33 ± 0.67 | 23.83 ± 0.6 |
| 50% AW | 8.5 ± 0.69 | 0.6 ± 0.06 | 2.25 ± 0.03 | 3.12 ± 0.16 | 10.08 ± 1.08 | 9.42 ± 1.39 | 15.11 ± 0.49 | 11 ± 0.58 | 24.67 ± 2.19 |
| 25% AW | 7.56 ± 0.34 | 0.71 ± 0.06 | 2.31 ± 0.01 | 3.36 ± 0.16 | 8.08 ± 0.65 | 6.25 ± 0.8 | 17.75 ± 0.43 | 13.5 ± 1.04 | 25 ± 0.5 |
| UTLA6 | |||||||||
| 100% AW | 7.55 ± 0.62 | 1.34 ± 0.16 | 2.43 ± 0.06 | 3.42 ± 0.16 | 11.39 ± 1.52 | 10.56 ± 1.44 | 15.37 ± 1.81 | 15.5 ± 1.32 | 29.33 ± 1.45 |
| 75% AW | 7.92 ± 0.52 | 0.96 ± 0.09 | 2.36 ± 0.04 | 3.51 ± 0.12 | 9.33 ± 0.95 | 8.83 ± 0.98 | 17.49 ± 1.5 | 17 ± 1.04 | 34.67 ± 0.73 |
| 50% AW | 7.04 ± 0.61 | 1.01 ± 0.1 | 2.48 ± 0.06 | 3.87 ± 0.13 | 12.18 ± 0.84 | 11.16 ± 0.63 | 15.48 ± 1.58 | 14.67 ± 0.73 | 30.17 ± 1.64 |
| 25% AW | 9.06 ± 0.48 | 1.33 ± 0.09 | 2.55 ± 0.01 | 3.7 ± 0.12 | 9.94 ± 1.06 | 8.44 ± 0.29 | 12.59 ± 0.6 | 15.83 ± 0.6 | 27.83 ± 1.3 |
| UTLA7 | |||||||||
| 100% AW | 8.49 ± 0.18 | 1.47 ± 0.15 | 2.46 ± 0.02 | 3.57 ± 0.15 | 8.94 ± 0.78 | 8.49 ± 0.7 | 16.45 ± 0.78 | 17.33 ± 0.6 | 31.5 ± 1.53 |
| 75% AW | 9.23 ± 0.31 | 2.79 ± 0.08 | 2.4 ± 0.07 | 3.43 ± 0.16 | 8.78 ± 0.91 | 7.89 ± 1.02 | 18.78 ± 0.94 | 21 ± 0.58 | 36.17 ± 0.73 |
| 50% AW | 8.59 ± 0.33 | 1.24 ± 0.13 | 2.48 ± 0.06 | 3.66 ± 0.08 | 8.87 ± 0.38 | 7.88 ± 0.06 | 15 ± 0.86 | 19.33 ± 1.09 | 31.83 ± 1.17 |
| 25% AW | 7.44 ± 0.06 | 0.55 ± 0.07 | 2.49 ± 0.07 | 3.39 ± 0.14 | 7.21 ± 0.74 | 6.07 ± 0.97 | 12.88 ± 0.74 | 17.67 ± 0.17 | 28.33 ± 0.33 |
| UTLA9 | |||||||||
| 100% AW | 8.96 ± 0.13 | 1.43 ± 0.13 | 2.31 ± 0.05 | 3.38 ± 0.12 | 12.81 ± 0.78 | 11.72 ± 0.64 | 23.25 ± 0.59 | 17.33 ± 0.88 | 36.5 ± 1.32 |
| 75% AW | 8.74 ± 0.41 | 2.27 ± 0.18 | 2.54 ± 0.07 | 3.84 ± 0.11 | 10.74 ± 0.9 | 10.3 ± 0.71 | 21.66 ± 1.28 | 18.5 ± 0.29 | 36.17 ± 1.88 |
| 50% AW | 6.92 ± 0.36 | 1.16 ± 0.14 | 2.42 ± 0.09 | 3.71 ± 0.04 | 10.92 ± 0.84 | 9.19 ± 0.91 | 19.87 ± 0.13 | 16.5 ± 1.04 | 34.67 ± 0.67 |
| 25% AW | 8.96 ± 0.14 | 1.91 ± 0.02 | 2.18 ± 0.05 | 2.98 ± 0.06 | 8.56 ± 0.29 | 6.73 ± 0.91 | 15.67 ± 1.18 | 14 ± 0.5 | 30.5 ± 1.76 |
| UTLA10 | |||||||||
| 100% AW | 8 ± 0.19 | 1.19 ± 0.08 | 2.37 ± 0.06 | 3.67 ± 0.04 | 9 ± 0.96 | 8.89 ± 0.87 | 17.2 ± 0.31 | 18.17 ± 0.88 | 31.5 ± 1 |
| 75% AW | 8.83 ± 0.33 | 1.32 ± 0.05 | 2.46 ± 0.08 | 3.67 ± 0.08 | 10.97 ± 0.51 | 9.72 ± 0.36 | 17.63 ± 0.59 | 18.83 ± 0.93 | 33.17 ± 0.93 |
| 50% AW | 8.44 ± 0.48 | 1.31 ± 0.13 | 2.39 ± 0.04 | 3.49 ± 0.09 | 8.78 ± 1.61 | 7.69 ± 0.54 | 14 ± 0.51 | 20.43 ± 0.74 | 30.17 ± 1.48 |
| 25% AW | 9.05 ± 0.22 | 1.48 ± 0.06 | 2.42 ± 0.01 | 3.86 ± 0.16 | 7.78 ± 0.22 | 6.41 ± 0.13 | 14.72 ± 1.07 | 17.83 ± 0.44 | 29.67 ± 1.3 |
| UTLA12 | |||||||||
| 100% AW | 8.39 ± 0.29 | 1.24 ± 0.1 | 2.39 ± 0.04 | 3.81 ± 0.15 | 9.58 ± 1.05 | 9.4 ± 0.97 | 18.97 ± 0.8 | 19.67 ± 0.88 | 36.17 ± 0.73 |
| 75% AW | 8.72 ± 0.24 | 1.38 ± 0.29 | 2.38 ± 0.07 | 3.93 ± 0.13 | 10.22 ± 0.67 | 9.44 ± 0.73 | 14.17 ± 0.73 | 13.33 ± 0.6 | 22.17 ± 1.01 |
| 50% AW | 8.86 ± 0.56 | 0.45 ± 0.06 | 2.46 ± 0.08 | 3.91 ± 0.08 | 7.42 ± 0.3 | 6.83 ± 0.17 | 16.92 ± 0.92 | 15.33 ± 0.88 | 28 ± 1.53 |
| 25% AW | 8.34 ± 0.6 | 0.59 ± 0.07 | 2.41 ± 0.07 | 3.68 ± 0.07 | 7.02 ± 0.39 | 6.11 ± 0.36 | 13.88 ± 0.51 | 15.83 ± 0.6 | 25.83 ± 1.42 |
| LSD 1% | 1.5 | 0.44 | 0.2 | 0.47 | 3.05 | 2.95 | 2.68 | 2.96 | 4.67 |
| UTLA1 | |||||||||
| 100% AW | 16.07 ± 1.27 | 5.33 ± 0.33 | 1.44 ± 0.29 | 34 ± 0.58 | 26.6 ± 0.81 | 21.85 ± 1.47 | 5.92 ± 0.42 | 24.11 ± 3.2 | 3.44 ± 0.32 |
| 75% AW | 19.08 ± 0.58 | 3 ± 0.58 | 1.56 ± 0.06 | 32.33 ± 0.67 | 23.5 ± 0.87 | 27.4 ± 0.35 | 7.68 ± 0.19 | 46.05 ± 2.42 | 5.19 ± 0.67 |
| 50% AW | 20.07 ± 0.23 | 2.33 ± 0.33 | 2.67 ± 0.17 | 34.33 ± 0.6 | 27.5 ± 0.87 | 17.38 ± 0.36 | 4.46 ± 0.86 | 21.17 ± 4.2 | 2.32 ± 0.62 |
| 25% AW | 19.98 ± 0.59 | 2 ± 0.58 | 4.22 ± 0.4 | 30.17 ± 0.44 | 32 ± 0.58 | 10 ± 1.15 | 3.7 ± 0.17 | 20.61 ± 0.8 | 3.05 ± 0.03 |
| UTLA6 | |||||||||
| 100% AW | 18.45 ± 1.12 | 5.83 ± 0.44 | 1.39 ± 0.46 | 32.78 ± 0.62 | 25 ± 0.58 | 28.19 ± 0.73 | 10.41 ± 0.43 | 56.9 ± 5.12 | 6.33 ± 0.88 |
| 75% AW | 21.97 ± 0.38 | 4.67 ± 0.88 | 1.22 ± 0.22 | 34.56 ± 0.68 | 27.05 ± 1.18 | 39.95 ± 1.7 | 14.05 ± 1.18 | 48.35 ± 5.98 | 8.1 ± 0.64 |
| 50% AW | 24.33 ± 0.45 | 4 ± 0.58 | 3 ± 0.58 | 31 ± 0.76 | 23.55 ± 0.89 | 26.6 ± 0.58 | 9.62 ± 0.07 | 48.06 ± 6.35 | 8.37 ± 0.21 |
| 25% AW | 21.07 ± 0.52 | 3.67 ± 0.88 | 5.28 ± 0.31 | 33.45 ± 0.48 | 26.57 ± 1.26 | 18.7 ± 0.81 | 7.78 ± 0.05 | 38 ± 4.09 | 5.93 ± 0.16 |
| UTLA7 | |||||||||
| 100% AW | 21.19 ± 0.74 | 6.33 ± 0.67 | 1.11 ± 0.4 | 36.87 ± 1.04 | 30.05 ± 1.18 | 56.8 ± 1.27 | 19.44 ± 1 | 62.47 ± 10.91 | 10.56 ± 1.17 |
| 75% AW | 23.78 ± 1.01 | 6.5 ± 0.5 | 1.78 ± 0.22 | 34.11 ± 0.67 | 29 ± 2 | 32.33 ± 1.59 | 13.63 ± 0.89 | 87.05 ± 26.03 | 9.36 ± 2.6 |
| 50% AW | 22.08 ± 1.29 | 4.67 ± 0.67 | 3.56 ± 0.29 | 33.17 ± 1.09 | 24.88 ± 1.07 | 32.8 ± 2.19 | 10.45 ± 0.2 | 56.66 ± 0.81 | 7.91 ± 1.12 |
| 25% AW | 21.36 ± 0.5 | 3.83 ± 0.73 | 4.67 ± 0.38 | 32.72 ± 1.07 | 35.15 ± 0.61 | 23.2 ± 1.33 | 7.85 ± 0.78 | 28.8 ± 0.8 | 5.4 ± 0.29 |
| UTLA9 | |||||||||
| 100% AW | 22.32 ± 0.22 | 4.5 ± 0.29 | 1.11 ± 0.11 | 41 ± 0.76 | 33.5 ± 1.44 | 42.03 ± 1.6 | 15.47 ± 1.63 | 65.69 ± 5.31 | 9.03 ± 0.56 |
| 75% AW | 20.9 ± 0.57 | 7.67 ± 0.33 | 1.89 ± 0.11 | 36.08 ± 1.23 | 28.05 ± 0.66 | 44.15 ± 0.66 | 15.91 ± 0.17 | 68.84 ± 5.45 | 11.26 ± 0.86 |
| 50% AW | 20.67 ± 1.15 | 4.67 ± 0.33 | 2.66 ± 0.33 | 38.07 ± 0.95 | 26.55 ± 1.82 | 19.15 ± 2.17 | 6.51 ± 1.03 | 27.23 ± 0.77 | 3.46 ± 0.31 |
| 25% AW | 20.57 ± 1.16 | 4.67 ± 0.33 | 3.78 ± 0.4 | 32.61 ± 0.81 | 30.88 ± 1.77 | 19.6 ± 2.66 | 6.45 ± 0.72 | 27.57 ± 7.34 | 3.79 ± 0.79 |
| UTLA10 | |||||||||
| 100% AW | 22.37 ± 0.9 | 3.67 ± 0.67 | 1.22 ± 0.4 | 32.72 ± 0.64 | 32.5 ± 0.87 | 43.76 ± 1.25 | 15.02 ± 1.58 | 91.24 ± 19.24 | 11.02 ± 1.63 |
| 75% AW | 23.48 ± 0.67 | 6.33 ± 0.67 | 1.39 ± 0.06 | 30.43 ± 0.74 | 31 ± 1.15 | 37.28 ± 0.85 | 10.86 ± 0.42 | 56.65 ± 0.4 | 8.02 ± 0.09 |
| 50% AW | 20 ± 0.75 | 5 ± 0.58 | 2.67 ± 0.44 | 35.28 ± 0.55 | 27.25 ± 0.43 | 14.55 ± 0.32 | 4.24 ± 0.14 | 28.45 ± 1.47 | 3.78 ± 0.15 |
| 25% AW | 24.77 ± 1.13 | 5.67 ± 0.67 | 4.17 ± 0.25 | 34.83 ± 0.88 | 25.75 ± 1.24 | 28.62 ± 2.08 | 9.9 ± 0.44 | 58.89 ± 6.21 | 8.92 ± 0.72 |
| UTLA12 | |||||||||
| 100% AW | 20.98 ± 0.28 | 4.67 ± 0.67 | 3.11 ± 0.49 | 34.5 ± 1.04 | 27.1 ± 0.52 | 25.78 ± 0.85 | 9.9 ± 0.76 | 38.02 ± 2.21 | 4.15 ± 0.24 |
| 75% AW | 21.68 ± 0.41 | 4.33 ± 0.67 | 3.22 ± 0.28 | 33.39 ± 0.56 | 26 ± 0.58 | 13.2 ± 0.64 | 4.66 ± 0.32 | 35.93 ± 3.89 | 4.53 ± 0.48 |
| 50% AW | 21.83 ± 0.93 | 1.67 ± 0.33 | 2.78 ± 0.29 | 31.5 ± 0.76 | 31.2 ± 0.69 | 13.65 ± 0.95 | 5.13 ± 0.38 | 37.85 ± 3.38 | 4.25 ± 0.37 |
| 25% AW | 22.97 ± 0.88 | 2.67 ± 0.33 | 4 ± 0.51 | 33.55 ± 0.78 | 28.55 ± 0.32 | 30.72 ± 1.36 | 11.31 ± 0.25 | 51.37 ± 1.11 | 6.62 ± 0.73 |
| LSD (1%) | 3.12 | 2.12 | 1.29 | 3.07 | 4.08 | 4.51 | 2.81 | 30.58 | 3.18 |
| Accession/Irrigation | RWC (%) | Chlorophyll-a (µg/mL) | Chlorophyll-b (µg/mL) | Carotenoids (µg/mL) | Proline (μmol/g FW) | Glycine Betaine (μmol/g DW) | Electrolyte Leakage (%) | MDA (μmol/g FW) | ABA (pmol/g DW) |
|---|---|---|---|---|---|---|---|---|---|
| UTLA1 | |||||||||
| 100% AW | 77.05 ± 1.98 | 19.3 ± 0.6 | 7.22 ± 0.25 | 5.45 ± 0.15 | 2.64 ± 0.04 | 228.75 ± 7.27 | 55.55 ± 3.71 | 0.102 ± 0.023 | 150.89 ± 3.45 |
| 75% AW | 80.74 ± 0.91 | 16.47 ± 1.48 | 5.51 ± 0.53 | 4.99 ± 0.35 | 5.62 ± 0.3 | 166.48 ± 5.82 | 65.7 ± 1.08 | 0.113 ± 0.0143 | 177.75 ± 11.72 |
| 50% AW | 80.67 ± 2.14 | 17.5 ± 0.19 | 5.55 ± 0.46 | 5.19 ± 0.12 | 3.67 ± 0.23 | 189.09 ± 10.66 | 78.21 ± 2.56 | 0.136 ± 0.0053 | 134.8 ± 13.83 |
| 25% AW | 85.11 ± 1.11 | 13.2 ± 1.82 | 4.72 ± 0.51 | 3.87 ± 0.41 | 5.78 ± 0.15 | 130.19 ± 5.25 | 65.59 ± 2.56 | 0.149 ± 0.005 | 340.01 ± 26.85 |
| UTLA6 | |||||||||
| 100% AW | 84.61 ± 0.54 | 20.38 ± 0.27 | 7.43 ± 0.1 | 5.47 ± 0.09 | 4.99 ± 0.22 | 169.59 ± 4.04 | 62.54 ± 0.83 | 0.175 ± 0.0021 | 101.59 ± 5.86 |
| 75% AW | 84.07 ± 0.73 | 19.3 ± 0.19 | 7.01 ± 0.11 | 5.37 ± 0.05 | 5.07 ± 0.21 | 122.69 ± 6.7 | 75.82 ± 0.64 | 0.151 ± 0.0088 | 89.62 ± 5.55 |
| 50% AW | 80.43 ± 0.62 | 19.18 ± 0.33 | 6.93 ± 0.15 | 5.37 ± 0.08 | 4.06 ± 0.32 | 141.62 ± 1.76 | 67.9 ± 1.06 | 0.155 ± 0.0109 | 135.71 ± 13.3 |
| 25% AW | 74.37 ± 1.16 | 20.44 ± 0.63 | 7.59 ± 0.11 | 5.72 ± 0.19 | 4.19 ± 0.05 | 168.04 ± 0.14 | 72.66 ± 1.16 | 0.154 ± 0.0108 | 112.73 ± 1.54 |
| UTLA7 | |||||||||
| 100% AW | 84.95 ± 0.92 | 22.68 ± 0.74 | 9.11 ± 0.05 | 5.97 ± 0.19 | 5.28 ± 0.2 | 199.09 ± 6.55 | 64.52 ± 1.89 | 0.184 ± 0.0085 | 136.87 ± 15.44 |
| 75% AW | 88.36 ± 0.94 | 20.88 ± 0.46 | 7.76 ± 0.19 | 6.12 ± 0.14 | 5.22 ± 0.12 | 164.51 ± 5.83 | 74.67 ± 1.28 | 0.12 ± 0.0101 | 137.79 ± 15.53 |
| 50% AW | 86.01 ± 1.1 | 20.15 ± 0.77 | 7 ± 0.32 | 6.47 ± 0.21 | 4.44 ± 0.18 | 152.06 ± 13.22 | 63.27 ± 1.55 | 0.174 ± 0.0102 | 109.44 ± 5.3 |
| 25% AW | 77.38 ± 1.23 | 18.88 ± 0.3 | 6.86 ± 0.36 | 5.76 ± 0.04 | 6.01 ± 0.13 | 209.33 ± 5.82 | 60.9 ± 2.78 | 0.128 ± 0.0099 | 113.19 ± 3.21 |
| UTLA9 | |||||||||
| 100% AW | 76.81 ± 0.42 | 21.19 ± 0.1 | 7.75 ± 0.29 | 6.13 ± 0.1 | 5.15 ± 0.08 | 227.11 ± 11.27 | 57.45 ± 1.65 | 0.157 ± 0.0099 | 103.23 ± 6.11 |
| 75% AW | 83.33 ± 1.44 | 21.62 ± 1 | 7.92 ± 0.19 | 5.96 ± 0.09 | 5.04 ± 0.14 | 193.48 ± 2.17 | 75.89 ± 3.42 | 0.139 ± 0.0079 | 90.38 ± 5.29 |
| 50% AW | 82.63 ± 1.25 | 18.99 ± 0.3 | 6.79 ± 0.2 | 5.28 ± 0.07 | 4.38 ± 0.21 | 171.31 ± 8.41 | 71.27 ± 1.53 | 0.208 ± 0.008 | 115.82 ± 4.89 |
| 25% AW | 75.82 ± 1.2 | 17.53 ± 0.33 | 5.81 ± 0.59 | 5.01 ± 0.17 | 5.4 ± 0.15 | 180.33 ± 4.68 | 67.71 ± 1.27 | 0.203 ± 0.014 | 139.39 ± 6.16 |
| UTLA10 | |||||||||
| 100% AW | 83.43 ± 0.52 | 21.44 ± 0.56 | 8.03 ± 0.19 | 5.5 ± 0.15 | 4.49 ± 0.14 | 182.79 ± 7.82 | 67.52 ± 1.11 | 0.112 ± 0.0006 | 101.87 ± 1.88 |
| 75% AW | 84.92 ± 0.18 | 20.42 ± 0.61 | 7.43 ± 0.43 | 5.64 ± 0.29 | 5.23 ± 0.17 | 185.98 ± 2.01 | 73.89 ± 1.4 | 0.085 ± 0.0073 | 122.38 ± 4.71 |
| 50% AW | 84.18 ± 1.06 | 17.58 ± 0.47 | 5.73 ± 0.28 | 5.1 ± 0.05 | 5.44 ± 0.16 | 190.73 ± 6.47 | 41.19 ± 3.54 | 0.18 ± 0.0039 | 117.18 ± 6.21 |
| 25% AW | 78.57 ± 0.42 | 16.82 ± 0.13 | 6.25 ± 0.35 | 5.44 ± 0.16 | 5.93 ± 0.15 | 249.64 ± 4.68 | 58.36 ± 0.9 | 0.182 ± 0.0066 | 155.81 ± 11.55 |
| UTLA12 | |||||||||
| 100% AW | 83.59 ± 2.92 | 17.58 ± 0.79 | 6.1 ± 0.32 | 5.25 ± 0.23 | 4.5 ± 0.1 | 168.78 ± 4 | 60 ± 1.85 | 0.131 ± 0.0079 | 117.36 ± 9.29 |
| 75% AW | 85.46 ± 0.92 | 17.14 ± 0.67 | 6.17 ± 0.09 | 4.64 ± 0.17 | 3.21 ± 0.05 | 215.52 ± 7.89 | 67.95 ± 1.57 | 0.122 ± 0.009 | 150.82 ± 11.34 |
| 50% AW | 87.5 ± 1.24 | 15.28 ± 0.65 | 5.58 ± 0.21 | 4.69 ± 0.19 | 5.72 ± 0.16 | 215.93 ± 8.4 | 80.17 ± 0.35 | 0.146 ± 0.0067 | 204.98 ± 10.91 |
| 25% AW | 82.18 ± 1.32 | 18.8 ± 0.48 | 6.72 ± 0.1 | 5.87 ± 0.14 | 5.46 ± 0.08 | 195.9 ± 7.14 | 85.14 ± 3.61 | 0.14 ± 0.0068 | 174.2 ± 7.06 |
| LSD (1%) | 4.536 | 2.011 | 1.156 | 0.562 | 0.658 | 25.7 | 7.768 | 0.0375 | 37.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiani, R.; Nazeri, V.; Shokrpour, M.; Hano, C. Morphological, Physiological, and Biochemical Impacts of Different Levels of Long-Term Water Deficit Stress on Linum album Ky. ex Boiss. Accessions. Agronomy 2020, 10, 1966. https://doi.org/10.3390/agronomy10121966
Kiani R, Nazeri V, Shokrpour M, Hano C. Morphological, Physiological, and Biochemical Impacts of Different Levels of Long-Term Water Deficit Stress on Linum album Ky. ex Boiss. Accessions. Agronomy. 2020; 10(12):1966. https://doi.org/10.3390/agronomy10121966
Chicago/Turabian StyleKiani, Reza, Vahideh Nazeri, Majid Shokrpour, and Christophe Hano. 2020. "Morphological, Physiological, and Biochemical Impacts of Different Levels of Long-Term Water Deficit Stress on Linum album Ky. ex Boiss. Accessions" Agronomy 10, no. 12: 1966. https://doi.org/10.3390/agronomy10121966
APA StyleKiani, R., Nazeri, V., Shokrpour, M., & Hano, C. (2020). Morphological, Physiological, and Biochemical Impacts of Different Levels of Long-Term Water Deficit Stress on Linum album Ky. ex Boiss. Accessions. Agronomy, 10(12), 1966. https://doi.org/10.3390/agronomy10121966






