Testing a Bovine Blood-Derived Compound as Iron Supply on Cucumis sativus L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Products
2.2. Recovery Assay
2.3. Assay on Fe-Reduction Capacity
2.4. Statistical Analysis
3. Results
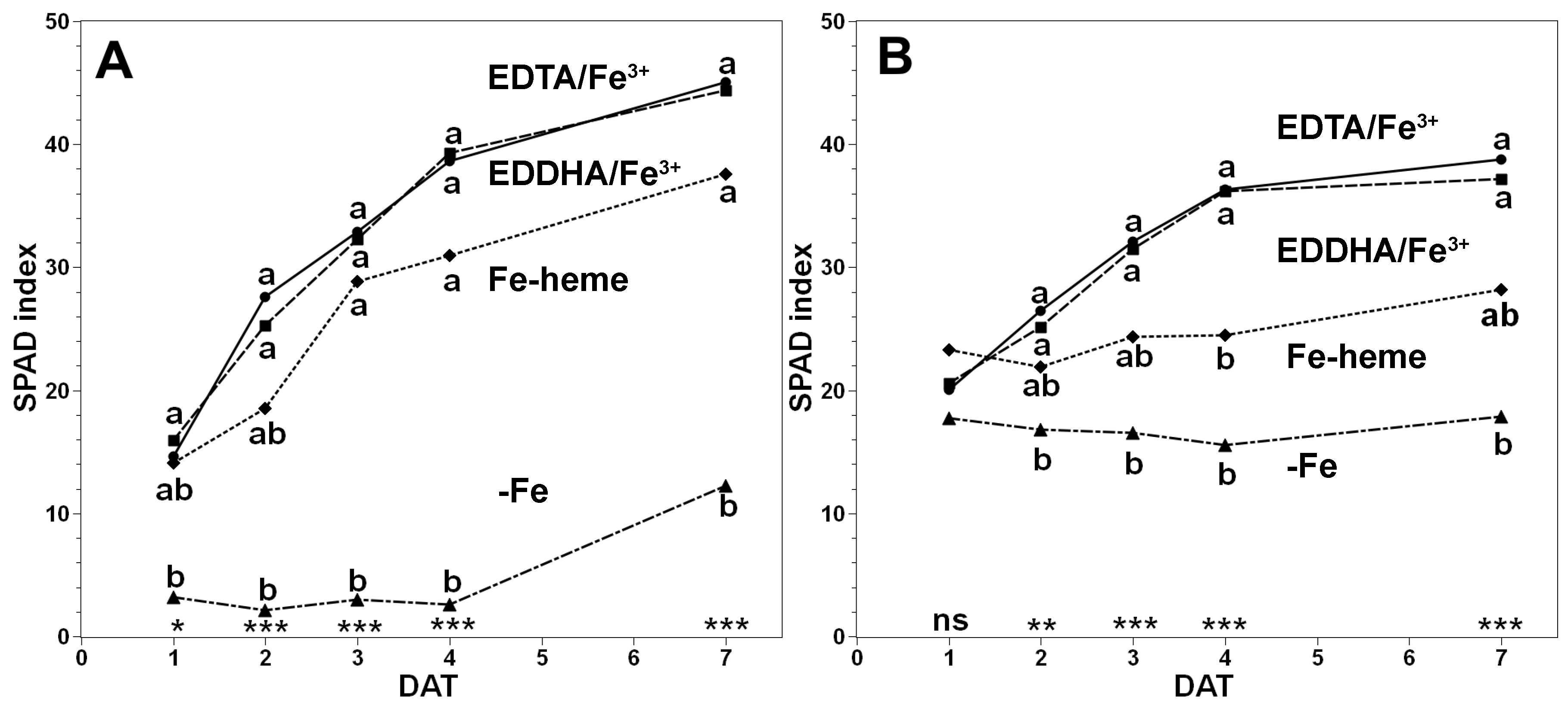
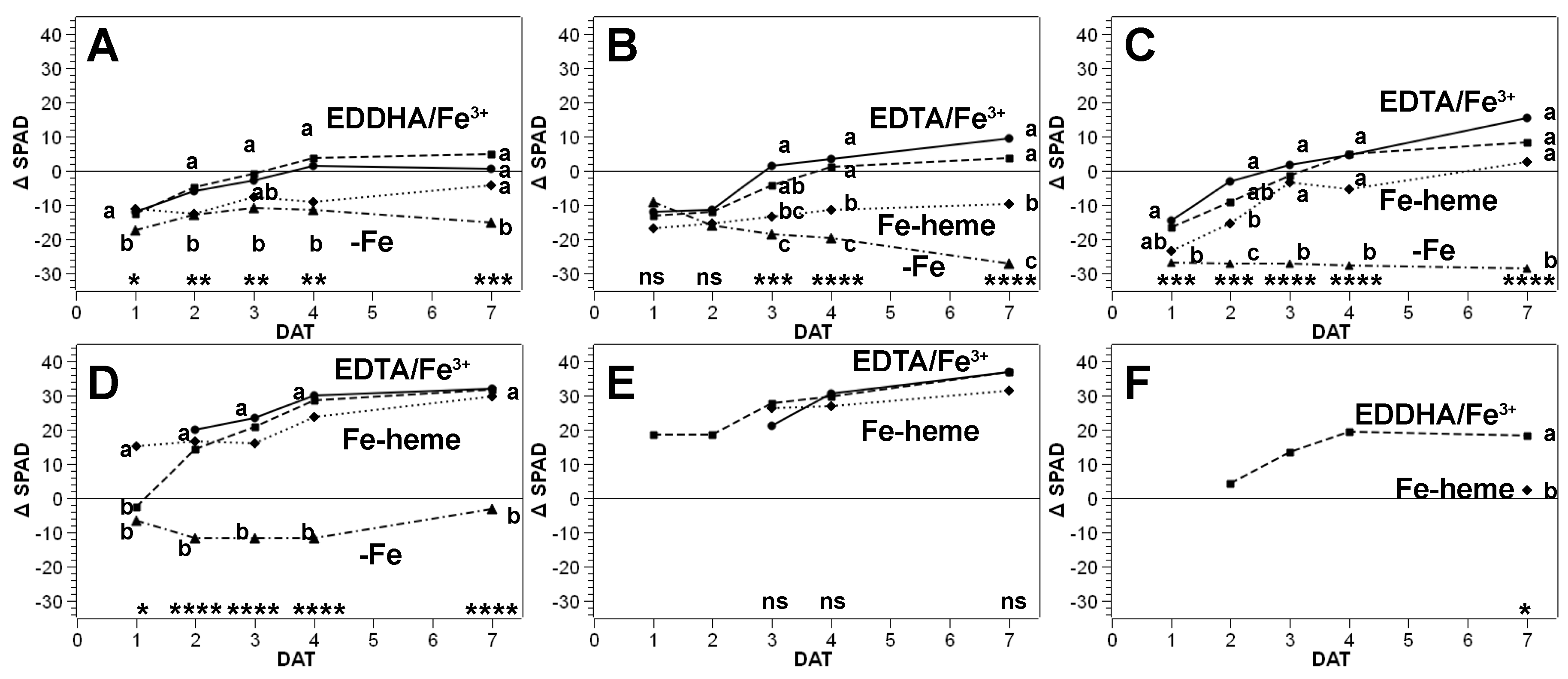
3.1. Recovery Assays
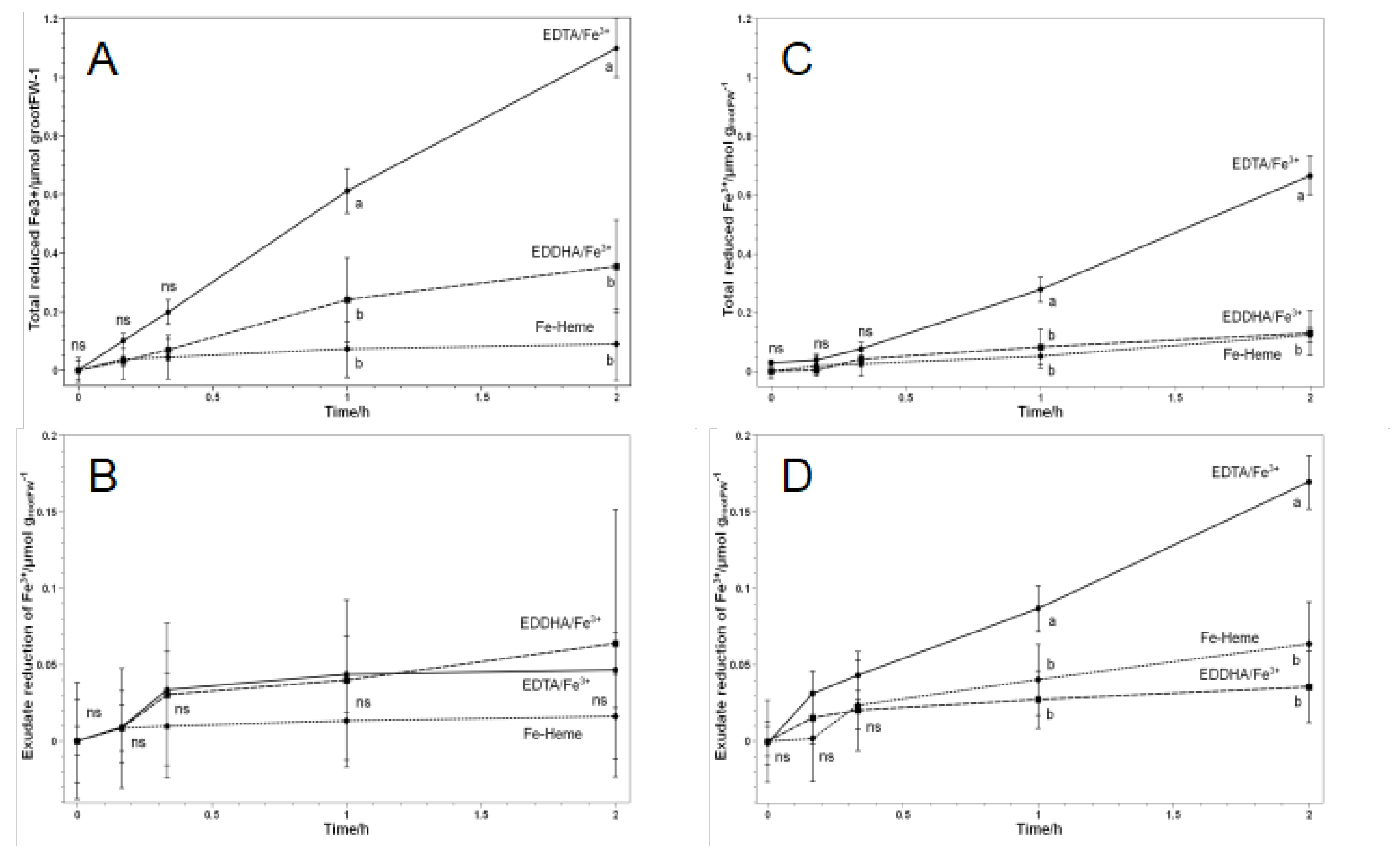
3.2. Fe Reduction by Roots and Root Exudates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tagliavini, M.; Rombolà, A.D. Iron deficiency and chlorosis in orchard and vineyard ecosystems. Eur. J. Agron. 2001, 15, 71–92. [Google Scholar] [CrossRef]
- Lucena, J.J. Synthetic Iron Chelates to Correct Iron Deficiency in Plants. In Iron Nutrition in Plants and Rhizospheric Microorganisms; Barton, L.L., Abadía, J., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 103–128. [Google Scholar] [CrossRef]
- Rombolà, A.D.; Tagliavini, M. Iron Nutrition of Fruit Tree Crops. In Iron Nutrition in Plants and Rhizospheric Microorganisms; Barton, L.L., Abadía, J., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 61–83. [Google Scholar] [CrossRef]
- Shenker, M.; Chen, Y. Increasing Iron Availability to Crops: Fertilizers, Organo-Fertilizers, and Biological Approaches. Soil Sci. Plant Nutr. 2005, 51, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Cesco, S.; Römheld, V.; Varanini, Z.; Pinton, R. Solubilization of iron by water-extractable humic substances. J. Plant Nutr. Soil Sci. 2000, 163, 285–290. [Google Scholar] [CrossRef]
- Carrasco, J.; Kovacs, K.; Czech, V.; Fodor, F.; Lucena, J.J.; Vértes, A.; Hernández-Apaolaza, L. Influence of pH, Iron Source, and Fe/Ligand Speciation in Lignosulfonate Complexes Studied Using Mössbauer Spectroscopy. Implications on Their Fertilizer Properties. J. Agric. Food Chem. 2012, 60, 3331–3340. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lucena, P.; Hernández-Apaolaza, L.; Lucena, J.J. Comparison of iron chelates and complexes supplied as foliar sprays and in nutrient solution to correct iron chlorosis of soybean. J. Plant Nutr. Soil Sci. 2009, 173, 120–126. [Google Scholar] [CrossRef]
- Chen, Y.; Barak, P. Iron Nutrition of Plants in Calcareous Soils. Adv. Agron. 1982, 35, 217–240. [Google Scholar] [CrossRef]
- Hargrove, M.S. The Structural Determinants of Myoglobin Stability. Ph.D. Thesis, Rice University, Houston, TX, USA, 1996. [Google Scholar]
- Yunta, F.; Di Foggia, M.; Bellido-Díaz, V.; Morales-Calderón, M.; Tessarin, P.; López-Rayo, S.; Tinti, A.; Kovacs, K.; Klencsár, Z.; Fodor, F.; et al. Blood Meal-Based Compound. Good Choice as Iron Fertilizer for Organic Farming. J. Agric. Food Chem. 2013, 61, 3995–4003. [Google Scholar] [CrossRef]
- Kalbasi, M.; Shariatmadari, H. Blood powder, a source of iron for plants. J. Plant Nutr. 1993, 16, 2213–2223. [Google Scholar] [CrossRef]
- Yunta, F.; García-Marco, S.; Lucena, J.J. Theoretical Speciation of Ethylenediamine-N-(o-hydroxyphenylacetic)-N’-(p-hydroxyphenylacetic) Acid (o,p-EDDHA) in Agronomic Conditions. J. Agric. Food Chem. 2003, 51, 5391–5399. [Google Scholar] [CrossRef]
- Nadal, P.; García-Marco, S.; Escudero, R.; Lucena, J.J. Fertilizer properties of DCHA/Fe3+. Plant Soil 2012, 356, 367–379. [Google Scholar] [CrossRef]
- Escudero, R.; Gómez-Gallego, M.; Romano, S.; Fernández, I.; Gutiérrez-Alonso, A.; Sierra, M.A.; López-Rayo, S.; Nadal, P.; Lucena, J.J. Biological activity of Fe(iii) aquo-complexes towards ferric chelate reductase (FCR). Org. Biomol. Chem. 2012, 10, 2272–2281. [Google Scholar] [CrossRef] [PubMed]
- Vigani, G.; Faoro, F.; Ferretti, A.M.; Cantele, F.; Maffi, D.; Marelli, M.; Maver, M.; Murgia, I.; Zocchi, G. Three-Dimensional Reconstruction, by TEM Tomography, of the Ultrastructural Modifications Occurring in Cucumis sativus L. Mitochondria under Fe Deficiency. PLoS ONE 2015, 10, e0129141. [Google Scholar] [CrossRef] [PubMed]
- Donnini, S.; Guidi, L.; DeglInnocenti, E.; Zocchi, G. Image changes in chlorophyll fluorescence of cucumber leaves in response to iron deficiency and resupply. J. Plant Nutr. Soil Sci. 2013, 176, 734–742. [Google Scholar] [CrossRef]
- Mori, S. Iron acquisition by plants. Curr. Opin. Plant Biol. 1999, 2, 250–253. [Google Scholar] [CrossRef]
- López-Rayo, S.; Di Foggia, M.; Bombai, G.; Yunta, F.; Moreira, E.R.; Filippini, G.; Pisi, A.; Rombolà, A. Blood-derived compounds can efficiently prevent iron deficiency in the grapevine. Aust. J. Grape Wine Res. 2014, 21, 135–142. [Google Scholar] [CrossRef]
- López-Rayo, S.; Di Foggia, M.; Moreira, E.R.; Donnini, S.; Bombai, G.; Filippini, G.; Pisi, A.; Rombolà, A. Physiological responses in roots of the grapevine rootstock 140 Ruggeri subjected to Fe deficiency and Fe-heme nutrition. Plant Physiol. Biochem. 2015, 96, 171–179. [Google Scholar] [CrossRef]
- Robinson, N.J.; Procter, C.M.; Connolly, E.L.; Guerinot, M.L. A ferric-chelate reductase for iron uptake from soils. Nature 1999, 397, 694–697. [Google Scholar] [CrossRef]
- Santi, S.; Schmidt, W. Laser microdissection-assisted analysis of the functional fate of iron deficiency-induced root hairs in cucumber. J. Exp. Bot. 2008, 59, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, D.; Pimentel, M.; Guerinot, M.L. To Improve Nutrition for the World’s Population. Science 2000, 288, 1966–1967. [Google Scholar] [CrossRef]
- Yi, Y.; Guerinot, M.L. Genetic evidence that induction of root Fe(III) chelate reductase activity is necessary for iron uptake under iron deficiency+. Plant J. 1996, 10, 835–844. [Google Scholar] [CrossRef]
- Mimmo, T.; Del Buono, D.; Terzano, R.; Tomasi, N.; Vigani, G.; Crecchio, C.; Pinton, R.; Zocchi, G.; Cesco, S. Rhizospheric organic compounds in the soil-microorganism-plant system: Their role in iron availability. Eur. J. Soil Sci. 2014, 65, 629–642. [Google Scholar] [CrossRef]
- Pavlovic, J.; Samardžić, J.; Maksimović, V.; Timotijevic, G.; Stevic, N.; Laursen, K.H.; Hansen, T.H.; Husted, S.; Schjoerring, J.K.; Liang, Y.; et al. Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytol. 2013, 198, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, A.; Zanin, L.; Tomasi, N.; Pezzotti, M.; Pinton, R.; Varanini, Z.; Cesco, S. Genome-wide microarray analysis of tomato roots showed defined responses to iron deficiency. BMC Genom. 2012, 13, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yunta, F.; García-Marco, S.; Lucena, J.J.; Gómez-Gallego, M.; Alcázar, R.; Sierra, M.A. Chelating Agents Related to Ethylenediamine Bis(2-hydroxyphenyl)acetic Acid (EDDHA): Synthesis, Characterization, and Equilibrium Studies of the Free Ligands and Their Mg2+, Ca2+, Cu2+, and Fe3+Chelates. Inorg. Chem. 2003, 42, 5412–5421. [Google Scholar] [CrossRef] [PubMed]
- Nadal, P.; Hernández-Apaolaza, L.; Lucena, J.J. Efficacy of HJB/Fe3+, a new iron chelate to supply Fe to plants. Plant Soil 2009, 325, 65–77. [Google Scholar] [CrossRef]
- Lucena, J.J.; Chaney, R.L. Synthetic Iron Chelates as Substrates of Root Ferric Chelate Reductase in Green Stressed Cucumber Plants. J. Plant Nutr. 2006, 29, 423–439. [Google Scholar] [CrossRef]
- Bityutskii, N.P.; Yakkonen, K.L.; Lukina, K.A.; Semenov, K.N.; Panova, G.G. Fullerenol can Ameliorate Iron Deficiency in Cucumber Grown Hydroponically. J. Plant Growth Regul. 2020, 1–15. [Google Scholar] [CrossRef]
- Susín, S.; Abadía, A.; González-Reyes, J.A.; Lucena, J.J.; Abadía, J. The pH requirement for in vivo activity of the iron-deficiency-induce “Turbo” ferric chelate reductase. Plant Physiol. 1996, 110, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Moog, P.R.; Van Der Kooij, T.A.W.; Brüggemann, W.; Schiefelbein, J.W.; Kuiper, P.J.C. Responses to iron deficiency in Arabidopsis thaliana: The Turbo iron reductase does not depend on the formation of root hairs and transfer cells. Planta 1995, 195, 505–513. [Google Scholar] [CrossRef] [Green Version]
- García-Marco, S.; Martinez, N.; Yunta, F.; Hernández-Apaolaza, L.; Lucena, J.J. Effectiveness of Ethylenediamine-N(o-hydroxyphenylacetic)-N′(p-hydroxyphenylacetic) acid (o,p-EDDHA) to Supply Iron to Plants. Plant Soil 2006, 279, 31–40. [Google Scholar] [CrossRef]
- Hernández-Apaolaza, L.; García-Marco, S.; Nadal, P.; Lucena, J.J.; Sierra, M.A.; Gómez-Gallego, M.; Ramírez-López, P.; Escudero, R. Structure and Fertilizer Properties of Byproducts Formed in the Synthesis of EDDHA. J. Agric. Food Chem. 2006, 54, 4355–4363. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, Y.; Hu, J.; Zhou, H.; Adeleye, A.S.; Keller, A.A. 1H NMR and GC-MS Based Metabolomics Reveal Defense and Detoxification Mechanism of Cucumber Plant under Nano-Cu Stress. Environ. Sci. Technol. 2016, 50, 2000–2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucena, C.; Porras, R.; Romera, F.J.; Alcántara, E.; García, M.J.; Pérez-Vicente, R. Similarities and Differences in the Acquisition of Fe and P by Dicot Plants. Agronomy 2018, 8, 148. [Google Scholar] [CrossRef] [Green Version]
- Bityutskii, N.P.; Pavlovic, J.; Yakkonen, K.; Maksimović, V.; Nikolić, M. Contrasting effect of silicon on iron, zinc and manganese status and accumulation of metal-mobilizing compounds in micronutrient-deficient cucumber. Plant Physiol. Biochem. 2014, 74, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Gattullo, C.E.; Pii, Y.; Allegretta, I.; Medici, L.; Cesco, S.; Mimmo, T.; Terzano, R. Iron Mobilization and Mineralogical Alterations Induced by Iron-Deficient Cucumber Plants ( Cucumis sativus L.) in a Calcareous Soil. Pedosphere 2018, 28, 59–69. [Google Scholar] [CrossRef]
- Römheld, V.; Marschner, H. Mechanism of iron uptake by peanuts plants: I. Fe reduction, chelate splitting and release of phenolics. Plant Physiol. 1983, 71, 949–954. [Google Scholar] [CrossRef] [Green Version]
- Donnini, S.; De Nisi, P.; Gabotti, D.; Tato, L.; Zocchi, G. Adaptive strategies of Parietaria diffusa (M.&K.) to calcareous habitat with limited iron availability*. Plant Cell Environ. 2012, 35, 1171–1184. [Google Scholar] [CrossRef]
- Fourcroy, P.; Sisó-Terraza, P.; Sudre, D.; Savirón, M.; Reyt, G.; Gaymard, F.; Abadia, A.; Abadía, J.; Álvarez-Fernández, A.; Briat, J.-F. Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives byArabidopsisroots in response to iron deficiency. New Phytol. 2013, 201, 155–167. [Google Scholar] [CrossRef]
- Sisó-Terraza, P.; Luis-Villarroya, A.; Fourcroy, P.; Briat, J.-F.; Abadía, A.; Gaymard, F.; Abadía, J.; Álvarez-Fernández, A. Accumulation and Secretion of Coumarinolignans and other Coumarins in Arabidopsis thaliana Roots in Response to Iron Deficiency at High pH. Front. Plant Sci. 2016, 1711. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, M.W.; Grusak, M.A. Morpho-physiological parameters affecting iron deficiency chlorosis in soybean (Glycine max L.). Plant Soil 2014, 374, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Michel, L.; Peña, Á.; Pastenes, C.; Berrios, P.; Rombolà, A.D.; Covarrubias, J.I. Sustainable Strategies to Prevent Iron Deficiency, Improve Yield and Berry Composition in Blueberry (Vaccinium spp.). Front. Plant Sci. 2019, 10, 255. [Google Scholar] [CrossRef] [Green Version]
| Iron Source | Molar Absorptivity Coefficients/L mol−1 cm−1 | |||
|---|---|---|---|---|
| a370 | a400 | a480 | a535 | |
| (BPDS)3/Fe2+ | 1.79 × 103 | 4.11 × 103 | 1.73 × 104 | 2.26 × 104 |
| o,oEDDHA/Fe3+ | 1.19 × 103 | 2.25 × 103 | 5.05 × 103 | 3.33 × 103 |
| EDTA/Fe3+ | 8.73 × 102 | 3.98 × 102 | 2.05 × 102 | 1.88 × 102 |
| Fe-heme | 4.35 × 104 | 1.04 × 104 | 4.41 × 104 | 1.33 × 104 |
| Treatments | FW/g | N° Leaves | ||||
|---|---|---|---|---|---|---|
| Total | Root | Stem | Leaves | Single Leaf | ||
| −Fe | 2.9 ± 0.9 b | 1.0 ± 0.1 b | 0.63 ± 0.09 b | 1.3 ± 0.7 b | 0.38 ± 0.19 b | 3.3 ± 0.5 b |
| o,oEDDHA/Fe3+ | 7.1 ± 3.2 a | 2.9 ± 1.3 a | 0.94 ± 0.39 a | 3.2 ± 1.6 a | 0.61 ± 0.18 a | 5.2 ± 1.5 a |
| EDTA/Fe3+ | 5.9 ± 2.5 ab | 2.4 ± 1.2 ab | 0.76 ± 0.18 ab | 2.7 ± 1.6 a | 0.56 ± 0.21 ab | 4.7 ± 1.4 ab |
| Fe-heme | 4.5 ± 2.6 ab | 1.5 ± 1.3 b | 0.77 ± 0.36 ab | 2.2 ± 1.1 ab | 0.46 ± 0.17 ab | 4.8 ± 1.0 a |
| Significance | ** | **** | * | ** | *** | ** |
| Treatments | DW/g | |||||
| Total | Root | Stem | Leaves | - | ||
| −Fe | 0.31 ± 0.10 b | 0.058 ± 0.021 b | 0.045 ± 0.011 b | 0.21 ± 0.07 b | - | |
| o,oEDDHA/Fe3+ | 0.59 ± 0.22 a | 0.104 ± 0.042 a | 0.069 ± 0.027 ab | 0.42 ± 0.18 a | - | |
| EDTA/Fe3+ | 0.48 ± 0.18 ab | 0.085 ± 0.024 ab | 0.057 ± 0.012 ab | 0.34 ± 0.16 ab | - | |
| Fe-heme | 0.45 ± 0.14 ab | 0.064 ± 0.032 b | 0.081 ± 0.013 a | 0.31 ± 0.10 ab | - | |
| Significance | ** | ** | *** | ** | - | |
| Time (h) | Fe Source | Total Fe-Reduction (µmolFe g−1 h−1) Slope ± Error [R2] | Fe Reduction by Root Exudates (µmolFe g−1 h−1) Slope ± Error [R2] | % Fe Reduction by Roots |
|---|---|---|---|---|
| pH = 6.0 | ||||
| 1/3 | EDTA/Fe3+ | 0.595 ± 0.024 [0.892] | 0.094 ± 0.025 [0.289] | 84.2 ± 4.8 |
| o,oEDDHA/Fe3+ | 0.203 ± 0.032 [0.642] | 0.086 ± 0.026 [0.422] | 57.6 ± 19.5 | |
| Fe-heme | 0.143 ± 0.038 [0.243] | 0.053 ± 0.041 [0.437] | 62.9 ± 38.5 | |
| 1 | EDTA/Fe3+ | 0.611 ± 0.011 [0.968] | 0.047 ± 0.008 [0.420] | 92.3 ± 1.5 |
| o,oEDDHA/Fe3+ | 0.239 ± 0.032 [0.609] | 0.043 ± 0.008 [0.565] | 82.0 ± 5.8 | |
| Fe-heme | 0.077 ± 0.012 [0.408] | 0.025 ± 0.018 [0.454] | 67.5 ± 28.4 | |
| 2 | EDTA/Fe3+ | 0.560 ± 0.008 [0.974] | 0.027 ± 0.004 [0.437] | 95.2 ± 0.8 |
| o,oEDDHA/Fe3+ | 0.185 ± 0.018 [0.667] | 0.034 ± 0.004 [0.680] | 81.6 ± 4.0 | |
| Fe-heme | 0.049 ± 0.006 [0.502] | 0.017 ± 0.008 [0.702] | 65.3 ± 20.6 | |
| pH = 7.5 | ||||
| 1/3 | EDTA/Fe3+ | 0.225 ± 0.031 [0.636] | 0.138 ± 0.039 [0.257] | 38.7 ± 25.8 |
| o,oEDDHA/Fe3+ | 0.110 ± 0.025 [0.500] | 0.065 ± 0.017 [0.506] | 40.9 ± 28.9 | |
| Fe-heme | 0.076 ± 0.032 [0.140] | 0.063 ± 0.027 [0.204] | 17.1 ± 70.4 | |
| 1 | EDTA/Fe3+ | 0.277 ± 0.009 [0.919] | 0.089 ± 0.040 [0.318] | 67.9 ± 15.5 |
| o,oEDDHA/Fe3+ | 0.083 ± 0.015 [0.515] | 0.030 ± 0.007 [0.482] | 63.9 ± 15.0 | |
| Fe-heme | 0.051 ± 0.013 [0.369] | 0.041 ± 0.013 [0.481] | 19.6 ± 46.0 | |
| 2 | EDTA/Fe3+ | 0.328 ± 0.006 [0.962] | 0.085 ± 0.021 [0.395] | 74.1 ± 6.9 |
| o,oEDDHA/Fe3+ | 0.067 ± 0.009 [0.581] | 0.020 ± 0.004 [0.500] | 70.2 ± 10.0 | |
| Fe-heme | 0.060 ± 0.005 [0.766] | 0.034 ± 0.007 [0.631] | 43.3 ± 16.4 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Foggia, M.; Yunta-Mezquita, F.; Tugnoli, V.; Rombolà, A.D.; Lucena, J.J. Testing a Bovine Blood-Derived Compound as Iron Supply on Cucumis sativus L. Agronomy 2020, 10, 1480. https://doi.org/10.3390/agronomy10101480
Di Foggia M, Yunta-Mezquita F, Tugnoli V, Rombolà AD, Lucena JJ. Testing a Bovine Blood-Derived Compound as Iron Supply on Cucumis sativus L. Agronomy. 2020; 10(10):1480. https://doi.org/10.3390/agronomy10101480
Chicago/Turabian StyleDi Foggia, Michele, Felipe Yunta-Mezquita, Vitaliano Tugnoli, Adamo Domenico Rombolà, and Juan José Lucena. 2020. "Testing a Bovine Blood-Derived Compound as Iron Supply on Cucumis sativus L." Agronomy 10, no. 10: 1480. https://doi.org/10.3390/agronomy10101480








