Impact of Drought Exerted during Spike Development on Tillering, Yield Parameters and Grain Chemical Composition in Semi-Dwarf Barley Mutants Deficient in the Brassinosteroid Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Plant Growth Conditions and Experimental Designs
2.2.1. Plant Vegetation under Conditions of Optimal Watering and Water Scarcity in the Pot Experiment
2.2.2. Vegetation and Plant Yield Analysis under the Field Conditions
2.3. Analysis of the Grain Chemical Composition
2.4. Statistical Analysis
3. Results
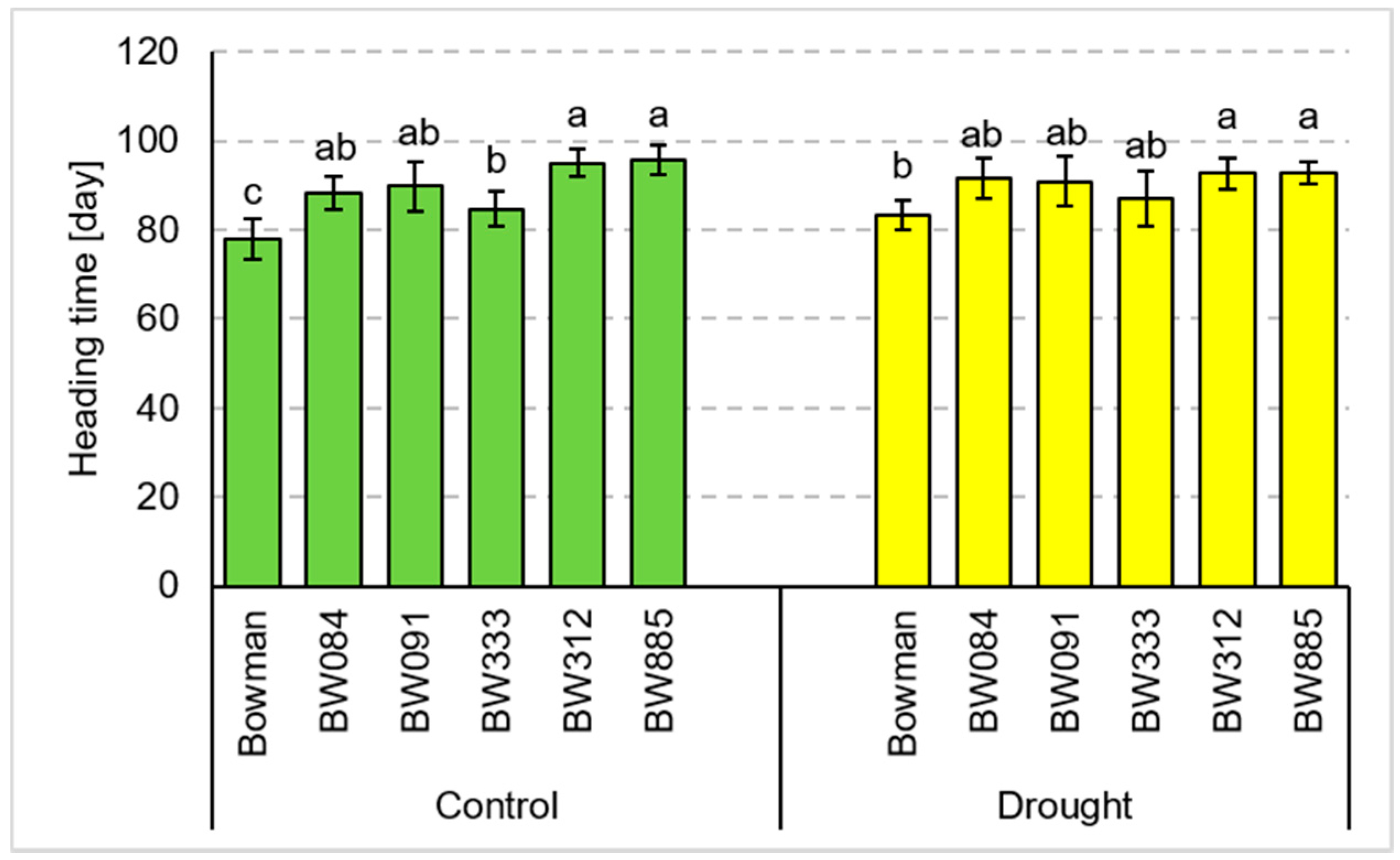
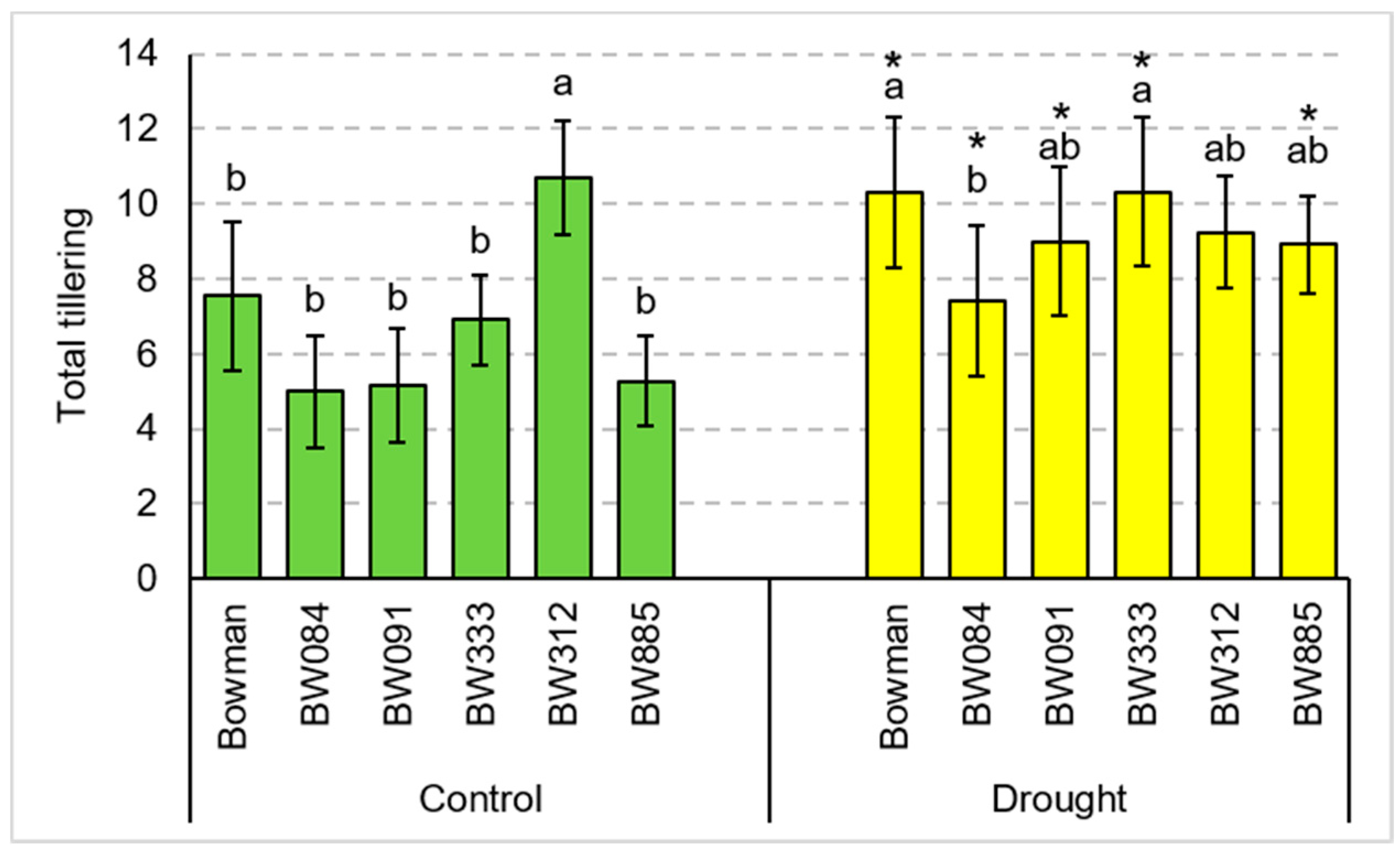
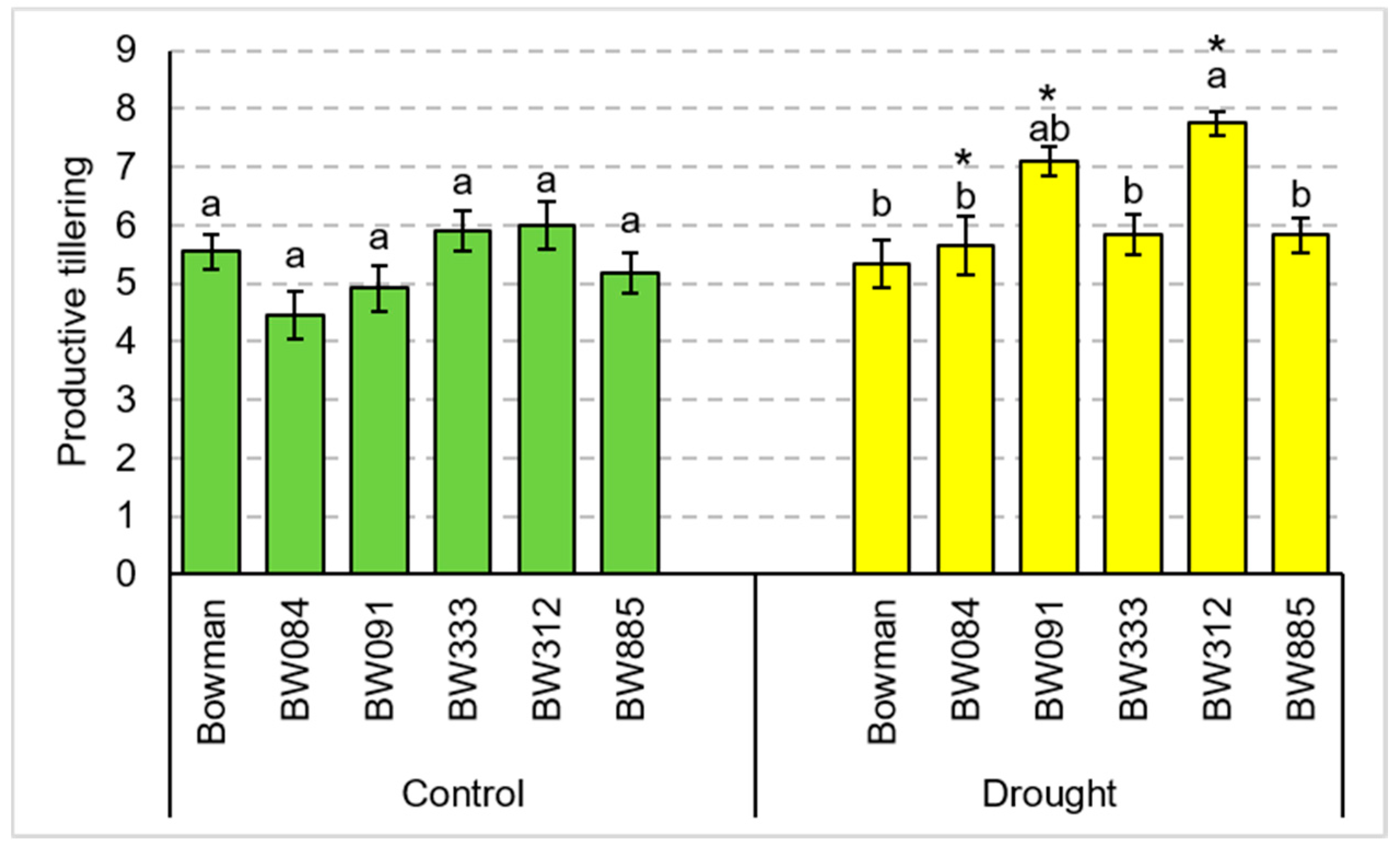
3.1. Total Tillering and Productive Tillering under the Conditions of Optimal Watering and Drought Exerted during Spike Development (the Pot Experiment)
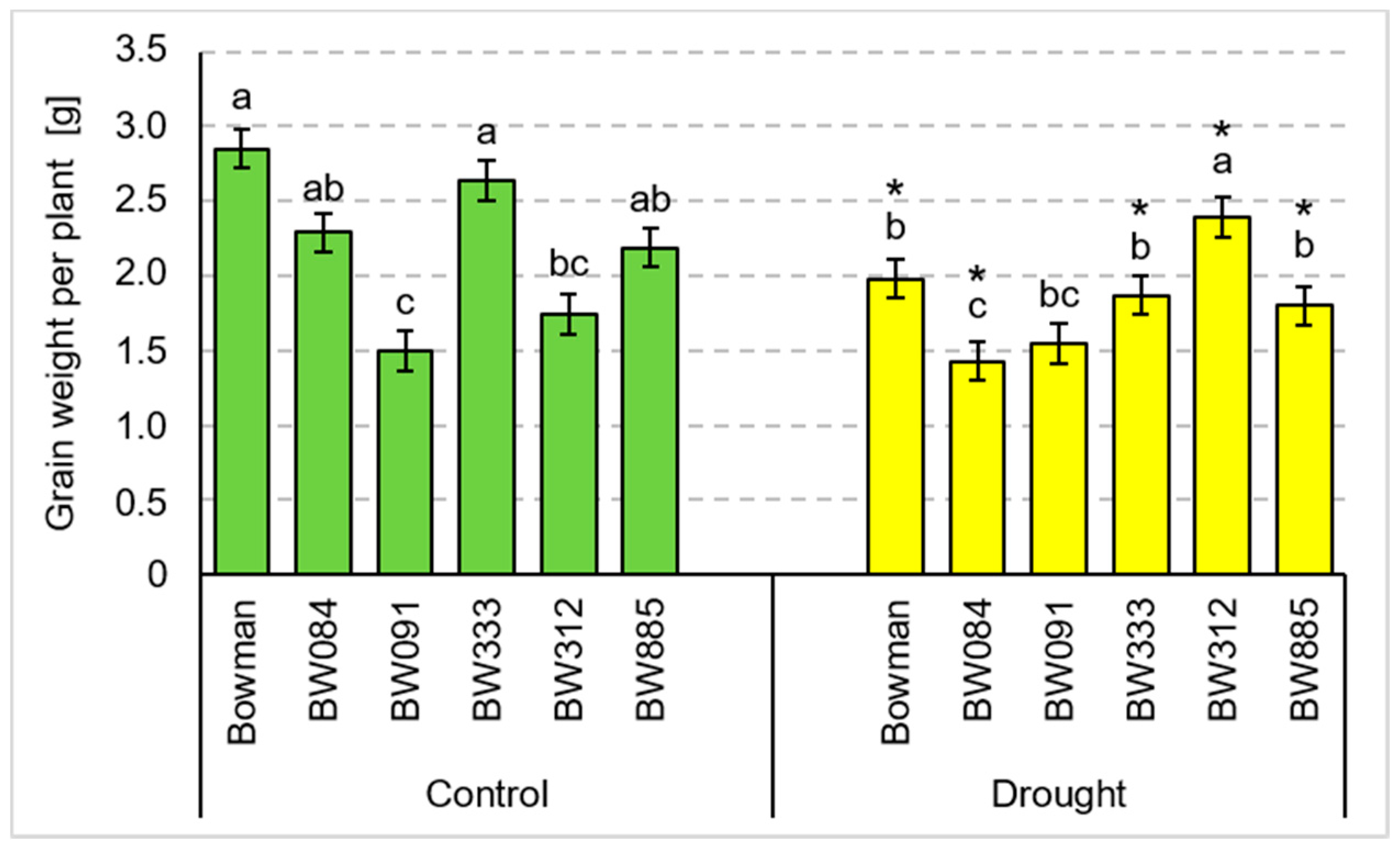
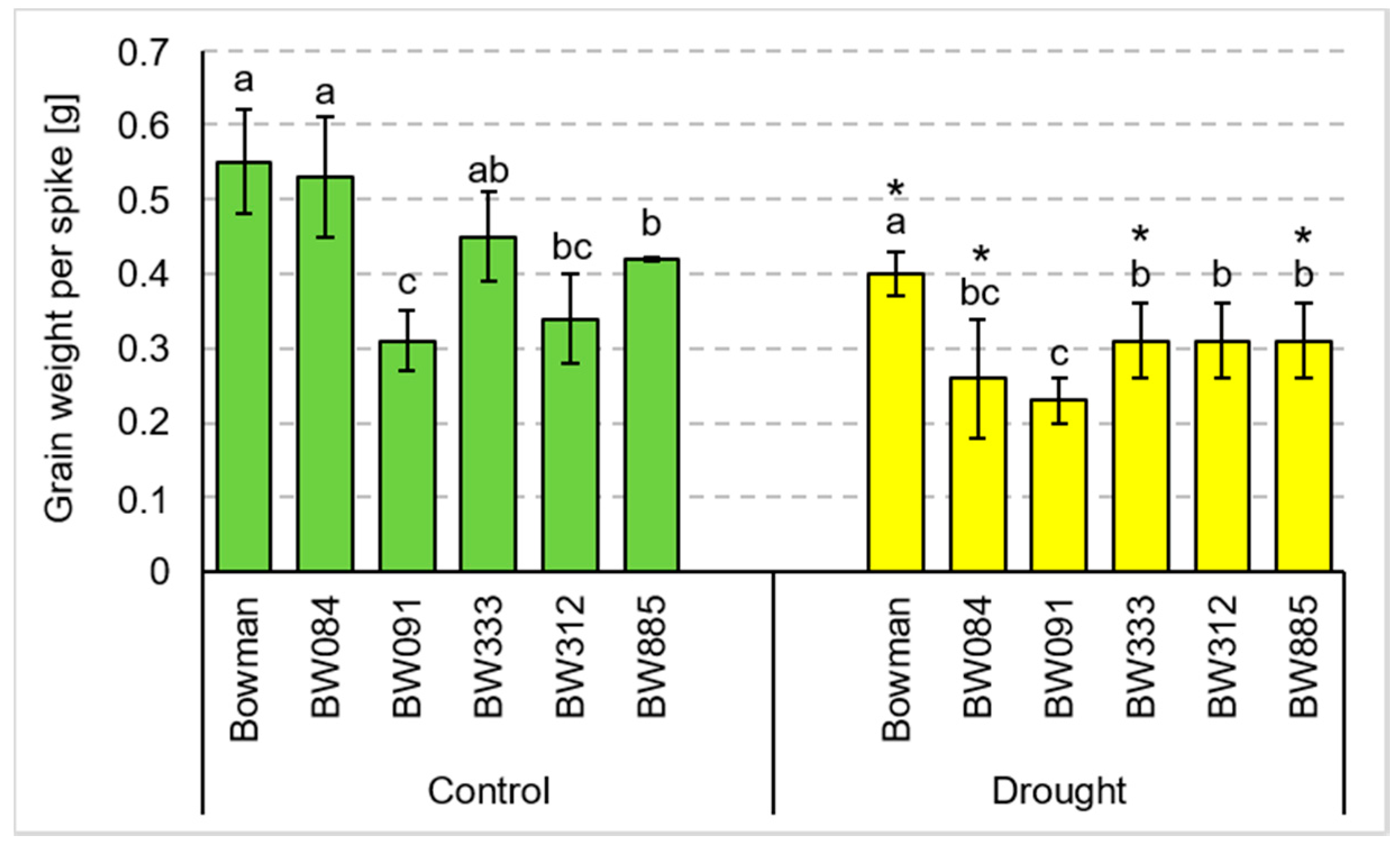
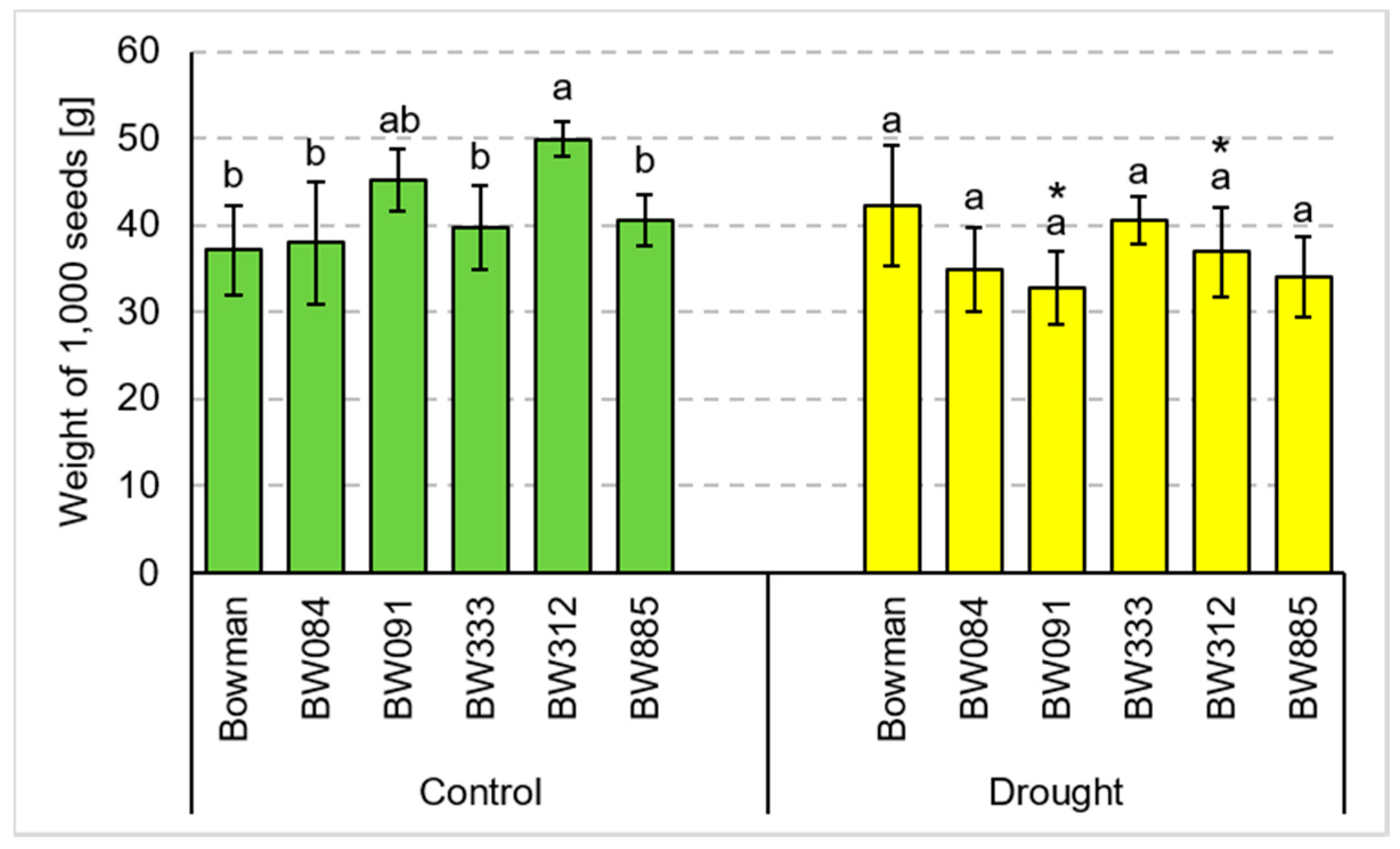
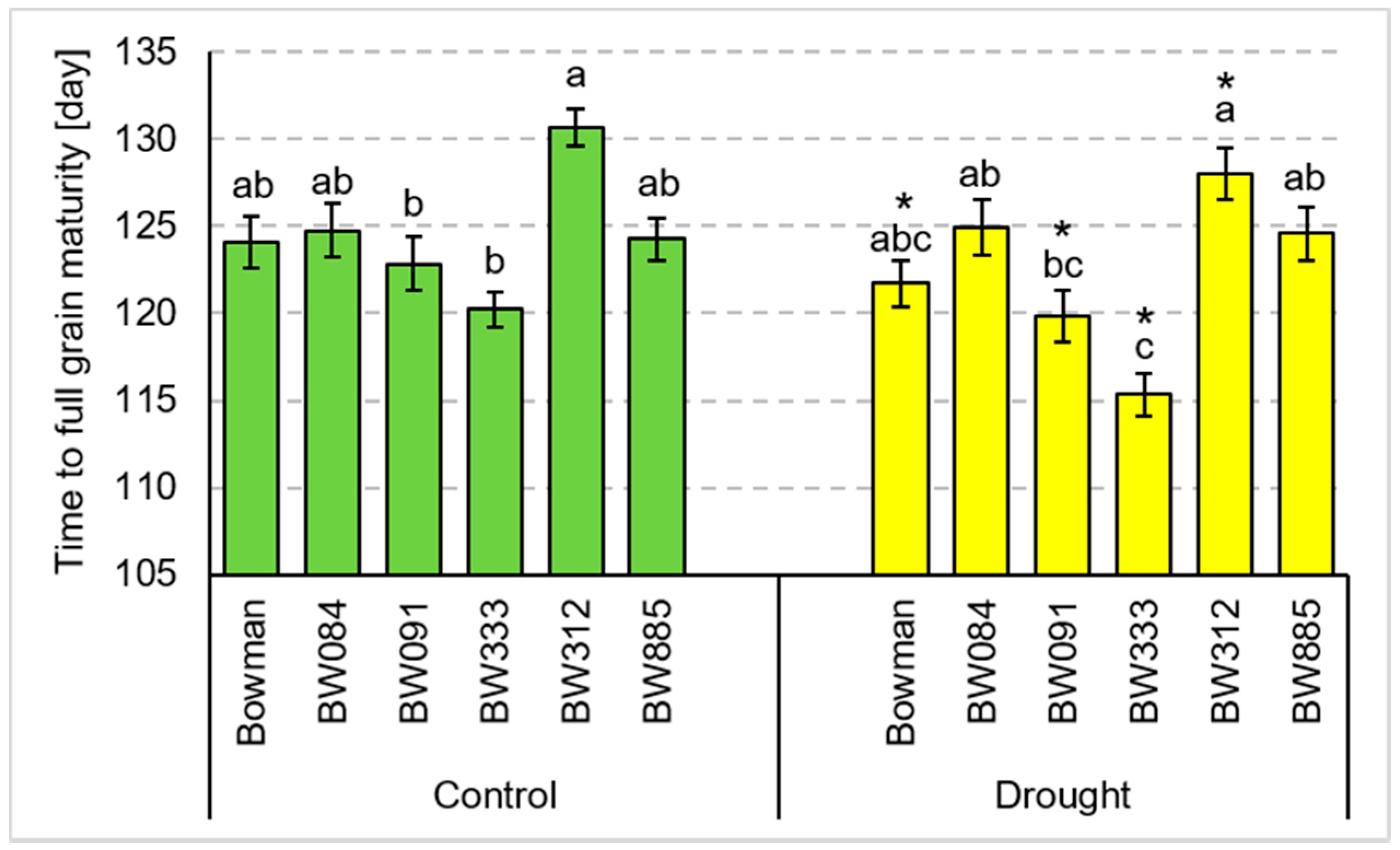
3.2. Yield Parameters under the Conditions of Optimal Watering and Drought Exerted during Spike Development (the Pot Experiment)
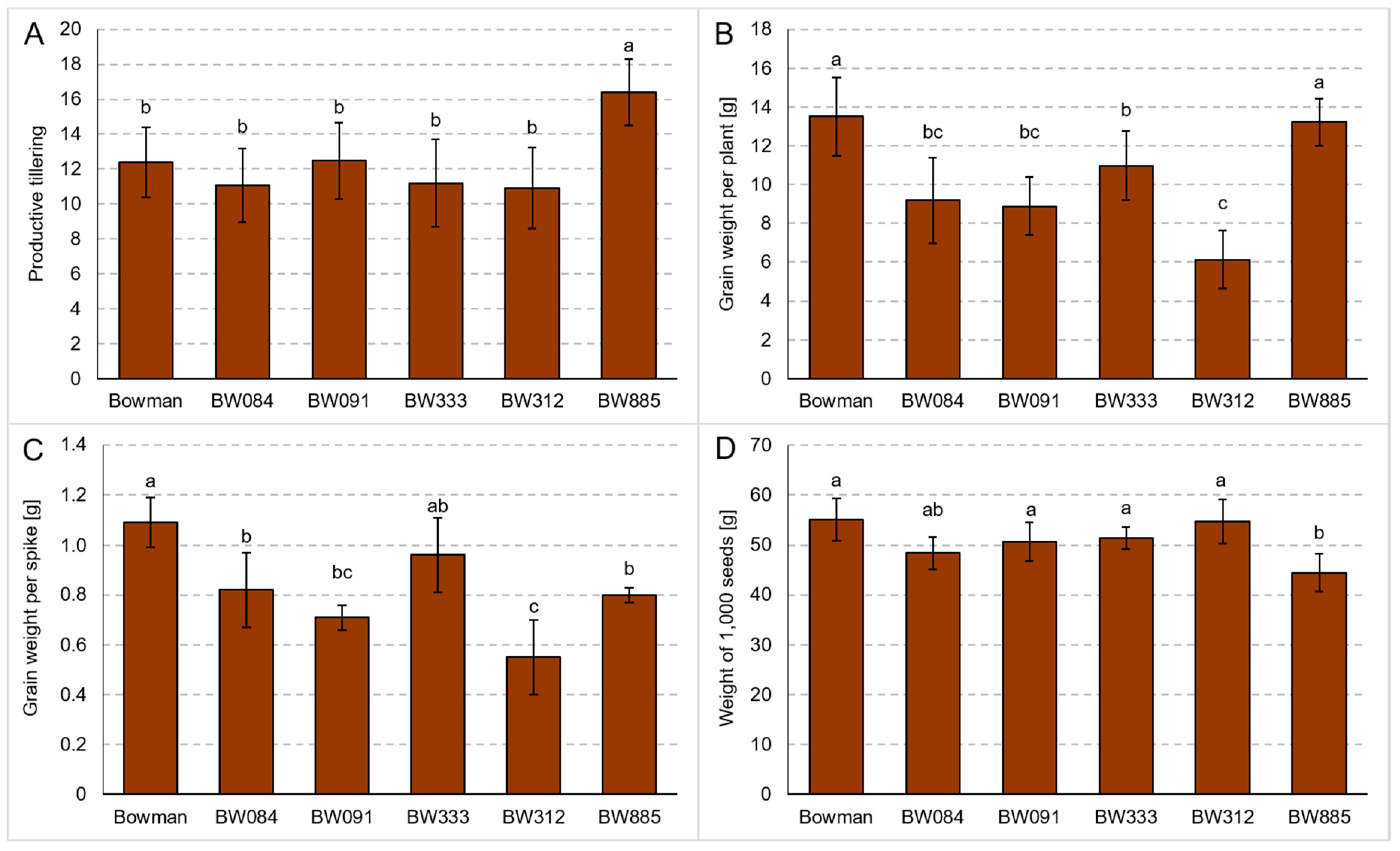
3.3. Tillering and Yield Parameters under the Field Conditions
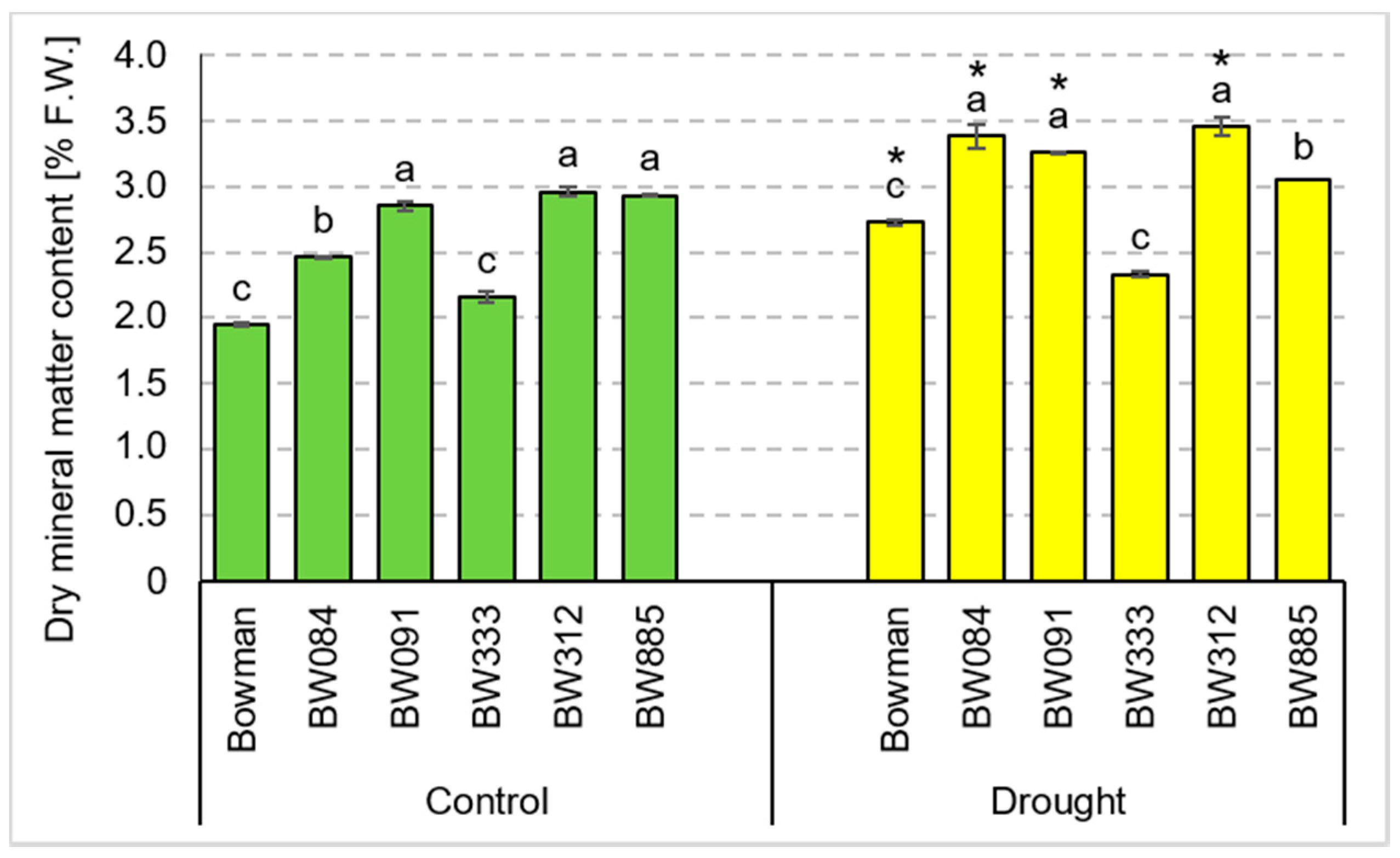
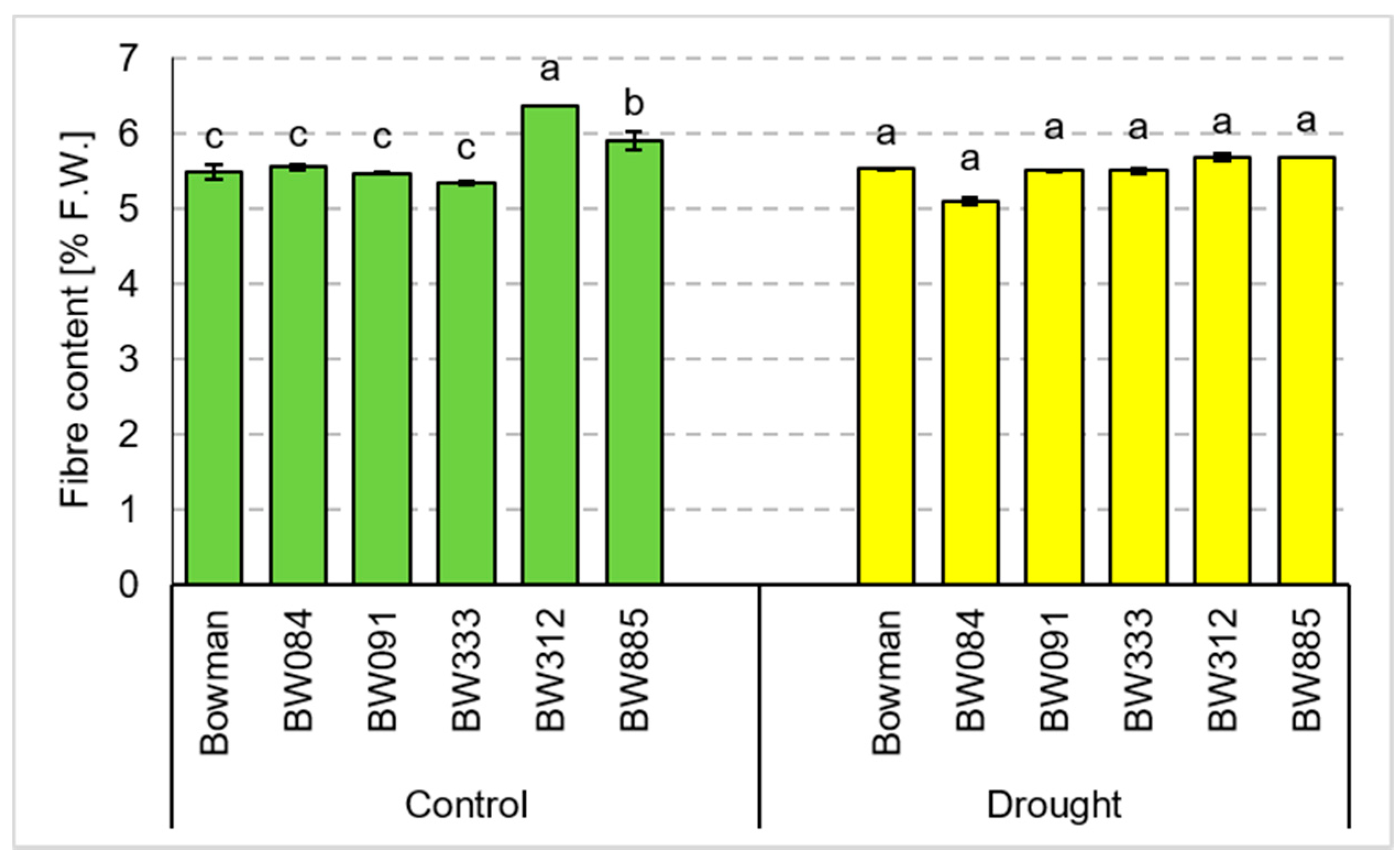
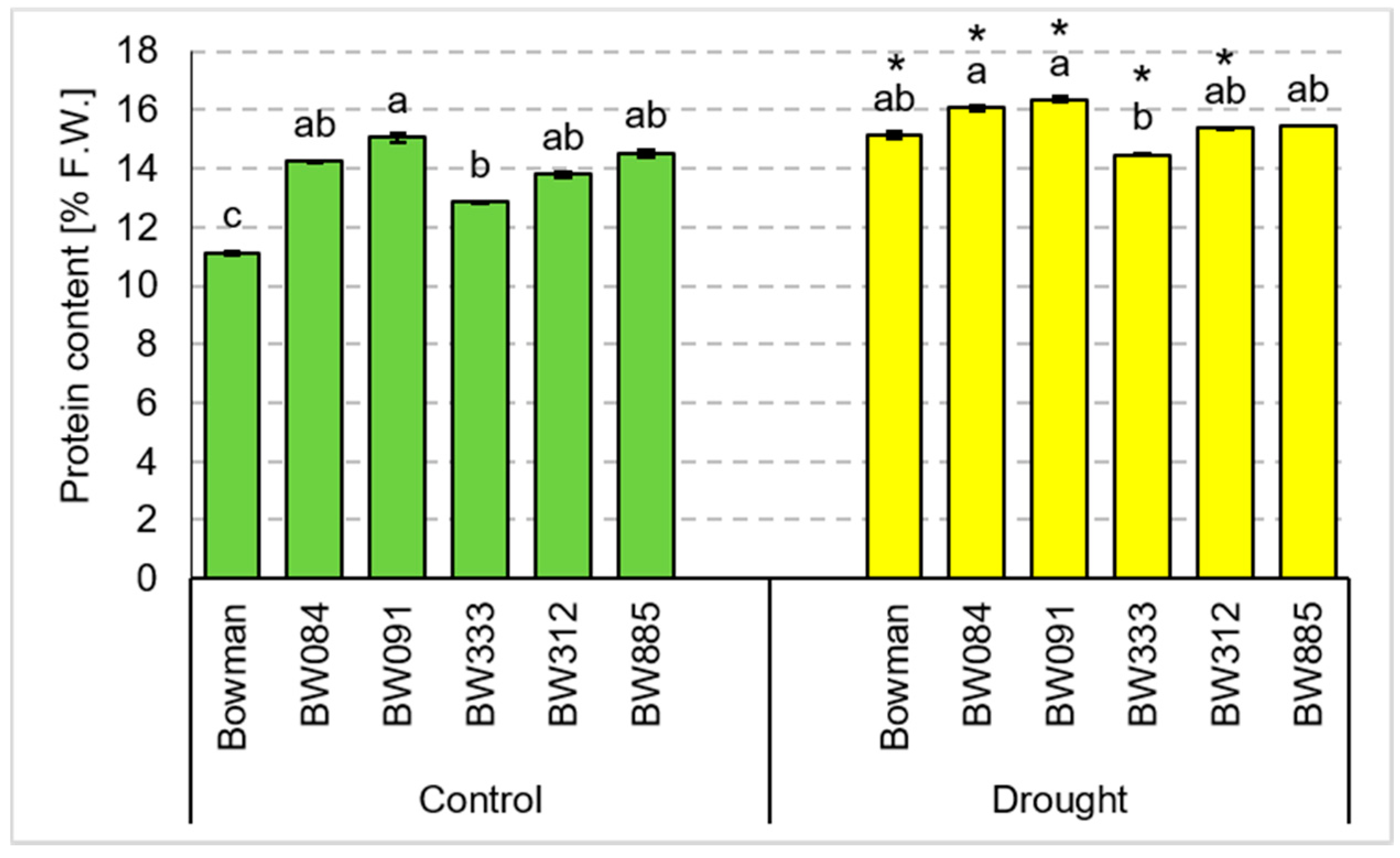
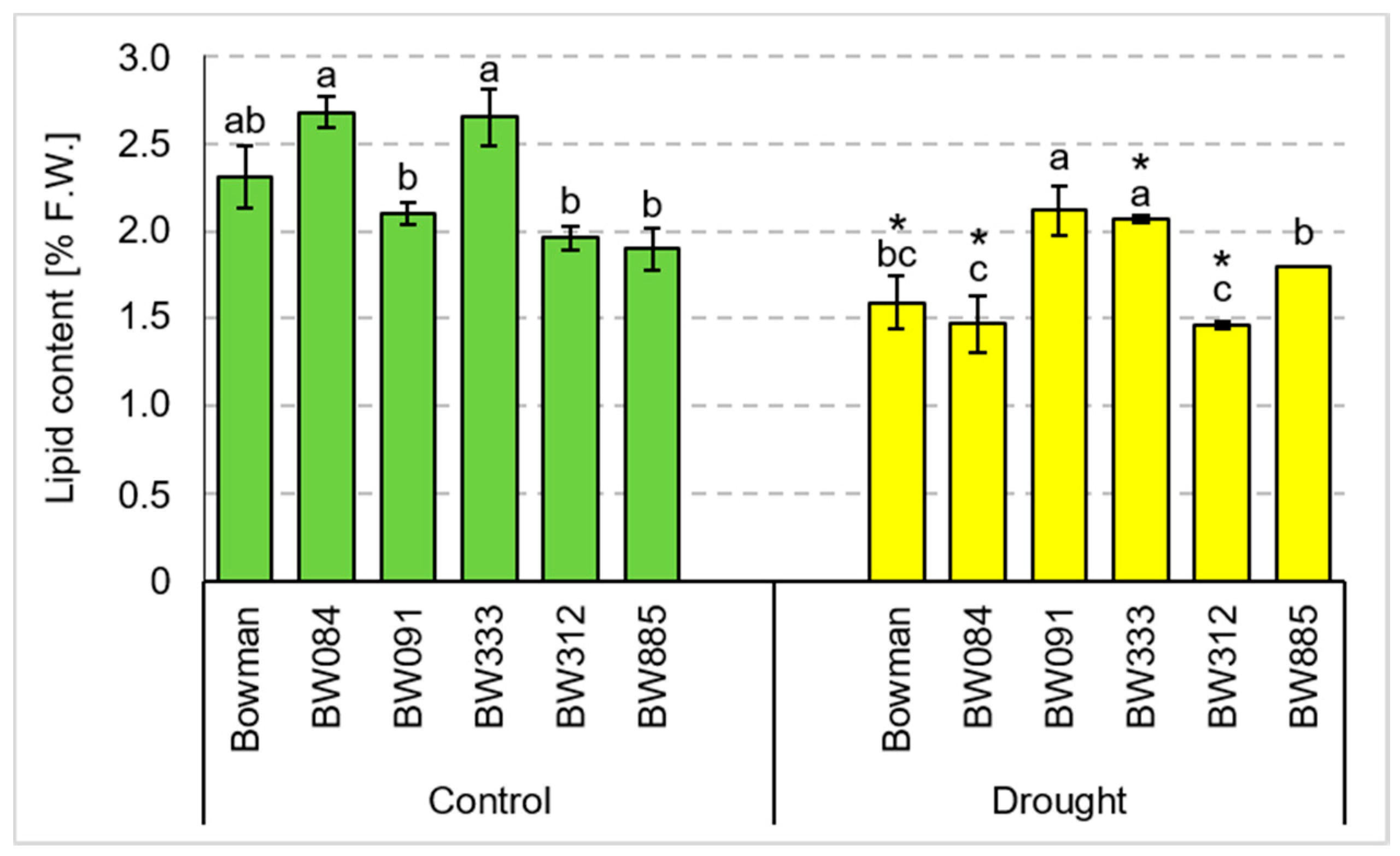
3.4. Chemical Composition of Grains Produced under the Conditions of Optimal Watering and Drought Exerted during Spike Development (the Pot Experiment)
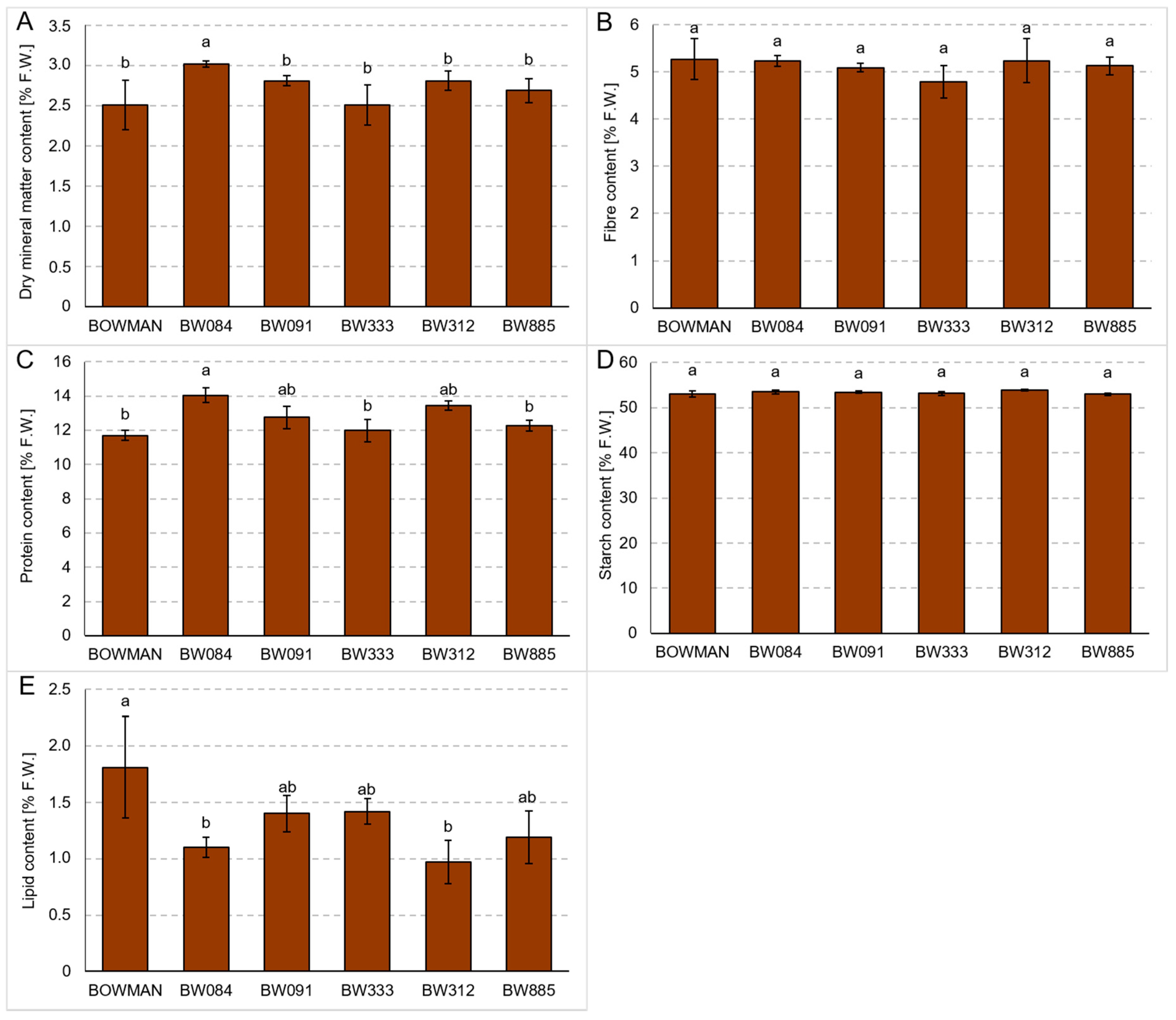
3.5. Chemical Composition of Grains Produced under the Field Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant Responses to Drought, Acclimation, and Stress Tolerance. Photosynthetica 2000, 38, 171–186. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Templer, S.E.; Ammon, A.; Pscheidt, D.; Ciobotea, O.; Schuy, C.; Mccollum, C.; Sonnewald, U.; Hanemann, A.; Förster, J.; Ordon, F.; et al. Metabolite profiling of barley flag leaves under drought and combined heat and drought stress reveals metabolic QTLs for metabolites associated with antioxidant defense. J. Exp. Bot. 2017, 68, 1697–1713. [Google Scholar] [CrossRef] [Green Version]
- Rao, D.E.; Chaitanya, K.V. Photosynthesis and antioxidative defense mechanisms in deciphering drought stress tolerance of crop plants. Biol. Plant. 2016, 60, 201–218. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Reddy, S.H.; Singhal, R.K.; Dacosta, M.; Kambalimath, S.K.; Rajanna, M.P.; Muthurajan, R.; Sevanthi, A.M.; Mohapatra, T.; Sarla, N.; Chinnusamy, V.; et al. Leaf mass area determines water use efficiency through its influence on carbon gain in rice mutants. Physiol. Plant. 2020, 169, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.A.; Stapper, M. Lodging effects on high-yielding crops of irrigated semidwarf wheat. Field Crop. Res. 1987, 17, 245–258. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, S.; Wu, W.; Chen, D.; Zhan, X.; Zhu, A.; Zhang, Y.; Cheng, S.; Cao, L.; Lou, X.; et al. Dissection of genetic architecture of rice plant height and heading date by multiple-strategy-based association studies. Sci. Rep. 2016, 6, 29718. [Google Scholar] [CrossRef] [Green Version]
- Gruszka, D. Exploring the Brassinosteroid Signaling in Monocots Reveals Novel Components of the Pathway and Implications for Plant Breeding. Int. J. Mol. Sci. 2020, 21, 354. [Google Scholar] [CrossRef] [Green Version]
- Gruszka, D. Crosstalk of the Brassinosteroid Signalosome with Phytohormonal and Stress Signaling Components Maintains a Balance between the Processes of Growth and Stress Tolerance. Int. J. Mol. Sci. 2018, 19, 2675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Wang, L.; Wang, M.; Xu, Y.Y.; Luo, W.; Liu, Y.J.; Xu, Z.H.; Li, J.; Chong, K. Engineering OsBAK1 gene as a molecular tool to improve rice architecture for high yield. Plant Biotechnol. J. 2009, 7, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Northey, J.G.B.; Liang, S.; Jamshed, M.; Deb, S.; Foo, E.; Reid, J.B.; McCourt, P.; Samuel, M.A. Farnesylation mediates brassinosteroid biosynthesis to regulate abscisic acid responses. Nat. Plants 2016, 2, 16114. [Google Scholar] [CrossRef]
- Feng, Y.; Yin, Y.; Fei, S. Down-regulation of BdBRI1, a putative brassinosteroid receptor gene produces a dwarf phenotype with enhanced drought tolerance in Brachypodium distachyon. Plant Sci. 2015, 234, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Ferrero-Serrano, A.; Assmann, S.M. The α-subunit of the rice heterotrimeric G protein, RGA1, regulates drought tolerance during the vegetative phase in the dwarf rice mutantd1. J. Exp. Bot. 2016, 67, 3433–3443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruszka, D.; Janeczko, A.; Dziurka, M.; Pociecha, E.; Oklestkova, J.; Szarejko, I. Barley Brassinosteroid Mutants Provide an Insight into Phytohormonal Homeostasis in Plant Reaction to Drought Stress. Front. Plant Sci. 2016, 7, 1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janeczko, A.; Gruszka, D.; Pociecha, E.; Dziurka, M.; Filek, M.; Jurczyk, B.; Kalaji, H.M.; Kocurek, M.; Waligorski, P. Physiological and biochemical characterisation of watered and drought-stressed barley mutants in the HvDWARF gene encoding C6-oxidase involved in brassinosteroid biosynthesis. Plant Physiol. Biochem. 2016, 99, 126–141. [Google Scholar] [CrossRef]
- Gruszka, D.; Pociecha, E.; Jurczyk, B.; Dziurka, M.; Oliwa, J.; Sadura, I.; Janeczko, A. Insights into Metabolic Reactions of Semi-Dwarf, Barley Brassinosteroid Mutants to Drought. Int. J. Mol. Sci. 2020, 21, 5096. [Google Scholar] [CrossRef]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Muller, B.; Pantin, F.; Genard, M.; Turc, O.; Freixes, S.; Piques, M.; Gibon, Y. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J. Exp. Bot. 2011, 62, 1715–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinclair, T.R.; Sheehy, J. EErect Leaves and Photosynthesis in Rice. Science 1999, 283, 1455. [Google Scholar] [CrossRef]
- Morinaka, Y.; Sakamoto, T.; Inukai, Y.; Agetsuma, M.; Kitano, H.; Ashikari, M.; Matsuoka, M. Morphological Alteration Caused by Brassinosteroid Insensitivity Increases the Biomass and Grain Production of Rice. Plant Physiol. 2006, 141, 924–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, T.; Morinaka, Y.; Ohnishi, T.; Sunohara, H.; Fujioka, S.; Ueguchi-Tanaka, M.; Mizutani, M.; Sakata, K.; Takatsuto, S.; Yoshida, S.; et al. Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat. Biotechnol. 2006, 24, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.M.B.; Fernandez, S.M.G. Differential manipulation of leaf angle throughout the canopy: Current status and prospects. J. Exp. Bot. 2017, 68, 5699–5717. [Google Scholar] [CrossRef] [Green Version]
- Dockter, C.; Gruszka, D.; Braumann, I.; Druka, A.; Druka, I.; Franckowiak, J.; Gough, S.P.; Janeczko, A.; Kurowska, M.; Lundqvist, J.; et al. Induced Variations in Brassinosteroid Genes Define Barley Height and Sturdiness, and Expand the Green Revolution Genetic Toolkit. Plant Physiol. 2014, 166, 1912–1927. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Wu, C.; Wang, C.; Roh, J.; Zhang, L.; Chen, J.; Zhang, S.; Zhang, H.; Yang, C.; Hu, J.; et al. SLG controls grain size and leaf angle by modulating brassinosteroid homeostasis in rice. J. Exp. Bot. 2016, 67, 4241–4253. [Google Scholar] [CrossRef] [Green Version]
- Dockter, C.; Hansson, M. Improving barley culm robustness for secured crop yield in a changing climate. J. Exp. Bot. 2015, 66, 3499–3509. [Google Scholar] [CrossRef] [Green Version]
- Porter, J.R.; Semenov, M.A. Crop responses to climatic variation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 2021–2035. [Google Scholar] [CrossRef]
- Braumann, I.; Urban, W.; Preuß, A.; Dockter, C.; Zakhrabekova, S.; Hansson, M. Semi-dwarf barley (Hordeum vulgare L.) brh2 and ari-l mutants are deficient in a U-box E3 ubiquitin ligase. Plant Growth Regul. 2018, 86, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Zhang, J.; Dell, B.; Biddulph, B.; Drake-Brockman, F.; Walker, E.; Khan, N.; Wong, D.; Hayden, M.; Appels, R. Wild-type alleles of Rht-B1 and Rht-D1 as independent determinants of thousand-grain weight and kernel number per spike in wheat. Mol. Breed. 2013, 32, 771–783. [Google Scholar] [CrossRef]
- Terao, T.; Hirose, T. Control of grain protein contents through SEMIDWARF1 mutant alleles: sd1 increases the grain protein content in Dee-geo-woo-gen but not in Reimei. Mol. Genet. Genom. 2014, 290, 939–954. [Google Scholar] [CrossRef]
- Derkx, A.P.; Harding, C.A.; Miraghazadeh, A.; Chandler, P.M. Overgrowth (Della) mutants of wheat: Development, growth and yield of intragenic suppressors of the Rht-B1c dwarfing gene. Funct. Plant Biol. 2017, 44, 525–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, H.; Mason, R.E.; Lozada, D.N.; Acuna, A.; Holder, A.; Larkin, D.; Winn, Z.; Murray, J.; Murphy, J.P.; Moon, D.E.; et al. Effects of allelic variation at Rht-B1 and Rht-D1 on grain yield and agronomic traits of southern US soft red winter wheat. Euphytica 2019, 215, 172. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, K.; Wang, Z.; Zhang, H.; Gu, J.; Liu, L.; Yang, J.; Zhang, J. Brassinosteroids function in spikelet differentiation and degeneration in rice. J. Integr. Plant Biol. 2019, 61, 943–963. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Sheng, J.; Xu, Y.; Xiong, F.; Wu, Y.; Wang, W.; Wang, Z.; Yang, J.; Zhang, J. Role of brassinosteroids in rice spikelet differentiation and degeneration under soil-drying during panicle development. BMC Plant Biol. 2019, 19, 409. [Google Scholar] [CrossRef]
- Zu, S.-H.; Jiang, Y.-T.; Hu, L.-Q.; Zhang, Y.-J.; Chang, J.-H.; Hue, H.-W.; Lin, W.-H. Effective Modulating Brassinosteroids Signal to Study Their Specific Regulation of Reproductive Development and Enhance Yield. Front. Plant Sci. 2019, 10, 980. [Google Scholar] [CrossRef] [Green Version]
- Druka, A.; Franckowiak, J.; Lundqvist, U.; Bonar, N.; Alexander, J.; Houston, K.; Radovic, S.; Shahinnia, F.; Vendramin, V.; Morgante, M.; et al. Genetic Dissection of Barley Morphology and Development. Plant Physiol. 2011, 155, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Salvi, S.; Druka, A.; Milner, S.G.; Gruszka, D. Induced Genetic Variation, TILLING and NGS-Based Cloning. In Biotechnological Approaches to Barley Improvement. Biotechnology in Agriculture and Forestry; Kumlehn, J., Stein, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 69, pp. 287–310. [Google Scholar]
- Chono, M.; Honda, I.; Zeniya, H.; Yoneyama, K.; Saisho, D.; Takeda, K.; Takatsuto, S.; Hoshino, T.; Watanabe, Y. A Semidwarf Phenotype of Barley uzu Results from a Nucleotide Substitution in the Gene Encoding a Putative Brassinosteroid Receptor. Plant Physiol. 2003, 133, 1209–1219. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-Y.; Trieu, A.; Radhakrishnan, P.; Kwok, S.F.; Harris, S.; Zhang, K.; Wang, J.; Wan, J.; Zhai, H.; Takatsuto, S.; et al. Brassinosteroids Regulate Grain Filling in Rice. Plant Cell 2008, 20, 2130–2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.-H. Designed Manipulation of the Brassinosteroid Signal to Enhance Crop Yield. Front. Plant Sci. 2020, 11, 854. [Google Scholar] [CrossRef]
- Rebetzke, G.; Richards, R. Gibberellic acid-sensitive dwarfing genes reduce plant height to increase kernel number and grain yield of wheat. Crop Pasture Sci. 2000, 51, 235–246. [Google Scholar] [CrossRef]
- Hu, X.; Cui, Y.; Dong, G.; Feng, A.; Wang, D.; Zhao, C.; Zhang, Y.; Hu, J.; Zeng, D.; Guo, L.; et al. Using CRISPR-Cas9 to generate semi-dwarf rice lines in elite landraces. Sci. Rep. 2019, 9, 19096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alghabari, F.; Ihsan, M.Z.; Khaliq, A.; Hussain, S.; Daur, I.; Fahad, S.; Nasim, W. Gibberellin-sensitive Rht alleles confer tolerance to heat and drought stresses in wheat at booting stage. J. Cereal Sci. 2016, 70, 72–78. [Google Scholar] [CrossRef]
- Gooding, M.J. The effects of growth environment and agronomy on grain quality. In Cereal Grains: Assessing and Managing Quality; Wrigley, C.W., Batey, I.L., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead: Oxford, UK, 2010; Volume 190, pp. 393–412. [Google Scholar]
- Janeczko, A.; Biesaga-Kościelniak, J.; Oklestkova, J.; Filek, M.; Dziurka, M.; Szarek-Łukaszewska, G.; Kościelniak, J. Role of 24-Epibrassinolide in Wheat Production: Physiological Effects and Uptake. J. Agron. Crop. Sci. 2010, 196, 311–321. [Google Scholar] [CrossRef]
- Fujii, S.; Saka, H. Distribution of Assimilates to Each Organ in Rice Plants Exposed to a Low Temperature at the Ripening Stage, and the Effect of Brassinolide on the Distribution. Plant Prod. Sci. 2001, 4, 136. [Google Scholar] [CrossRef]
- Divi, U.K.; Krishna, P. Brassinosteroid: A biotechnological target for enhancing crop yield and stress tolerance. New Biotechnol. 2009, 26, 131–136. [Google Scholar] [CrossRef]
- Sadura, I.; Pociecha, E.; Dziurka, M.; Oklestkova, J.; Novak, O.; Gruszka, D.; Janeczko, A. Mutations in the HvDWARF, HvCPD and HvBRI1 Genes-Involved in Brassinosteroid Biosynthesis/Signalling: Altered Photosynthetic Efficiency, Hormonal Homeostasis and Tolerance to High/Low Temperatures in Barley. J. Plant Growth Regul. 2019, 38, 1062–1081. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruszka, D.; Janeczko, A.; Puła, J.; Lepiarczyk, A.; Pociecha, E. Impact of Drought Exerted during Spike Development on Tillering, Yield Parameters and Grain Chemical Composition in Semi-Dwarf Barley Mutants Deficient in the Brassinosteroid Metabolism. Agronomy 2020, 10, 1595. https://doi.org/10.3390/agronomy10101595
Gruszka D, Janeczko A, Puła J, Lepiarczyk A, Pociecha E. Impact of Drought Exerted during Spike Development on Tillering, Yield Parameters and Grain Chemical Composition in Semi-Dwarf Barley Mutants Deficient in the Brassinosteroid Metabolism. Agronomy. 2020; 10(10):1595. https://doi.org/10.3390/agronomy10101595
Chicago/Turabian StyleGruszka, Damian, Anna Janeczko, Joanna Puła, Andrzej Lepiarczyk, and Ewa Pociecha. 2020. "Impact of Drought Exerted during Spike Development on Tillering, Yield Parameters and Grain Chemical Composition in Semi-Dwarf Barley Mutants Deficient in the Brassinosteroid Metabolism" Agronomy 10, no. 10: 1595. https://doi.org/10.3390/agronomy10101595
APA StyleGruszka, D., Janeczko, A., Puła, J., Lepiarczyk, A., & Pociecha, E. (2020). Impact of Drought Exerted during Spike Development on Tillering, Yield Parameters and Grain Chemical Composition in Semi-Dwarf Barley Mutants Deficient in the Brassinosteroid Metabolism. Agronomy, 10(10), 1595. https://doi.org/10.3390/agronomy10101595






