Bacteria Associated with Winter Wheat Degrade Fusarium Mycotoxins and Triazole Fungicide Residues
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin and Identification of Bacterial Isolates of the Genus Sphingomonas
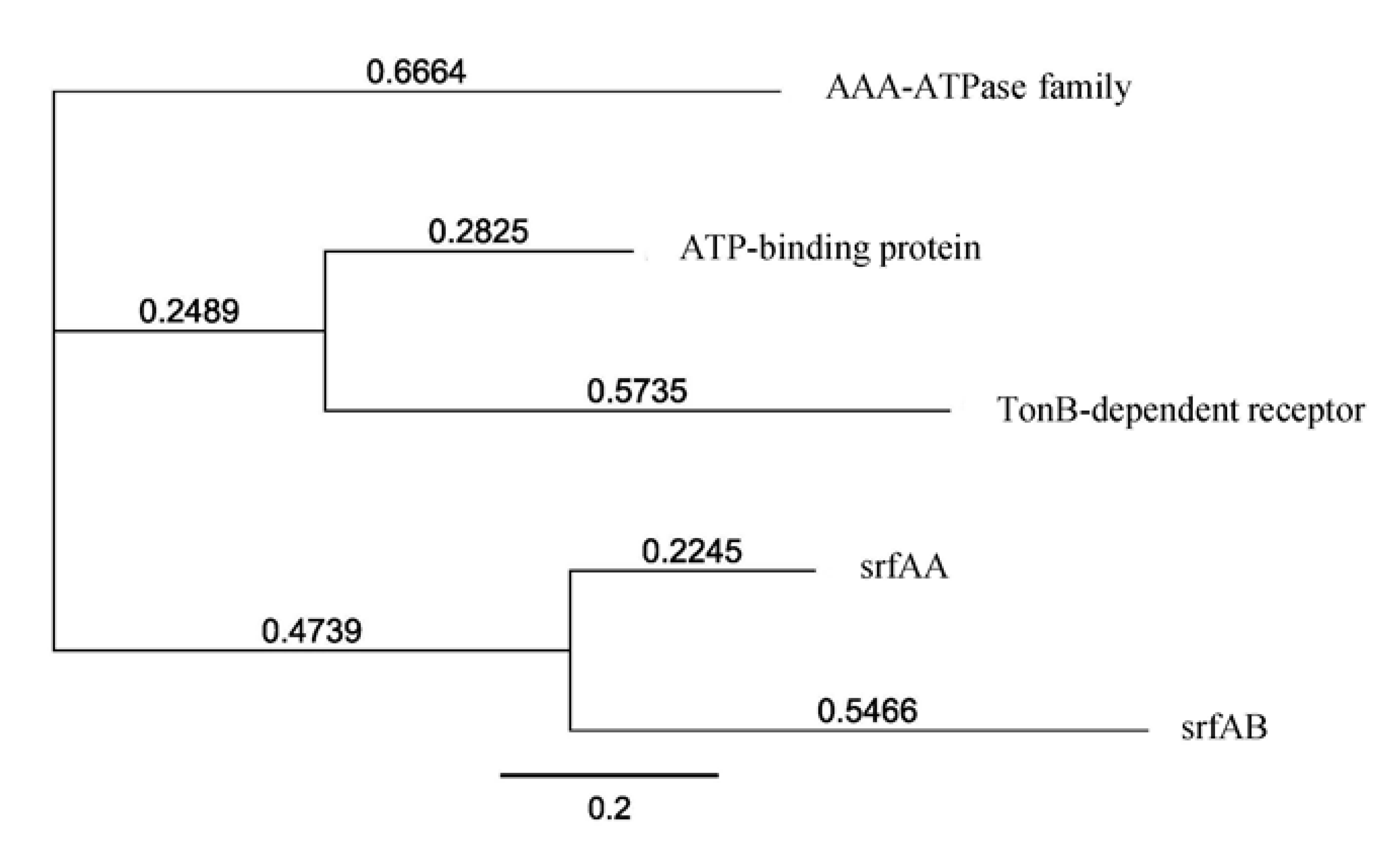
2.2. Molecular Screening for Natural Product Biosynthetic Gene Clusters
2.3. Extraction and Purification of Surfactin
2.4. Isolate Screening for Antifungal Properties
2.5. Selection of Sphingomonas sp. Isolates Capable of Biodegrading Propiconazole
2.6. Survival Rates of Sphingomonas Bacteria on the Leaves of Wheat Seedlings
2.7. Preparation of the Bacterial Suspension for the Field Treatment
2.8. Spike Inoculation with Fusarium Culmorum
2.9. Antifungal Properties of Sphingomonas Strain S11 during the Growing Season of Wheat
2.10. Evaluation of Spike Health
2.11. Grain Colonization by Fungi of the Genus Fusarium
2.12. Chemical Analysis of Ergosterol and Trichothecene
2.13. Statistical Analysis
3. Results
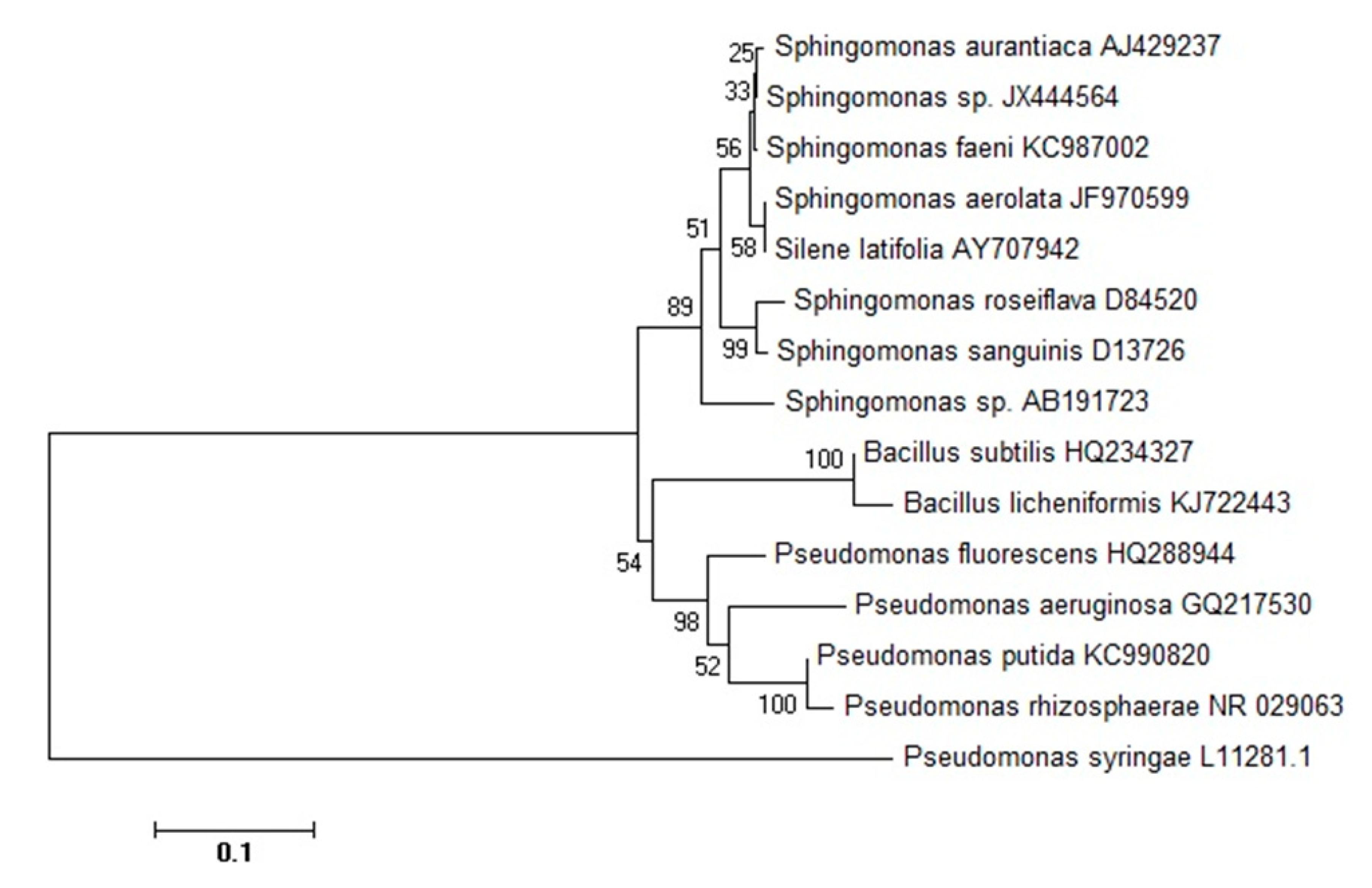
3.1. Identification and Phylogenetic Analysis of Sphingomonas sp.
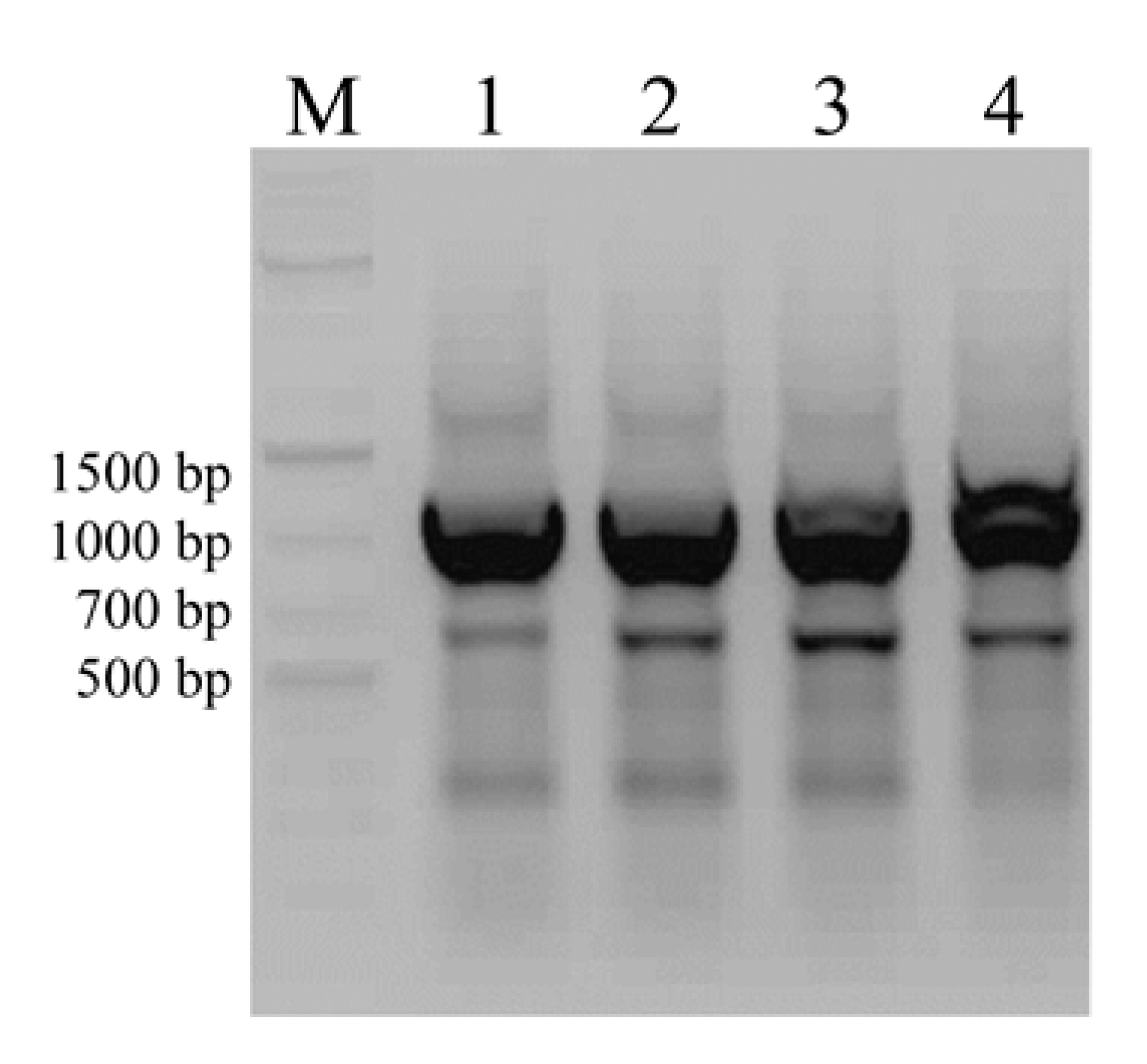
3.2. Screening for Natural Product Biosynthetic Gene Clusters by PCR
3.3. Surfactin Production by Sphingomonas Isolates
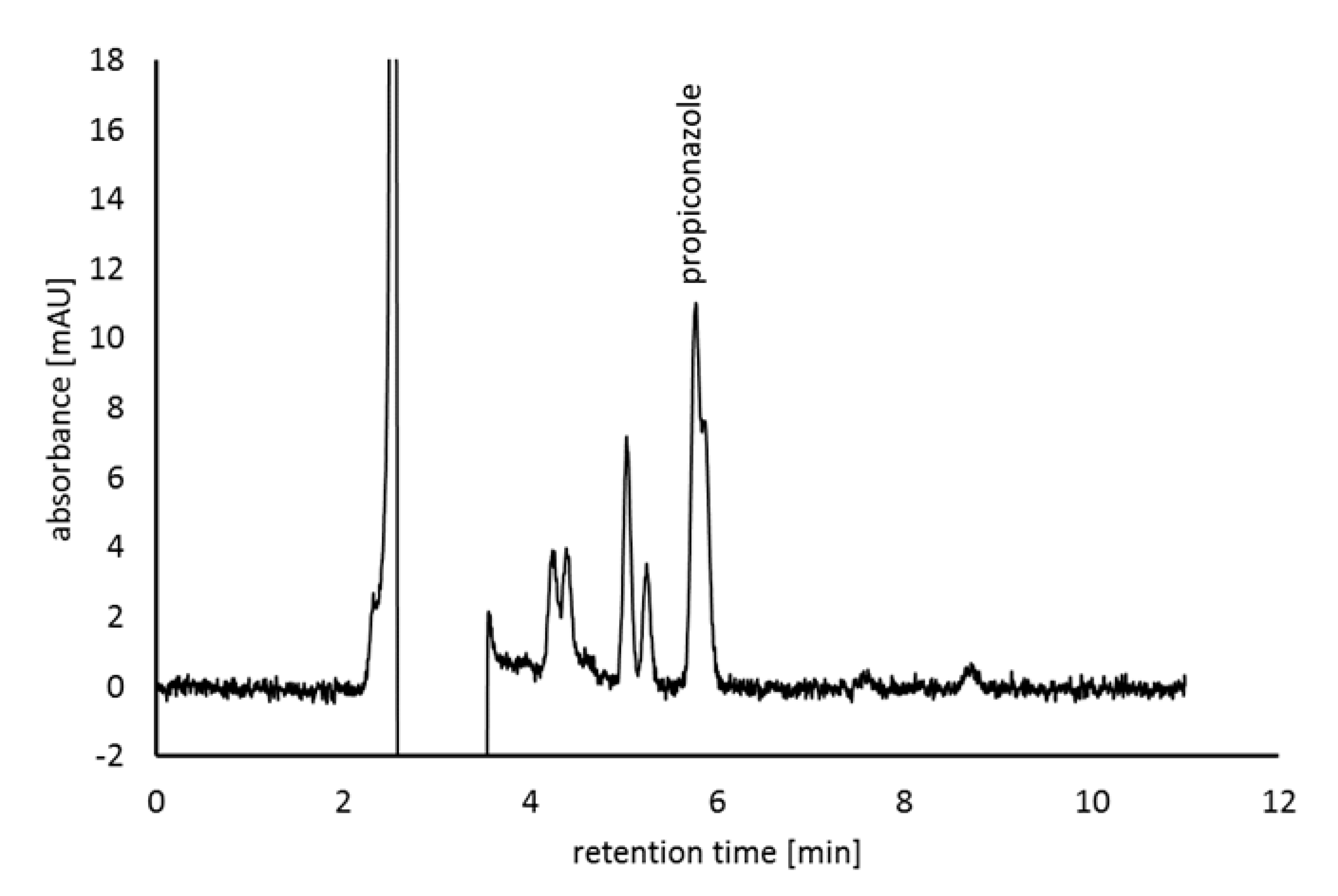
3.4. Propiconazole-Degrading Ability of Sphingomonas Isolates
3.5. Isolate Activity in Dual Cultures
3.6. Survival of Bacteria on Wheat Leaves
3.7. The Effectiveness of Sphingomonas S11 in Inhibiting FHB
3.8. Trichothecene Content of Grain
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palazzini, J.M.; Torres, A.M.; Chulze, S.N. Tolerance of triazole–based fungicides by biocontrol agents used to control Fusarium head blight in wheat in Argentina. Lett. Appl. Microbiol. 2018, 66, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Mionetto, A.; Tiscornia, S.; Bettucci, L. Endophytic bacteria from wheat grain as biocontrol agents of Fusarium graminearum and deoxynivalenol production in wheat. Mycotoxin Res. 2015, 31, 137. [Google Scholar] [CrossRef] [PubMed]
- Minati, M.H.; Mohammed-Ameen, M.K. Novel report on six Fusarium species associated with head blight and crown rot of wheat in Basra province, Iraq. Bull. Natl. Res. Cent. 2019, 43, 139. [Google Scholar] [CrossRef]
- Qiu, H.; Zhao, X.; Fang, W.; Wu, H.; Abubakar, Y.S.; Lu, G.; Wang, Z.; Zheng, W. Spatiotemporal nature of Fusarium graminearum—Wheat coleoptile interactions. Phytopathol. Res. 2019, 1, 26. [Google Scholar] [CrossRef]
- Rojas, E.C.; Sapkota, R.; Jensen, B.; Jørgensen, H.J.L.; Henriksson, T.; Jørgensen, L.N.; Nicolaisen, M.; Collinge, D.B. Fusarium head blight modifies fungal endophytic communities during infection of wheat spikes. Microb. Ecol. 2019, 79, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Abdallah-Nekache, N.; Laraba, I.; Ducos, C.; Barreau, C.; Bouznad, Z.; Boureghda, H. Occurrence of Fusarium head blight and Fusarium crown rot in Algerian wheat: Identification of associated species and assessment of aggressiveness. Eur. J. Plant Pathol. 2019, 154, 499–512. [Google Scholar] [CrossRef]
- Matny, O.; Shamsallah, S.; Fahad, M.A.; Haas, M. Genetic diversity study of Fusarium culmorum: Causal agent of wheat crown rot in Iraq. J. Plant Protec. Res. 2017, 57, 43–49. [Google Scholar] [CrossRef]
- Xu, X.M.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Cooke, B.M.; Doohan, F.M.; Edwards, S.G. Relationship between the fungal complex causing Fusarium head blight of wheat and environmental conditions. Phytopathology 2008, 98, 69–78. [Google Scholar] [CrossRef]
- Góral, T.; Wiśniewska, H.; Ochodzki, P.; Nielsen, L.K.; Walentyn-Góral, D.; Stępień, Ł. Relationship between fusarium head blight, kernel damage, concentration of fusarium biomass, and Fusarium toxins in grain of winter wheat inoculated with Fusarium culmorum. Toxins 2019, 11, 2. [Google Scholar] [CrossRef]
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium head blight, mycotoxins and strategies for their reduction. Agronomy 2020, 10, 509. [Google Scholar] [CrossRef]
- Venkataramana, M.; Selvakumar, G.; Chandranayaka, S. Fusarium Mycotoxin: Toxicity and Detection. In Microbial Toxins. Toxinology; Gopalakrishnakone, P., Stiles, B., Alape-Girón, A., Dubreuil, J., Mandal, M., Eds.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Berthiller, F.; Krska, R.; Domig, K.J.; Kneifel, W.; Juge, N.; Schuhmacher, R.; Adam, G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 2011, 206, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process. Nutr. 2019, 1, 6. [Google Scholar] [CrossRef]
- He, W.; Zhang, L.; Yi, S.; Tang, X.L.; Yuan, Q.S.; Guo, M.W.; Wu, A.B.; Qu, B.; Li, H.P.; Liao, Y.C. An aldo-keto reductase is responsible for Fusarium toxin-degrading activity in a soil Sphingomonas strain. Sci. Rep. 2017, 7, 9549. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Sato, I.; Ishizaka, M.; Yoshida, S.; Koitabashi, M.; Yoshida, S.; Tsushima, S. Bacterial cytochrome P450 system catabolizing the Fusarium toxin deoxynivalenol. Appl. Environ. Microbiol. 2013, 79, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Baffoni, L.; Gaggia, F.; Dalanaj, N.; Prodi, A.; Nipoti, P.; Pisi, A.; Biavati, B.; Gioia, D. Microbial inoculants for the biocontrol of Fusarium spp. in durum wheat. BMC Microbiol. 2015, 15, 242. [Google Scholar]
- Cromey, M.G.; Lauren, D.R.; Parkes, R.A.; Sinclair, K.I.; Shorter, S.C.; Wallaceet, A.R. Control of fusarium head blight of wheat with fungicides. Australas. Plant Pathol. 2001, 30, 301–308. [Google Scholar] [CrossRef]
- Kim, I.S.; Shim, J.H.; Suh, Y.T. Laboratory studies on formation of bound residues and degradation of propiconazole in soils. Pest Manag. Sci. 2003, 59, 324–330. [Google Scholar] [CrossRef]
- Riise, G.; Lundekvam, H.; Wu, Q.L.; Haugen, L.E.; Mulder, J. Loss of pesticides from agricultural fields in SE Norway—Runoff through surface and drainage water. Environ. Geochem. Health. 2004, 26, 269–276. [Google Scholar] [CrossRef]
- Satapute, P.; Sanakal, R.D.; Mulla, S.I.; Kaliwal, B. Molecular interaction of the triazole fungicide propiconazole with homology modeled superoxide dismutase and catalase. Environ. Sustain. 2019, 2, 429–439. [Google Scholar] [CrossRef]
- Skolness, S.Y.; Blanksma, C.A.; Cavallin, J.E.; Churchill, J.J.; Durhan, E.J.; Jensen, K.M.; Johnson, R.D.; Kahl, M.D.; Makynen, E.A.; Villeneuve, D.L.; et al. Propiconazole inhibits steroidogenesis and reproduction in the Fathead Minnow (Pimephales promelas). Toxicol. Sci. 2013, 132, 284–297. [Google Scholar] [CrossRef]
- Wang, W.D.; Niu, J.L.; Cui, Z.J. Biodegradation of pesticides: A review. J. Heilongjiang August First Land Reclam. Univ. 2005, 17, 18. [Google Scholar]
- Jain, R.K.; Kapur, M.; Labana, S.; Lal, B.; Sarma, P.M.; Bhattacharya, D.; Thakur, I.S. Microbial diversity: Application of microorganisms for the biodegradation of xenobiotics. Curr. Sci. 2005, 89, 101–112. [Google Scholar]
- Mulla, S.I.; Bharagava, R.N.; Belhaj, D.; Saratale, G.D.; Bagewadi, Z.K.; Saxena, G.; Kumar, A.; Mohan, H.; Yu, C.P.; Ninnekar, H.Z. An overview of nitro group-containing compounds and herbicides degradation in microorganisms. In Microbial Metabolism of Xenobiotic Compounds Microorganisms for Sustainability; Arora, P.K., Ed.; Springer: Singapore, 2019; pp. 310–335. [Google Scholar]
- Porto, A.M.; Melgar, G.Z.; Kasemodel, M.C.; Nitschke, M. Biodegradation of Pesticides, Pesticides in Modern World-Pesticides Use and Management. 2011. Available online: http://www.intechopen.com/books/pesticides-in-the-modern-world-pesticides-use-and-management/biodegradation-of-pesticides (accessed on 12 October 2015).
- Satapute, P.; Kaliwal, B. Burkholderia sp. Strain BBK_9: A potent agent for propiconazole degradation. In Toxicity and Biodegradation Testing, Methods in Pharmacology and Toxicology; Bidoia, E.D., Montagnolli, R.N., Eds.; Humana Press: New York, NY, USA, 2018; pp. 87–108. [Google Scholar]
- Srinivasan, S.; Lee, J.; Kim, M.K. Sphingomonas rosea sp. nov. and Sphingomonas swuensis sp. nov., rosy colored beta-glucosidase-producing bacteria isolated from soil. J. Microbiol. 2011, 49, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, E.; Yano, I.; Oyaizu, H.; Hashimoto, Y.; Ezaki, T.; Yamamoto, H. Proposals of Sphingomonas paucimobilis gen. nov. and comb. nov., Sphingomonas parapaucimobilis sp. nov., Sphingomonas yanoikuyae sp. nov., Sphingomonas adhaesiva sp. nov., Sphingomonas capsulata comb. nov., and two genospecies of the genus Sphingomonas. Microbiol. Immunol. 1990, 34, 99–119. [Google Scholar] [CrossRef]
- Available online: www.bacterio.net/sphingomonas.html (accessed on 14 August 2019).
- Asaf, S.; Khan, A.L.; Khan, M.A.; Al-Harrasi, A.; Lee, I.J. Complete genome sequencing and analysis of endophytic Sphingomonas sp. LK11 and its potential in plant growth. Biotech 2018, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Futamura, A.; Miyauchi, K.; Takagi, M. Two different types of dehalogenases, Lina and Linb, involved in γ-hexachlorocyclohexane degradation in Sphingomonas paucimobilis Ut26 are localized in the periplasmic space without molecular processing. J. Bacteriol. 1999, 181, 5409–5413. [Google Scholar] [CrossRef] [PubMed]
- Thelusond, J.R.; Strathmann, T.J.; Cupples, A.M. Carbamazepine, triclocarban, triclosan biodegradation and the phylotypes and functional genes associated with xenobiotic degradation in four agricultural soils. Sci. Total Environ. 2019, 657, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Leys, N.M.; Ryngaert, A.; Bastiaens, L.; Top, E.M.; Verstraete, W.; Springael, D. Culture independent detection of Sphingomonas sp. EPA 505 related strains in soils contaminated with polycyclic aromatic hydrocarbons (PAHs). Microb. Ecol. 2005, 49, 443–450. [Google Scholar] [CrossRef]
- Davison, A.D.; Gillings, M.R.; Jardine, D.R.; Karuso, P.; Nouwens, A.S.; French, J.J.; Veal, D.A.; Altavilla, N. Sphingomonas paucimobilis BPSI-3 mutant AN2 produces a red catabolite during biphenyl degradation. J. Ind. Microbiol. Biotechnol. 1999, 23, 314–319. [Google Scholar] [CrossRef]
- Pan, T.; Wang, R.; Xiao, K.; Ye, W.; Dong, W.; Xu, M. Continous degradation of phenanthrene in cloud point system by reuses of Sphingomonas polyaromaticivorans cells. AMB Express 2019, 9, 8. [Google Scholar] [CrossRef]
- Sorensen, S.R.; Ronen, Z.; Aamand, J. Isolation from agricultural soil and characterization of a Sphingomonas sp. able to mineralize the phenylurea herbicide isoproturon. Appl. Environ. Microbiol. 2001, 67, 5403–5409. [Google Scholar] [CrossRef]
- Sarkar, S.; Seenivasan, S.; Premkumar, R. Biodegradation of propiconazole by Pseudomonas putina isolated from tea rhizosphere. Plant Soil Environ. 2009, 55, 196–201. [Google Scholar] [CrossRef]
- Innerebner, G.; Knief, C.; Vorholt, J.A. Sphingomonas strains protect Arabidopsis thaliana against leaf-pathogenic Pseudomonas syringae in a controlled model system. Appl. Environ. Microbiol. 2011, 77, 3202–3210. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, S.; Mehnaz, S.; Malik, K.A. Microbial diversity in the rhizosphere of plants growing under extreme environments and its impact on crop improvement. Environ. Sustain. 2019, 2, 329–338. [Google Scholar] [CrossRef]
- Shimoi, S.; Inoue, K.; Kitagawa, H.; Yamasaki, M.; Tsushima, S.; Park, P.; Ikeda, K. Biological control for rice blast disease by employing detachment action with gelatinolytic bacteria. Biol. Control 2010, 55, 85–91. [Google Scholar] [CrossRef]
- Vogel, C.; Innerebner, G.; Zingg, J.; Guder, J.; Vorholt, J.A. Forward genetic in planta screen for identification of plant-protective traits of Sphingomonas sp. strain Fr1 against Pseudomonas syringae DC3000. Appl. Environ. Microbiol. 2012, 78, 5529–5535. [Google Scholar] [CrossRef]
- Wachowska, U.; Irzykowski, W.; Jędryczka, M.; Stasiulewicz-Paluch, A.D.; Głowacka, K. Biological control of winter wheat pathogens with the use of antagonistic Sphingomonas bacteria under greenhouse conditions. Biocontrol Sci. Technol. 2013, 23, 1110–1122. [Google Scholar] [CrossRef]
- Adhikari, T.B.; Joseph, C.M.; Yang, G.; Phillips, D.A.; Nelson, L.M. Evaluation of bacteria isolated from rice for plant growth promotion and biological control of seedling disease of rice. Can. J. Microbiol. 2001, 47, 916–924. [Google Scholar] [CrossRef]
- Cottyn, B.; Debode, J.; Regalado, E.; Mew, T.W.; Swings, J. Phenotypic and genetic diversity of rice seed-associated bacteria and their role in pathogenicity and biological control. J. Appl. Microbiol. 2009, 107, 885–889. [Google Scholar] [CrossRef]
- Laca, A.; Mousia, Z.; Diaz, M.; Webb, C.; Pandiella, S. Distribution of microbial contamination within cereal grains. J. Food Eng. 2006, 72, 332–338. [Google Scholar] [CrossRef]
- Wachowska, U. Activity of fungicides against epiphytic yeast-like fungi of winter wheat. Pol. J. Environ. Stud. 2009, 18, 1169–1174. [Google Scholar]
- King, E.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar]
- Buck, J.D. Nonstaining (KOH) method for determination of gram reactions of marine bacteria. Appl. Environ. Microbiol. 1982, 44, 992–993. [Google Scholar] [CrossRef] [PubMed]
- NCBI. Available online: www.ncbi.nlm.nih.gov (accessed on 5 May 2020).
- Wachowska, U.; Popielarczyk, C.; Duba, A.; Goriewa, K.; Kucharska, K. Evaluation of usefulness of yeast and chemical fungicides to reduce development of fungi colonizing Scot pine seedlings (in Polish). Sylwan 2016, 7, 556–563. [Google Scholar]
- Jasim, B.; Sreelakshmi, K.S.; Mathew, J.; Radhakrishnan, E.K. Surfactin, iturin, and fengycin biosynthesis by endophytic Bacillus sp. from Bacopa monnieri. Microb. Ecol. 2016, 72, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Tapi, A.; Chollet-Imbert, M.; Scherens, B.; Jacques, P. New approach for the detection of non-ribosomal peptide synthetase genes in Bacillus strains by polymerase chain reaction. Appl. Gen. Mol. Biotechnol. 2010, 85, 1521–1531. [Google Scholar] [CrossRef]
- Mubarak, M.Q.E.; Hassan, A.R.; Hamid, A.A.; Khalil, S.; Isa, M.H.M. A simple and effective isocratic HPLC method for fast identification and quantification of surfactin. Sains Malays. 2015, 44, 115–120. [Google Scholar] [CrossRef]
- Wiwart, M.; Suchowilska, E.; Kandler, W.; Sulyok, M.; Wachowska, U.; Krska, R. The response of selected Triticum spp. genotypes with different ploidy levels to head blight caused by Fusarium culmorum (W.G.Smith) Sacc. Toxins 2016, 8, 112. [Google Scholar] [CrossRef]
- Meier, U. Phenological growth stages. In Phenology: An Integrative Science; Task for vegetation science; Schwarz, M.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2003; Volume 39. [Google Scholar]
- EPPO Bulletin. 1998. Available online: https://bip.minrol.gov.pl/.../fung%20-%20choroby%20liści%20zbóż (accessed on 1 May 2009).
- Martin, J.P. Use of acid, rose Bengal and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1950, 38, 215–220. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Professional: Ames, IA, USA, 2006. [Google Scholar]
- Wachowska, U.; Stuper-Szablewska, K.; Perkowski, J. Yeasts isolated from wheat grain can suppress fusarium head blight and decrease trichothecene concentrations in bread wheat and durum wheat grain. Pol. J. Environ. Stud. 2020, 29, 4345–4360. [Google Scholar] [CrossRef]
- Statsoft, Inc. Statistica (Data Analysis Software System), Version 12. 2015. Available online: www.statsoft.com (accessed on 30 November 2019).
- Zhao, Y.; Selvaraj, J.N.; Xing, F.; Zhou, L.; Wang, Y.; Song, H.; Tan, X.; Sun, L.; Sangare, L.; Folly, Y.M.E.; et al. Antagonistic action of Bacillus subtilis strain SG6 on Fusarium graminearum. PLoS ONE 2014, 9, e92486. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhou, Y.; Feng, J. Analysis of both chitinase and chitosanase produced by Sphingomonas sp. CJ-5. J. Zhejiang Univ. Sci. 2007, 8, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yang, J.; Nie, Q.; Huang, D.; Yu, C.; Zheng, L.; Cai, M.; Thomashow, L.S.; Weller, D.M.; Yu, Z.; et al. Volatile organic compounds from Paenibacillus polymyxa KM2501-1 control Meloidogyne incognita by multiple strategies. Sci. Rep. 2017, 7, 16213. [Google Scholar] [CrossRef]
- Mannaa, M.; Oh, J.Y.; Kim, K.D. Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus flavus and aflatoxin production on stored rice grains. Mycobiology 2017, 45, 213–219. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Aboulwafa, M.M.; Hassouna, N.A. Characterization of surfactin produced by Bacillus subtilis isolate BS5. Appl. Biochem. Biotechnol. 2008, 150, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Hussein, W.; Fahim, S. Detection of synthetases genes involved in non ribosomal lipopeptides (NRLPS) biosynthesis from Bacillus species by bioinformatics and PCR degenerated primers and estimation of their production. Int. J. Pharm. Bio. Sci. 2017, 8, 116–125. [Google Scholar]
- Farzaneh, M.; Shi, Z.Q.; Ahmadzadeh, M.; Hu, L.B.; Ghassempour, A. Inhibition of the Aspergillus flavus growth and aflatoxin B1 contamination on pistachio nut by fengycin and surfactin-producing Bacillus subtilis UTBSP1. Plant Pathol. J. 2016, 32, 209–215. [Google Scholar] [CrossRef]
- Sarwar, A.; Hassan, M.N.; Imran, M.; Iqbal, M.; Majeed, S.; Brader, G.; Sessitsch, A.; Hafeez, F.Y. Biocontrol activity of surfactin A purified from Bacillus NH-100 and NH-217 against rice bakanae disease. Microbiol. Res. 2018, 209, 1–13. [Google Scholar] [CrossRef]
- Tendulkar, S.R.; Saikumari, Y.K.; Patel, V.; Raghotama, S.; Munshi, T.K.; Balaram, P.; Chattoo, B.B. Isolation, purification and characterization of an antifungal molecule produced by Bacillus licheniformis BC98, and its effect on phytopathogen Magnaporthe grisea. J. Appl. Microbiol. 2007, 103, 2331–2339. [Google Scholar] [CrossRef]
- Desmyttere, H.; Deweer, C.; Muchembled, J.; Sahmer, K.; Jacquin, J.; Coutte, F.; Jacques, P. Antifungal activities of Bacillus subtilis lipopeptides to two Venturia inaequalis strains possessing different tebuconazole sensitivity. Front. Microbiol. 2019, 10, 2327. [Google Scholar] [CrossRef]
- Krishnan, N.; Velramar, B.; Velu, R.K. Investigation of antifungal activity of surfactin against mycotoxigenic phytopathogenic fungus Fusarium moniliforme and its impact in seed germination and mycotoxicosis. Pestic. Biochem. Physiol. 2019, 155, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Nam, J.; Kim, J.; Song, J.; Kim, P.I.; Min, H.J.; Lee, C.W. Structure and mechanism of surfactin peptide from Bacillus velezensis antagonistic to fungi plant pathogens. Bull. Korean Chem. Soc. 2019, 40, 704–709. [Google Scholar] [CrossRef]
- Le Mire, G.; Siah, A.; Brisset, M.-N.; Gaucher, M.; Deleu, M.; Jijakli, M.H. Surfactin protects wheat against Zymoseptoria tritici and activates both salicylic acid- and jasmonic acid-dependent defense responses. Agriculture 2018, 8, 11. [Google Scholar] [CrossRef]
- Farace, G.; Fernandez, O.; Jacquens, L.; Coutte, F.; Krier, F.; Jacques, P.; Clément, C.; Barka, E.A.; Jacquard, C.; Dorey, S. Cyclic lipopeptides from Bacillus subtilis activate distinct patterns of defence responses in grapevine. Mol. Plant Pathol. 2015, 16, 177–187. [Google Scholar] [CrossRef]
- Jourdan, E.; Henry, G.; Duby, F.; Dommes, J.; Barthelemy, J.P.; Thonart, P.; Ongena, M.A.R.C. Insights into the defense-related events occurring in plant cells following perception of surfactin-type lipopeptide from Bacillus subtilis. Mol. Plant Microbe Interact. 2009, 22, 456–468. [Google Scholar] [CrossRef]
- Cawoy, H.; Mariutto, M.; Henry, G.; Fisher, C.; Vasilyeva, N.; Thonart, P.; Dommes, J.; Ongena, M. Plant defense stimulation by natural isolates of Bacillus depends on efficient surfactin production. Mol. Plant Microbe Interact. 2014, 27, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.M.; Magnuson, R.; Myers, A.; Curry, J.; Grossman, A.D.; Zuber, P. SrfA is an operon required for surfactin production, competence development, and efficient sporulation in Bacillus subtilis. J. Bacteriol. 1991, 173, 1770–1778. [Google Scholar] [CrossRef]
- Nakano, M.M.; Corbell, N.; Besson, J.; Zuber, P. Isolation and characterization of sfp a gene that functions in the production of the lipopeptide biosurfactant, surfactin, in Bacillus subtilis. Mol. Gen. Genet. 1992, 232, 313–321. [Google Scholar] [CrossRef] [PubMed]


| Treatment | First Node BBCH 31 | Middle of Heading BBCH 55 | Full Flowering BBCH 65 |
|---|---|---|---|
| Experiment 1, cv. Bogatka | |||
| Integrated (Integ) | fenpropimorph 1 (1 dm3 per ha) | propiconazole 2 (1 dm3 per ha) | Sphingomonas sp. S11 JX444564 |
| Experiment 2, cv. Tonacja | |||
| Biological (Bac) | Sphingomonas sp. S11 JX444564 | Sphingomonas sp. S11 JX444564 | Sphingomonas sp. S11 JX444564 |
| Sphingomonas sp. Strains | Surfactin Content (g/L) | Inhibition Zones in the Disc Diffusion Test (cm2) | Biodegradation of Propiconazole (%) |
|---|---|---|---|
| B4 | 3.89 a | 0.98 c | 4.52 |
| B6 | 2.36 b | 0 e | 0 |
| M2 | 1.86 c | 0.67 d | 7.52 |
| M3 | 1.70 d | 0.45 d | 7.91 |
| K2 | 0.05 e | 0.74 c,d | 7.23 |
| S11 | 0.02 e | 2.87 a | 15.13 |
| Pathogen | Sphingomonas sp. | Colony Area in cm2 | Colony Ellipticity Ratio |
|---|---|---|---|
| Fusarium culmorum | Control | 8.69 a,b | 0.89 a |
| B4 | 9.77 a | 0.79 a,b,c,d | |
| B6 | 6.92 a,b | 0.67 d,e | |
| M2 | 10.00 a | 0.83 a,b | |
| M3 | 6.79 a,b | 0.85 a,b | |
| K2 | 4.59 c | 0.83 a,b | |
| S11 | 4.88 c | 0.93 a | |
| Fusarium graminearum | Control | 7.99 a,b | 0.92 a |
| B4 | 7.05 a,b | 0.81 a,b,c | |
| B6 | 4.89 b | 0.67 c,d,e | |
| M2 | 8.83 a,b | 0.94 a | |
| M3 | 5.57 b | 0.64 e | |
| K2 | 5.49 b | 0.65 e | |
| S11 | 7.55 a,b | 0.87 a |
| Inoculation | Treatment | Yield (g m−2) | FHB Severity | ERG Content (mg kg−1) | Fusarium spp. | ||
|---|---|---|---|---|---|---|---|
| CFU × 10 2 per 10 g of Grain a | Percentage b | ||||||
| Epiphytes | Endophytes | ||||||
| Experiment 1, cv. Bogatka | |||||||
| Without inoculation | Control * | 479.40 b | 0.08 | 2.06 | 3.05 a | 15.28 a | 12.50 |
| Integ | 560.00 a | 0 | 6.39 | 3.09 a | 8.33 a,b | 15.28 | |
| Inoculation F. culmorum | Control | 384.50 c | 0.18 | 19.41 | 1.59 b | 15.29 a | 12.5 |
| Integ | 337.70 d | 0 | 4.26 | 2.06 a,b | 0.10 b | 6.94 | |
| Experiment 2, cv. Tonacja | |||||||
| Without inoculation | Control | 303.30 | 1.36 | 1.11 | 2.95 a | 1.39 b | 8.33 |
| Bac | 291.06 | 0.44 | 10.06 | 2.54 a | 2.78 a,b | 12.50 | |
| Inoculation F. culmorum | Control | 258.51 | 1.78 | 12.36 | 3.15 a | 9.72 a,b | 0.10 |
| Bac | 268.01 | 0.11 | 9.61 | 2.68 a | 12.50 a | 4.17 | |
| Inoculation | Treatment | DON | FUS-X | 3AcDON | 15AcDON | NIV | Sum B | STO | T-2 | HT-2 | Sum A |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experiment 1, cv. Bogatka | |||||||||||
| Control | Control | 0.106 | 0.001 | 0.003 | 0.001 | 0.025 | 0.136 | <LOD | 0.006 | <LOD | 0.006 |
| Integ | 0.039 | 0.001 | 0.001 | 0.001 | 0.063 | 0.105 | 0.006 | 0.012 | <LOD | 0.018 | |
| Inoculation F.culmorum | Control | 6.128 | 0.026 | 0.314 | 0.042 | 0.064 | 6.575 | 0.017 | 0.006 | <LOD | 0.023 |
| Integ | 1.889 | 0.009 | 0.036 | 0.011 | 0.366 | 2.311 | 0.018 | 0.039 | 0.007 | 0.066 | |
| Experiment 2, cv. Tonacja | |||||||||||
| Control | Control | 1.099 | 0.012 | 0.044 | 0.013 | 0.453 | 1.621 | 0.007 | 0.009 | 0.004 | 0.020 |
| Bac | 1.086 | 0.017 | 0.024 | 0.009 | 0.112 | 1.248 | <LOD | <LOD | <LOD | <LOD | |
| Inoculation F.culmorum | Control | 7.808 | 0.027 | 0.143 | 0.048 | 0.167 | 8.193 | 0.010 | <LOD | <LOD | 0.010 |
| Bac | 0.352 | 0.005 | 0.015 | 0.004 | 0.036 | 0.413 | <LOD | <LOD | <LOD | <LOD | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wachowska, U.; Kucharska, K.; Pluskota, W.; Czaplicki, S.; Stuper-Szablewska, K. Bacteria Associated with Winter Wheat Degrade Fusarium Mycotoxins and Triazole Fungicide Residues. Agronomy 2020, 10, 1673. https://doi.org/10.3390/agronomy10111673
Wachowska U, Kucharska K, Pluskota W, Czaplicki S, Stuper-Szablewska K. Bacteria Associated with Winter Wheat Degrade Fusarium Mycotoxins and Triazole Fungicide Residues. Agronomy. 2020; 10(11):1673. https://doi.org/10.3390/agronomy10111673
Chicago/Turabian StyleWachowska, Urszula, Katarzyna Kucharska, Wioletta Pluskota, Sylwester Czaplicki, and Kinga Stuper-Szablewska. 2020. "Bacteria Associated with Winter Wheat Degrade Fusarium Mycotoxins and Triazole Fungicide Residues" Agronomy 10, no. 11: 1673. https://doi.org/10.3390/agronomy10111673
APA StyleWachowska, U., Kucharska, K., Pluskota, W., Czaplicki, S., & Stuper-Szablewska, K. (2020). Bacteria Associated with Winter Wheat Degrade Fusarium Mycotoxins and Triazole Fungicide Residues. Agronomy, 10(11), 1673. https://doi.org/10.3390/agronomy10111673






