Gene Expression in 1-Methylcyclopropene (1-MCP) Treated Tomatoes during Pre-Climacteric Ripening Suggests Shared Regulation of Methionine Biosynthesis, Ethylene Production and Respiration
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. 1-MCP Treatment
2.3. RNA Extraction, Gene Chip Analysis, and Data Analysis
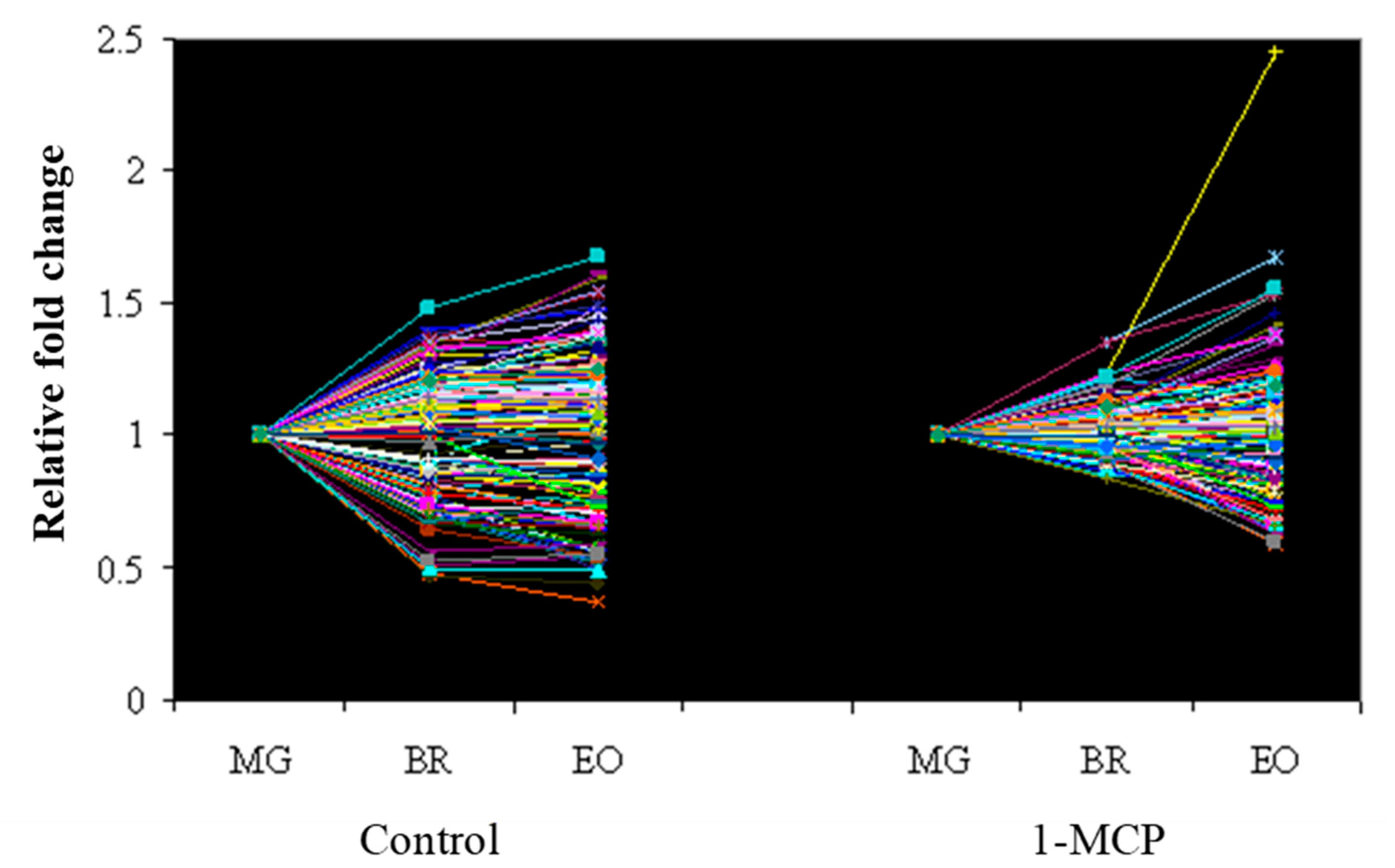
2.4. Real-Time PCR Validation of Microarray Data
2.5. Extraction and Analysis of Free Amino Acids and Derivatives
2.6. Detection of the AtSR1 Binding Motif Sequense
2.7. Gas Chromatography Analysis of Ethylene Production
3. Results
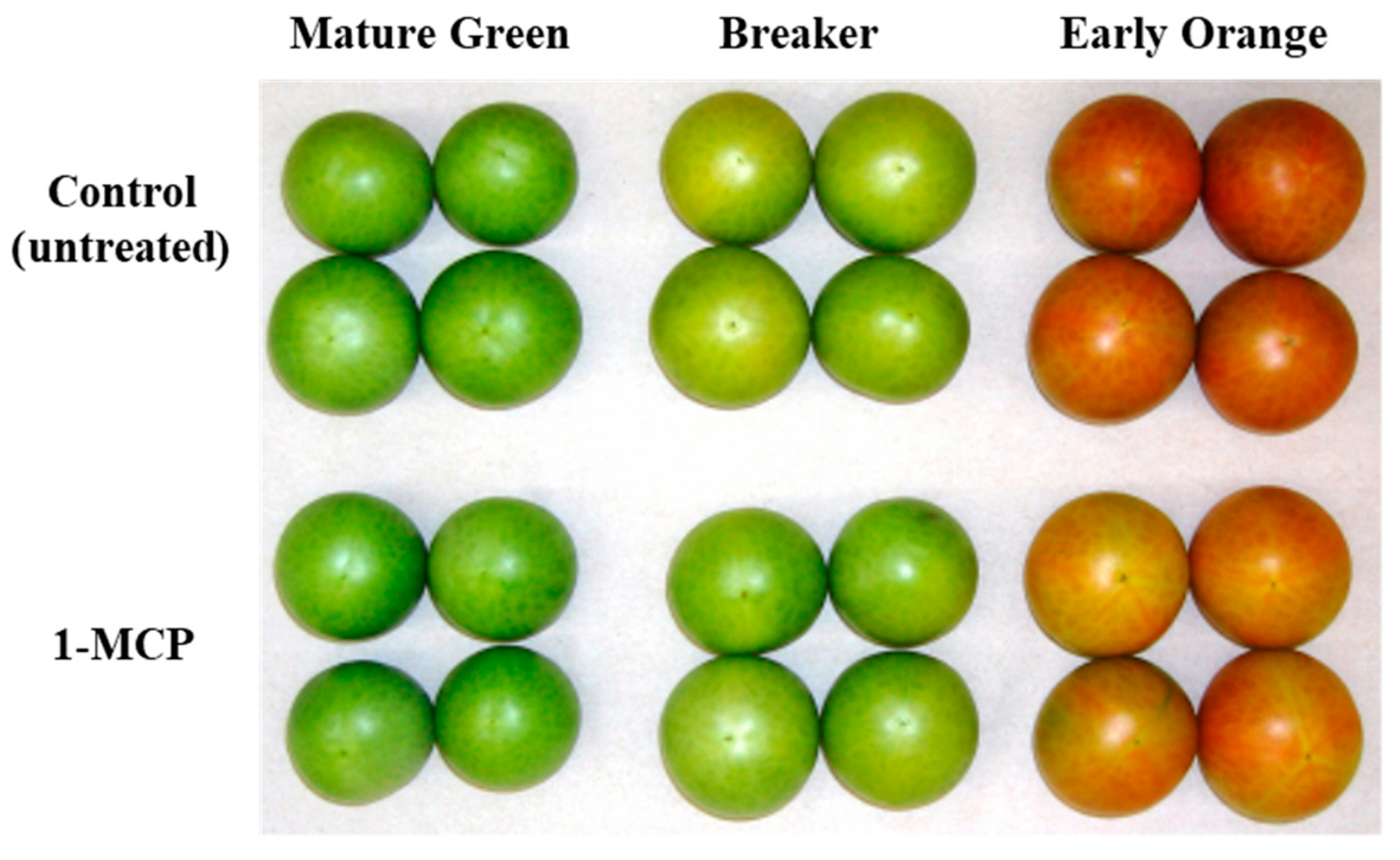
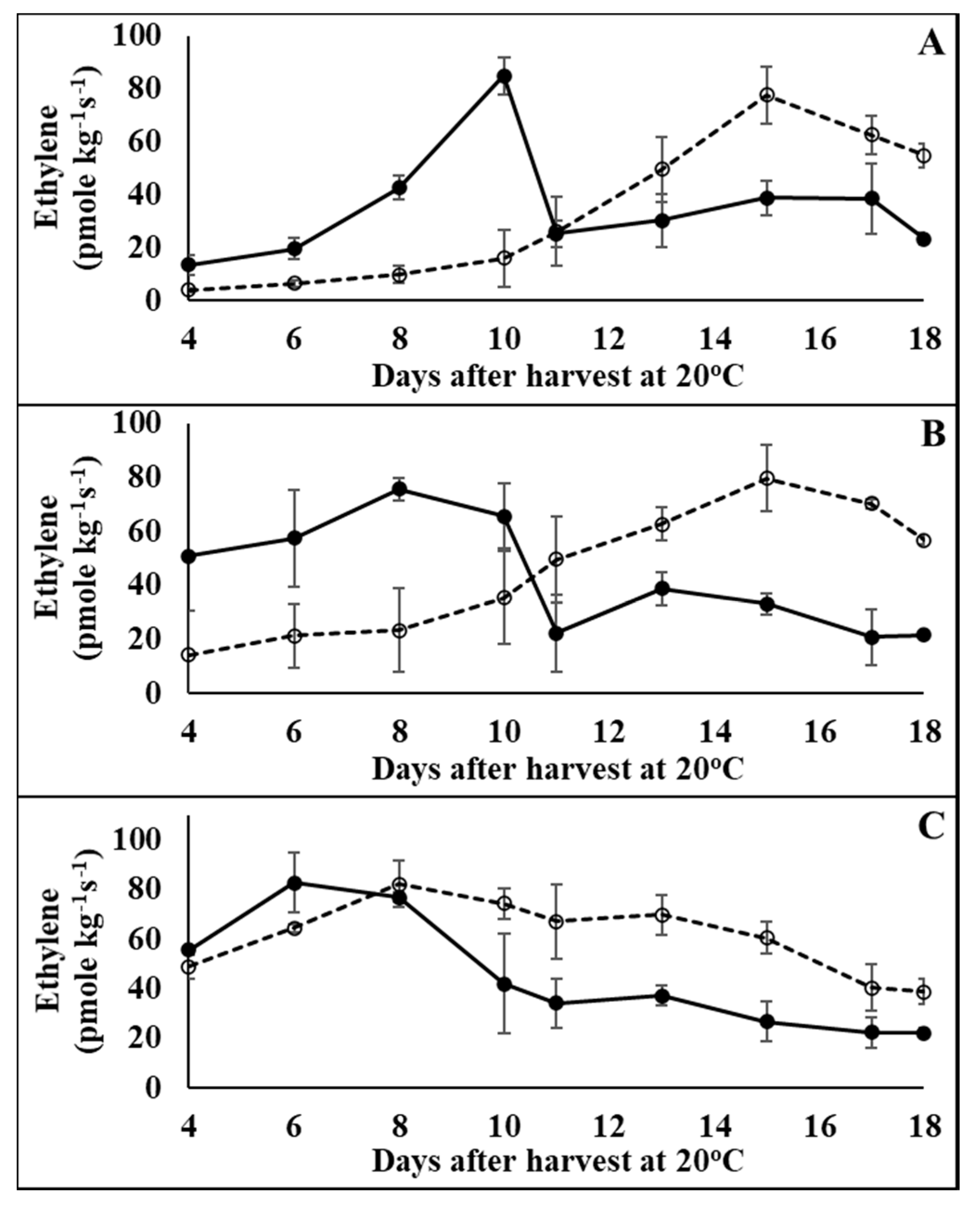
3.1. Physiological Effects of 1-MCP Treatment on Tomatoes at Pre-Climacteric Stages
3.2. The Effect of 1-MCP Treatment on the Expression Pattern of Tomatoes at Pre- Climacteric Stages
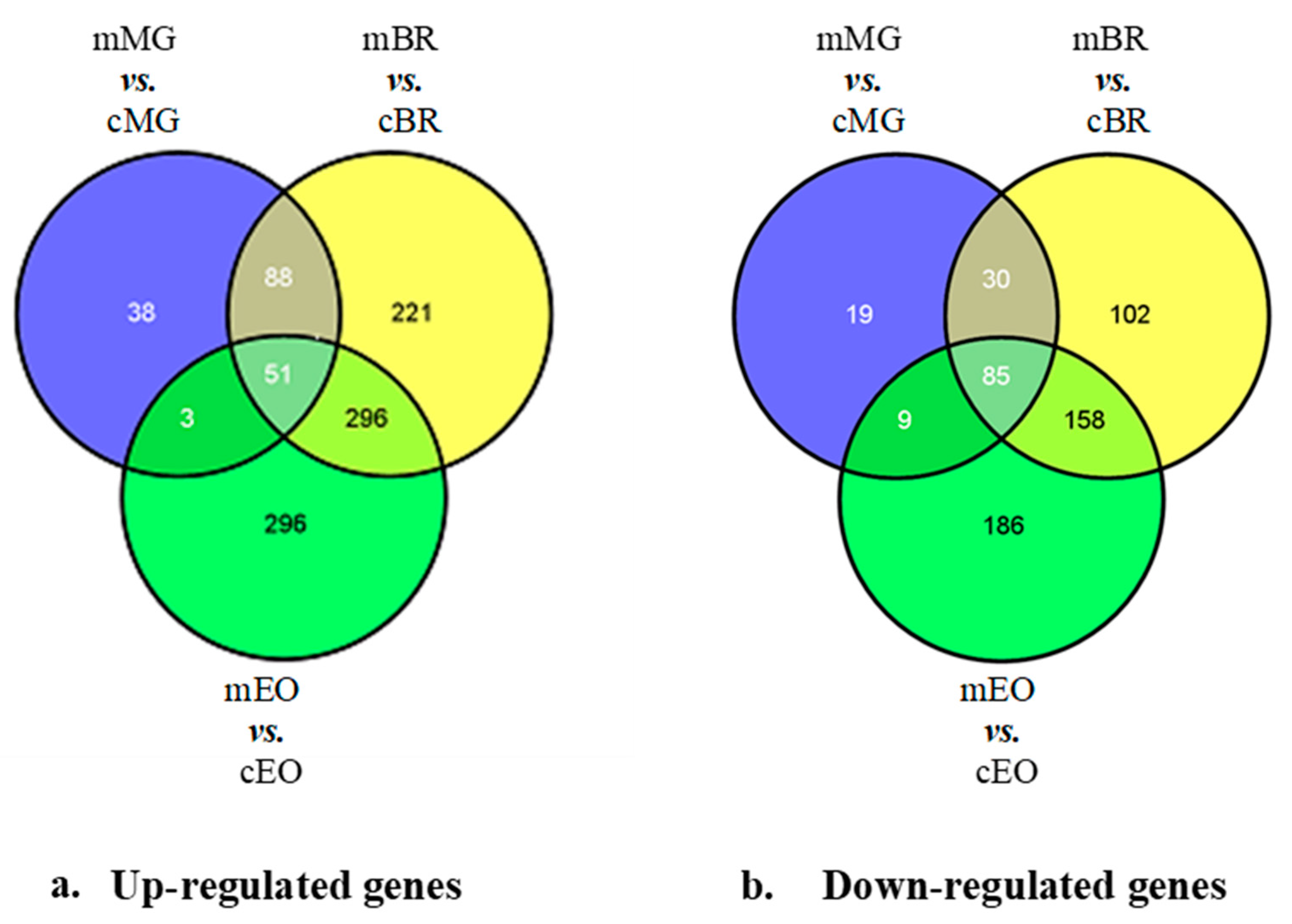
3.3. 1-MCP Responsive Transcripts Common to Tomatoes at Pre- Climacteric Stages
3.4. Gene Ontology (GO) Analysis for 1-MCP Responsive Transcripts Shared at Different Ripening Stages
3.5. Ripening Related Genes Common to Tomatoes at Pre-Climacteric Stages Affected by 1-MCP Treatment
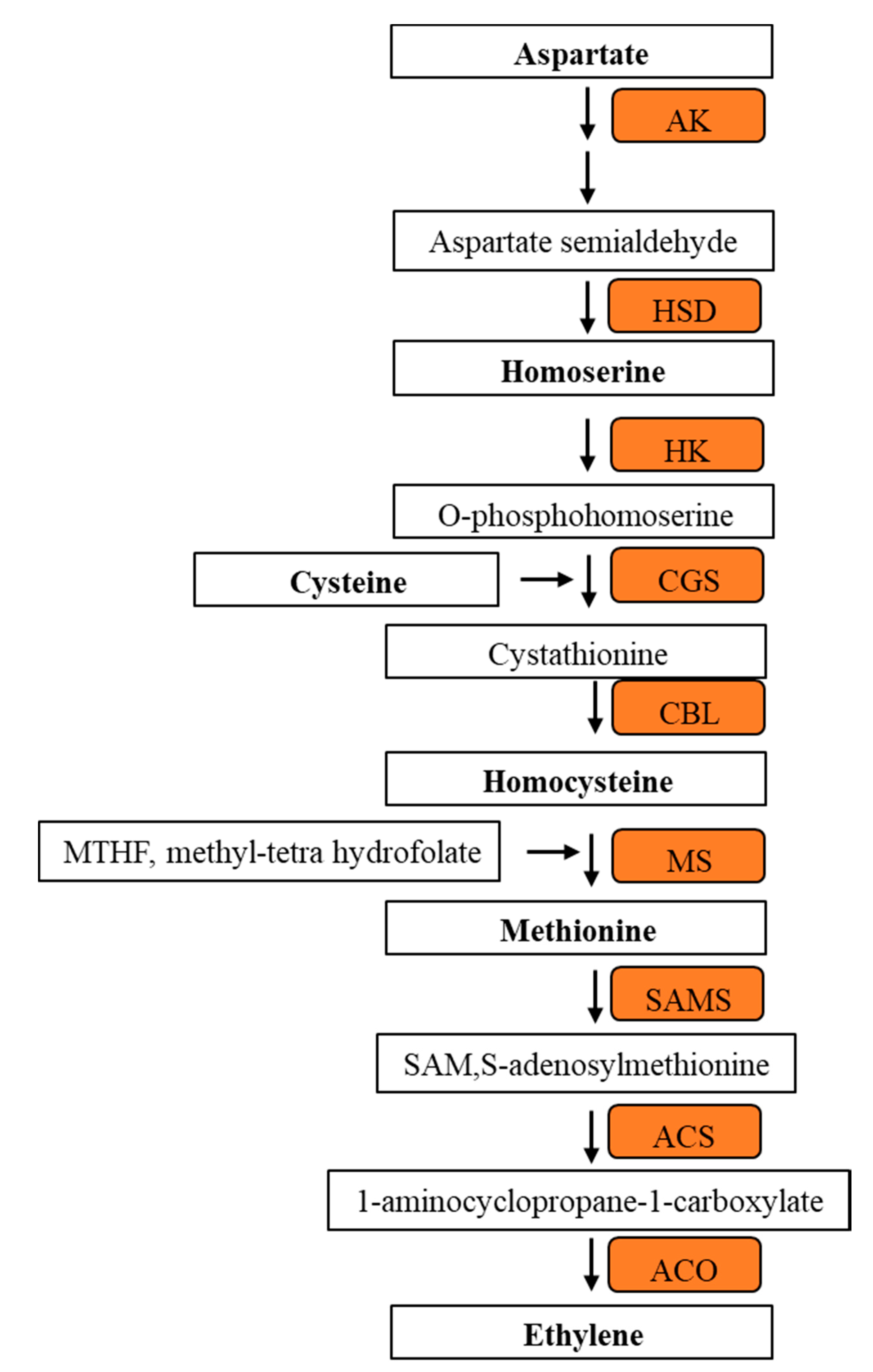
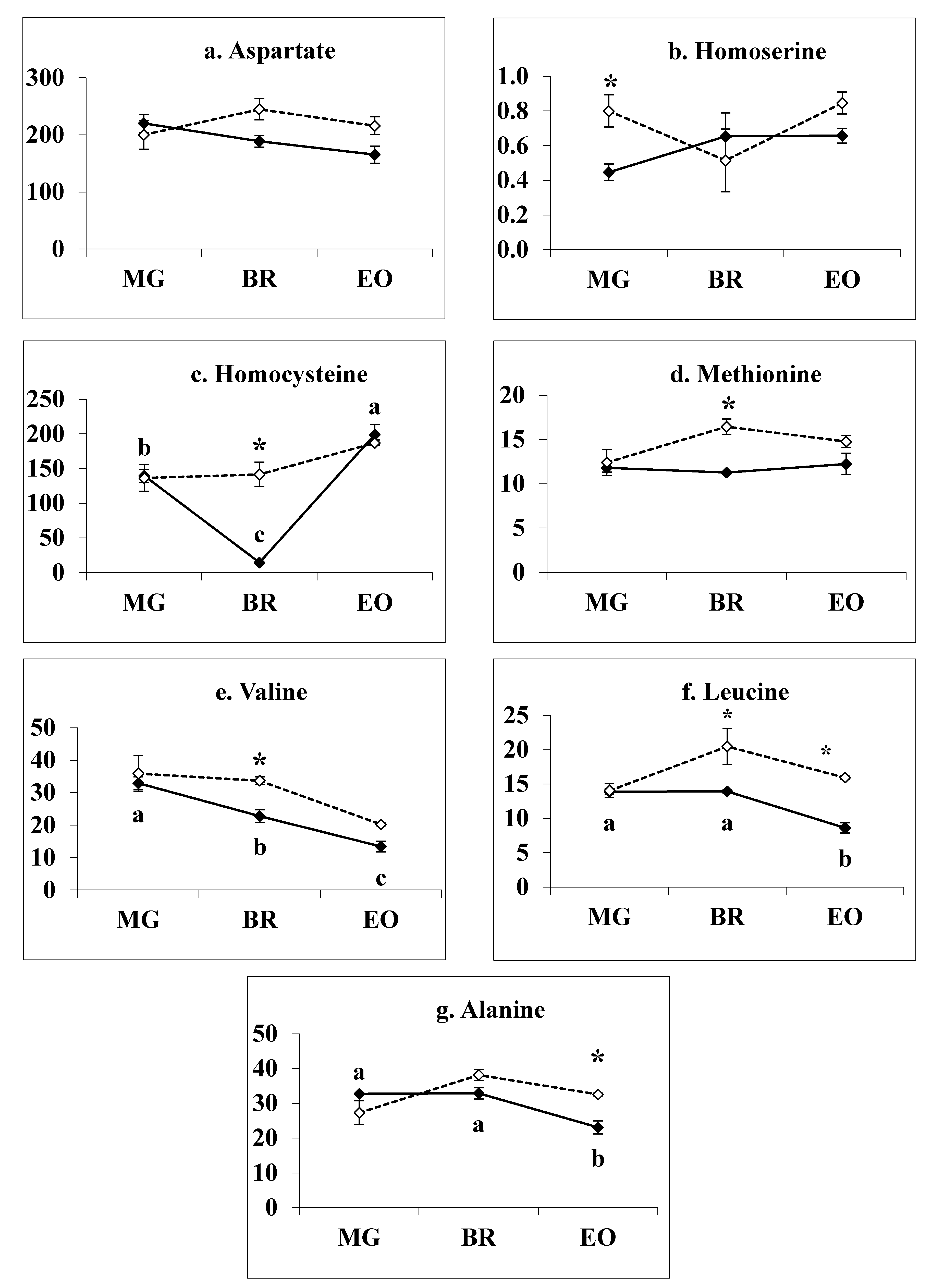
3.6. Methionine Related Metabolites in 1-MCP Treated Tomatoes at Pre- Climacteric Stages
3.7. Branch-Chain Amino Acids in 1-MCP Treated Tomatoes during Pre-Climacteric Ripening
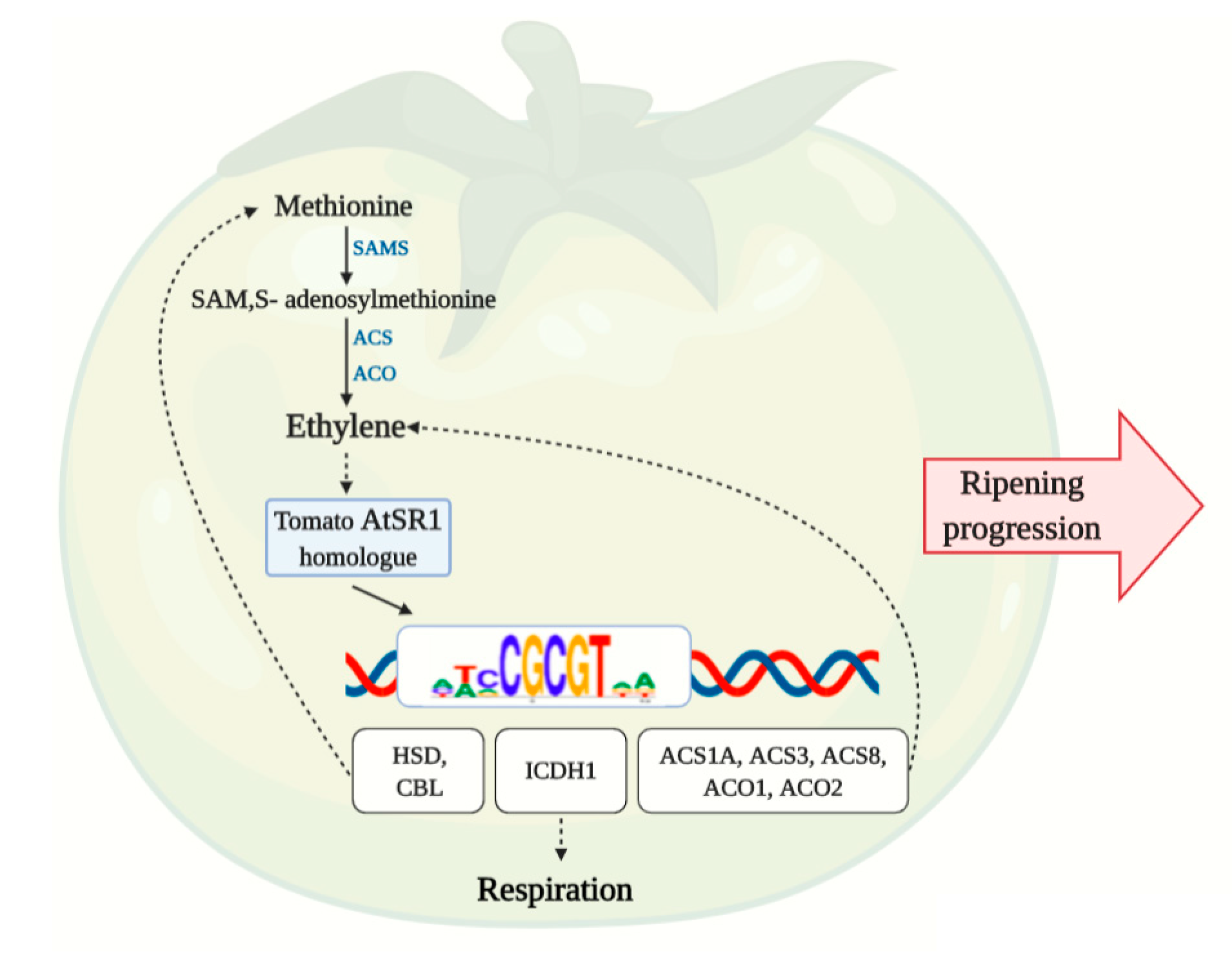
3.8. AtSR1 Binding Motif in the Promotors of Methionine and Ethylene Biosynthesis Pathway Genes
4. Discussion
4.1. The Genetic Tomato Pre-Climacteric Response to 1-MCP
4.2. Methionine Biosynthesis Metabolites Involved in Climacteric Ethylene Induction
4.3. Possible Shared Regulation of Respiration and Ethylene Production
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mcmurchie, E.J.; Mcglasson, W.B.; Eaks, I.L. Treatment of fruit with propylene gives information about the biogenesis of ethylene. Nature 1972, 237, 235–236. [Google Scholar] [CrossRef]
- Alexander, L.; Grierson, D. Ethylene biosynthesis and action in tomato: A model for climacteric fruit ripening. J. Exp. Bot. 2002, 53, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, E.E.; Huberman, M.; Goren, R. Probing the role of endogenous ethylene in the degreening of citrus fruit with ethylene antagonists. Plant Growth Regul. 1993, 12, 325–329. [Google Scholar] [CrossRef]
- Hiwasa, K.; Kinugasa, Y.; Amano, S.; Hashimoto, A.; Nakano, R.; Inaba, A.; Kubo, Y. Ethylene is required for both the initiation and progression of softening in pear (Pyrus communis L.) fruit. J. Exp. Bot. 2003, 54, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, A.; O’Connell, A.P. Gene expression analysis of strawberry achene and receptacle maturation using DNA microarrays. J. Exp. Bot. 2002, 53, 2073–2087. [Google Scholar] [CrossRef]
- Costa, F.; Alba, R.; Schouten, H.; Soglio, V.; Gianfranceschi, L.; Serra, S.; Musacchi, S.; Sansavini, S.; Costa, G.; Fei, Z.; et al. Use of homologous and heterologous gene expression profiling tools to characterize transcription dynamics during apple fruit maturation and ripening. BMC Plant Biol. 2010, 10, 229. [Google Scholar] [CrossRef]
- Lee, S.; Chung, E.J.; Joung, Y.H.; Choi, D. Non-climacteric fruit ripening in pepper: Increased transcription of EIL-like genes normally regulated by ethylene. Funct. Integr. Genom. 2010, 10, 135–146. [Google Scholar] [CrossRef]
- Giovannoni, J.J. Fruit ripening mutants yield insights into ripening control. Curr. Opin. Plant Biol. 2007, 10, 283–289. [Google Scholar] [CrossRef]
- Sozzi, G.; Beaudry, R. The potential to retard postharvest senescence using biotechnology. Stewart Postharvest Rev. 2007, 3, 1–16. [Google Scholar] [CrossRef]
- Gamrasni, D.; Ben-Arie, R.; Goldway, M. 1-Methylcyclopropene (1-MCP) application to Spadona pears at different stages of ripening to maximize fruit quality after storage. Postharvest Biol. Technol. 2010, 58, 104–112. [Google Scholar] [CrossRef]
- Tiwari, K.; Paliyath, G. Microarray analysis of ripening-regulated gene expression and its modulation by 1-MCP and hexanal. Plant Physiol. Biochem. 2011, 49, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Yokotani, N.; Yamaoka, T.; Ushijima, K.; Nakano, R.; Yano, K.; Aoki, K.; Kubo, Y. Characterization of ripening-associated genes using a tomato DNA macroarray, 1-methylcyclopropene, and ripening-impaired mutants. Postharvest Biol. Technol. 2013, 86, 159–170. [Google Scholar] [CrossRef]
- Bobokalonov, J.; Liu, Y.; Shahrin, T.; Liu, L. Transcriptomic Analysis on the Regulation of Tomato Ripening by the Ethylene Inhibitor 1-methylcyclopropene. J. Plant Stud. 2018, 7, 49. [Google Scholar] [CrossRef]
- Gamrasni, D.; Goldway, M.; Stern, Y.; Breitel, D.; Aharoni, A. 1-MCP (1-methylcyclopropene) Treatment Protocol for Fruit or Vegetables. Bio-Protocol. 2017, 7, 8–11. [Google Scholar] [CrossRef]
- Itkin, M.; Seybold, H.; Breitel, D.; Rogachev, I.; Meir, S.; Aharoni, A. TOMATO AGAMOUS-LIKE 1 is a component of the fruit ripening regulatory network. Plant J. 2009, 60, 1081–1095. [Google Scholar] [CrossRef]
- Verwoerd, T.C.; Dekker, B.M.; Hoekema, A. Nucleic Acids Research. Nucleic Acids Res. 1989, 17, 2362. [Google Scholar] [CrossRef]
- Irizarry, R.A.; Bolstad, B.M.; Collin, F.; Cope, L.M.; Hobbs, B.; Speed, T.P. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003, 31, e15. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Amira, G.; Ifat, M.; Tal, A.; Hana, B.; Shmuel, G.; Rachel, A. Soluble methionine enhances accumulation of a 15 kDa zein, a methionine-rich storage protein, in transgenic alfalfa but not in transgenic tobacco plants. J. Exp. Bot. 2005, 56, 2443–2452. [Google Scholar] [CrossRef]
- Katz, Y.S.; Galili, G.; Amir, R. Regulatory role of cystathionine-γ-synthase and de novo synthesis of methionine in ethylene production during tomato fruit ripening. Plant Mol. Biol. 2006, 61, 255–268. [Google Scholar] [CrossRef]
- Yang, T.; Poovaiah, B.W. A calmodulin-binding/CGCG box DNA-binding protein family involved in multiple signaling pathways in plants. J. Biol. Chem. 2002, 277, 45049–45058. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Kwok, S.F.; Bleecker, A.B.; Meyerowitz, E.M. Arabidopsis ethylene-response gene ETR1: Similarity of product to two-component regulators. Science 1993, 262, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Randlett, M.D.; Findell, J.L.; Schallert, G.E. Localization of the ethylene receptor ETR1 to the endoplasmic reticulum of Arabidopsis. J. Biol. Chem. 2002, 277, 19861–19866. [Google Scholar] [CrossRef]
- Gamrasni, D.; Erov, M.; Saar, L.; Raz, A.; Glikman, M.; Sonawane, P.D.; Aharoni, A.; Goldway, M. The isocitrate dehydrogenase 1 gene is associated with the climacteric response in tomato fruit ripening. Postharvest Biol. Technol. 2020, 166, 111219. [Google Scholar] [CrossRef]
- Tcherkez, G.; Gauthier, P.; Buckley, T.N.; Busch, F.A.; Barbour, M.M.; Bruhn, D.; Heskel, M.A.; Gong, X.Y.; Crous, K.Y.; Griffin, K.; et al. Leaf day respiration: Low CO2 flux but high significance for metabolism and carbon balance. New Phytol. 2017, 216, 986–1001. [Google Scholar] [CrossRef]
- Santner, A.; Estelle, M. Recent advances and emerging trends in plant hormone signalling. Nature 2009, 459, 1071–1078. [Google Scholar] [CrossRef]
- Huber, D.J. Suppression of Ethylene Responses through Application of 1-Methylcyclopropene: A Powerful Tool for Elucidating Ripening and Senescence Mechanisms in Climacteric and Nonclimacteric Fruits and Vegetables. HortScience 2008, 43, 106–111. [Google Scholar] [CrossRef]
- Mata, C.I.; Van de Poel, B.; Hertog, M.L.A.T.M.; Tran, D.; Nicolai, B.M. Transcription analysis of the ethylene receptor and CTR genes in tomato: The effects of on and off-vine ripening and 1-MCP. Postharvest Biol. Technol. 2018, 140, 67–75. [Google Scholar] [CrossRef]
- Kevany, B.M.; Tieman, D.M.; Taylor, M.G.; Cin, V.D.; Klee, H.J. Ethylene receptor degradation controls the timing of ripening in tomato fruit. Plant J. 2007, 51, 458–467. [Google Scholar] [CrossRef]
- Mata, C.I.; Fabre, B.; Parsons, H.T.; Hertog, M.L.A.T.M.; Van Raemdonck, G.; Baggerman, G.; Van de Poel, B.; Lilley, K.S.; Nicolaï, B.M. Ethylene receptors, CTRS and EIN2 target protein identification and quantification through parallel reaction monitoring during tomato fruit ripening. Front. Plant Sci. 2018, 9, 1626. [Google Scholar] [CrossRef]
- Adams, D.O.; Yang, S.F. Methionine metabolism in apple tissue. Plant Physiol. 1977, 60, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Martin, M.N.; Lee, J.; Muhitch, M.J.; Leustek, T.; Brunswick, N. Methionine and threonine synthesis are limited by homoserine availability and not the activity of homoserine kinase in Arabidopsis thaliana. Plant J. 2005, 41, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Galili, G.; Amir, R.; Fernie, A.R. The Regulation of Essential Amino Acid Synthesis and Accumulation in Plants. Annu. Rev. Plant Biol. 2016, 67, 153–178. [Google Scholar] [CrossRef]
- Hacham, Y.; Matityahu, I.; Schuster, G.; Amir, R.; Galilee, M. Overexpression of mutated forms of aspartate kinase and cystathionine c -synthase in tobacco leaves resulted in the high accumulation of methionine and threonine. Plant J. 2008, 54, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Licausi, F.; Araújo, W.L.; Nunes-Nesi, A.; Sodek, L.; Fernie, A.R.; van Dongen, J.T. Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol. 2010, 152, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Araújo, W.L.; Ishizaki, K.; Nunes-Nesi, A.; Larson, T.R.; Tohge, T.; Krahnert, I.; Witt, S.; Obata, T.; Schauer, N.; Graham, I.A.; et al. Identification of the 2-hydroxyglutarate and isovaleryl-CoA dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of Arabidopsis mitochondria. Plant Cell 2010, 22, 1549–1563. [Google Scholar] [CrossRef] [PubMed]

| Gene Ontology Terms Classification | Corrected p-Value |
|---|---|
| Molecular function | |
| two-component response regulator activity | <0.001 |
| protein histidine kinase activity | <0.001 |
| isocitrate dehydrogenase (NADP+) activity | <0.001 |
| ion binding | 0.041 |
| oxidoreductase activity | 0.009 |
| monooxygenase activity | 0.009 |
| Biological process | |
| peptidyl-histidine phosphorylation | 0.001 |
| isocitrate metabolic process | 0.002 |
| glyoxylate cycle | 0.028 |
| response to hormone stimulus | 0.034 |
| response to endogenous stimulus | 0.008 |
| anatomical structure development | 0.024 |
| Cellular component | |
| endoplasmic reticulum membrane | 0.02 |
| extracellular region | <0.001 |
| Affymetrix Gene Code | MG | BR | EO | Annotation |
|---|---|---|---|---|
| les.3662.1.s1_at | −3.7 | −5.1 | −2.8 | ACS2, 1−aminocyclopropane-1-carboxylate synthase 2 |
| les.5917.1.s1_at | −7.1 | −2.9 | −6.0 | ACO5, 1-aminocyclopropane-1-carboxylate oxidase 1 |
| les.132.1.s1_at | −4.1 | −12.8 | −11.2 | ACO4,1-aminocyclopropane-1-carboxylate oxidase 4 |
| les.244.2.s1_at | −5.6 | −3.1 | −2.3 | 1-aminocyclopropane-1-carboxylate oxidase homolog |
| lesaffx.18025.1.s1_at | −2.8 | −7.8 | −67.1 | oxidoreductase, 2OG-Fe(II) oxygenase family protein |
| les.3465.1.s1_at, | −24.8 | −19.8 | −8.2 | ETR2, ethylene response 2 receptor |
| les.36.1.s1_at, | −2.5 | −3.0 | −3.5 | ETR4, ethylene response 4 receptor |
| les.35.1.s1_at | −3.7 | −2.2 | −2.3 | ETR5, ethylene response 5 receptor |
| les.85.1.s1_at | −4.9 | −7.2 | −3.4 | ERS1, ethylene-responsive sensor 1 |
| les.876.1.a1_at | 3.7 | 21.4 | 2.6 | ERF1, Ethylene response factor 1 |
| les.4101.2.s1_a_at | 2.6 | 4.2 | 3.9 | ERF2, Ethylene response factor 2 |
| les.3311.3.s1_at | −2.6 | −4.8 | −5.6 | ICDH1, Isocitrate dehydrogenase (NADP+) |
| les.2518.1.a1_at, | −2.3 | −5.7 | −4.8 | SAT-1, serine O-acetyltransferase |
| les.2518.2.a1_at, | −2.4 | −5.9 | −8.6 | SAT-1, serine O-acetyltransferase |
| les.2518.3.a1_at | −2.3 | −5.0 | −13.0 | SAT-1, serine O-acetyltransferase |
| les.3323.1.s1_at | −3.3 | −3.5 | −2.9 | CGS, cystathionine gamma synthase |
| LesAffx.67643.1.S1_at | −3.4 | −19.5 | −10.4 | L-asparaginase |
| Les.290.1.S1_at | −69.9 | −307.0 | −17.3 | HDC, histidine decarboxylase |
| Description | Locus Name | Upstream to ATG |
|---|---|---|
| Aspartokinase/homoserine dehydrogenase | Solyc11g040390 | −1004 |
| Aspartokinase/homoserine dehydrogenase | Solyc11g072010 | −1361 |
| Cystathionine beta-lyase | Solyc04g055230 | −691 |
| Cystathionine beta-lyase | Solyc08g066620 | −1598 |
| * 1-aminocyclopropane-1-carboxylate synthase 1a | Solyc08g081550 | −2191 |
| 1-aminocyclopropane-1-carboxylate synthase 3 | Solyc02g091990 | −352 |
| 1-aminocyclopropane- 1-carboxylate synthase 8 | Solyc03g043890 | −309 |
| * 1-aminocyclopropane-1-carboxylate oxidase 1 | Solyc07g049530 | −653 |
| 1-aminocyclopropane-1-carboxylate oxidase 2 | Solyc12g005940 | −630 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamrasni, D.; Feldmesser, E.; Ben-Arie, R.; Raz, A.; Tabatznik Asiag, A.; Glikman, M.; Aharoni, A.; Goldway, M. Gene Expression in 1-Methylcyclopropene (1-MCP) Treated Tomatoes during Pre-Climacteric Ripening Suggests Shared Regulation of Methionine Biosynthesis, Ethylene Production and Respiration. Agronomy 2020, 10, 1669. https://doi.org/10.3390/agronomy10111669
Gamrasni D, Feldmesser E, Ben-Arie R, Raz A, Tabatznik Asiag A, Glikman M, Aharoni A, Goldway M. Gene Expression in 1-Methylcyclopropene (1-MCP) Treated Tomatoes during Pre-Climacteric Ripening Suggests Shared Regulation of Methionine Biosynthesis, Ethylene Production and Respiration. Agronomy. 2020; 10(11):1669. https://doi.org/10.3390/agronomy10111669
Chicago/Turabian StyleGamrasni, Dan, Ester Feldmesser, Ruth Ben-Arie, Amir Raz, Amit Tabatznik Asiag, Michal Glikman, Asaph Aharoni, and Martin Goldway. 2020. "Gene Expression in 1-Methylcyclopropene (1-MCP) Treated Tomatoes during Pre-Climacteric Ripening Suggests Shared Regulation of Methionine Biosynthesis, Ethylene Production and Respiration" Agronomy 10, no. 11: 1669. https://doi.org/10.3390/agronomy10111669
APA StyleGamrasni, D., Feldmesser, E., Ben-Arie, R., Raz, A., Tabatznik Asiag, A., Glikman, M., Aharoni, A., & Goldway, M. (2020). Gene Expression in 1-Methylcyclopropene (1-MCP) Treated Tomatoes during Pre-Climacteric Ripening Suggests Shared Regulation of Methionine Biosynthesis, Ethylene Production and Respiration. Agronomy, 10(11), 1669. https://doi.org/10.3390/agronomy10111669





