Chlorophyll-a Fluorescence Analysis Reveals Differential Response of Photosynthetic Machinery in Melatonin-Treated Oat Plants Exposed to Osmotic Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth and Experimental Design
2.2. Determination of Morphological Parameters and Chlorophyll Content
2.3. Stomatal Conductance, Electrolyte Leakage, and Malondialdehyde (MDA) Measurements
2.4. Chl-a Fluorescence Measurements
2.5. Estimation of Proline and Expression Analysis of the Genes Involved in Proline Biosynthesis
2.6. Expression Analyses of the Genes Encoding the PSII Core Proteins and ROS-Scavenging Enzymes
2.7. Statistical Analysis
3. Results
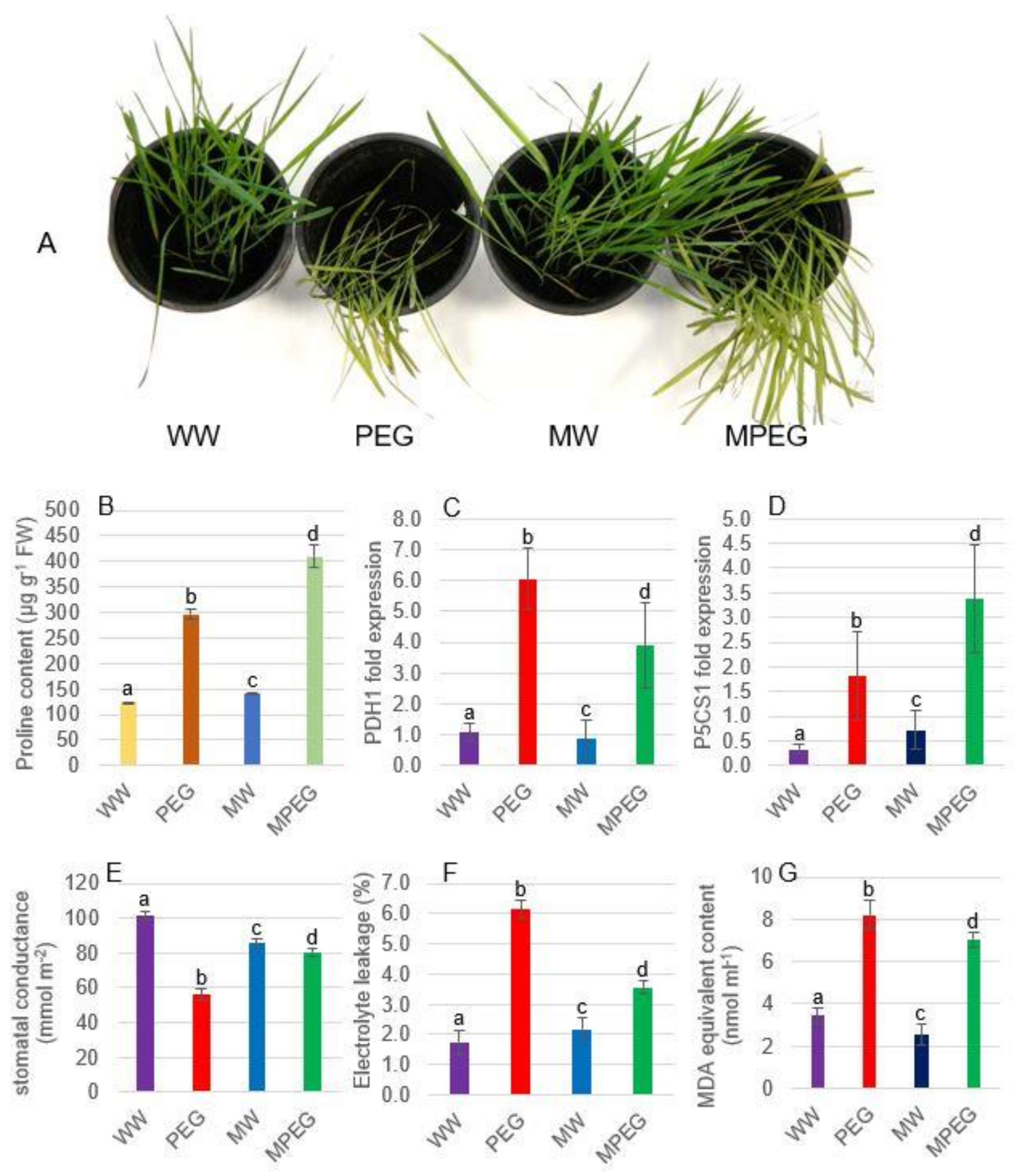
3.1. Morphological, Physiological, and Biochemical Changes in Oat Plants Subjected to Osmotic Stress
3.2. Melatonin Induces the Expression of Major PSII Genes under Osmotic Stress
3.3. Melatonin Upregulates the Expression of the Major ROS-Scavenging Enzyme Genes in Oat Plants under Osmotic Stress
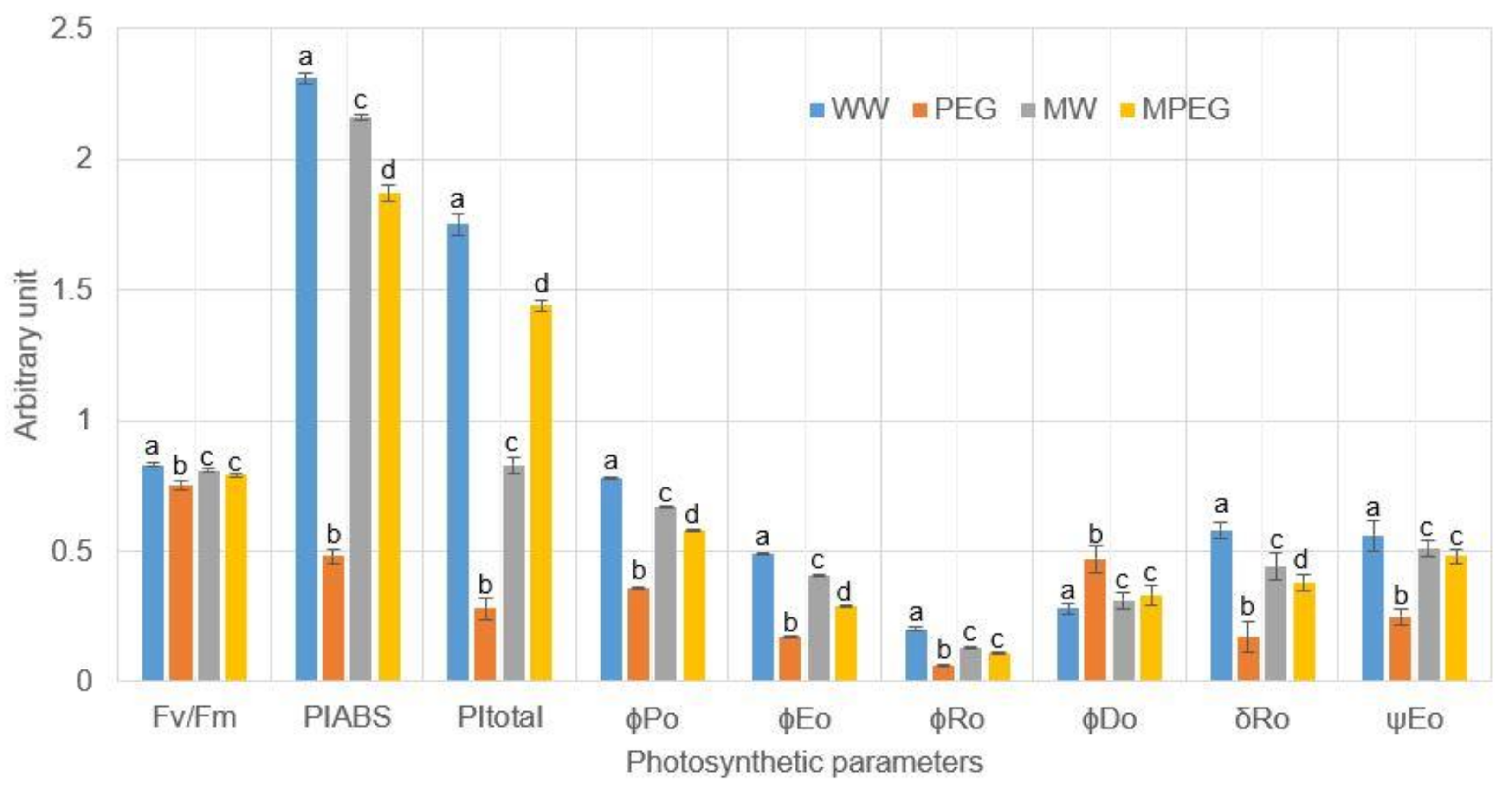
3.4. Melatonin-Pretreated Plants Were Photosynthetically more Efficient under Osmotic Stress
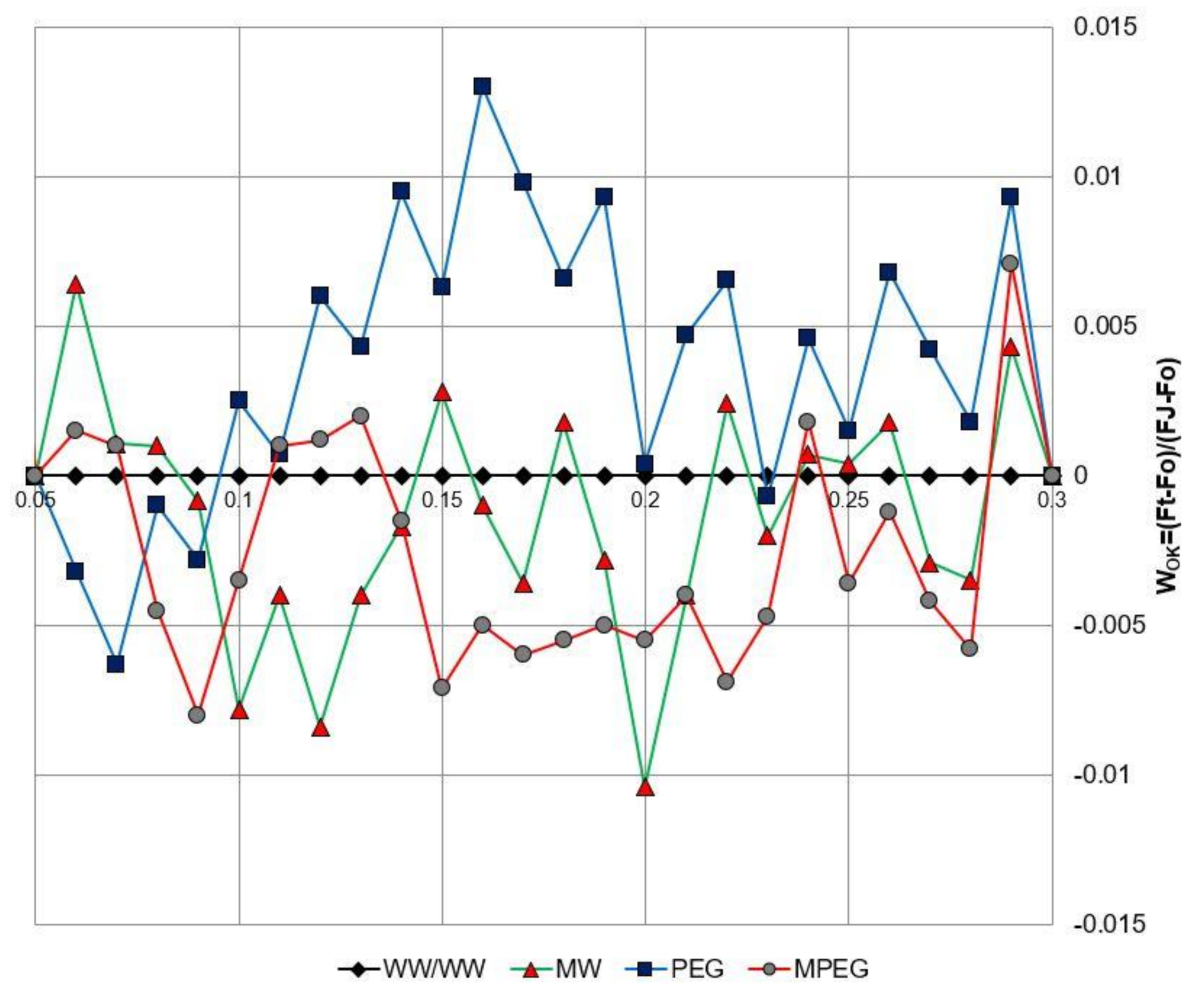
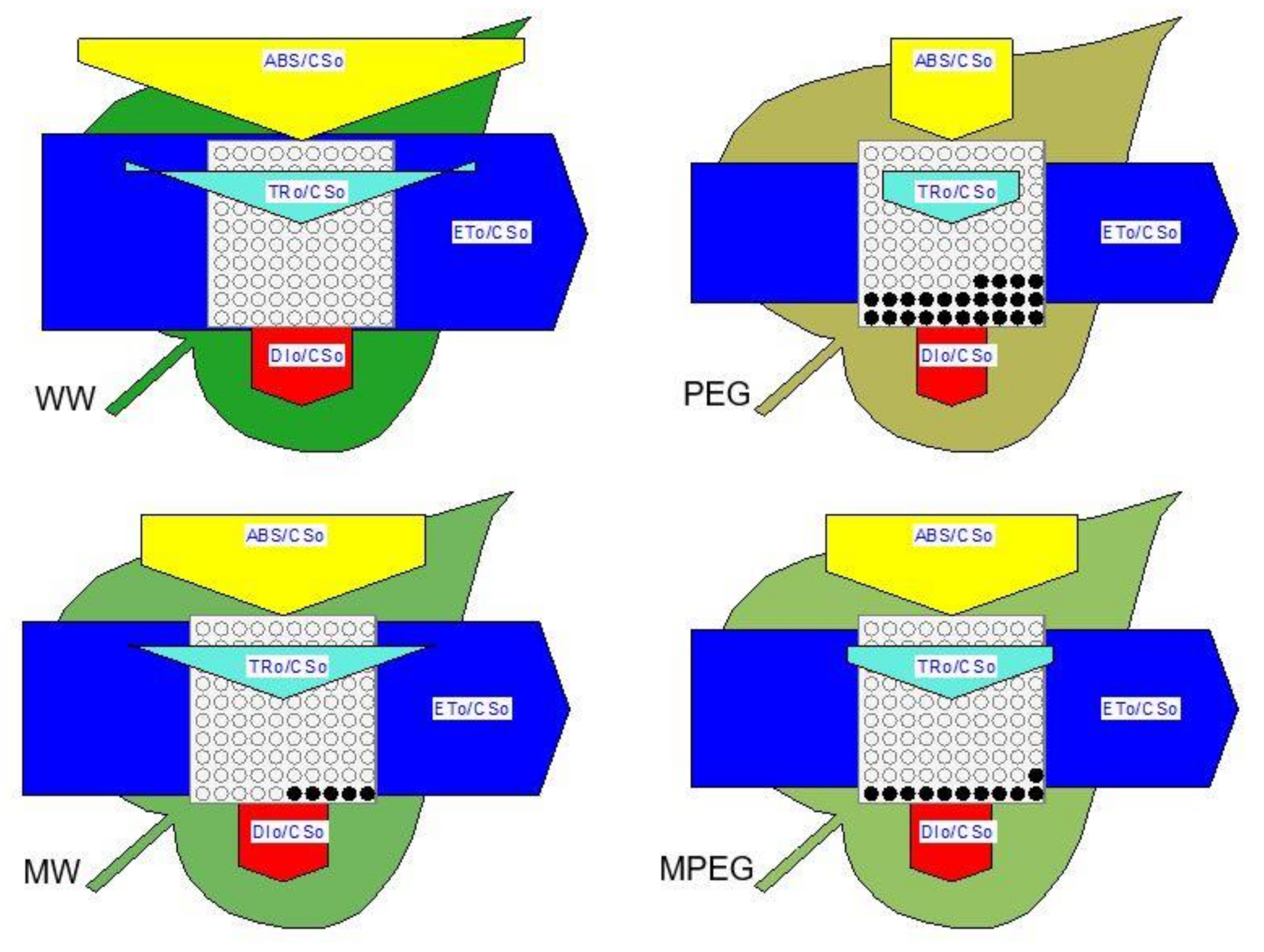
3.5. Melatonin-Induced Changes in the Biophysical Parameters of PSII as Revealed by the JIP-test Equations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | Absorbance |
| APX | Ascorbate peroxidase |
| CAT | Catalase |
| ET | Electron transport |
| FW | Fresh weight |
| GR | Glutathione reductase |
| MDA | Malondialdehyde |
| OEC | Oxygen evolving complex |
| PDH | Proline dehydrogenase |
| PEG | Polyethylene glycol |
| PI | Performance index |
| PSI | Photosystem I |
| PSII | Photosystem II |
| RC | Reaction left |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| WW | Well-watered |
References
- Dhankher, O.P.; Foyer, C.H. Climate resilient crops for improving global food security and safety. Plant Cell Environ. 2018, 41, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gururani, M.A.; Upadhyaya, C.P.; Strasser, R.J.; Yu, J.W.; Park, S.W. Evaluation of abiotic stress tolerance in transgenic potato plants with reduced expression of PSII manganese stabilizing protein. Plant Sci. 2013, 198, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Pawłowicz, I.; Kosmala, A.; Rapacz, M. Expression pattern of the psbO gene and its involvement in acclimation of the photosynthetic apparatus during abiotic stresses in Festuca arundinacea and F. pratensis. Acta Physiol. Plant. 2012, 34, 1915–1924. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a chemical substance or as phytomelatonin rich-extracts for use as plant protector and/or biostimulant in accordance with EC legislation. Agronomy 2019, 9, 570. [Google Scholar] [CrossRef] [Green Version]
- Galano, A.; Tan, D.; Reiter, R. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Turk, H.; Erdal, S.; Genisel, M.; Atici, O.; Demir, Y.; Yanmis, D. The regulatory effect of melatonin on physiological, biochemical and molecular parameters in cold-stressed wheat seedlings. Plant Growth Regul. 2014, 74, 139–152. [Google Scholar] [CrossRef]
- Weeda, S.; Zhang, N.; Zhao, X.; Ndip, G.; Guo, Y.; Buck, G.A.; Fu, C.; Ren, S. Arabidopsis Transcriptome Analysis Reveals Key Roles of Melatonin in Plant Defense Systems. PloS ONE 2014, 9, e93462. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C. Exogenous Melatonin Confers Salt Stress Tolerance to Watermelon by Improving Photosynthesis and Redox Homeostasis. Front. Plant Sci. 2017, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Varghese, N.; Alyammahi, O.; Nasreddine, S.; Alhassani, A.; Gururani, M.A. Melatonin positively influences the photosynthetic machinery and antioxidant system of avena sativa during salinity stress. Plants 2019, 8, 610. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Zhang, Y.; Feng, Z.; Bai, Q.; He, J.; Wang, Y. Effects of Melatonin on Antioxidant Capacity in Naked Oat Seedlings under Drought Stress. Molecules 2018, 23, 1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gururani, M.A.; Ganesan, M.; Song, I.-J.; Han, Y.; Kim, J.-I.; Lee, H.-Y.; Song, P.-S. Transgenic Turfgrasses Expressing Hyperactive Ser599Ala Phytochrome A Mutant Exhibit Abiotic Stress Tolerance. J. Plant Growth Regul. 2016, 35, 11–21. [Google Scholar] [CrossRef]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A. Govindjee Polyphasic Chlorophyll a fluorescence transient in plant and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Strasser, R.J. The grouping model of plant photosynthesis: Heterogeneity of photosynthetic units in thylakoids. In Photosynthesis III; Structure and Molecular Organisation of Photosynthetic Apparatus: Balaban, PHL, USA, 1981; pp. 727–737. [Google Scholar]
- Gururani, M.A.; Upadhyaya, C.P.; Strasser, R.J.; Woong, Y.J.; Park, S.W. Physiological and biochemical responses of transgenic potato plants with altered expression of PSII manganese stabilizing protein. Plant Physiol. Biochem. 2012, 58, 182–194. [Google Scholar] [CrossRef]
- Ghosh, R.; Gururani, M.A.; Ponpandian, L.N.; Mishra, R.C.; Park, S.-C.; Jeong, M.-J.; Bae, H. Expression analysis of sound vibration-regulated genes by touch treatment in arabidopsis. Front. Plant Sci. 2017, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, G.C. Govindjee Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Demmig-Adams, B., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–44. ISBN 978-94-017-9031-4. [Google Scholar]
- Toth, S.Z.; Nagy, V.; Puthur, J.T.; Kovacs, L.; Garab, G. The Physiological Role of Ascorbate as Photosystem II Electron Donor: Protection against Photoinactivation in Heat-Stressed Leaves. Plant Physiol. 2011, 156, 382–392. [Google Scholar] [CrossRef] [Green Version]
- Mathur, S.; Jajoo, A.; Mehta, P.; Bharti, S. Analysis of elevated temperature-induced inhibition of photosystem II using chlorophyll a fluorescence induction kinetics in wheat leaves (Triticum aestivum). Plant Biol. (Stuttg.) 2011, 13, 1–6. [Google Scholar] [CrossRef]
- Yin, Z.; Lu, J.; Meng, S.; Liu, Y.; Mostafa, I.; Qi, M.; Li, T. Exogenous melatonin improves salt tolerance in tomato by regulating photosynthetic electron flux and the ascorbate–glutathione cycle. J. Plant Interact. 2019, 14, 453–463. [Google Scholar] [CrossRef]
- Nawaz, M.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z. Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 2018, 220, 115–127. [Google Scholar] [CrossRef]
- Kabiri, R.; Hatami, A.; Oloumi, H.; Naghizadeh, M.; Nasibi, F.; Tahmasebi, Z. Foliar application of melatonin induces tolerance to drought stress in Moldavian balm plants (Dracocephalum moldavica) through regulating the antioxidant system. Folia Hortic. 2018, 30, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Gururani, M.A.; Venkatesh, J.; Ghosh, R.; Strasser, R.J.; Ponpandian, L.N.; Bae, H. Chlorophyll-a fluorescence evaluation of PEG-induced osmotic stress on PSII activity in Arabidopsis plants expressing SIP1. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2017, 3504, 1–8. [Google Scholar] [CrossRef]
- Lazár, D.; Murch, S.J.; Beilby, M.J.; Al Khazaaly, S. Exogenous melatonin affects photosynthesis in characeae Chara australis. Plant Signal. Behav. 2013, 8, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.X.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.Y.; Liu, J.L.; Wang, W.X.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumbers under salinity-induced stress. Photosynthetica 2015, 53, 1–10. [Google Scholar] [CrossRef]
- Dobrá, J.; Vanková, R.; Havlová, M.; Burman, A.J.; Libus, J.; Storchová, H. Tobacco leaves and roots differ in the expression of proline metabolism-related genes in the course of drought stress and subsequent recovery. J. Plant Physiol. 2011, 168, 1588–1597. [Google Scholar] [CrossRef]
- Soni, P.; Abdin, M.Z. Water deficit-induced oxidative stress affects artemisinin content and expression of proline metabolic genes in Artemisia annua L. FEBS Open Bio 2017, 7, 367–381. [Google Scholar] [CrossRef]
- Mutava, R.N.; Prince, S.J.K.; Syed, N.H.; Song, L.; Valliyodan, B.; Chen, W.; Nguyen, H.T. Understanding abiotic stress tolerance mechanisms in soybean: A comparative evaluation of soybean response to drought and flooding stress. Plant Physiol. Biochem. 2015, 86, 109–120. [Google Scholar] [CrossRef]
- Sun, L.; Li, X.; Wang, Z.; Sun, Z.; Zhu, X.; Liu, S.; Song, F.; Liu, F.; Wang, Y. Cold priming induced tolerance to subsequent low temperature stress is enhanced by melatonin application during recovery in wheat. Molecules 2018, 23, 1091. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cao, Y.; Guo, Y. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA 4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar]
- Byeon, Y.; Back, K. Low melatonin production by suppression of either serotonin N-acetyltransferase or N-acetylserotonin methyltransferase in rice causes seedling growth retardation with yield penalty, abiotic stress susceptibility, and enhanced coleoptile growth under anoxi. J. Pineal Res. 2016, 60, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Sen, G.; Eryilmaz, I.E.; Ozakca, D. The effect of aluminium-stress and exogenous spermidine on chlorophyll degradation, glutathione reductase activity and the photosystem II D1 protein gene (psbA) transcript level in lichen Xanthoria parietina. Phytochemistry 2014, 98, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Wang, M.; Wei, Y.; Xia, Z. Overexpression of the Maize psbA Gene enhances drought tolerance through regulating antioxidant system, photosynthetic capability, and stress defense Gene expression in tobacco. Front. Plant Sci. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasi, S.; Venkatesh, J.; Daneshi, R.F.; Gururani, M.A. Photosystem II extrinsic proteins and their putative role in abiotic stress tolerance in higher plants. Plants 2018, 7, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, Y.; Aminaka, R.; Yoshioka, M.; Khatoon, M.; Komayama, K.; Takenaka, D.; Yamashita, A.; Nijo, N.; Inagawa, K.; Morita, N.; et al. Quality control of photosystem II: Impact of light and heat stresses. Photosynth. Res. 2008, 98, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Bricker, T.M.; Roose, J.L.; Fagerlund, R.D.; Frankel, L.K.; Eaton-rye, J.J. Biochimica et Biophysica Acta the extrinsic proteins of Photosystem II. BBA Bioenerg. 2012, 1817, 121–142. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.; Hargett, S.R.; Frankel, L.K.; Bricker, T.M. The effects of simultaneous RNAi suppression of PsbO and PsbP protein expression in photosystem II of Arabidopsis. Photosynth. Res. 2008, 98, 439–448. [Google Scholar] [CrossRef]
- Roose, J.L.; Frankel, L.K.; Bricker, T.M. Developmental Defects in Mutants of the PsbP Domain Protein 5 in Arabidopsis thaliana. PLoS ONE 2011, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Bueno, M.L.; Barón, M.; García-Luque, I. PsbO, PsbP, and PsbQ of photosystem II are encoded by gene families in Nicotiana benthamiana. Structure and functionality of their isoforms. Photosynthetica 2011, 49, 573–580. [Google Scholar] [CrossRef]
- Akilan, S.; Halima, T.H.; Sasi, S.; Kappachery, S.; Baniekal-Hiremath, G.; Venkatesh, J.; Gururani, M.A. Evaluation of osmotic stress tolerance in transgenic Arabidopsis plants expressing Solanum tuberosum D200 gene. J. Plant Interact. 2019, 14, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Puyang, X.; An, M.; Han, L.; Zhang, X. Protective effect of spermidine on salt stress induced oxidative damage in two Kentucky bluegrass (Poa pratensis L.) cultivars. Ecotoxicol. Environ. Saf. 2015, 117, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tan, D.; Dong, J.; Liu, F. Melatonin enhances cold tolerance in drought—Type deficient mutant barley. J. Pineal Res. 2016, 61, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, Q.; Shah, F.A.; Liu, W.; Wang, D.; Huang, S.; Fu, S.; Wu, L. Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 2018, 23, 799. [Google Scholar] [CrossRef] [Green Version]
- Zushi, K.; Kajiwara, S.; Matsuzoe, N. Chlorophyll a fluorescence OJIP transient as a tool to characterize and evaluate response to heat and chilling stress in tomato leaf and fruit. Sci. Hortic. (Amst.) 2012, 148, 39–46. [Google Scholar] [CrossRef]
- Camejo, D.; Nicolás, E.; Torres, W.; Alarcón, J.J. Differential heat-induced changes in the CO2 assimilation rate and electron transport in tomato (Lycopersicon esculentum Mill.). J. Hortic. Sci. Biotechnol. 2010, 85, 137–143. [Google Scholar] [CrossRef]
- Chen, L.S.; Cheng, L. The sun-exposed peel of apple fruit has a higher photosynthetic capacity than the shaded peel. Funct. Plant Biol. 2007, 34, 1038–1048. [Google Scholar] [CrossRef]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fl uorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef]
- Jedmowski, C.; Ashoub, A.; Brüggemann, W. Reactions of Egyptian landraces of Hordeum vulgare and Sorghum bicolor to drought stress, evaluated by the OJIP fluorescence transient analysis. Acta Physiol. Plant. 2013, 35, 345–354. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of gamma-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. Biochim. Biophys. Acta 2010, 1797, 1428–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 38. [Google Scholar] [CrossRef] [Green Version]
- Oukarroum, A.; El Madidi, S.; Strasser, R.J. Exogenous glycine betaine and proline play a protective role in heat-stressed barley leaves (Hordeum vulgare L.): A chlorophyll a fluorescence study. Plant Biosyst. 2012, 146, 1037–1043. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by invivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Hasan, S.A.; Ali, B.; Hayat, S.; Fariduddin, Q.; Ahmad, A. Effect of salicylic acid on salinity-induced changes in Brassica juncea. J. Integr. Plant Biol. 2008, 50, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Vuletic, V.; Spanic, V. Characterization of photosynthetic performance during natural leaf senescence in winter wheat: Multivariate analysis as a tool for phenotypic characterization Characterization of photosynthetic performance during natural leaf senescence in winter wheat. Photosynthetica 2019, 57, 116–128. [Google Scholar]




| No. | Primer Name | Sequence 5′ to 3′ |
|---|---|---|
| 1 | As-PsbA-F | TCG CTT CTG CAA CTG GAT AAC |
| As-PsbA-R | GCA GCG ATG AAG GCG ATA ATA | |
| 2 | As-PsbD-F | GCC CTT GGT AGA GTT CCT AAA G |
| As-PsbD-R | CGA AAT AAG CGC AAG GAA AGA G | |
| 3 | As-PsbB-F | GCG GGT ACG TTG GGT ATA TTA G |
| As-PsbB-R | CAG CAG CGA TAC TAC TGG AAA G | |
| 4 | As-PsbC-F | AAC GGT TTG GAC TTG AGT AGG |
| As-PsbC-R | CTA CGC CAC CCA CAG AAT TTA | |
| 5 | As-PsbO-F | AGA GAG GCT CGG TGA AAT AGA |
| As-PsbO-R | CCA ATC CCA GGG AAC AGT AAA G | |
| 6 | AsGAPDH-F | GGTGGTGCCAAGAAGGTTAT |
| AsGAPDH-R | GGAGACAATGGTGATGTCAGAG | |
| 7 | As-P5Cs1-F | TGTCCTCTGGGTGTTCTCTTGAT |
| As-P5Cs1-R | CGAATGGCTAAAGACGCAATC | |
| 8 | As-PDH1-F | CCCCGTGGAGCACATCAT |
| As-PDH1-R | AAGGTTGAAGCAGAGAGCAATCC | |
| 9 | As-CAT1-F | CAGGCTGGCGAGAGATTCC |
| As-CAT1-R | AGCATCCGTGAGTGCATCAA | |
| 10 | As-APX-F | GCTCCGTGAAGTAAGTGTTATCAAAC |
| As-APX-R | CCTGGGAAGGTGCCACAA | |
| 11 | As-SOD-F | CACAAGCACTTCACAGGAACAGT |
| As-SOD-R | TGCCACTCTGAACATTTCATCAC |
| WW | PEG | MW | MPEG | |
|---|---|---|---|---|
| Root length (cm) | 10.84 ± 1 a | 5.4 ± 0.3 b | 7.4 ± 0.5 c | 6.86 ± 0.4 d |
| Shoot length (cm) | 20.36 ± 1.3 a | 9.6 ± 0.5 b | 16.4 ± 0.9 c | 15.08 ± 0.3 d |
| F.W. (g) | 0.55 ± 0.04 a | 0.17 ± 0.02 b | 0.37 ± 0.03 c | 0.33 ± 0.09 c |
| Chl-A (mg/g dwb) | 5.68 ± 0.2 a | 2.15 ± 0.2 b | 4.69 ± 0.2 c | 4.51 ± 0.1 c |
| Chl-B (mg/g dwb) | 6.36 ± 0.5 a | 3.17 ± 0.7 b | 5.09 ± 0.7 c | 4.49 ± 0.9 d |
| Total Chl (mg/g dwb) | 12.05 ± 0.5 a | 5.32 ± 0.5 b | 9.78 ± 0.8 c | 9.01 ± 1.0 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alyammahi, O.; Gururani, M.A. Chlorophyll-a Fluorescence Analysis Reveals Differential Response of Photosynthetic Machinery in Melatonin-Treated Oat Plants Exposed to Osmotic Stress. Agronomy 2020, 10, 1520. https://doi.org/10.3390/agronomy10101520
Alyammahi O, Gururani MA. Chlorophyll-a Fluorescence Analysis Reveals Differential Response of Photosynthetic Machinery in Melatonin-Treated Oat Plants Exposed to Osmotic Stress. Agronomy. 2020; 10(10):1520. https://doi.org/10.3390/agronomy10101520
Chicago/Turabian StyleAlyammahi, Onoud, and Mayank Anand Gururani. 2020. "Chlorophyll-a Fluorescence Analysis Reveals Differential Response of Photosynthetic Machinery in Melatonin-Treated Oat Plants Exposed to Osmotic Stress" Agronomy 10, no. 10: 1520. https://doi.org/10.3390/agronomy10101520
APA StyleAlyammahi, O., & Gururani, M. A. (2020). Chlorophyll-a Fluorescence Analysis Reveals Differential Response of Photosynthetic Machinery in Melatonin-Treated Oat Plants Exposed to Osmotic Stress. Agronomy, 10(10), 1520. https://doi.org/10.3390/agronomy10101520






