Nutritional Relationships in Bitter Pit-Affected Fruit and the Feasibility of Vis-NIR Models to Determine Calcium Concentration in ‘Fuji’ Apples
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Mineral Sampling
2.3. Soil Sampling
2.4. Bitter Pit Assessment
2.5. NIR Approach to Predict Ca Concentration
2.6. Statistical Analyses
3. Results
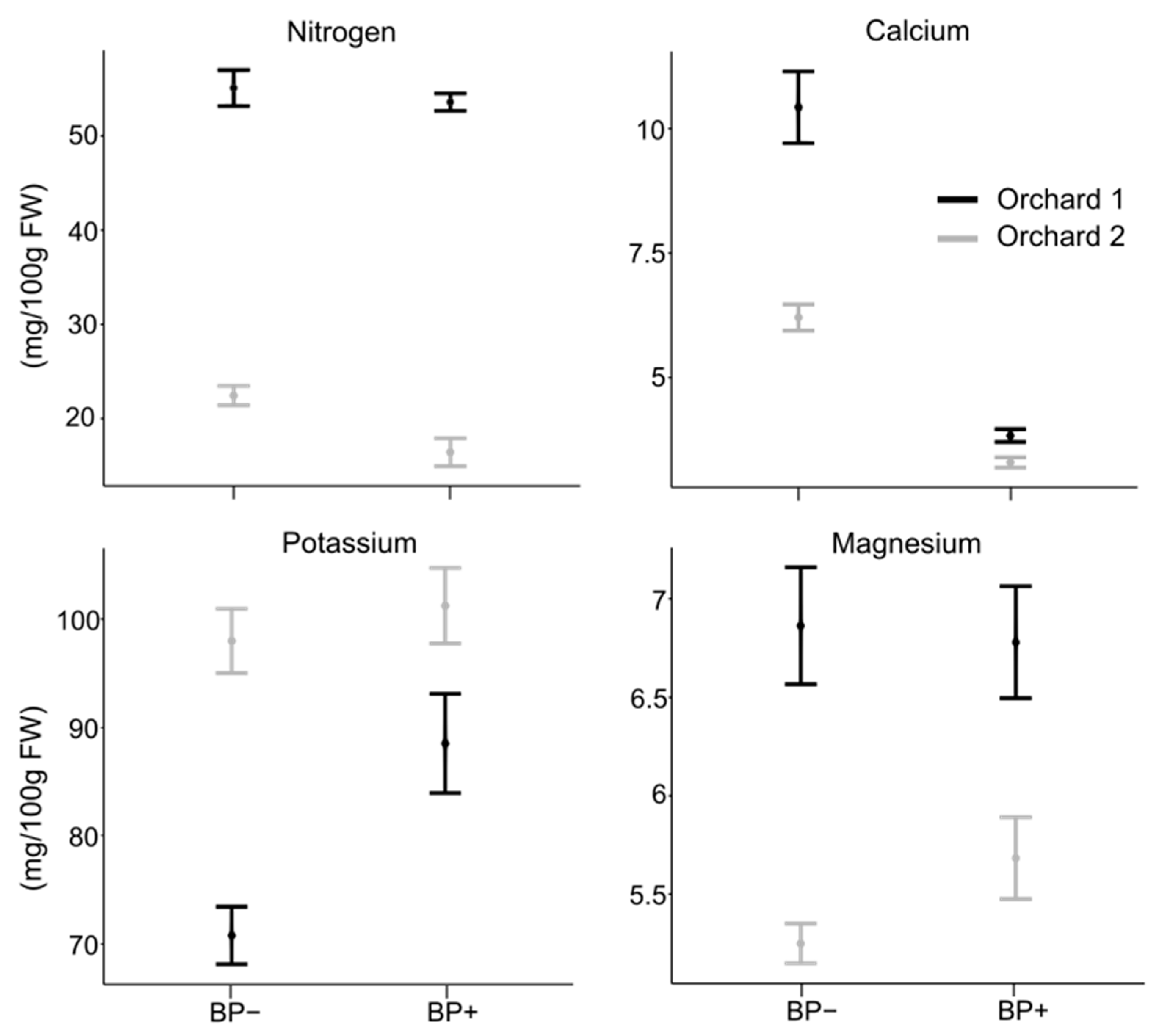
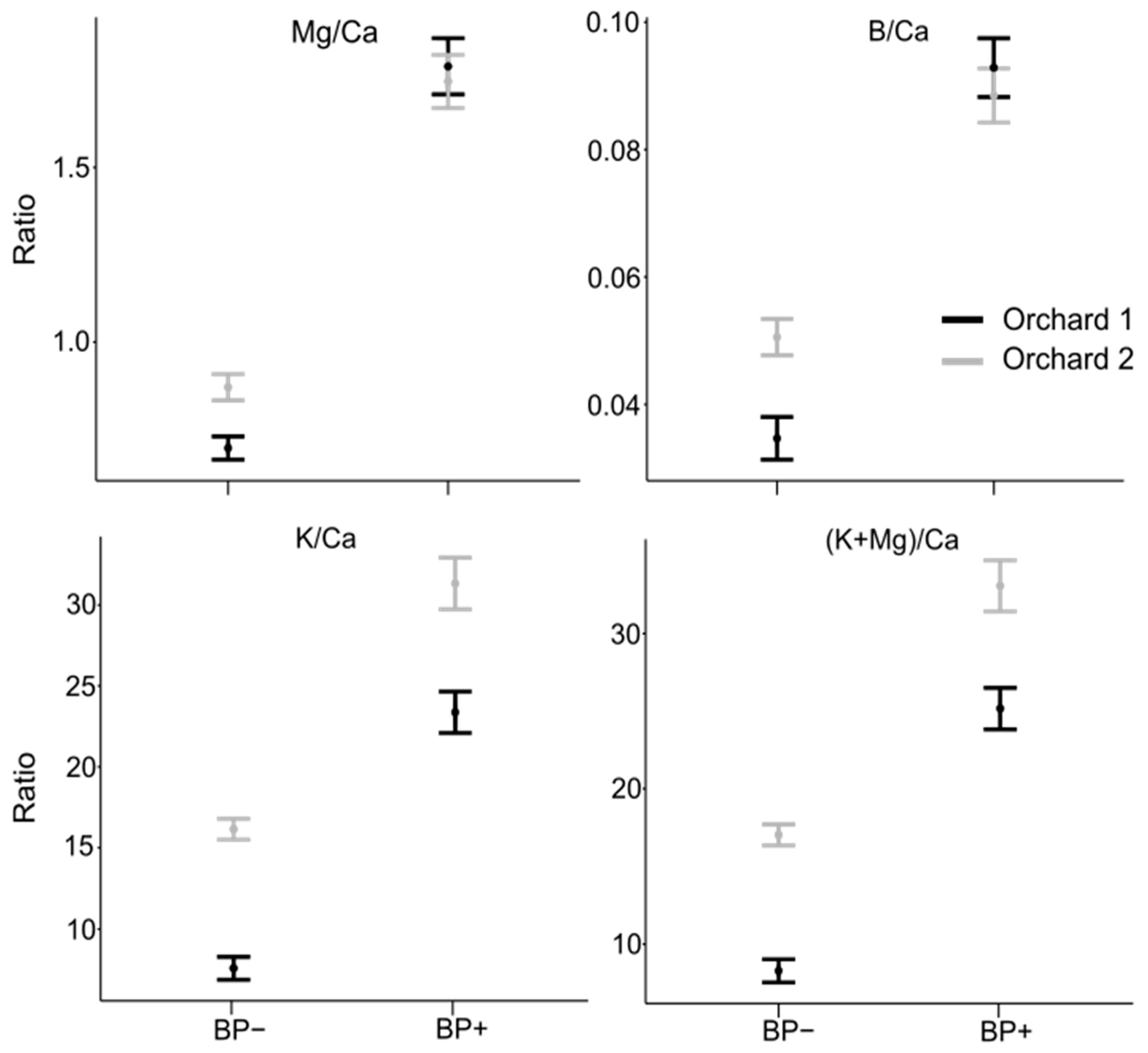
3.1. Mineral Concentration in Healthy and BP-Affected Fruit
3.2. Principal Component Analysis (PCA) for Mineral Concentrations
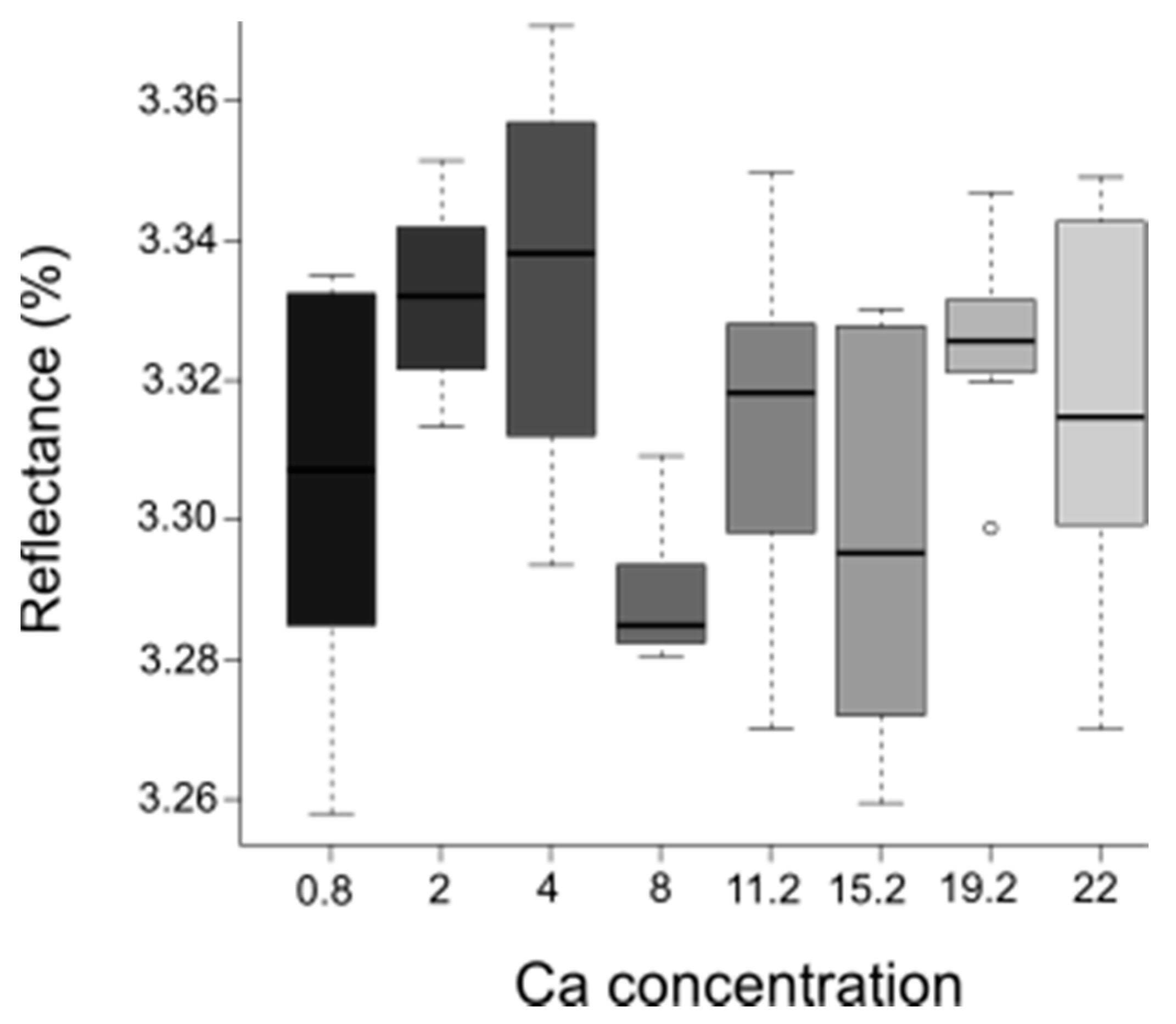
3.3. NIR Approach to Predict Ca Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Rourke, D. World Production, Trade, Consumption, and Economic Outlook for Apples. In Apples: Botany, Production and Uses; Ferree, D.C., Warrington, I.J., Eds.; CABI Publishing: Wallingford, UK, 2003; pp. 15–29. [Google Scholar]
- USDA. Available online: https://apps.fas.usda.gov/psdonline/circulars/fruit.pdf (accessed on 7 September 2020).
- De Freitas, S.T.; do Amarante, C.V.T.; Mitcham, E.J. Mechanisms regulating apple cultivar susceptibility to bitter pit. Sci. Hortic. 2015, 186, 54–60. [Google Scholar] [CrossRef]
- Fiorabanço, J.C.; Costa, A.B. Biennal bearing in apple cultivars. Rev. Ceres. 2018, 65, 144–149. [Google Scholar] [CrossRef]
- Smock, R.; Van Doren, A. The histology of bitter pit in apples. Proc. Am. Soc. Hort. Sci. 1937, 35, 176–179. [Google Scholar]
- Bünemann, G. Zusammenhänge zwischen Stickstoff- und Kationengehalt und Auftreten von Stippigkeit bei Äpfeln verschiedener Sorten und Herkünfte (Vorläufige Mitteilung). Die Gart. 1959, 24, 330–333. [Google Scholar]
- Askew, H.; Chittenden, E.; Monk, R.; Watson, J. Chemical investigations on bitter pit of apples. N. Z. J. Agric. Res. 1960, 3, 169–178. [Google Scholar] [CrossRef][Green Version]
- Martin, D.; Wade, G.; Starkborne, K. Bitter pit in the apple variety Sturmer in a pot experiment using low levels of major elements. Aust. J. Exp. Agric. Anim. Husb. 1962, 2, 92–96. [Google Scholar] [CrossRef]
- De Freitas, S.T.; do Amarante, C.V.T.; Labavitch, J.; Mitcham, E.J. Cellular approach to understand bitter pit development in apple fruit. Postharvest Biol. Technol. 2010, 57, 6–13. [Google Scholar] [CrossRef]
- Hocking, B.; Tyerma, S.; Burton, R.; Gilliham, M. Fruit Calcium: Transport and Physiology. Front. Plant Sci. 2016, 7, 1–17. [Google Scholar] [CrossRef]
- Fallahi, E.; Conway, W.S.; Hickey, K.D.; Sams, C.E. The role of calcium and nitrogen in postharvest quality and disease resistance of apples. HortScience 1997, 30, 751. [Google Scholar] [CrossRef]
- Al Shoffe, Y.; Nock, J.; Zhang, Y.; Zhu, L.; Watkins, C. Comparisons of mineral and non-mineral prediction methods for bitter pit in ‘Honeycrisp’ apples. Sci. Hortic. 2019, 254, 116–123. [Google Scholar] [CrossRef]
- Al Shoffe, Y.; Nock, J.; Baugher, T.; Marini, R.; Watkins, C. Bitter pit and soft scald development during storage of unconditioned and conditioned ‘Honeycrisp’apples in relation to mineral contents and harvest indices. Postharvest Biol. Technol. 2020, 160, 111044. [Google Scholar] [CrossRef]
- Do Amarante, C.; Steffens, C.; Ernani, P. Identificação pré-colheita do risco de ocorrência de “bitter pit” em maçãs ‘gala’ por meio de infiltração com magnésio e análise dos teores de cálcio e nitrogênio nos frutos. Rev. Bras. Frutic. 2010, 32, 27–34. [Google Scholar] [CrossRef]
- Torres, E.; Recasens, I.; Peris, J.M.; Alegre, S. Induction of symptoms pre-harvest using the “passive method”: An easy way to predict bitter pit. Postharvest Biol. Technol. 2015, 101, 66–72. [Google Scholar] [CrossRef]
- Al Shoffe, Y.; Nock, J.; Watkins, C. Non-Mineral Prediction of Bitter Pit in ‘Honeycrisp’ Apples. Fruit Q. 2018, 26, 21–24. [Google Scholar]
- Do Amarante, C.V.T.; Silveira, J.P.G.; Steffens, C.A.; de Freitas, S.T.; Mitcham, E.J.; Miqueloto, A. Post-bloom and preharvest treatment of ‘Braeburn’ apple trees with prohexadione-calcium and GA4+7 affects vegetative growth and postharvest incidence of calcium-related physiological disorders and decay in the fruit. Sci. Hortic. 2020, 261, 108919. [Google Scholar] [CrossRef]
- Zhang, Y.; Nock, J.; Al Shoffe, Y.; Watkins, C. Non-destructive prediction of soluble solids and dry matter contents in eight apple cultivars using near-infrared spectroscopy. Postharvest Biol. Technol. 2019, 151, 111–118. [Google Scholar] [CrossRef]
- Ciavarella, S.; Batten, G.D.; Blakeney, A.B. Measuring potassium in plant tissues using near infrared spectroscopy. J. Near Infrared Spec. 1998, 66, 63–66. [Google Scholar] [CrossRef]
- García-Sánchez, F.; Gálvez-Sola, L.; Martínez-Nicolás, J.; Muelas-Domingo, R.; Nieves, M. Using Near-Infrared Spectroscopy in Agricultural Systems. In Developments in Near-Infrared Spectroscopy; Kyprianidis, K., Ed.; IntechOpen: London, UK, 2017; pp. 97–127. [Google Scholar] [CrossRef]
- Gálvez-Sola, L.; García-Sánchez, F.; Pérez-Pérez, J.G.; Gimeno, V.; Navarro, J.M.; Moral, R.; Martínez-Nicolás, J.J.; Nieves, M. Rapid estimation of nutritional elements on citrus leaves by near infrared reflectance spectroscopy. Front. Plant Sci. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Fukumoto, M.; Nagai, K. Possible role of calcium and ammonium in the development of bitter pit in apple. Physiol. Plant. 1983, 59, 171–176. [Google Scholar] [CrossRef]
- Rossa, Ü.B.; Angelo, A.C.; Nisgoski, S.; Westphalen, D.J.; Frizon, C.N.T.; Hoffmann-Ribani, R. Application of the NIR method to determine nutrients in yerba mate (Ilex paraguariensis A. St.-Hill) leaves. Commun. Soil Sci. Plant Anal. 2015, 46, 2323–2331. [Google Scholar] [CrossRef]
- Ryan, J.; Estefan, G.; Rashid, A. Soil and Plant Analysis Laboratory Manual, 2nd ed.; International Center for Agricultural Research in the Dry Areas (ICARDA): Aleppo, Syria, 2001; p. 172. [Google Scholar]
- Sparks, D.L. Methods of Soil Analysis, Part 3. In Chemical Methods SSSA Book Series, 3rd ed.; Soil Science Society of America: Madison, WI, USA, 1996; p. 1424. [Google Scholar]
- De Freitas, S.T.; Mitcham, E.I. Factors involved in fruit calcium deficiency disorders. Hortic. Rev. 2012, 40, 107–146. [Google Scholar]
- Siddiqi, M.Y.; Malhotra, B.; Min, X.; Glass, A.D.M. Effects of ammonium and inorganic carbon enrichment on growth and yield of a hydroponic tomato crop. J. Plant. Nutr. Soil Sci. 2002, 165, 191–197. [Google Scholar] [CrossRef]
- Zharare, G.; Asher, C.; Blamey, F. Magnesium antagonizes pod-zone calcium and zinc uptake by developing peanut pods. J. Plant Nutr. 2011, 34, 1–11. [Google Scholar] [CrossRef]
- Do Amarante, C.V.T.; Miqueloto, A.; Steffens, C.A.; Maciel, T.M.; Denardi, V.; Argenta, L.C.; de Freitas, S.T. Optimization of fruit tissue sampling method to quantify calcium, magnesium and potassium contents to predict bitter pit in apples. Acta Hortic. 2018, 1194, 487–492. [Google Scholar] [CrossRef]
- Brown, P.; Bellaloui, N.; Wimmer, M.A.; Bassil, E.S.; Ruiz, J.; Hu, H.; Pfeffer, H.; Dannel, F.; Römheld, V. Boron in plant biology. Plant Biol. 2002, 4, 205–223. [Google Scholar] [CrossRef]
- Hosseini, S.M.E.; Maftoun, M.; Karimian, N.; Rounagui, A.; Emam, Y. Effect of zinc sulfate on corn resistance to boron toxicity. Iran. J. Soil Water Sci. 2004, 18, 125–134. [Google Scholar]
- Eraslan, F.; Inal, A.; Gunes, A.; Alpaslan, M. Boron toxicity alters nitrate reductase activity, proline accumulation, membrane permeability, and mineral constituents of tomato and pepper plants. J. Plant Nutr. 2007, 30, 981–994. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Mai, J.; Tao, L.; Qu, M.; Liu, J.; Shen, R.; Xu, G.; Feng, Y.; Xiao, H.; et al. Boron alleviates aluminum toxicity by promoting root alkalization in transition zone via polar auxin transport. Plant Physiol. 2018, 177, 1254–1266. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, A.L.; Dikilitas, M.; Ashraf, M.; Koskeroglu, S.; Guneri, M. Supplementary phosphorus can alleviate boron toxicity in tomato. Sci. Hortic. 2009, 121, 284–288. [Google Scholar] [CrossRef]
- Ghaffari, S.; Etesami, H. The Importance of Boron in Plant Nutrition. In Metalloids in Plants: Advances and Future Prospects; Deshmukh, R., Tripathi, D., Guerriero, G., Eds.; John Wiley & Sons, Inc. Press: Hoboken, NJ, USA, 2019; pp. 431–447. [Google Scholar]
- Sotiropoulos, T.E.; Therios, I.N.; Dimassi, K.N. Calcium application as a means to improve tolerance of kiwifruit (Actinidia deliciosa L.) to boron toxicity. Sci. Hortic. 1999, 81, 443–449. [Google Scholar] [CrossRef]
- Fang, K.; Zhang, W.; Xing, Y.; Zhang, Q.; Yang, L.; Cao, Q.; Qin, L. Boron toxicity causes multiple effects on Malus domestica pollen tube growth. Front. Plant Sci. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Du, C.; Xu, F.; Yang, Y. Effects of boron and calcium supply on calcium fractionation in plants and suspension cells of rape cultivars with different boron efficiency. J. Plant Nutr. 2003, 26, 789–806. [Google Scholar] [CrossRef]
- Fazio, G.; Lordan, L.; Grusak, M.; Francescatto, P.; Robinson, T. Mineral nutrient profiles and relationships of ‘Honeycrisp’ grown on a genetically diverse set of rootstocks under Western New York climatic conditions. Sci. Hortic. 2020, 266, 108477. [Google Scholar] [CrossRef]
- Oertli, J.; Richardson, W.F. The mechanism of boron immobility in plants. Physiol. Plant. 2006, 23, 108–116. [Google Scholar] [CrossRef]
- Wang, N.; Yang, C.; Pan, Z.; Liu, Y.; Peng, S. Boron deficiency in woody plants: Various responses and tolerance mechanisms. Front. Plant Sci. 2015, 6, 916. [Google Scholar] [CrossRef] [PubMed]
- Picchioni, G.A.; Weinbaum, S.A.; Brown, P.H. Retention and the kinetics of uptake and export of foliage-applied, labeled boron by apple, pear, prune, and sweet cherry leaves. J. Am. Soc. Hortic. Sci. 1995, 120, 28–35. [Google Scholar] [CrossRef]
- Brown, P.; Bellaloui, N.; Hu, H.; Dandekar, A. Transgenically enhanced sorbitol synthesis facilitates phloem boron transport and increases tolerance of tobacco to boron deficiency. Plant Physiol. 1999, 119, 17–20. [Google Scholar] [CrossRef]
- Brown, P.; Hu, H. Phloem Mobility of Boron is Species Dependent: Evidence for Phloem Mobility in Sorbitol-rich Species. Ann. Bot. 1996, 77, 497–506. [Google Scholar] [CrossRef]
- Hu, H.; Penn, S.G.; Lebrilla, C.B.; Brown, P.H. Isolation and characterization of soluble boron complexes in higher plants (the mechanism of phloem mobility of boron). Plant Physiol. 1997, 113, 649–655. [Google Scholar] [CrossRef]
- Brown, P.; Shelp, B. Boron mobility in plants. Plant Soil 1997, 193, 85–101. [Google Scholar] [CrossRef]
- Coskun, Y.; Olgunsoy, P.; Karatas, N. Mannitol application alleviates boron toxicity in wheat seedlings. Commun. Soil Sci. Plan. 2014, 45, 944–952. [Google Scholar] [CrossRef]
- Minchin, P.; Thorp, T.; Boldingh, H.; Gould, N.; Cooney, J.; Negm, F.; Focht, E.; Arpaia, M.L.; Hu, H.; Brown, P. A possible mechanism for phloem transport of boron in ‘Hass’ avocado (Persea americana Mill.) trees. J. Hortic. Sci. Biotechnol. 2012, 87, 23–28. [Google Scholar] [CrossRef]



| Orchard 1 | Orchard 2 | ||||
|---|---|---|---|---|---|
| Evaluation Date (Days) | Bitter Pit Incidence (%) | Bitter Pit Severity (Average Number of Pits) | Bitter Pit Incidence (%) | Bitter Pit Severity (Average Number of Pits) | P Value (*) |
| 0 | 0 | 0 | 0 | 0 | - |
| 10 | 0 | 0 | 0 | 0 | - |
| 30 | 0 | 0 | 0 | 0 | - |
| 50 | 0.4 | 4 | 0.5 | 3 | 0.469 |
| 70 | 1.9 | 3.8 | 0.7 | 4.6 | 0.485 |
| 90 | 3.2 | 5 | 2.8 | 4.5 | 0.641 |
| 110 | 4.1 | 4.8 | 3.3 | 4.4 | 0.655 |
| 130 | 5.3 | 4.5 | 3.5 | 4.3 | 0.816 |
| 150 | 5.6 | 4.7 | 3.6 | 4.2 | 0.528 |
| Orchard 1 | Orchard 2 | ||||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | P Value | |
| Dry matter | 14.65 | 0.44 | 14.61 | 0.44 | 0.915 |
| N | 27.45 | 1.12 | 31.98 | 1.06 | 0.006 |
| Ca | 7.44 | 0.32 | 7.91 | 0.22 | 0.247 |
| K | 67.18 | 2.59 | 99.26 | 1.91 | 0.000 |
| Mg | 5.48 | 0.15 | 5.49 | 0.09 | 0.976 |
| P | 10.77 | 0.24 | 10.95 | 0.33 | 0.647 |
| B | 0.29 | 0.01 | 0.24 | 0.01 | 0.001 |
| Cu | 0.09 | 0.01 | 0.07 | 0.01 | 0.021 |
| Fe | 0.13 | 0.01 | 0.14 | 0.01 | 0.160 |
| Mn | 0.02 | 0.00 | 0.04 | 0.00 | 0.000 |
| Zn | 0.05 | 0.00 | 0.05 | 0.00 | 0.421 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonomelli, C.; Mogollón, R.; Tonetto de Freitas, S.; Zoffoli, J.P.; Contreras, C. Nutritional Relationships in Bitter Pit-Affected Fruit and the Feasibility of Vis-NIR Models to Determine Calcium Concentration in ‘Fuji’ Apples. Agronomy 2020, 10, 1476. https://doi.org/10.3390/agronomy10101476
Bonomelli C, Mogollón R, Tonetto de Freitas S, Zoffoli JP, Contreras C. Nutritional Relationships in Bitter Pit-Affected Fruit and the Feasibility of Vis-NIR Models to Determine Calcium Concentration in ‘Fuji’ Apples. Agronomy. 2020; 10(10):1476. https://doi.org/10.3390/agronomy10101476
Chicago/Turabian StyleBonomelli, Claudia, René Mogollón, Sergio Tonetto de Freitas, Juan Pablo Zoffoli, and Carolina Contreras. 2020. "Nutritional Relationships in Bitter Pit-Affected Fruit and the Feasibility of Vis-NIR Models to Determine Calcium Concentration in ‘Fuji’ Apples" Agronomy 10, no. 10: 1476. https://doi.org/10.3390/agronomy10101476
APA StyleBonomelli, C., Mogollón, R., Tonetto de Freitas, S., Zoffoli, J. P., & Contreras, C. (2020). Nutritional Relationships in Bitter Pit-Affected Fruit and the Feasibility of Vis-NIR Models to Determine Calcium Concentration in ‘Fuji’ Apples. Agronomy, 10(10), 1476. https://doi.org/10.3390/agronomy10101476






