Temporal Responses to Direct and Induced Iron Deficiency in Parietaria judaica
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Biomass Parameters
2.3. Optical Measurements of Leaf Chlorophyll and Flavonoids
2.4. Determination of Apoplastic Fe
2.5. Collection of Root Exudates
2.6. Determination of Organic Acids
2.7. Determination of Total Phenolic Content
2.8. Enzyme Activity Assays
2.9. Statistical Analysis
3. Results
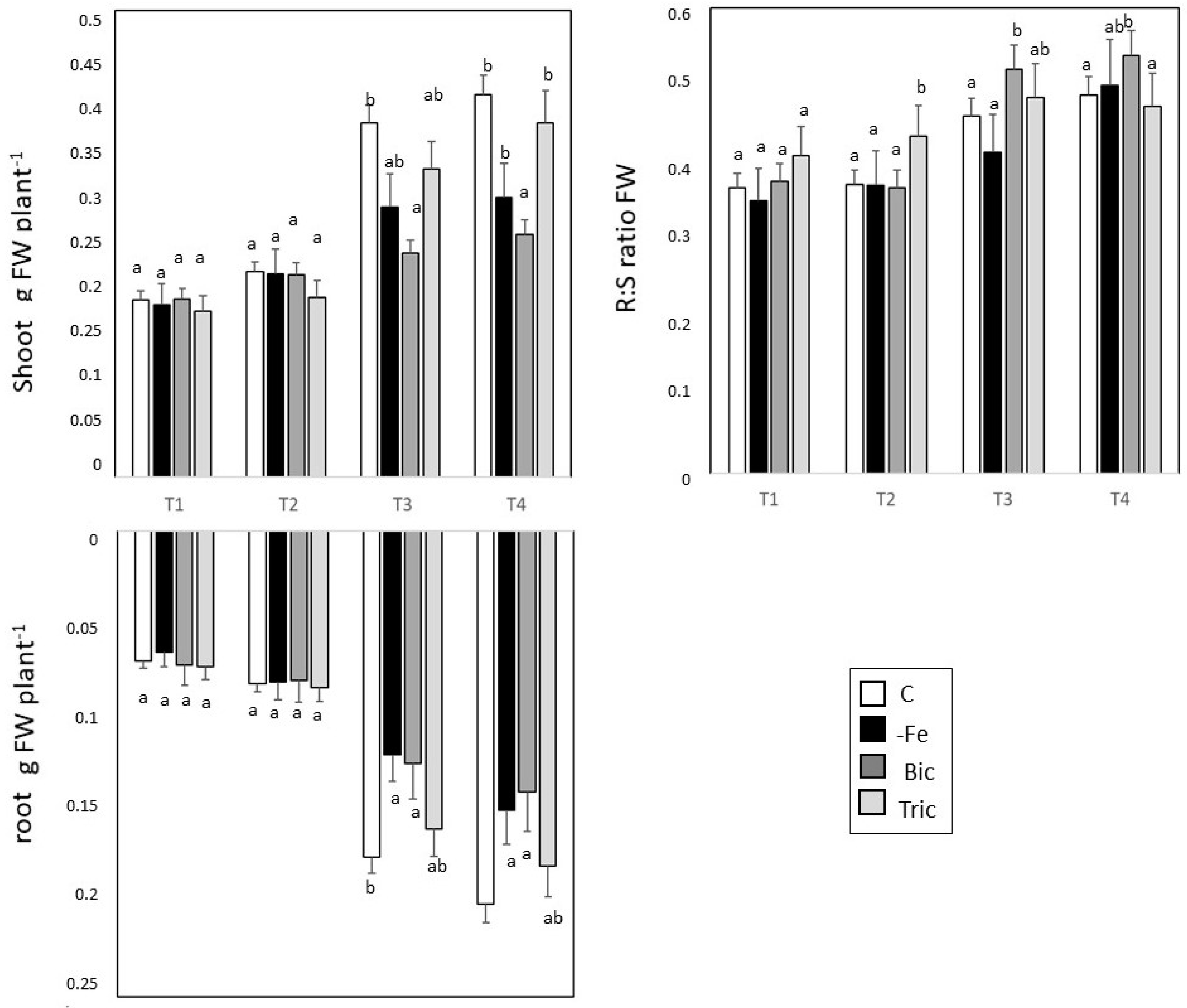
3.1. Direct and Induced Fe Deficiency Affect Plant Growth during Time
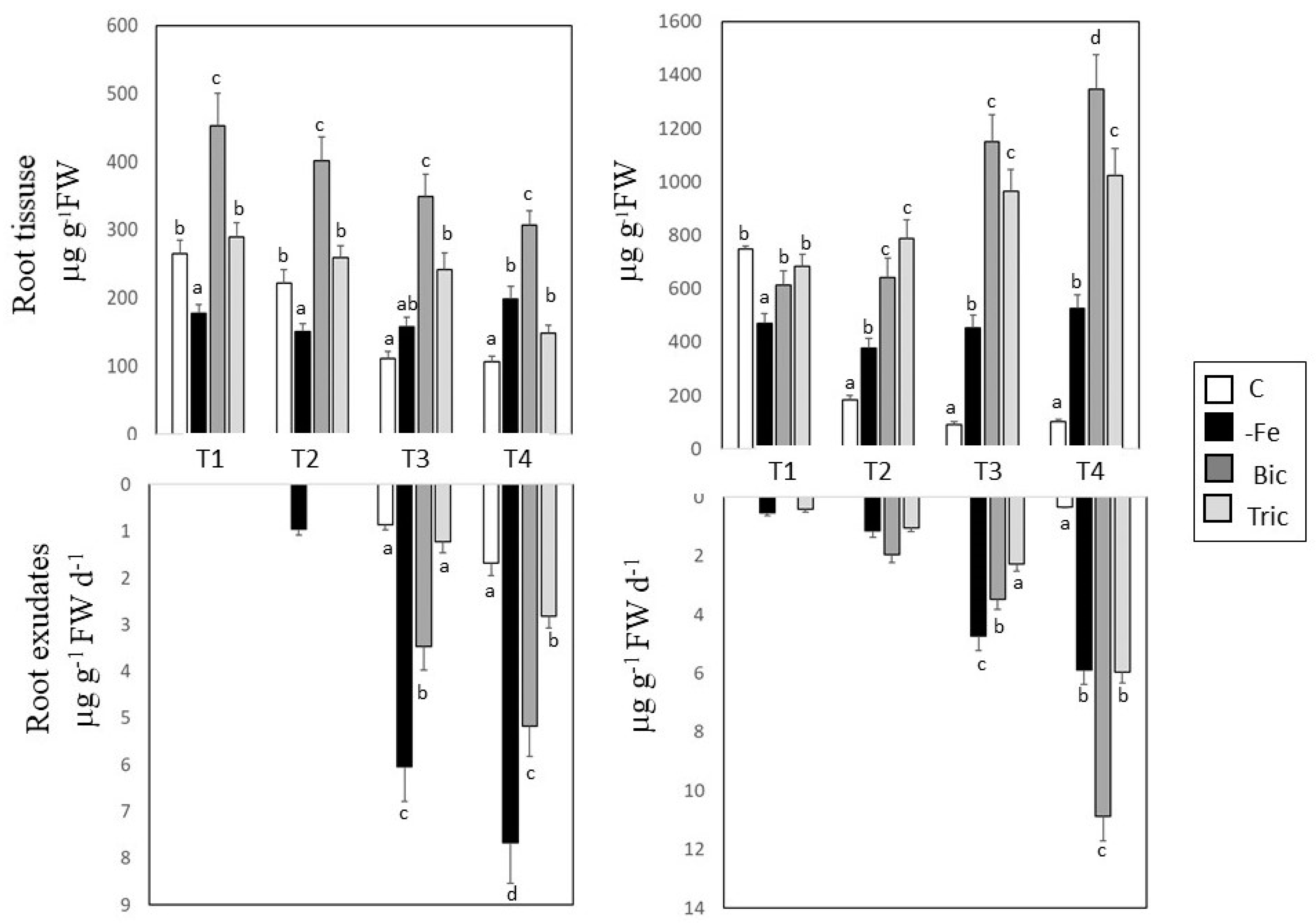
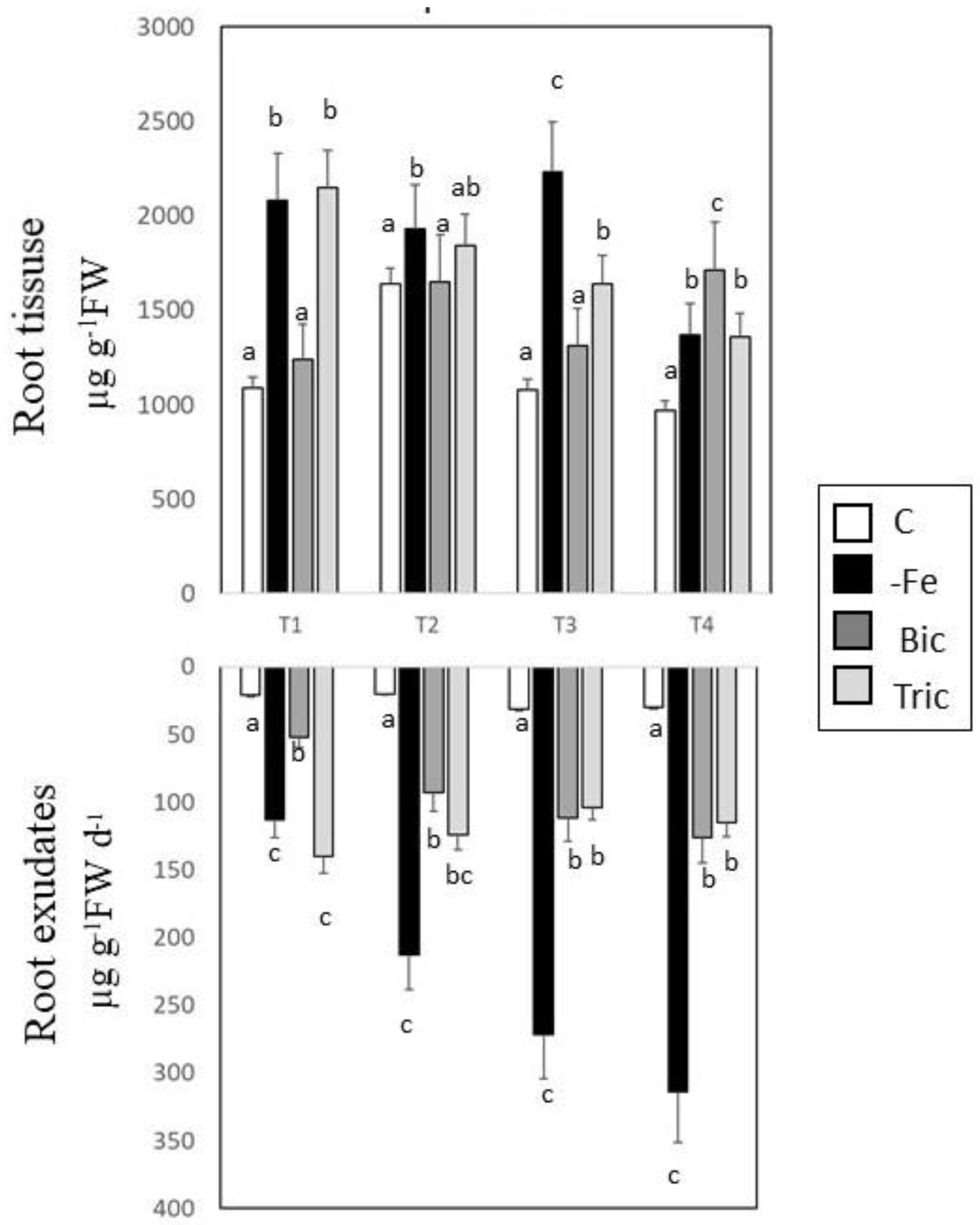
3.2. Malic and Citric Acid Contents in Root Tissues and Exudates
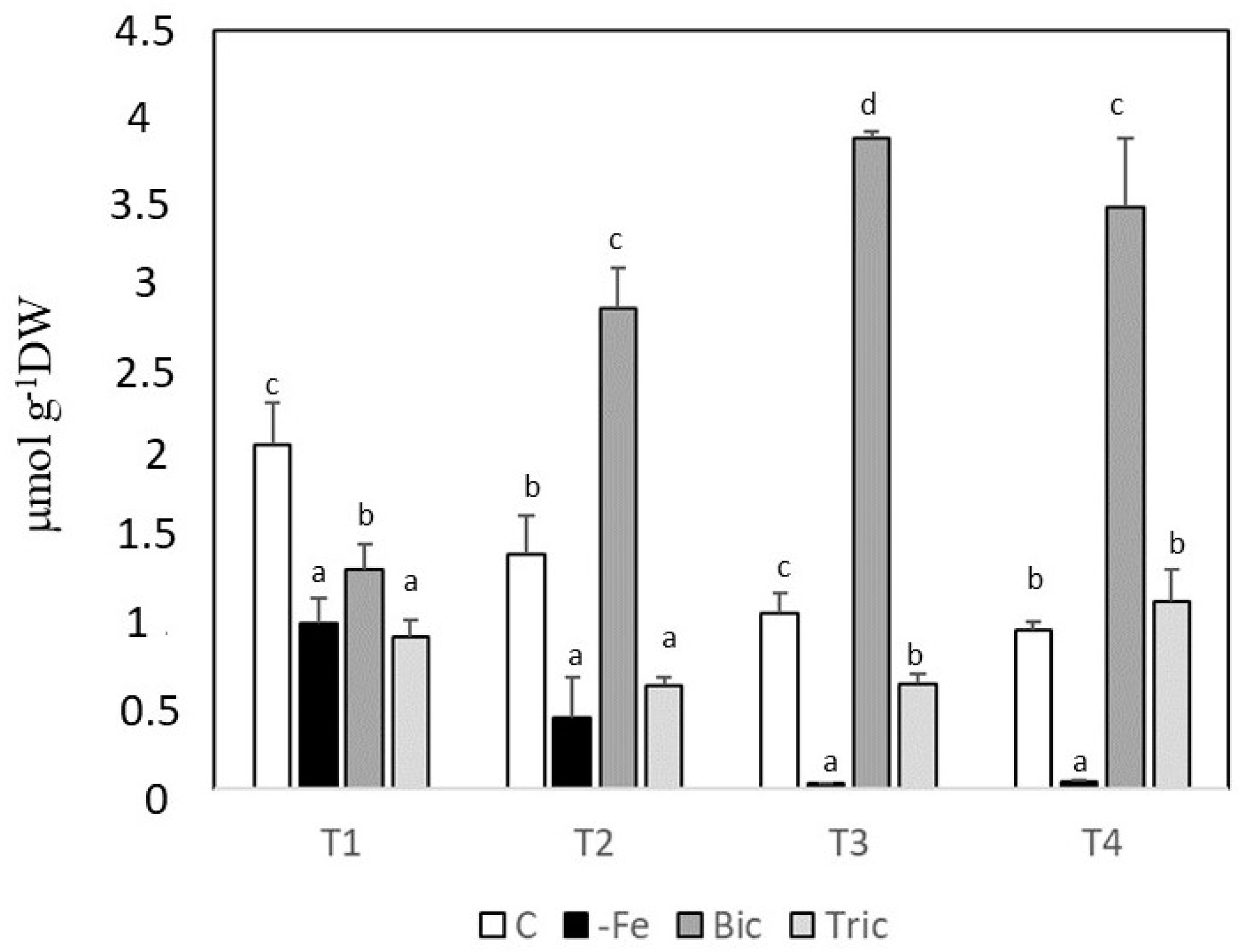
3.3. Phenolic Compounds in Root Extracts and Exudates
3.4. Root Apoplastic Fe
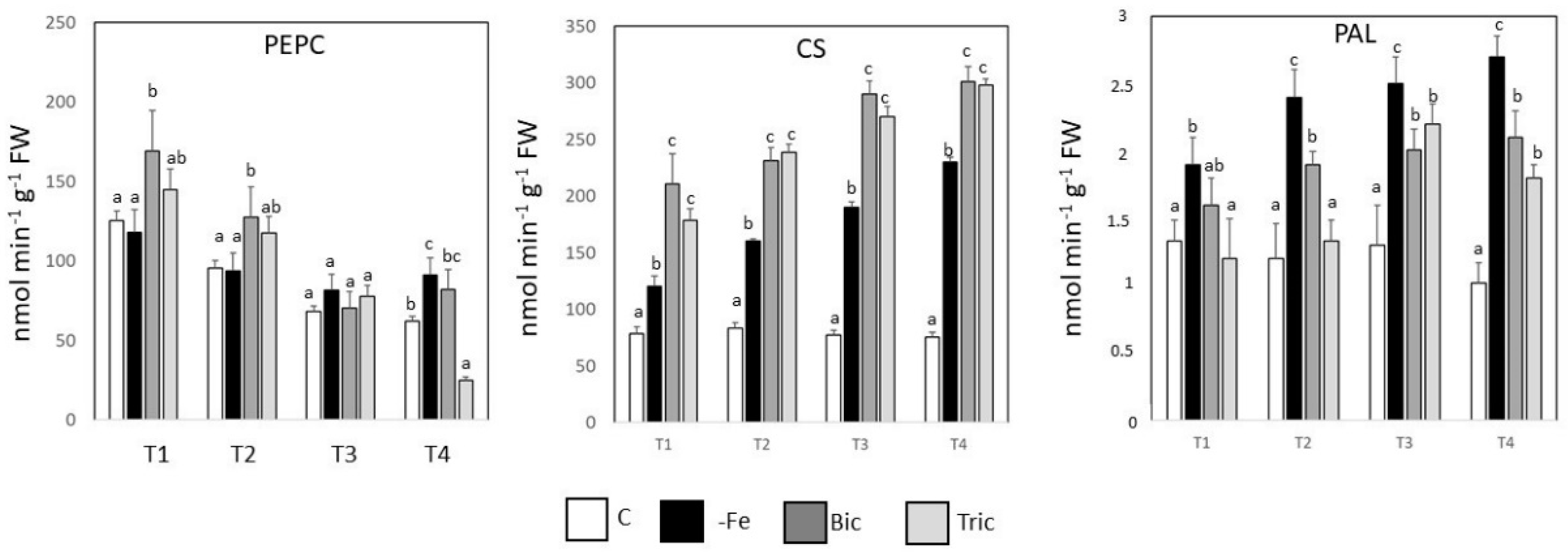
3.5. PEPCase, CS, and PAL Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1995. [Google Scholar]
- Kim, S.A.; Guerinot, M.L. Mining iron: Iron uptake and transport in plants. FEBS Lett. 2007, 581, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Rabotti, G.; Zocchi, G. Plasma membrane-bound H+-ATPase and reductase activities in Fe-deficient cucumber roots. Physiol. Plant. 1994, 90, 779–785. [Google Scholar] [CrossRef]
- Dell’Orto, M.; Santi, S.; De Nisi, P.; Cesco, S.; Varanini, Z.; Zocchi, G. Development of Fe deficiency responses in cucumber (Cucumis sativus L.) roots: Involvement of plasma membrane H+-ATPase activity. J. Exp. Bot. 2001, 51, 695–701. [Google Scholar]
- Santi, S.; Cesco, S.; Varanini, Z.; Pinton, R. Two plasma membrane H+-ATPase genes are differentially expressed in iron-deficient cucumber plants. Plant Physiol. Biochim. 2005, 43, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Santi, S.; Schmidt, W. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol. 2009, 183, 1072–1084. [Google Scholar] [CrossRef]
- Zocchi, G. Metabolic changes in iron-stressed dicotyledonous plants. In Iron Nutrition in Plants and Rhizospheric Microorganisms; Barton, L.L., Abadía, J., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 359–370. [Google Scholar]
- Abadía, J.; López-Millán, A.F.; Rombolà, A.; Abadía, A. Organic acids and Fe deficiency: A review. Plant Soil 2002, 241, 75–86. [Google Scholar] [CrossRef]
- Jelali, N.; Wissala, M.; Dell’Orto, M.; Abdelly, C.; Gharsalli, M.; Zocchi, G. Changes of metabolic responses to direct and induced Fe deficiency of two Pisum sativum cultivars. Environ. Exp. Bot. 2010, 68, 238–246. [Google Scholar] [CrossRef]
- López-Millán, A.F.; Morales, F.; Abadía, A.; Abadía, J. Effects of iron deficiency on the composition of the leaf apoplastic fluid and xylem sap in sugar beet. Implications for iron and carbon transport. Plant Physiol. 2000, 124, 873–884. [Google Scholar] [CrossRef]
- Rellán-Álvarez, R.; Andaluz, S.; Rodríguez-Celma, J.; Wohlgemuth, G.; Zocchi, G.; Álvarez-Fernández, A.; Fiehn, O.; López-Millán, A.F.; Abadía, J. Changes in the proteomic and metabolic profiles of Beta vulgaris root tips in response to iron deficiency and resupply. BMC Plant Biol. 2010, 10, 120. [Google Scholar] [CrossRef]
- López-Bucio, J.; Nieto-Jacobo, M.F.; Ramírez-Rodríguez, V.; Herrera-Estrella, L. Organic acid metabolism in plants: From adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef]
- Reid, R.; Smith, F.A. The cytoplasmic pH Stat. In Handbook of Plant Growth: pH as the Master Variable; Rengel, Z., Ed.; Marcel Dekker: New York, NY, USA, 2002; pp. 47–67. [Google Scholar]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanism and their roles in plant responses to environmental stimuli. Front Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Tyler, G.; Strom, L. Differing organic acid exudation patterns explain calcifuge and acidifuge behaviour of plants. Ann. Bot. 1995, 75, 75–78. [Google Scholar] [CrossRef]
- Bush, D.S. Calcium regulation in plant cells and its role in signaling. Annu. Rev. Plant Phys. 1995, 46, 95–122. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Cesco, S.; Neumann, G.; Tommasi, N.; Pinton, R.; Weisskopf, L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 2010, 329, 1–25. [Google Scholar] [CrossRef]
- Jin, C.W.; You, G.Y.; He, Y.F.; Tang, C.; Wu, P.; Zheng, S.J. Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol. 2007, 144, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Voges, M.J.E.E.E.; Bai, Y.; Schulze-Lefert, P.; Sattely, E.S. Plant-derived coumarins shape the composition of an Arabidopsis synthetic root microbiome. Proc. Natl. Acad. Sci. USA 2019, 116, 12558–12565. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Schwab, A.P. The chemistry of iron in soils and its availability to plants. J. Plant Nutr. 1982, 5, 821–840. [Google Scholar] [CrossRef]
- Schenkeveld, W.D.C.; Kraemer, S.M. Constrints to Synergistic Fe mobilization from calcareous soil by a phytosiderophore and a reductant. Soil Syst. 2018, 2, 67. [Google Scholar] [CrossRef]
- Mengel, K. Iron availability in plant tissues—iron chlorosis on calcareous soils. Plant Soil 1994, 165, 275–283. [Google Scholar] [CrossRef]
- Kosegarten, H.; Hoffmann, D.; Rroco, E.; Grolig, F.; Glüsenkamp, F.K.; Mengel, K. Apoplastic pH and FeIII reduction in young sunflower (Helianthus annuus) roots. Physiol. Plant 2004, 122, 95–106. [Google Scholar] [CrossRef]
- Alhendawi, R.A.; Römheld, V.; Kirkby, E.A.; Marschner, H. Influence of increasing bicarbonate concentrations on plant growth, organic acid accumulation in roots and iron uptake by barley, sorghum, and maize. J. Plant Nutr. 1997, 20, 1731–1753. [Google Scholar] [CrossRef]
- Zohlen, A.; Tyler, G. Immobilization of tissue iron on calcareous soil: Differences between calcicole and calcifuge plants. Oikos 2000, 89, 95–106. [Google Scholar] [CrossRef]
- Zohlen, A. Chlorosis in wild plants: Is it a sign of iron deficiency? J. Plant Nutr. 2002, 25, 2205–2228. [Google Scholar] [CrossRef]
- Lucena, C.; Romera, F.J.; Rojas, C.L.; García, M.J.; Alcántara, E.; Pérez-Vicente, R. Bicarbonate blocks the expression of several genes involved in the physiological responses to Fe deficiency of Strategy I plants. J. Funct. Plant Biol. 2007, 34, 1002–1009. [Google Scholar] [CrossRef]
- Donnini, S.; De Nisi, P.; Gabotti, G.; Tato, L.; Zocchi, G. Adaptive strategies of Parietaria diffusa (M.K.) to calcareous habitat with limited iron availability. Plant Cell Environ. 2012, 35, 1171–1184. [Google Scholar] [CrossRef]
- Dell’Orto, M.; De Nisi, P.; Pontiggia, A.; Zocchi, G. Fe deficiency responses in Parietaria diffusa: A calcicole plant. J. Plant Nutr. 2003, 26, 10–11. [Google Scholar] [CrossRef]
- Tato, L.; De Nisi, P.; Donnini, S.; Zocchi, G. Low iron availability and phenolic metabolism in a wild plant species (Parietaria judaica L.). Plant Physiol. Biochem. 2013, 72, 145–153. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophyll and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Bienfait, H.F.; van den Briel, W.; Mesland-Mul, N.T. Free space iron pools in roots. Generation and mobilization. Plant Physiol. 1985, 78, 596–600. [Google Scholar] [CrossRef]
- De Nisi, P.; Zocchi, G. Phosphoenolpyruvate carboxylase in cucumber (Cucumis sativus L.) roots under iron deficiency: Activity and kinetic characterization. J. Exp. Bot. 2000, 352, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Vigani, G.; Bashir, K.; Ishimaru, Y.; Lehmann, M.; Casiraghi, F.M.; Nakanishi, H.; Seki, M.; Geigenberger, P.; Zocchi, G.; Nishizawa, N.K. Knocking down Mitochondrial Iron Transporter (MIT) reprograms primary and secondary metabolism in rice plants. J. Exp. Bot. 2016, 67, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.; Kastori, R. Effect of bicarbonate and Fe supply on Fe nutrition of grapevine. J. Plant Nutr. 2000, 23, 1619–1627. [Google Scholar] [CrossRef]
- Tagliavini, M.; Rombolà, A.D. Iron deficiency and chlorosis in orchard and vineyard ecosystems. Eur. J. Agron. 2001, 15, 71–92. [Google Scholar] [CrossRef]
- Mora-Macias, J.; Ojeda-Rivera, J.O.; Gutierrez-Alanis, D.; Yong-Villalobos, L.; Oropeza-Aburto, A.; Raya-Gonzalez, J.; Jiménez-Domínguez, G.; Chávez-Calvillo, G.; Rellán-Álvarez, R.; Herrera-Estrella, L. Malate-dependent Fe accumulation is a critical checkpoint in the root developmental response to low phosphate. Proc. Natl. Acad. Sci. USA 2017, 114, E3563–E3572. [Google Scholar] [CrossRef]
- Farrar, J.F.; Jones, D.L. The control of carbon acquisition by roots. New Phytol. 2000, 147, 43–53. [Google Scholar] [CrossRef]
- Ström, L.; Owen, A.G.; Godbold, D.L.; Jones, D.L. Organic acid behavior in calcareous soil implication for rhizosphere nutrient cycling. Soil Biol. Biochem. 2005, 37, 2046–2054. [Google Scholar] [CrossRef]
- Abadía, J.; Vázquez, S.; Rellán-Álvarez, R.; El-Jendoubi, E.; Abadía, A.; Álvarez-Fernández, A.; López-Millán, A.F. Towards a knowledge-based correction of iron chlorosis. Plant Physiol. Biochem. 2011, 49, 471–482. [Google Scholar] [CrossRef]
- Terzano, R.; Cuccovillo, G.; Gattullo, C.E.; Medici, L.; Tomasi, N.; Pinton, R.; Mimmo, T.; Cesco, R. Combined effect of organic acids and flavonoids on the mobilization of major and trace elements from soils. Biol. Fert. Soil 2015, 51, 685–695. [Google Scholar] [CrossRef]
- Mimmo, T.; Hann, S.; Jaitz, L.; Cesco, S.; Gessa, C.E.; Puschenreiter, M. Time and substrate dependent exudation of carboxylates by Lupinus albus and Brassica napus L. Plant Physiol. Biochem. 2011, 49, 1272–1278. [Google Scholar] [CrossRef]
- Vigani, G.; Zocchi, G.; Bashir, K.; Philippar, K.; Briat, J.F. Signals from chloroplasts and mitochondria for iron homeostasis regulation. Trends Plant Sci. 2013, 18, 305–311. [Google Scholar] [CrossRef]
- Vigani, G.; Pii, Y.; Celletti, S.; Maver, M.; Mimmo, T.; Cesco, S.; Astolfi, S. Mitochondrial dysfunction under Fe and S deficiency: Is citric acid involved in the regulation of nutrient-responsive genes? Plant Physiol Biochem. 2018, 2018 126, 86–96. [Google Scholar] [CrossRef]
- Horst, W.J.; Wang, Y.; Eticha, D. The role of the root apoplast in aluminium induced inhibition of root elongation and in aluminium resistance of plants: A review. Ann. Bot. 2010. 106, 185–197. [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Van Camp, J.; De Meuleraer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Oxidative stress and antioxidant responses in young leaves of mulberry plants grown under nitrogen, phosphorus or potassium deficiency. J. Integr. Plant Biol. 2007, 49, 313–322. [Google Scholar] [CrossRef]
- Hajiboland, R. Effect of micronutrient deficiencies on plant stress responses. In Abiotic Stress Responses in Plants; Hyderabad, A.P., Parvaiz, A., Eds.; Springer: New York, NY, USA, 2007; pp. 330–389. [Google Scholar]
- Zhang, F.S.; Romheld, V.; Marschner, H. Role of the root apoplasm for iron acquisition by wheat plants. Plant Physiol. 1991, 97, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Schmid, N.B.; Giehl, R.F.; Döll, S.; Mock, H.P.; Strehmel, N.; Scheel, D.; Kong, X.; Hider, R.C.; von Wirén, N. Feruloyl-CoA 6′-Hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in Arabidopsis. Plant Physiol. 2014, 164, 160–172. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tato, L.; Islam, M.; Mimmo, T.; Zocchi, G.; Vigani, G. Temporal Responses to Direct and Induced Iron Deficiency in Parietaria judaica. Agronomy 2020, 10, 1037. https://doi.org/10.3390/agronomy10071037
Tato L, Islam M, Mimmo T, Zocchi G, Vigani G. Temporal Responses to Direct and Induced Iron Deficiency in Parietaria judaica. Agronomy. 2020; 10(7):1037. https://doi.org/10.3390/agronomy10071037
Chicago/Turabian StyleTato, Liliana, Monirul Islam, Tanja Mimmo, Graziano Zocchi, and Gianpiero Vigani. 2020. "Temporal Responses to Direct and Induced Iron Deficiency in Parietaria judaica" Agronomy 10, no. 7: 1037. https://doi.org/10.3390/agronomy10071037
APA StyleTato, L., Islam, M., Mimmo, T., Zocchi, G., & Vigani, G. (2020). Temporal Responses to Direct and Induced Iron Deficiency in Parietaria judaica. Agronomy, 10(7), 1037. https://doi.org/10.3390/agronomy10071037







