Effects of Salt Stress on Fruit Antioxidant Capacity of Wild (Solanum chilense) and Domesticated (Solanum lycopersicum var. cerasiforme) Tomatoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Stress Treatments
2.2. Fruit Weight and Quality
2.3. Proline and Malondialdehyde Content
2.4. Antioxidant Capacity
2.5. Non-Enzymatic Antioxidant Content
2.6. Ascorbate Peroxidase and Superoxide Dismutase Activities
2.7. Statistical Analysis
3. Results
3.1. Fruit Weight and Quality
3.2. Antioxidant Capacity of Fruit
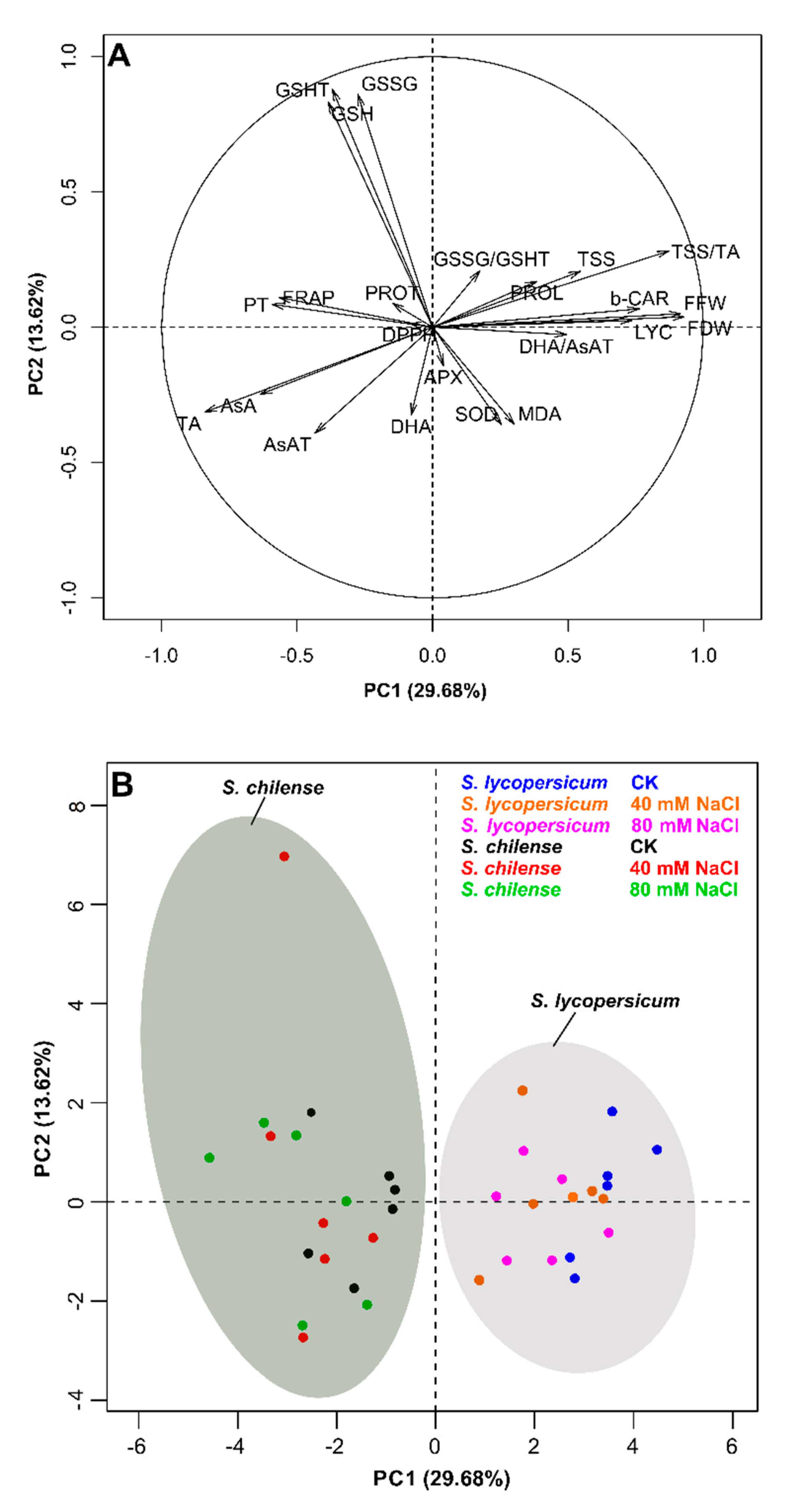
3.3. PCA Analysis of the Antioxidant Capacity and Quality Fruit at the Harvest
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marti, C.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Dueñas, M.; Pinela, J.; Carvalho, A.; Buelga, C.; Ferreira, I. Charcaterization and quatntification of phenolic compounds in four tomato (Lycopersicum esculentum L.) farmers’ varieties in Norheastern Portugal homegardens. Plant Foods Hum. Nutr. 2012, 67, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Del Río, D.; Borges, G.; Crozier, A. Berry flavonoids and phenolics: Bioavailability and evidence of protective effects. Br. J. Nutr. 2010, 104, S67–S90. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Isayenkov, S.; Maathuis, J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Libutti-Cammerino, A.; Monteleone, M. Risk assessment of soil salinization due to tomato cultivation in Mediterranean climate conditions. Water 2018, 10, 1503. [Google Scholar] [CrossRef]
- Flowers, T.; Yeo, A.R. Breeding for salinity resistance in crop plants: Where next? Austr. J. Plant Physiol. 1995, 22, 875–884. [Google Scholar] [CrossRef]
- Cuartero, J.; Bolarín, M.; Asins, M.; Moreno, M. Increasing salt tolerance in the tomato. J. Exp. Bot. 2006, 57, 1045–1058. [Google Scholar] [CrossRef]
- Ghanem, M.; van Elteren, J.; Albacete, A.; Quinet, M.; Martinez-Andujar, C.C.; Kinet, J.M.; Pérez-Alfoceà, F.; Lutts, S. Impact of salinity on early reproductive physiology of tomato (Solanum lycopersicum L.) in relation to a heterogeneous distribution of toxic ions in flower organs. Funct. Plant Biol. 2009, 36, 125–136. [Google Scholar] [CrossRef]
- Zhang, P.; Senge, M.; Dai, Y. Effects of salinity stress at different growth stages on tomato growth, yield, and water-use efficiency. Comm. Soil. Sci. Plant Anal. 2017, 48, 624–634. [Google Scholar] [CrossRef]
- Dorai, M.; Papadopoulos, A.; Gosselin, A. Influence of electric conductivity management on greenhouse tomato yield and fruit quality. Agronomie 2001, 21, 367–383. [Google Scholar] [CrossRef]
- Martínez, J.P.; Antúnez, A.; Petrtuzé, R.; Acosta, M.D.P.; Palma, X.; Fuentes, L.; Ayala, A.; Araya, H.; Lutts, S. Saline water irrigation effects on water status, yield and fruit quality of wild (Solanum chilense) and cropped (Solanum lycopersicum var. cerasiforme) tomatoes. Exp. Agric. 2012, 48, 573–586. [Google Scholar] [CrossRef]
- Nouck, A.; Taffouo, V.D.; Tsoata, E.; Dibong, D.S.; Nguemezi, S.T.; Gouado, I.; Youmbi, E. Growth, biochemical constituents, micronutrient uptake and yield response of six tomato (Lycopersicum esculentum L.) cultivars grown under salinity stress. J. Agron. 2016, 15, 58–67. [Google Scholar] [CrossRef]
- Diouf, I.; Derivot, L.; Bitton, F.G.; Pascual, L.; Causse, M. Water deficit and salinity stress reveal many specific QTL for plant growth and fruit quality traits in tomato. Front. Plant Sci. 2018, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Matsukura, C.; Ban, Y.; Shoji, K.; Sugiyama, M.; Fukuda, N.; Nishimura, S. Salinity stress affects assimilate metabolism at the gene-expression level during fruit development and improves fruit quality in tomato (Solanum lycopersicum L.). J. Jap. Soc. Hort. Sci. 2008, 77, 61–68. [Google Scholar] [CrossRef]
- Ruiz, M.; Yasuor, H.; Ben-Gal, A.; Yermiyahu, U.; Saranga, Y.; Elbaum, R. Salinity induced fruit hypodermis thickening alters the structure of tomato (Solanum lycopersicum Mill) fruits. Sci. Hortic. 2015, 192, 244–249. [Google Scholar] [CrossRef]
- Cuartero, J.; Férnandez-Muñoz, R. Tomato and salinity. Sci. Hortic. 1998, 78, 83–125. [Google Scholar] [CrossRef]
- Van Meulebroek, L.; Hanssens, J.; Steppe, K.; Vanhaecke, L. Metabolic fingerprinting to assess the impact of salinity on carotenoid content in developing tomato fruits. Int. J. Mol. Sci. 2016, 17, 821. [Google Scholar] [CrossRef]
- Johkan, M.; Nagatsuka, A.; Yoshitoni, A.; Nakagawa, T.; Maruo, T.; Satoru, T.; Hohjo, M.; Nakaminami, A.; Tsuchiya, K.; Shinohar, Y. Effect of moderate salinity stress on the sugar concentration and fruit yield in single-truss, high-density tomato production system. J. Jpn. Soc. Hort. Sci. 2014, 83, 229–234. [Google Scholar] [CrossRef]
- Gough, C.; Hobson, G.E. A comparison of the productivity, quality, shelf-life characteristics and consumer reaction to the crop from cherry tomato plants grown at different levels of salinity. J. Hort. Sci. 1990, 65, 431–439. [Google Scholar] [CrossRef]
- El-Mogy, M.; Garchery, C.; Stevens, R. Irrigation with salt water affects growth, yield, fruit quality, storability and marker-gene expression in cherry tomato. Acta Agric. Scand. B Soil Plant Sci. 2018, 68, 727–737. [Google Scholar] [CrossRef]
- Islam, M.; Mele, M.A.; Choi, K.Y.; Kang, H.M. Nutrient and salinity concentrations effects on quality and storability of cherry tomato fruits grown by hydroponic system. Bragantia 2018, 77, 385–393. [Google Scholar] [CrossRef]
- Rick, C. Tomato genetic resources of South America reveal many genetic treasures. Diversity 1991, 7, 54–56. [Google Scholar]
- Razali, R.; Bougouffa, S.; Morton, M.; Lightfoot, D.; Essack, A.I.M.; Arold, S. The genome sequence of the wild tomato Solanum pimpinellifolium provides insights into salinity tolerance. Front. Plant Sci. 2018, 9, 1402. [Google Scholar] [CrossRef]
- Nosenko, T.; Böndel, K.B.; Kumpfmüller, G.; Stephan, W. Adaptation to low temperatures in the wild tomato species Solanum chilense. Mol. Ecol. 2016, 25, 2853–2869. [Google Scholar] [CrossRef]
- Tapia, G.; Méndez, J.; Inostroza, L. Different combinations of morpho-physiological traits are responsible for tolerance to drought in wild tomatoes Solanum chilense and Solanum peruvianum. Plant Biol. 2016, 18, 406–416. [Google Scholar] [CrossRef]
- Gharbi, E.; Martínez, J.P.; Benahmed, H.; Fauconnier, M.L.; Lutts, S.; Quinet, M. Salicylic acid differently impacts ethylene and polyamine synthesis in the glycophyte Solanum lycopersicum and the wild-related halophyte Solanum chilense exposed to mild salt stress. Physiol. Plant. 2016, 158, 152–167. [Google Scholar] [CrossRef]
- Gharbi, E.; Martínez, J.P.; Benahmed, E.; Dailly, H.; Lutts, S. The salicylic acid analog 2,5-dichloroisonicotinic acid has specific impact on the response of the halophyte plant species Solanum chilense to salinity. Plant Growth Regul. 2017, 82, 517–525. [Google Scholar] [CrossRef]
- Gharbi, E.; Martínez, J.P.; Benahmed, H.; Hichri, I.; Dobrev, P.I.; Motyka, V.; Lutts, S. Phytohormone profiling in relation to osmotic adjustment in NaCl-treated plants of the halophyte tomato wild relative species Solanum chilense comparatively to the cultivated glycophyte Solanum lycopersicum. Plant Sci. 2017, 258, 77–89. [Google Scholar] [CrossRef]
- Gharbi, E.; Martínez, J.P.; Benahmed, H.; Lepoint, G.; Vanpee, B.; Quinet, M.; Lutts, S. Inhibition of ethylene synthesis reduces salt-tolerance in tomato wild relative species Solanum chilense. J. Plant Physiol. 2017, 210, 24–37. [Google Scholar] [CrossRef]
- Martínez, J.P.; Antúnez, A.; Araya, H.; Pertuzé, R.; Acosta, M.D.P.; Fuentes, L.; Lizana, C.; Lutts, S. Salt stress differently affects growth, water status and antioxidant enzyme activities in Solanum lycopersicum L. and its wild-relative Solanum chilense Dun. Aust. J. Bot. 2014, 62, 359–368. [Google Scholar] [CrossRef]
- Almasoum, A.A. Effect of planting depth on growth and productivity of tomatoes using drip irrigation with semi-saline water. Acta Hort. 2000, 573, 773–778. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of freee radical method to evaluate antioxidant activity. Food Science Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic and phosphotungstic acid reagents. Am. J. Enol. Vit. 1965, 16, 144–148. [Google Scholar]
- Galati, E.; Mondello, M.R.; Giuffrida, D.; Dugo, G.; Miceli, N.; Pergolizzi, S.; Taviano, M.F. Chemical characterization and biological effects of Sicilian Opuntia ficus indica (L.) Mill. fruit juice: Antioxidant and antiulcerogenic activity. J. Agric. Food Chem. 2003, 51, 4903–4908. [Google Scholar] [CrossRef]
- Clinton, S.; Emenhisher, C.; Scwartz, S.J.; Bostwick, D.G.; Williams, A.W.; Moore, B.J.; Erdman, J.W. Cis-trans lycopene isomers, carotenoids, and retinol in the human prostrate. Cancer Epidem. Biomar. Prev. 1996, 5, 823–833. [Google Scholar]
- Barba, A.; Hurtado, M.C.; Mata, M.C.S.; Ruiz, V.F.; Sáenz de Tejada, M.L. Application of UV-vis detection-HPLC method for a rapide determination of lycopene and β-carotene in vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- Griffith, A. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpryridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Wang, S.; Jiao, H.J.; Faust, M. Changes in ascorbate, glutathione and related enzyme activities during thidiazuron-induced bud break of apple. Physiol. Plant. 1991, 82, 231–236. [Google Scholar] [CrossRef]
- Gianno-Politis, C.N.; Ries, S.K. Superoxide dismutase I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Asada, K.; Takahashi, M.A.; Nagate, M. Assay and inhibitors of spinach superoxide dismutase. Agric. Biol. Chem. 1974, 38, 471–473. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid sensitive method for the quantification of microquantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 161, 559–566. [Google Scholar] [CrossRef]
- Sumaan, R.M.; Ciulca, S.I.; Poiana, M.A.; Moigradean, D.; Radulov, I.; Negrea, M.; Crisan, M.E.; Copolovici, L.; Samulan, R.L. The antioxidant profile evaluation of some tomato landraces with soil salinity tolerance correlated with high nutraceutical and functional values. Agronomy 2020, 10, 500. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martínez, J.P.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Ntagkas, N.; Woltering, E.; Nicole, C.; Labrie, C.; Marcelis, L.F.M. Light regulation of vitamin C in tomato fruit is mediated through photosynthesis. Environ. Exp. Bot. 2019, 158, 180–188. [Google Scholar] [CrossRef]
- Abdelgawad, K.F.; El-Mogy, M.M.; Mohamed, M.I.A.; Garchery, C.; Stevens, R.G. Increasing ascorbic acid content and salinity tolerance of cherry tomato plants by supressed expression of the ascorbate oxidase gene. Agronomy 2019, 9, 51. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and glutathione reductase: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Bioch. 2013, 70, 204–212. [Google Scholar] [CrossRef]
- Zushi, K.; Matsuzoe, N. Seasonal and cultivar differences in salt-induced changes in antioxidant system in tomato. Sci. Hortic. 2009, 120, 181–187. [Google Scholar] [CrossRef]
- Sánchez-González, M.; Schouten, R.E.; Tijskens, L.M.M.; Sánchez-Guerrero, M.C.; Medrano, E.; del Rio-Celestino, M.; Lorenzo, P. Salinity and ripening on/off the plant effects on lycopene synthesis and chlorophyll breakdown in hybrid Raf tomato. Sci. Hortic. 2016, 211, 203–212. [Google Scholar] [CrossRef]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Effect of salt stress on tomato fruit antioxidant systems depends on fruit development stage. Physiol. Mol. Biol. Plants 2014, 20, 15–29. [Google Scholar] [CrossRef]
- Azuma, R.; Ito, N.; Nakayama, N.; Suwa, R.; Nguyen, N.T.; Larrinaga-Mayoral, J.A.; Esaka, M.; Fujiyamac, H.; Hirofumi Saneoka, H. Fruits are more sensitive to salinity than leaves and stems in pepper plants (Capsicum annuum L.). Sci. Hortic. 2010, 125, 171–178. [Google Scholar] [CrossRef]
- Sánchez-González, M.; Sánchez-Guerrero, M.C.; Medrano, E.; Porras, M.E.; Baeza, E.J.; Lorenzo, P. Influence of pre-harvest factors on quality of a winter cycle high commercial value tomato cultivar. Sci. Hortic. 2015, 189, 104–111. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, H.; Du, H.; Bao, Z.; Shi, Q. Sugar metabolic and N)-glycosylated profiles unveil the regulatory mechanisms of tomato quality under salt stress. Environ. Exp. Bot. 2020, 177, 104145. [Google Scholar] [CrossRef]
- Rodríguez-Ortega, W.M.; Martínez, V.; Nieves, M.; Simón, I.; Lidón, V.; Fernandez-Zapata, J.C.; Martinez-Nicolas, J.J.; Cámara-Zapata, J.M.; García-Sánchez, F. Agricultural and physiological responses of tomato plants grown in different soilless culture systems with saline water under greenhouse conditions. Sci. Rep. 2019, 9, 6733. [Google Scholar] [CrossRef]
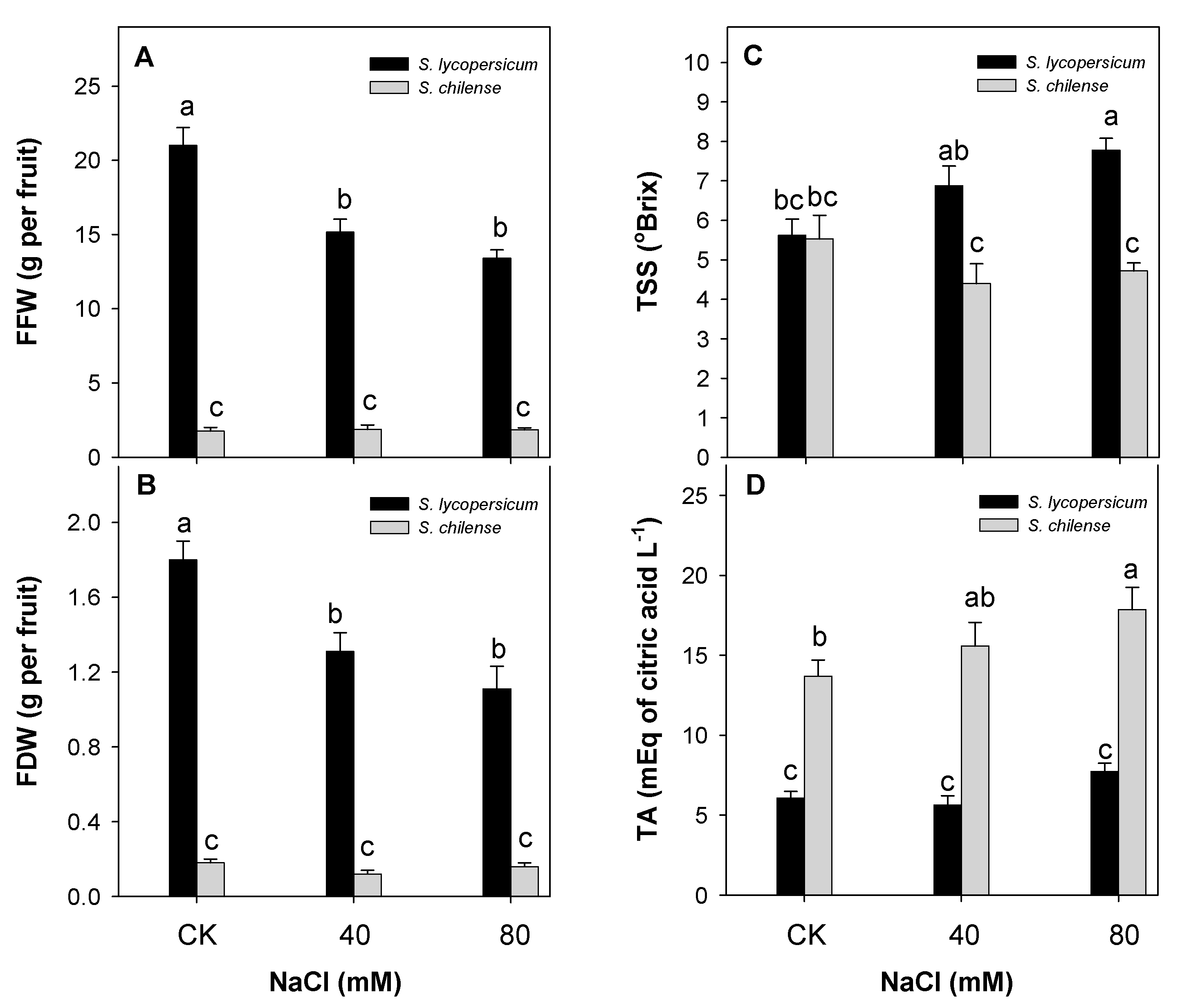
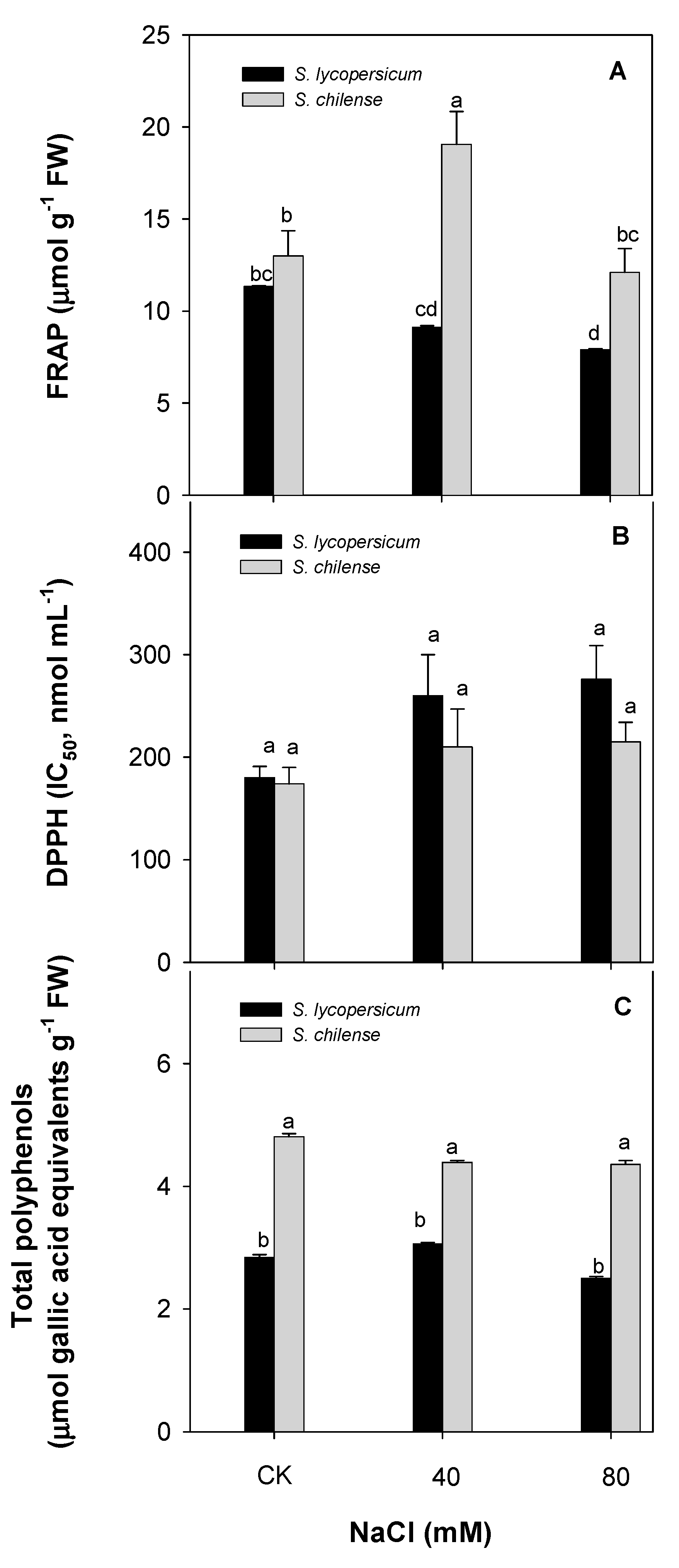
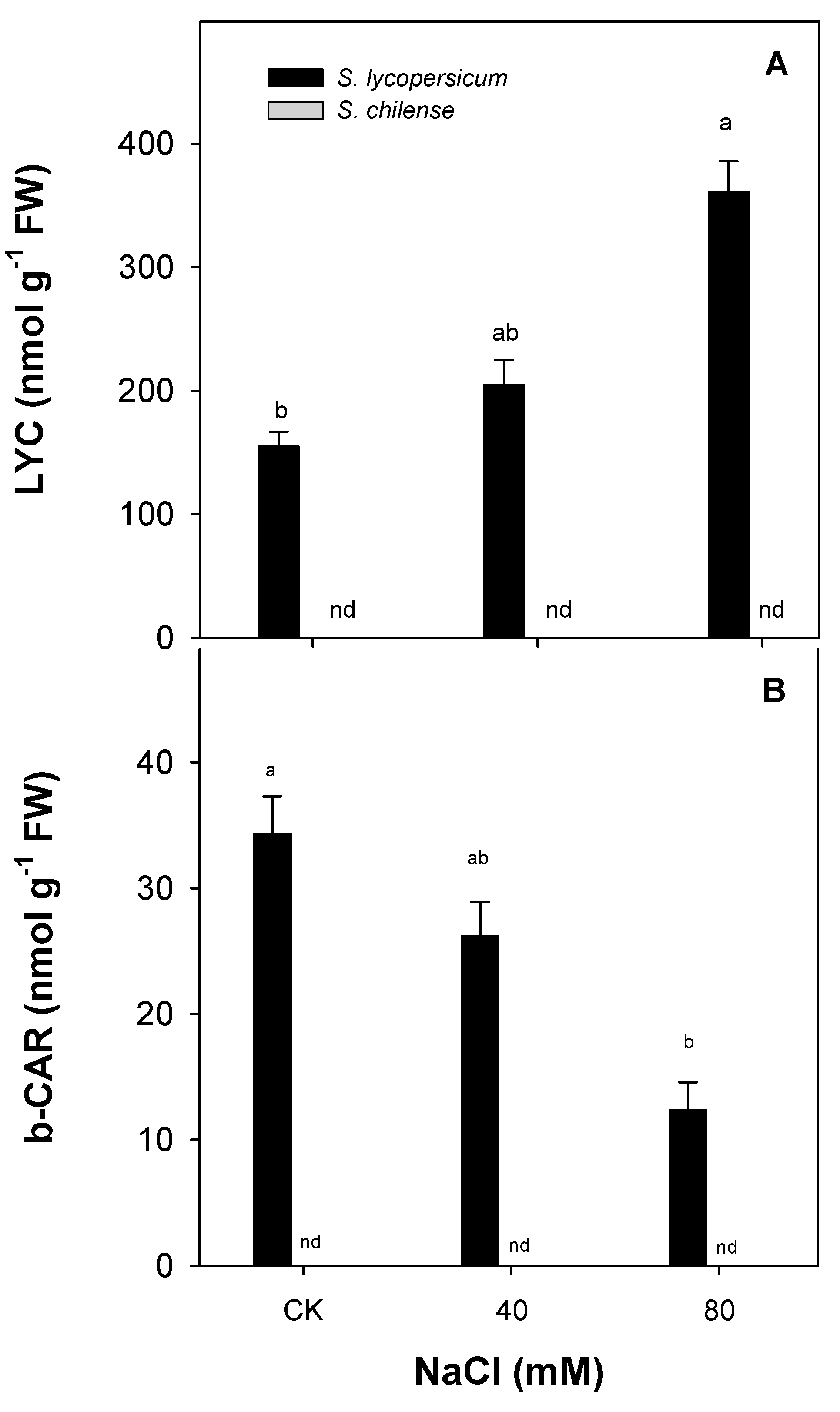
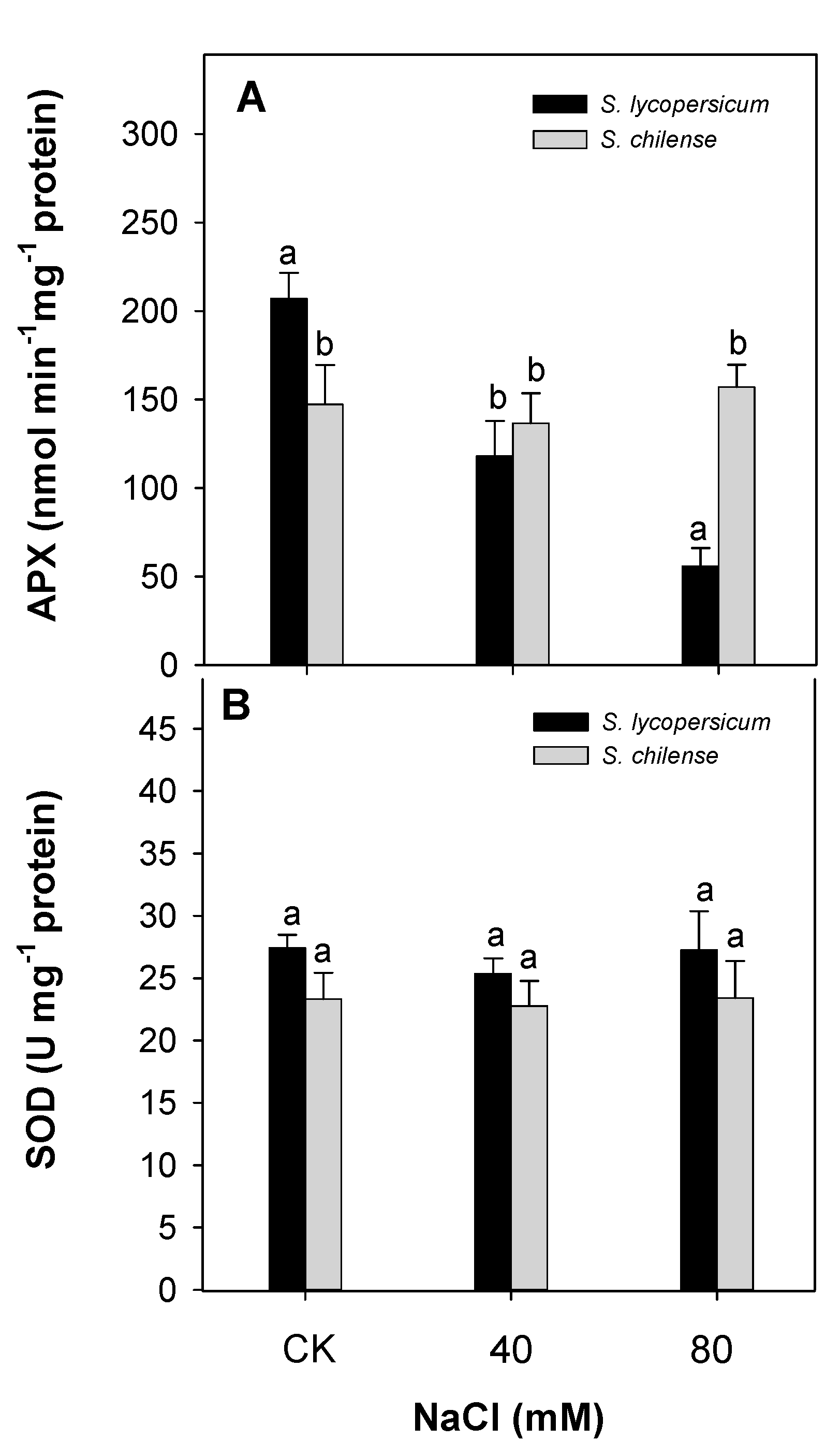
| Parameter | Solanum lycopersicum | Solanum chilense | ||||
|---|---|---|---|---|---|---|
| NaCl (mM) | NaCl (mM) | |||||
| CK | 40 | 80 | 0 | 40 | 80 | |
| PROT (mg g−1 FW) | 0.75 ± 0.04 a 1 | 0.92 ± 0.21 a | 1.15 ± 0.12 a | 1.16 ± 0.07 a | 1.25 ± 0.13 a | 0.96 ± 0.21 a |
| PROL (μmol g−1 FW) | 16.9 ± 2.93 bc | 34.98 ± 4.90 a | 34.46 ± 4.00 a | 12.57 ± 3.74 c | 20.85 ± 3.92 b | 14.73 ± 4.50 c |
| MDA (nmol g−1 FW) | 13.25 ± 1.48 a | 13.78 ± 1.00 a | 12.91 ± 2.31 a | 8.57 ± 1.11 b | 12.69 ± 1.95 a | 11.36 ± 1.85 a |
| Treatment (NaCl, mM) | Specie | GSH | GSSG | GSSG/(GSH + GSSG) | AsA | DHA | DHA/(DHA + AsA) |
|---|---|---|---|---|---|---|---|
| CK | S. lycopersicum | 675 ± 111 b | 333 ± 38 b | 0.34 ± 0.03 a | 190 ± 32 e | 609 ± 44 a | 0.76 ± 0.04 a |
| S. chilense | 1520 ± 421 a 1 | 683 ± 173 a | 0.35 ± 0.05 a | 680± 83 ab | 729 ± 169 a | 0.50 ± 0.07 c | |
| 40 | S. lycopersicum | 586 ± 62 b | 322 ± 66 b | 0.33± 0.05 a | 343 ± 39 d | 863 ± 86 a | 0.72 ± 0.01 a |
| S. chilense | 892 ± 217 ab | 543 ± 110 ab | 0.38 ± 0.05 a | 506 ± 24 c | 1085 ± 158 a | 0.67 ± 0.03 ab | |
| 80 | S. lycopersicum | 601 ± 145 b | 360 ± 103 ab | 0.30 ± 0.04 a | 638 ± 83 bc | 795 ± 193 a | 0.54 ± 0.05 bc |
| S. chilense | 702 ± 143 b | 259 ± 41 b | 0.28 ± 0.01 a | 799 ± 42 a | 649 ± 197 a | 0.42 ± 0.06 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, J.P.; Fuentes, R.; Farías, K.; Lizana, C.; Alfaro, J.F.; Fuentes, L.; Calabrese, N.; Bigot, S.; Quinet, M.; Lutts, S. Effects of Salt Stress on Fruit Antioxidant Capacity of Wild (Solanum chilense) and Domesticated (Solanum lycopersicum var. cerasiforme) Tomatoes. Agronomy 2020, 10, 1481. https://doi.org/10.3390/agronomy10101481
Martínez JP, Fuentes R, Farías K, Lizana C, Alfaro JF, Fuentes L, Calabrese N, Bigot S, Quinet M, Lutts S. Effects of Salt Stress on Fruit Antioxidant Capacity of Wild (Solanum chilense) and Domesticated (Solanum lycopersicum var. cerasiforme) Tomatoes. Agronomy. 2020; 10(10):1481. https://doi.org/10.3390/agronomy10101481
Chicago/Turabian StyleMartínez, Juan Pablo, Raúl Fuentes, Karen Farías, Carolina Lizana, Juan Felipe Alfaro, Lida Fuentes, Nicola Calabrese, Servane Bigot, Muriel Quinet, and Stanley Lutts. 2020. "Effects of Salt Stress on Fruit Antioxidant Capacity of Wild (Solanum chilense) and Domesticated (Solanum lycopersicum var. cerasiforme) Tomatoes" Agronomy 10, no. 10: 1481. https://doi.org/10.3390/agronomy10101481
APA StyleMartínez, J. P., Fuentes, R., Farías, K., Lizana, C., Alfaro, J. F., Fuentes, L., Calabrese, N., Bigot, S., Quinet, M., & Lutts, S. (2020). Effects of Salt Stress on Fruit Antioxidant Capacity of Wild (Solanum chilense) and Domesticated (Solanum lycopersicum var. cerasiforme) Tomatoes. Agronomy, 10(10), 1481. https://doi.org/10.3390/agronomy10101481






