Jerusalem Artichoke: Quality Response to Potassium Fertilization and Irrigation in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.2. Determination of Tocopherol and β-Carotene Content
2.3. Determination of Amino Acid Content and the Nutritional Value of Protein
2.4. Statistical Analysis
2.5. Weather Conditions
3. Results and Discussion
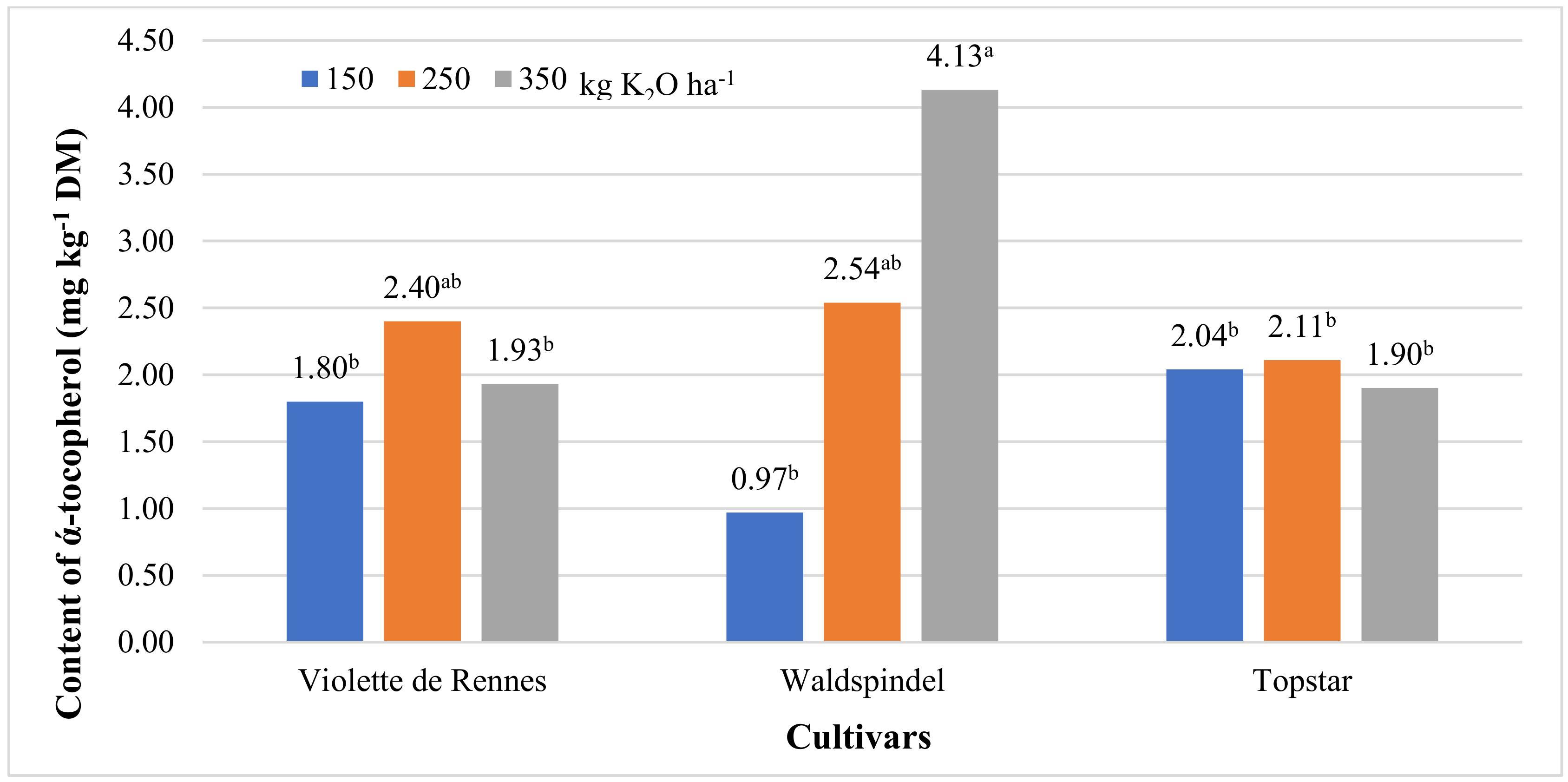
3.1. Content of ά-Tocopherol and β-Carotene
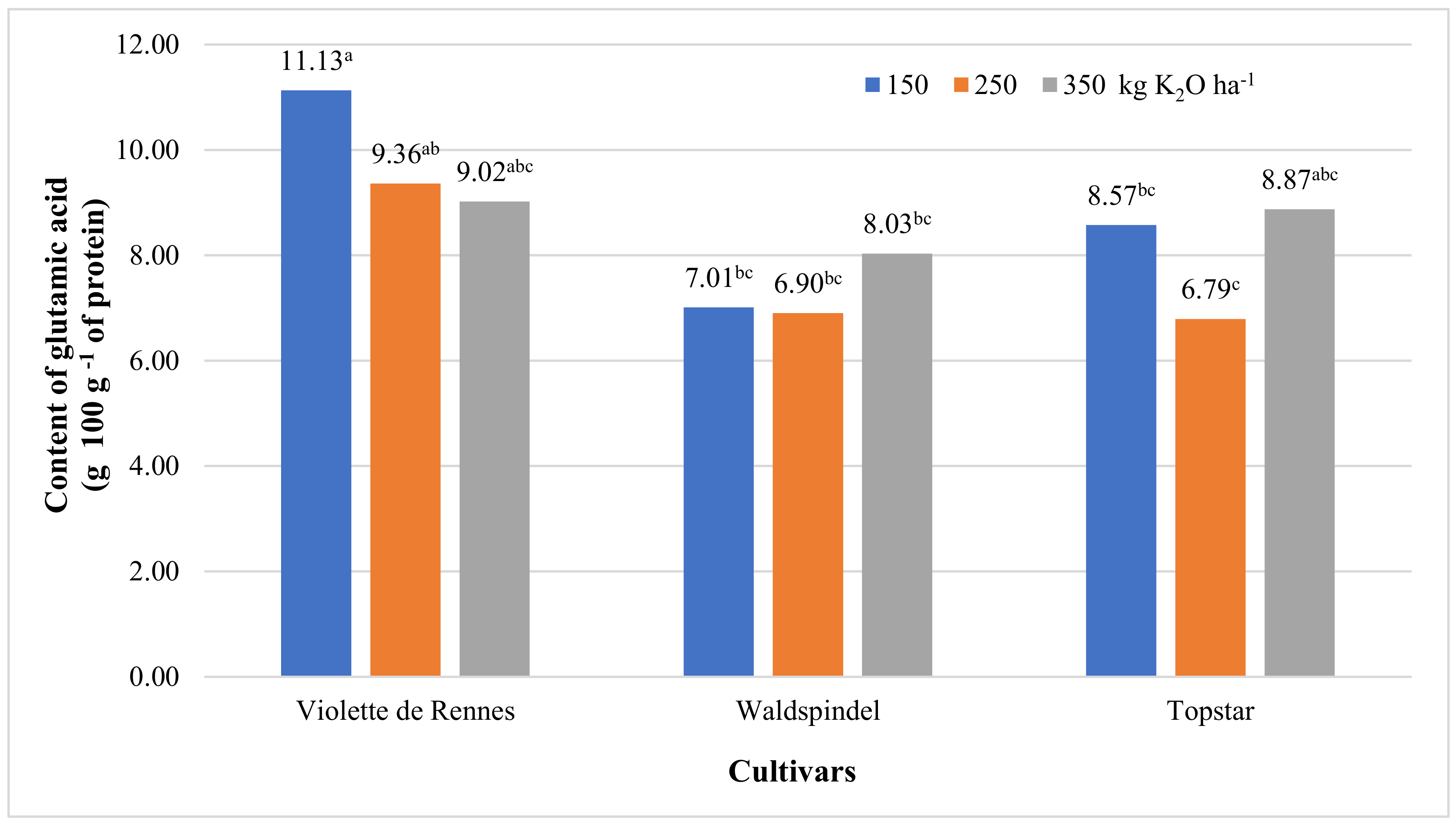
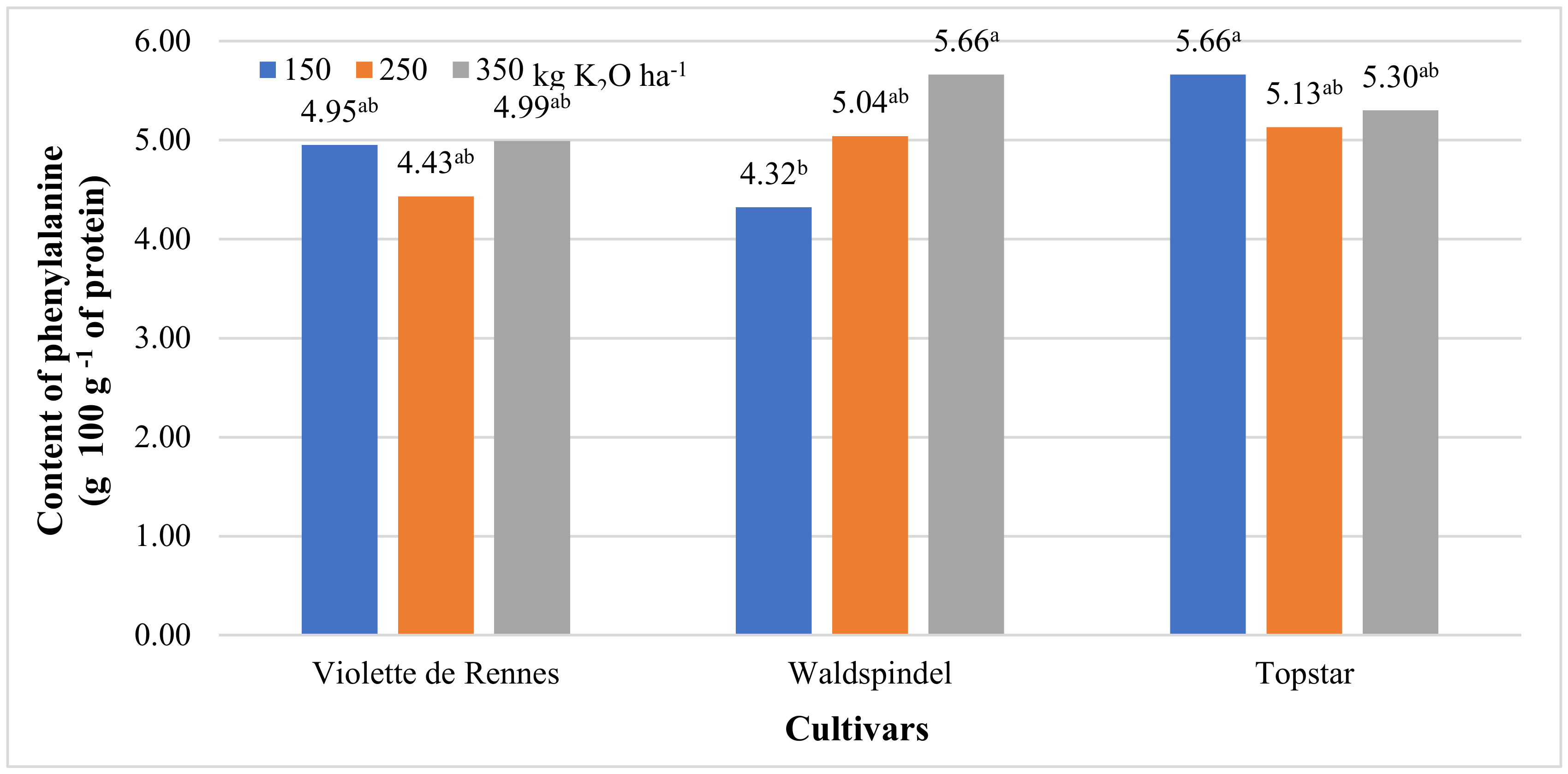
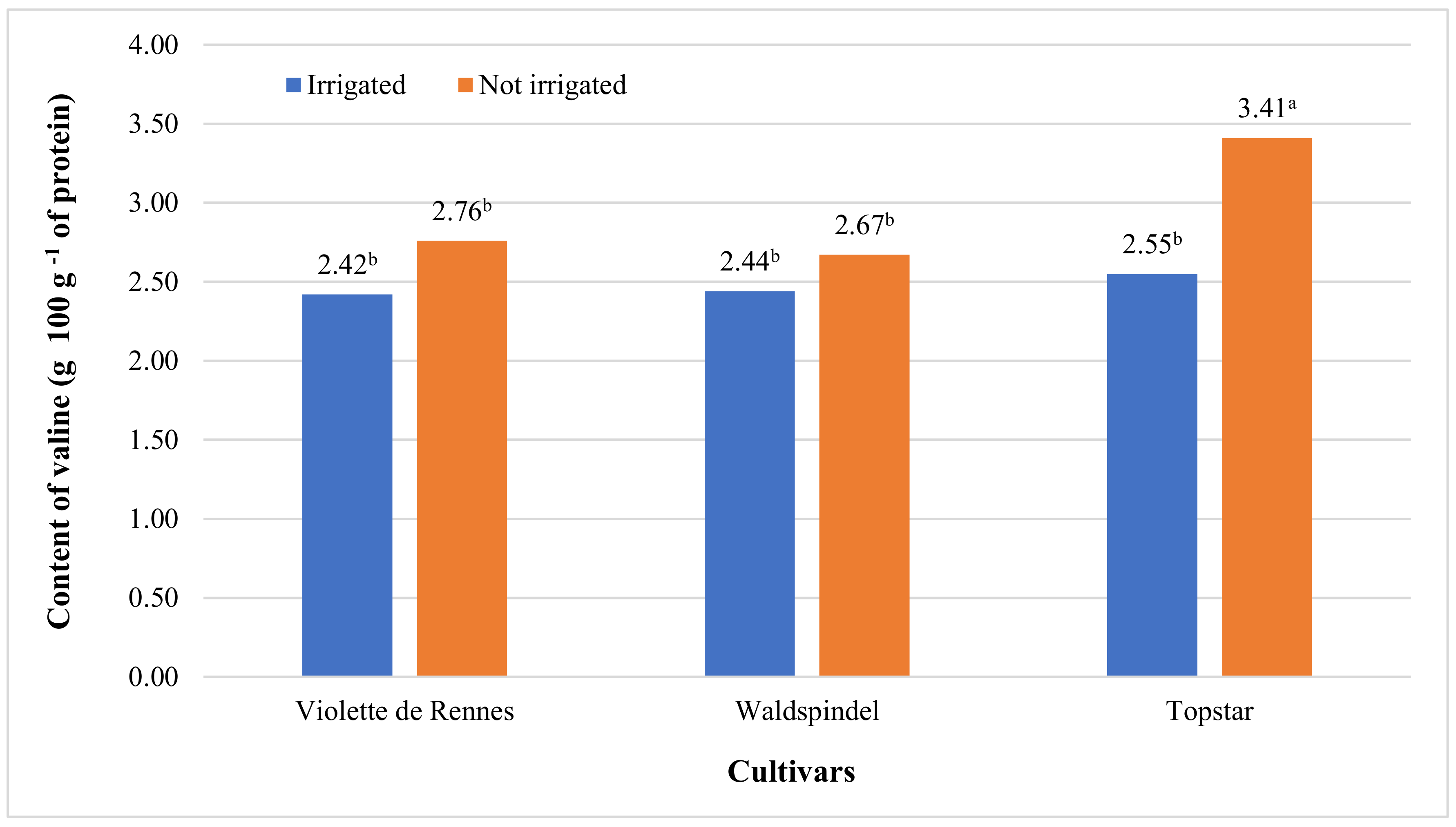
3.2. Amino acid Content and Nutritional Value of Protein
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, L.; He, Q.S.; Corscadden, K.; Udenigwe, C.C. The prospects of Jerusalem artichoke in functional food ingredients and bioenergy production. Biotechnol. Rep. 2015, 5, 77–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, N.S.; Ferreira, J.F.S.; Liu, X.L.; Suarez, D.L. Jerusalem artichoke (Helianthus tuberosus L.) maintains high inulin, tuber yield, and antioxidant capacity under moderately-saline irrigation waters. Ind. Crop. Prod. 2016, 94, 1009–1024. [Google Scholar] [CrossRef]
- Matias, J.; Encinar, J.M.; Gonzales, J.; Gonzales, J.F. Optimisation of ethanol fermentation of Jerusalem artichoke tuber juice using simple technology for a decentralised and sustainable ethanol production. Energy Sustain. Dev. 2015, 25, 34–39. [Google Scholar] [CrossRef]
- Fang, Y.R.; Liu, J.A.; Steiberger, Y.; Xie, G.H. Energy use efficiency and economic feasybility of Jerusalem artichoke production on arid and coastal saline lands. Ind. Crop. Prod. 2018, 117, 131–139. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Dubis, B.; Sokólski, M.M.; Załuski, D.; Bórawski, P.; Szempliński, W. Biomass yield and energy balance of Virginia fanpetals in different production technologies in north-eastern Poland. Energy 2019, 185, 612–623. [Google Scholar] [CrossRef]
- Lewandowski, I.; Bahrs, E.; Dahmen, N.; Hirth, T.; Rausch, T.; Weidtmann, A.W. Biobased value chains for a growing bioeconomy. GCB Bioenergy 2019, 11, 4–8. [Google Scholar] [CrossRef]
- Dubis, B.; Jankowski, K.J.; Sokólski, M.M.; Załuski, D.; Bórawski, P.; Szempliński, W. Biomass yield and energy balance of fodder galega in different production technologies: An 11-year field experiment in a large-area farm in Poland. Renew. Energ. 2020, 154, 813–825. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, Z.; Zhu, T.; Coulter, J.A. The influence of flower removal on tuber yield and biomass characteristics of Helianthus tuberosus L. in a semi-arid area. Ind. Crop. Prod. 2020, 150, 112374. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Sokólski, M.M.; Dubis, B.; Załuski, D.; Szempliński, W. Sweet sorghum—Biomass production and energy balance at different levels of agricultural inputs. A six-year field experiment in north-eastern Poland. Eur. J. Agron. 2020, 119, 126119. [Google Scholar] [CrossRef]
- Piskier, T. Labour inputs and costs of topinambour growing. Inżynieria Rol. 2006, 11, 359–365. (In Polish) [Google Scholar]
- Piskier, T. A model of topinambour cultivation for fuel purposes. Inżynieria Rol. 2010, 7, 183–190. (In Polish) [Google Scholar]
- Sawicka, B.; Skiba, D.; Michałek, W. Jerusalem artichoke as an alternative source of biomass on the fallowed land in the Lublin region. Zesz. Probl. PNR 2009, 542, 465–479. (In Polish) [Google Scholar]
- Sawicka, B. Energy value Jerusalem artichoke (Helianthus tuberosus L.) as a source of biomass. Zesz. Nauk. UP Wrocław Rol. 2010, 47, 245–256. (In Polish) [Google Scholar]
- Bolibok, Ł. Energy value of the straw of selected Jerusalem artichoke cultivars. Episterme 2011, 12, 15–20. (In Polish) [Google Scholar]
- Stolarski, M.J.; Krzyżaniak, M.; Warmiński, K.; Tworkowski, J.; Szczukowski, S.; Olba-Zięty, E.; Gołaszewski, J. Energy efficiency of herbaceous crops production depending on the type of digestate and mineral fertilizers. Energy 2017, 134, 50–60. [Google Scholar] [CrossRef]
- Paixão, S.M.; Alves, L.; Pacheco, R.; Silva, C.M. Evaluation of Jerusalem artichoke as a sustainable energy crop to bioethanol: Energy and CO2eq emissions modeling for an industrial scenario. Energy 2018, 150, 468–481. [Google Scholar] [CrossRef] [Green Version]
- Denoroy, P. The crop physiology of Helianthus tuberosus L.: A model orientated view. Biomass Bioenerg. 1996, 11, 11–32. [Google Scholar] [CrossRef]
- Kim, S.; Kim, C.H. Evaluation of whole Jerusalem artichoke (Helianthus tuberosus L.) for consolidated bioprocessing ethanol production. Renew. Energ. 2014, 65, 83–91. [Google Scholar] [CrossRef]
- Sawicka, B.; Skiba, D.; Bienia, B.; Kieltyka-Dadasiewicz, A.; Danilcenko, H. Jerusalem artichoke (Helianthus tuberosus L.) as energy raw material. In Proceedings of the 9th International Scientific Conference Rural Development, Research and Innovation for Bioeconomy, Kaunas, Lithuania, 26–28 September 2019; pp. 336–342. [Google Scholar]
- Diederichsen, A. Phenotypic diversity of Jerusalem artichoke (Helianthus tuberosus L.) germplasmpreserved by the Canadian genebank. Helia 2010, 33, 1–16. [Google Scholar] [CrossRef]
- Swanton, C.J.; Cavers, P.B.; Clementsl, D.R.; Mooret, J.M. The biology of Canadian weeds. 101. Helianthus tuberosus L. Can. J. Plant Sci. 1992, 72, 1367–1382. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, C.; Zhang, Y.; Hu, Z.; Wang, C.; Zong, Y.; Zhang, L.; Zou, J. Annual net greenhouse gas balance in a halophyte (Helianthus tuberosus) bioenergy cropping system under various soil practices in Southeast China. Bioenergy 2015, 7, 690–703. [Google Scholar] [CrossRef]
- Sawicka, B. Jerusalem Artichoke Helianthus tuberosus L.—Biology, Cultivation and Applications; University of Life Sciences: Lublin, Poland, 2016; p. 223. (In Polish) [Google Scholar]
- Monti, A.; Amaducci, M.T.; Venturi, G. Growth response, leaf gas exchange and fructans accumulation of Jerusalem artichoke (Helianthus tuberosus L.) as affected by different water regimes. Eur. J. Agron. 2005, 23, 136–145. [Google Scholar] [CrossRef]
- Niness, R.K. Inulin and Oligofructose. J. Nutr. 1999, 129, 1402–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalska-Ciechanowska, A.; Wojdyło, A.; Bogucka, B.; Dubis, B. Moderation of inulin and polyphenolics contents in three cultivars of Helianthus tuberosus L. by potassium fertilization. Agronomy 2019, 9, 884. [Google Scholar] [CrossRef] [Green Version]
- Nair, K.K.; Kharb, K.; Thompkinson, D.K. Inulin Dietary Fiber with Functional and Health Attributes. Food Rev. Int. 2010, 26, 189–203. [Google Scholar] [CrossRef]
- Sawicka, B. The effect of nitrogen fertilization of the biological value of Helianthus tuberosus L. tubers. Rocz. AR Pozn. 2000, 323, 447–451. (In Polish) [Google Scholar]
- Mystkowska, I.; Zarzecka, K. Nutritional value andpro-healthy properties of jerusalem artichoke (Helianthus tuberosus L.). Post. Fitoter. 2013, 2, 123–126. (In Polish) [Google Scholar]
- Sawicka, B.; Kalembasa, D.; Skiba, D. Variability in macroelement content in theaboveground part of Helianthus tuberosus L. at different nitrogen fertilization levels. Plant Soil Environ. 2015, 61, 158–163. [Google Scholar] [CrossRef]
- Góral, S. Morphological variation and yield of selected Jerusalem artichoke (Helianthus tuberosus L.) clones. Hod. Roślin Nasienn. 1998, 2, 6–11. (In Polish) [Google Scholar]
- Praznik, W.; Cieślik, E.; Filipiak, A. The influence of harvest time on the content of nutritional components in tubers of Jerusalem artichoke (Helianthus tuberosus L.). In Proceedings of the Seventh Seminar on Inulin, Leuven, Belgium, 22–23 January 1998; pp. 154–157. [Google Scholar]
- Matias, J.; Gonzales, J.; Cabanillas, J.; Royano, L. Influence of NPK fertilisation and harvest date on agronomic performance of Jerusalem artichoke crop in the Guadiana Basin (Southwestern Spain). Ind. Crop. Prod. 2013, 48, 191–197. [Google Scholar] [CrossRef]
- Gao, K.; Zhu, T.; Wang, Q. Nitrogen fertilization, irrigation, and harvest times affect biomass and energy value of Helianthus tuberosus L. J. Plant Nutr. 2016, 39, 1906–1914. [Google Scholar]
- Soja, G.; Dersch, G.; Praznik, W.I. Harvest dates, fertilizer and varietal effects on yield, concentration and molecular distribution of fructan in Jerusalem artichoke (Helianfhus tuberosus L.). J. Agron. Crop Sci. 1990, 165, 181–189. [Google Scholar] [CrossRef]
- Westermann, D.T.; James, D.W.; Tindall, T.A.; Hurst, R.L. Nitrogen and potassium fertilization of potatoes: Sugars and starch. Am. Potato J. 1994, 71, 433–453. [Google Scholar] [CrossRef]
- Johnston, A.E.; Milford, G.F.J. Potassium and Nitrogen Interactions in Crops; Potash Development Association: York, UK, 2000; p. 16. [Google Scholar]
- Haverkort, A.; Verhagen, A. Climate change and its repercussions for the potato supply chain. Potato Res. 2008, 51, 223–237. [Google Scholar] [CrossRef]
- Pandey, A.D.; Ostrowski, V.M.; Pandey, R.P. Simulation and optimization for irrigation and cropplanning. Irrig. Drain. 2012, 61, 178–188. [Google Scholar]
- PIORiN. Integrated Production Method for Potatoes. Main Inspectorate of Plant Health and Seed Inspection; 2014. Available online: http://www.piorin.gov.pl/30.01.2020 (accessed on 15 June 2020). (In Polish)
- IUSS Working Group WRB. World Reference Base for Soil Resources. In World Soil Resources Reports, 2nd ed.; FAO: Roma, Italy, 2006. [Google Scholar]
- Houba, V.J.G.; Van der Lee, J.J.; Novozamsky, I. Soil and Plant Analysis. Part 5B. Soil Analysis Procedure other Procedure; Wageningen Agriculture University: Wageningen, The Netherlands, 1995. [Google Scholar]
- Polish Standard PN-77-R-64820. In Animal Feed. Determination of Tryptophan Content; Polish Standard ISO 6867: 2002; Animal Feed. Determination of Vitamin E Content. High-Performance Liquid Chromatography—Tocopherols in Animal Feed; Polish Committee for Standardization: Warsaw, Poland, 1977.
- Manz, U. Assay Methods for Beta-Carotene in ROVIMIX-β-Carotene 10% and in Mixed Animal Feeds, and of Carotene in Animal Feedstuffs, Blood Plasma and MilkInformation Services Animal Nutrition Department, Chalcombe PublicationsChalcombe Publications: Basel, Switzerland, 1986.
- Rodriquez-Bernaldo de Quiros, A.; Costa, H.S. Analysis of carotenoids in vegetable and plasma samples. J. Food Compos. Anal. 2006, 19, 97–111. [Google Scholar] [CrossRef]
- Raju, M.; Varakumar, S.; Lakshminarayana, R.; Krishnakantha, T.P.; Baskaran, V. Carotenoid composition and vitamin A activity of medicinally important green leafy vegetables. Food Chem. 2007, 101, 1598–1605. [Google Scholar] [CrossRef]
- Mitchell, H.H.; Block, R.J. Some relationship between the amino acid contents of proteins and their nutritive values for the rat. J. Biol. Chem. 1946, 163, 599–620. [Google Scholar] [CrossRef]
- Oser, B.L. An integrated essential amino acid index for predicting biological value of proteins. In Protein Amino Acid Nutrition; Academic Press: Cambridge, MA, USA, 1959; pp. 295–311. [Google Scholar]
- Statsoft Inc. Statistica (Data Analysis Software System), 10th ed.; Statsoft Inc.: Tulsa, OK, USA, 2011; Available online: http:/www.statsoft.com (accessed on 1 April 2020).
- Sram, R.J.; Binkova, B.; Topinka, J.; Kotesovec, F.; Fojtikova, I.; Hanel, I.; Klaschka, J.; Kocisova, J.; Prosek, M.; Machalek, J. Effect of antioxidant supplementation in an elderly population. Basic Life Sci. 1993, 61, 459–477. [Google Scholar]
- Martin, A.; Youdim, K.; Szprengiel, A.; Shukitt-Hale, B.; Joseph, J. Roles of Vitamins E and C on Neurodegenerative Diseasesand Cognitive Performance. Nutr. Rev. 2002, 60, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Moszczyński, P.; Pyć, R. Biochemistry of Vitamins. Part 2: Lipophilic Vitamins and Ascorbic Acid; Polish Scientific Publishers: Warszaw, Poland, 1999; p. 162. (In Polish) [Google Scholar]
- Krist, S. Vegetable Fats and Oils; Springer: Cham, Switzerland, 2000; p. 774. [Google Scholar]
- Mao, X.; Chen, W.; Huyan, Z.; Sherazi, S.T.H. Impact of linolenic acid on oxidative stability of rapeseed oils. J. Food Sci. Technol. 2020, 57, 3184–3192. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.; Galvin, K.; O’Connor, T.P.; Maguire, A.R.; O’Brien, N.M. Phytosterol, Squalene, Tocopherol Content and Fatty Acid Profile of Selected Seeds, Grains, and Legumes. Plant Foods Hum. Nutr. 2007, 62, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Hamouz, K.; Orsak, M.; Pivec, V. Potato tubers as a significant source of antioxidants in human nutrition. Rost. Vyroba 2000, 46, 231–236. [Google Scholar]
- Andre, C.M.; Qufir, M.; Guignard, C.; Hoffmann, L.; Hausman, J.F.; Evers, D.; Larondelle, Y. Antioxidant Profiling of Native Andean Potato Tubers (Solanum tuberosum L.) Reveals Cultivars with High Levels of β-Carotene, ά-Tocopherol, Chlorogenic Acid, and Petanin. J. Agric. Food Chem. 2007, 55, 10839–10849. [Google Scholar] [CrossRef]
- Gilbert, C. How to manage children with the eye signs of vitamin A deficiency. Community Eye Health J. 2013, 26, 68. [Google Scholar]
- Toti, E.; Chen, C.Y.O.; Palmery, M.; Valencia, D.V.; Peluso, I. Non-Provitamin A and Provitamin A Carotenoids as Immunomodulators: Recommended Dietary Allowance, Therapeutic Index, or Personalized Nutrition? Oxid. Med. Cell. Longev. 2018, 2018, 20. [Google Scholar] [CrossRef]
- Staniaszek, K.; Goździcka-Józefiak, A. The influence of β-carotene, retinoids, RAR and RXR receptors proliferation and neoplastic transformation of cells. Biotechnologia 2008, 3, 28–45. (In Polish) [Google Scholar]
- Wierzbicka, A.; Hallmann, E. The prevalence and role of carotenoids in potatoes. Ziemn. Pol. 2015, 3, 42–46. (In Polish) [Google Scholar]
- Preedy, V.R. Vitamin A and Carotenoids. Chemistry, Analysis, Function and Effects; Royal Society and Chemistry: Cambridge, UK, 2012; p. 555. [Google Scholar]
- Cieślik, E.; Filipiak-Florkiewicz, A. Perspective usage of Jerusalem artichoke (Helianthus tuberosus L.) for producing functional food. Żywność Nauka Technol. Jakość 2000, 1, 73–81. (In Polish) [Google Scholar]
- Eppendorfer, W.H. Free and total amino acid composition of edible parts of beans, kale, spinach, cauliflower and potatoes as influenced by nitrogen fertilization and phosphorus and potassium deficiency. J. Sci. Food Agric. 1996, 71, 449–458. [Google Scholar] [CrossRef]
- Pęksa, A.; Rytel, E.; Tajner-Czopek, A.; Kita, A.; Pytlarz-Kozicka, M.; Miedzianka, J. The comparision of amino acid composition of the protein in preparation obtained from potato tu bers cultivated within ecological and conventional systems. Biuletyn IHAR 2012, 266, 307–317. (In Polish) [Google Scholar]
- WHO; FAO; UNU. Protein and Amino Acid Requirements in Human Nutrition; United Nations University: Tokyo, Japan, 2007. [Google Scholar]
- Mitrus, J.; Stankiewicz, C.; Steć, E.; Kamecki, M.; Starczewski, J. The influence of selected cultivation on the content of total protein and amino acids in the potato tubers. Plant Soil Environ. 2003, 49, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Danilcenko, H.; Jariene, E.; Gajewska, M.; Sawicka, B.; Kulaitien, J.; Cerniauskiene, J.; Aleknaviciene, P. Changes in amino acids content in tuber of Jerusalem artichoke (Helianthus tuberosus L.) cultivars during storage. Acta Sci. Pol. Hortorum Cultus 2013, 12, 97–105. [Google Scholar]
- Pęksa, A.; Kita, A.; Jariene, E.; Danilcenko, H.; Gryszkin, A.; Figiel, A.; Kulaitiene, J.; Cerniauskiene, J.; Aniołowska, M. Amino acid improving and physical qualities of extruded corn snacks using flours made from Jerusalem artichoke (Helianthus tuberosus L.), Amaranth (Amaranthus cruentus L.) and Pumpkin (Cucurbita maxima L.). J. Food Qual. 2016, 38, 580–589. [Google Scholar] [CrossRef]
- Ciborowska, H.; Rudnicka, A. Nutrition—Dietary Guidelines for Healthy and Sick Individuals; Medical Publishing House: Warsaw, Poland, 2014; p. 658. (In Polish) [Google Scholar]
- Zhu, F.; Cai, Y.Z.; Ke, J.; Corke, H. Compositions of phenolic compounds, amino acid and reducing sugars in commercial potato varieties and their effects on acrylamide formation. J. Sci. Food Agric. 2010, 90, 2254–2262. [Google Scholar] [CrossRef]

| Parameter | Cv. | K | Irrigation (IR) | Cv. × K | Cv. × IR | K × IR | Cv. × K × IR |
|---|---|---|---|---|---|---|---|
| α-Tocopherol | 1.899 ns | 6.254 ** | 2.850 ns | 6.161 ** | 0.053 ns | 7.787 ** | 2.426 ns |
| % α-Tocopherol in total tocopherols | 1.428 ns | 5.678 ** | 0.004 ns | 2.830 ns | 1.766 ns | 1.098 ns | 1.541 ns |
| β-Carotene | 1.558 ns | 0.932 ns | 10.066 ** | 0.371 ns | 0.010 ns | 1.370 ns | 1.370 ns |
| Retinol equivalents | 1.578 ns | 0.928 ns | 10.061 ** | 0.376 ns | 0.010 ns | 1.395 ns | 0.496 ns |
| Alanine | 4.001 * | 0.711 ns | 14.589 ** | 0.563 ns | 1.253 ns | 0.879 ns | 1.653 ns |
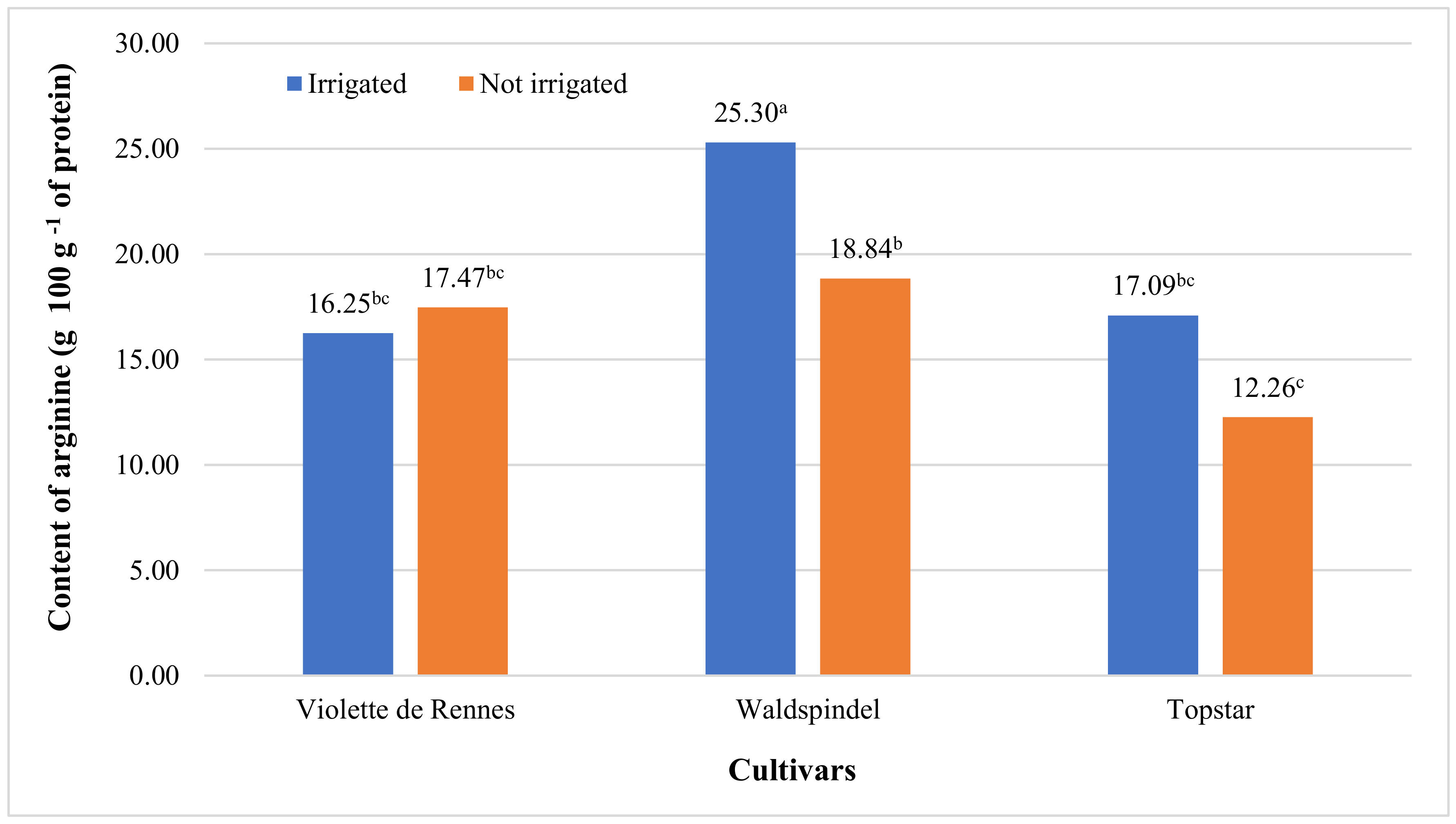
| Arginine | 17.560 *** | 0.199 ns | 10.283 ** | 1.113 ns | 5.001 * | 0.396 ns | 2.395 ns |
| Glycine | 5.258 * | 0.717 ns | 23.100 ** | 1.329 ns | 2.885 ns | 0.119 ns | 2.127 ns |
| Histidine | 0.482 ns | 0.607 ns | 13.105 ** | 0.519 ns | 1.017 ns | 0.151 ns | 1.236 ns |
| Aspartic acid | 7.032 ** | 1.674 ns | 0.944 ns | 1.463 ns | 0.729 ns | 0.144 ns | 1.710 ns |
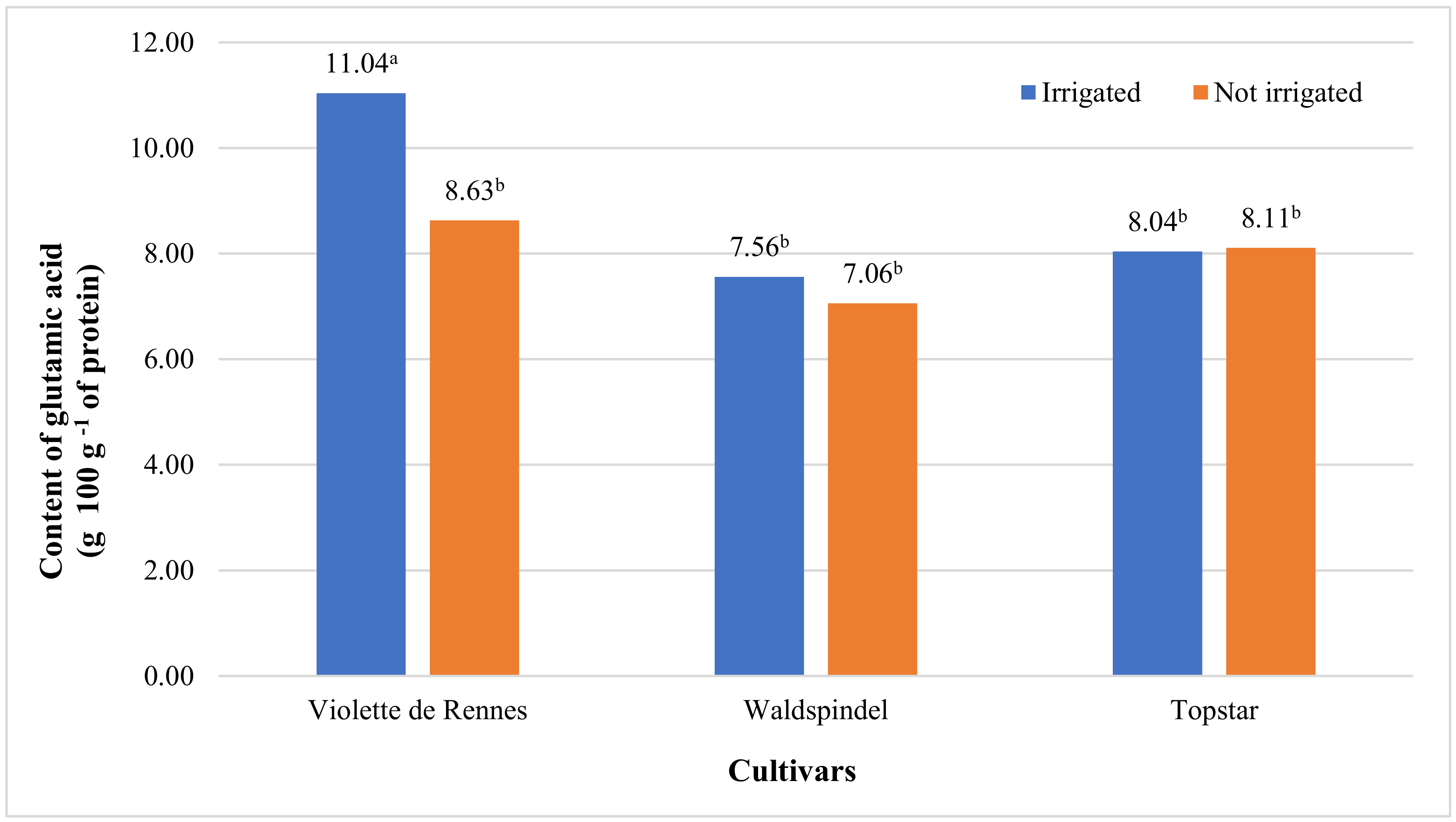
| Glutamic acid | 20.094 ** | 4.917 * | 8.103 * | 3.383 * | 5.050 * | 1.457 ns | 1.505 ns |
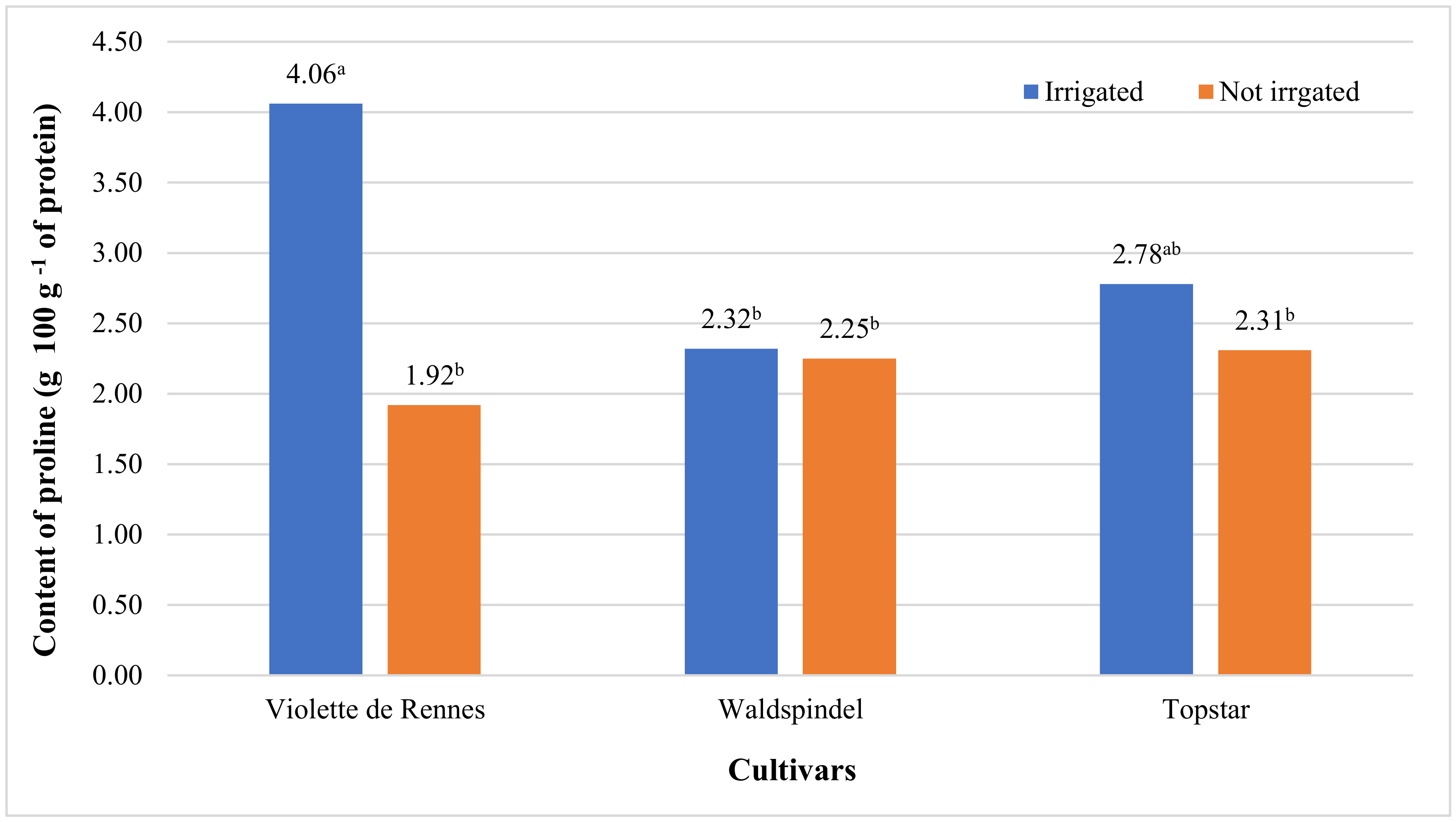
| Proline | 2.472 ns | 1.399 ns | 11.632 ** | 2.266 ns | 5.884 * | 1.765 ns | 1.611 ns |
| Serine | 2.120 ns | 0.276 ns | 9.025 ** | 0.620 ns | 1.495 ns | 0.235 ns | 1.078 ns |
| Threonine | 4.229 * | 0.532 ns | 17.101 ** | 0.712 ns | 1.564 ns | 0.068 ns | 1.669 ns |
| Isoleucine | 6.208 ** | 0.196 ns | 17.926 ** | 1.614 ns | 1.617 ns | 0.131 ns | 1.562 ns |
| Leucine | 8.587 ** | 0.803 ns | 21.939 ** | 1.109 ns | 3.340 ns | 0.389 ns | 2.297 ns |
| Lysine | 1.004 ns | 0.160 ns | 4.445 * | 0.543 ns | 0.677 ns | 0.018 ns | 0.400 ns |
| Methionine | 1.369 ns | 0.141 ns | 0.435 ns | 0.265 ns | 0.381 ns | 0.494 ns | 0.690 ns |
| Cystine | 1.695 ns | 0.231 ns | 1.326 ns | 0.232 ns | 0.338 ns | 0.980 ns | 0.716 ns |
| Phenylalanine | 3.666 * | 2.447 ns | 25.538 ** | 3.391 * | 3.268 ns | 0.481 ns | 1.215 ns |
| Thyrosine | 29.488 *** | 0.061 ns | 1.565 ns | 0.817 ns | 0.921 ns | 0.154 ns | 1.911 ns |
| Tryptophan | 1.610 ns | 0.093 ns | 0.127 ns | 0.093 ns | 0.129 ns | 0.219 ns | 0.261 ns |
| Valine | 7.553 ** | 0.501 ns | 23.428 ** | 1.121 ns | 3.981 * | 0.442 ns | 1.734 ns |
| Specification | Month | |||||||
|---|---|---|---|---|---|---|---|---|
| April | May | June | July | August | September | October | November | |
| Mean air temperature (°C) | 10.8 | 15.7 | 17.2 | 19.7 | 19.2 | 14.5 | 8.7 | 3.3 |
| 1981–2010 | 7.7 | 13.5 | 16.1 | 18.7 | 17.9 | 12.8 | 8.0 | 2.9 |
| Rainfall total (mm) | 33.5 | 25.0 | 53.7 | 141.0 | 44.6 | 20.3 | 84.7 | 16.0 |
| 1981–2010 | 33.3 | 58.5 | 80.4 | 74.2 | 59.4 | 56.9 | 42.6 | 44.8 |
| Water requirements of late-maturing potato varieties [40] | - | 62 | 74 | 97 | 79 | 50 | - | - |
| Parameter | α-Tocopherol (mg kg−1 Lyophilisate) | α-Tocopherol (% Total Tocopherols) | β-Carotene (mg kg−1 Lyophilisate) | Retinol Equivalents |
|---|---|---|---|---|
| Cultivar | ||||
| Violette de Rennes | 2.04 | 76.1 | 0.82 | 137 |
| Waldspindel | 2.55 | 70.2 | 0.83 | 138 |
| Topstar | 2.02 | 71.2 | 0.97 | 162 |
| Potassium fertilization (kg K2O ha−1) | ||||
| 150 | 1.60 b | 68.0 b | 0.81 | 135 |
| 250 | 2.35 ab | 69.8 b | 0.94 | 157 |
| 350 | 2.65 a | 79.6 a | 0.88 | 147 |
| Irrigation | ||||
| Irrigated | 2.57 a | 72.4 | 0.75 b | 125 b |
| Not irrigated | 1.83 b | 72.6 | 1.00 a | 167 a |
| Parameter | Isoleucine | Leucine | Lysine | Methionine | Cysteine | Phenylalanine | Thyrosine | Tryptophan | Valine |
|---|---|---|---|---|---|---|---|---|---|
| Cultivar | |||||||||
| Violette de Rennes | 2.12 b | 2.99 b | 3.72 | 0.59 | 0.433 | 4.79 b | 1.41 b | 0.67 | 2.59 b |
| Waldspindel | 2.09 b | 2.97 b | 3.94 | 0.67 | 0.532 | 5.01 ab | 2.44 a | 0.82 | 2.56 b |
| Topstar | 2.49 a | 3.48 a | 4.23 | 0.76 | 0.570 | 5.36 a | 2.82 a | 0.68 | 2.98 a |
| Potassium fertilization (kg ha−1) | |||||||||
| 150 | 2.20 | 3.08 | 3.99 | 0.69 | 0.523 | 4.98 | 2.22 | 0.71 | 2.70 |
| 250 | 2.22 | 3.11 | 3.86 | 0.64 | 0.482 | 4.87 | 2.20 | 0.72 | 2.65 |
| 350 | 2.28 | 3.25 | 4.06 | 0.69 | 0.531 | 5.32 | 2.26 | 0.75 | 2.77 |
| Irrigation | |||||||||
| Irrigated | 2.02 b | 2.88 b | 3.66 b | 0.65 | 0.476 | 4.62 b | 2.32 | 0.71 | 2.47 b |
| Not irrigated | 2.45 a | 3.41 a | 4.28 a | 0.70 | 0.548 | 5.49 a | 2.13 | 0.74 | 2.95 a |
| Parameter | Alanine | Arginine | Glycine | Histidine | Aspartic acid | Glutamic acid | Proline | Serine | Threonine |
|---|---|---|---|---|---|---|---|---|---|
| Cultivar | |||||||||
| Violette de Rennes | 3.04 b | 16.86 b | 2.69 b | 2.10 | 8.92 a | 9.84 a | 2.99 | 2.18 | 2.63 b |
| Waldspindel | 3.23 ab | 22.07 a | 2.75 ab | 2.14 | 7.34 b | 7.31 b | 2.28 | 2.24 | 2.73 ab |
| Topstar | 3.50 a | 14.68 b | 3.11 a | 2.22 | 7.43 b | 8.08 b | 2.55 | 2.50 | 3.11 a |
| Potassium fertilization (kg K2O ha−1) | |||||||||
| 150 | 3.23 | 17.83 | 2.81 | 2.11 | 8.40 | 8.90 a | 2.52 | 2.26 | 2.76 |
| 250 | 3.18 | 17.48 | 2.79 | 2.13 | 7.67 | 7.68 b | 2.91 | 2.29 | 2.78 |
| 350 | 3.37 | 18.29 | 2.95 | 2.23 | 7.62 | 8.64 ab | 2.39 | 2.38 | 2.93 |
| Irrigation | |||||||||
| Irrigated | 3.00 b | 19.55 a | 2.58 b | 1.98 b | 7.71 | 8.88 a | 3.05 a | 2.11 b | 2.52 b |
| Not irrigated | 3.51 a | 16.19 b | 3.13 a | 2.33 a | 8.09 | 7.93 b | 2.16 b | 2.51 a | 3.12 a |
| Amino Acids (g 100 g−1 of Protein) | cv. Topstar | cv. Waldspindel | cv. Violette de Rennes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | CS | EAAI | Mean | CS | EAAI | Mean | CS | EAAI | |
| Isoleucine | 2.49 | 88.90 | 78.00 | 2.09 | 74.60 | 71.00 | 2.12 | 78.20 | 66.00 |
| Leucine | 3.48 | 52.70 | 2.97 | 45.00 | 2.99 | 45.30 | |||
| Lysine | 4.23 | 72.90 | 3.94 | 67.90 | 3.72 | 64.30 | |||
| Methionine + Cysteine | 1.33 | 53.20 | 1.20 | 48.00 | 1.02 | 40.80 | |||
| Phenylalanine + Tyrosine | 8.18 | 129.80 | 7.45 | 118.20 | 6.20 | 98.40 | |||
| Tryptophan | 0.68 | 61.80 | 0.82 | 74.50 | 0.67 | 60.90 | |||
| Valine | 2.98 | 85.10 | 2.56 | 73.10 | 2.59 | 74.00 | |||
| Amino Acids (g 100 g−1 of Protein) | 150 kg K2O ha−1 | 250 kg K2O ha−1 | 350 kg K2O ha−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | CS | EAAI | Mean | CS | EAAI | Mean | CS | EAAI | |
| Isoleucine | 2.20 | 78.57 | 71.00 | 2.22 | 79.28 | 70.00 | 2.28 | 81.43 | 74.00 |
| Leucine | 3.08 | 46.67 | 3.11 | 47.12 | 3.25 | 49.24 | |||
| Lysine | 3.99 | 68.79 | 3.86 | 66.55 | 4.06 | 70.00 | |||
| Methionine + Cysteine | 1.21 | 48.40 | 1.12 | 44.80 | 1.22 | 48.80 | |||
| Phenylalanine + Thyrosine | 7.20 | 114.28 | 7.07 | 112.22 | 7.58 | 120.32 | |||
| Tryptophan | 0.71 | 64.54 | 0.72 | 65.45 | 0.75 | 68.18 | |||
| Valine | 2.70 | 77.14 | 2.65 | 75.71 | 2.77 | 79.14 | |||
| Amino Acids (g 100 g−1 of protein) | Irrigation | No Irrigation | ||||
|---|---|---|---|---|---|---|
| Mean | CS | EAAI | Mean | CS | EAAI | |
| Isoleucine | 2.02 | 72.14 | 67.00 | 2.45 | 87.50 | 76.00 |
| Leucine | 2.88 | 43.64 | 3.41 | 51.67 | ||
| Lysine | 3.66 | 63.10 | 4.28 | 73.79 | ||
| Methionine + Cysteine | 1.13 | 45.20 | 1.25 | 50.00 | ||
| Phenylalanine + Thyrosine | 6.94 | 110.16 | 7.62 | 120.95 | ||
| Tryptophan | 0.71 | 64.54 | 0.74 | 67.27 | ||
| Valine | 2.47 | 70.57 | 2.95 | 84.28 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogucka, B.; Jankowski, K. Jerusalem Artichoke: Quality Response to Potassium Fertilization and Irrigation in Poland. Agronomy 2020, 10, 1518. https://doi.org/10.3390/agronomy10101518
Bogucka B, Jankowski K. Jerusalem Artichoke: Quality Response to Potassium Fertilization and Irrigation in Poland. Agronomy. 2020; 10(10):1518. https://doi.org/10.3390/agronomy10101518
Chicago/Turabian StyleBogucka, Bożena, and Krzysztof Jankowski. 2020. "Jerusalem Artichoke: Quality Response to Potassium Fertilization and Irrigation in Poland" Agronomy 10, no. 10: 1518. https://doi.org/10.3390/agronomy10101518
APA StyleBogucka, B., & Jankowski, K. (2020). Jerusalem Artichoke: Quality Response to Potassium Fertilization and Irrigation in Poland. Agronomy, 10(10), 1518. https://doi.org/10.3390/agronomy10101518





