Effect of Bacterial Inoculum and Fertigation Management on Nursery and Field Production of Lettuce Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Nursery Production
2.2. Lettuce Plant Cultivation
2.3. Statistics and Principal Component Analysis
3. Results
3.1. Nursery Production of Lettuce Seedlings
3.2. Lettuce Plants Cultivation
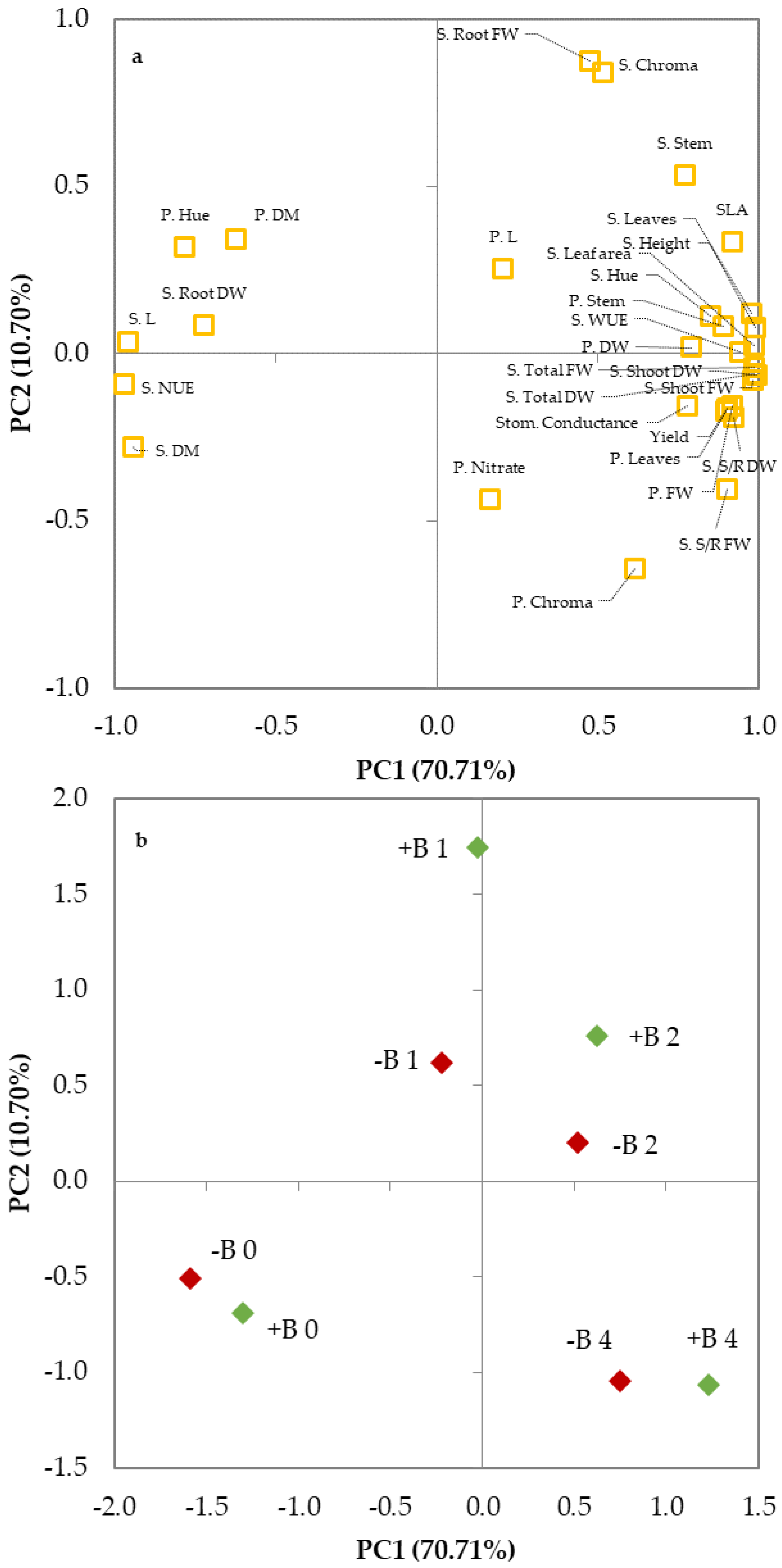
3.3. Principal Components Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sterrett, S. Composts as Horticultural Substrates for Vegetable Transplant Production. In Compost Utilization in Horticultural Cropping Systems; Stoffella, P.J., Kahn, B.A., Eds.; Lewis Publication: Boca Raton, FL, USA, 2001; pp. 227–240. ISBN 1420026224. [Google Scholar]
- Nicola, S.; Cantliffe, D.J. Increasing Cell Size and Reducing Medium Compression Enhance Lettuce Transplant Quality and Field Production. HortScience 1996, 31, 184–189. [Google Scholar] [CrossRef]
- Iapichino, G.; Vetrano, F.; Moncada, A.; Fascella, S.; Incalcaterra, G. Effects of plastic mulch and floating cover on lettuce production in sicily. Acta Hortic. 2012, 936, 491–494. [Google Scholar] [CrossRef]
- Kubota, C.; McClure, M.A.; Kokalis-Burelle, N.; Bausher, M.G.; Rosskopf, E.N. Vegetable Grafting: History, Use, and Current Technology Status in North America. HortScience 2008, 43, 1664–1669. [Google Scholar] [CrossRef]
- Caracciolo, G.; Moncada, A.; Prinzivalli, C.; D’Anna, F. Effects of thr plantinig dates on strawberry plug plant performance in sicly. Acta Hortic. 2009, 155–158. [Google Scholar] [CrossRef]
- Swiader, J.M.; Ware, G.W.; McCollum, J.P. Producing Vegetable Crops: Teacher’s Manual; Interstate Publishers: Crete, IL, USA, 1992; ISBN 0813429048. [Google Scholar]
- Herrera, F.; Castillo, J.; Chica, A.; Bellido, L.L. Use of municipal solid waste compost (MSWC) as a growing medium in the nursery production of tomato plants. Bioresour. Technol. 2008, 99, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.M. Biological amendment, fertilizer rate, and irrigation frequency for organic Bell pepper transplant production. HortScience 2006, 41, 1402–1407. [Google Scholar] [CrossRef]
- McCall, D. Effect of supplementary light on tomato transplant growth, and the after-effects on yield. Sci. Hortic. 1992, 51, 65–70. [Google Scholar] [CrossRef]
- Masson, J.; Tremblay, N.; Gosselin, A. Nitrogen Fertilization and HPS Supplementary Lighting Influence Vegetable Transplant Production. I. Transplant Growth. J. Am. Soc. Hortic. Sci. 1991, 116, 594–598. [Google Scholar] [CrossRef]
- Masson, J.; Tremblay, N.; Gosselin, A. Effects of Nitrogen Fertilization and HPS Supplementary Lighting on Vegetable Transplant Production. II. Yield. J. Am. Soc. Hortic. Sci. 1991, 116, 599–602. [Google Scholar] [CrossRef]
- Miceli, A.; Romano, C.; Vetrano, F.; D’Anna, F. Effects of a Brassica juncea cover crop on a mono-succession of melon. Acta Hortic. 2013, 447–451. [Google Scholar] [CrossRef]
- Schrader, W.L. Using Transplants in Vegetable Production; University of California Agriculture and Natural Resources (UC ANR): St. Davis, CA, USA, 2000. [Google Scholar]
- Dufault, R.J. Vegetable Transplant Nutrition. HortTechnology 1998, 8, 515–523. [Google Scholar] [CrossRef]
- Liptay, A.; Nicholls, S. Nitrogen Supply during Greenhouse Transplant Production Affects Subsequent Tomato Root Growth in the Field. J. Am. Soc. Hortic. Sci. 1993, 118, 339–342. [Google Scholar] [CrossRef]
- Vetrano, F.; Iapichino, G.; Poma, M.; Fascella, S.; Incalcaterra, G. Use of organic fertilizers for lettuce plug plant production. Acta Hortic. 2009, 607–612. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Caldwell, R.D. Best Management Practices for Minimizing Nitrate Leaching from Container-Grown Nurseries. Sci. WorldJ. 2001, 1, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Moncada, A.; Miceli, A.; Vetrano, F. Use of plant growth-promoting rhizobacteria (PGPR) and organic fertilization for soilless cultivation of basil. Sci. Hortic. 2020, 275, 109733. [Google Scholar] [CrossRef]
- Shereni, C. Use of Biostimulants as an Alternate Approach to Achieve Plant Performance and Fruit Quality, Stellenbosch; Stellenbosch University: Cape Town, South Africa, 2019. [Google Scholar]
- Bashan, Y.; De-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.-P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Glick, B.R. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Kloepper, J.; Reddy, M.; Rodríguez-Kábana, R.; Kenney, D.; Kokalis-Burelle, N.; Martinez-Ochoa, N.; Vavrina, C.S. Application for Rhizobacteria in transplant production and yield enhancement. Acta Hortic. 2004, 631, 219–229. [Google Scholar] [CrossRef]
- Gul, A.; Kidoglu, F.; Tüzel, Y.; Tüzel, I.H. Effects of nutrition and “Bacillus amyloliquefaciens” on tomato (“Solanum lycopersicum L.”) growing in perlite. Span. J. Agric. Res. 2008, 6, 422–429. [Google Scholar] [CrossRef]
- Akram, W.; Anjum, T.; Ali, B. Co-cultivation of tomato with two Bacillus strains: Effects on growth and yield. J. Anim. Plant Sci. 2015, 25, 1644–1651. [Google Scholar]
- Kokalis–Burelle, N.; Vavrina, C.S.; Rosskopf, E.N.; Shelby, R.A. Field evaluation of plant growth-promoting Rhizobacteria amended transplant mixes and soil solarization for tomato and pepper production in Florida. Plant Soil 2002, 238, 257–266. [Google Scholar] [CrossRef]
- Kokalis-Burelle, N.; Vavrina, C.; Reddy, M.; Kloepper, J. Amendment of Muskmelon and Watermelon Transplant Media with Plant Growth-Promoting Rhizobacteria: Effects on Seedling Quality, Disease, and Nematode Resistance. HortTechnology 2003, 13, 476–482. [Google Scholar] [CrossRef]
- Yan, Z.; Reddy, M.S.; Kloepper, J.W. Survival and colonization of rhizobacteria in a tomato transplant system. Can. J. Microbiol. 2003, 49, 383–389. [Google Scholar] [CrossRef][Green Version]
- Ekinci, M.; Turan, M.; Yildirim, E.; Güneş, A.; Kotan, R.; Dursun, A. Effect of plant growth promoting rhizobacteria on growth, nutrient, organic acid, amino acid and hormone content of cauliflower (Brassica oleracea L. var. botrytis) transplants. Acta Sci. Pol. Hortorum Cultus 2014, 13, 71–85. [Google Scholar]
- Fageria, N.K.; Baligar, V.C.; Li, Y. The Role of Nutrient Efficient Plants in Improving Crop Yields in the Twenty First Century. J. Plant Nutr. 2008, 31, 1121–1157. [Google Scholar] [CrossRef]
- Miceli, C.; Moncada, A.; Vetrano, F.; Iapichino, G.; D’Anna, F.; Miceli, A. Effect of Agronomic Practices on Yield and Quality of Borage at Harvest and During Storage as Minimally-Processed Produce. Agronomy 2020, 10, 242. [Google Scholar] [CrossRef]
- Rodrigo, M.C.; Ramos, C. Nitrate sap analysis as a tool to assess nitrogen nutrition in artichoke. In Proceedings of the VI International Symposium on Artichoke, Cardoon and Their Wild Relatives 730, Lorca, Spain, 28–31 March 2006; pp. 251–256. [Google Scholar]
- Miceli, A.; Miceli, C. Effect of Nitrogen Fertilization on the Quality of Swiss Chard at Harvest and during Storage as Minimally Processed Produce. J. Food Qual. 2014, 37, 125–134. [Google Scholar] [CrossRef]
- Caracciolo, G.; D’Anna, E.; Moncada, A.; D’Anna, F. Evaluation of the quality and antioxidant capacity of woodland strawberry biotypes in Sicily. J. Food Agric. Environ. 2013, 11, 522–525. [Google Scholar]
- Soundy, P.; Cantliffe, D.J.; Hochmuth, G.J.; Stoffella, P.J. Management of Nitrogen and Irrigation in Lettuce Transplant Production affects Transplant Root and Shoot Development and Subsequent Crop Yields. HortScience 2005, 40, 607–610. [Google Scholar] [CrossRef]
- Sahin, F.; Cakmakci, R.; Kantar, F. Sugar beet and barley yields in relation to inoculation with N2-fixing and phosphate solubilizing bacteria. Plant Soil 2004, 265, 123–129. [Google Scholar] [CrossRef]
- Shao, J.; Xu, Z.; Zhang, N.; Shen, Q.; Zhang, R. Contribution of indole-3-acetic acid in the plant growth promotion by the rhizospheric strain Bacillus amyloliquefaciens SQR9. Boil. Fertil. Soils 2014, 51, 321–330. [Google Scholar] [CrossRef]
- Joo, G.-J.; Kim, Y.-M.; Lee, S.-U.; Song, K.-S.; Rhee, I.-K. Growth promotion of red pepper plug seedlings and the production of gibberellins by Bacillus cereus, Bacillus macroides and Bacillus pumilus. Biotechnol. Lett. 2004, 26, 487–491. [Google Scholar] [CrossRef]
- Ekin, Z.; Faruk, O.; Erman, M.; Erdal, Ö. The effect of Bacillus sp. OSU-142 inoculation at various levels of nitrogen fertilization on growth, tuber distribution and yield of potato (Solanum tuberosum L.). Afr. J. Biotechnol. 2009, 8, 4418–4424. [Google Scholar]
- Welbaum, G.E.; Sturz, A.V.; Dong, Z.; Nowak, J. Managing Soil Microorganisms to Improve Productivity of Agro-Ecosystems. Crit. Rev. Plant Sci. 2004, 23, 175–193. [Google Scholar] [CrossRef]
- Marschner, P.; Gerendás, J.; Sattelmacher, B. Effect of N concentration and N source on root colonization by Pseudomonas fluorescens 2-79RLI. Plant Soil 1999, 215, 135–141. [Google Scholar] [CrossRef]
- Rovira, A.D.; Davey, C.B. Biology of the rhizosphere. In The Plant Root and its Environment; Carson, E., Ed.; University of Virginia Press: Charlottesville, VR, USA, 1974; ISBN 978-0813904115. [Google Scholar]
- Oliveira, A.L.M.; Urquiaga, S.; Döbereiner, J.; Baldani, J. The effect of inoculating endophytic N2-fixing bacteria on micropropagated sugarcane plants. Plant Soil 2002, 242, 205–215. [Google Scholar] [CrossRef]
- Soundy, P.; Cantliffe, D.J.; Hochmuth, G.J.; Stoffella, P.J.; Hall, F.; Box, P.O. Nutrient Requirements for Lettuce Transplants Using a Floatation Irrigation System II. Potassium. HortScience 2001, 36, 1071–1074. [Google Scholar] [CrossRef]
- Soundy, P.; Cantliffe, D.J.; Hochmuth, G.J.; Stoffella, P.J.; Hall, F.; Box, P.O. Nutrient Requirements for Lettuce Transplants Using a Floatation Irrigation System. I. Phosphorus. HortScience 2001, 36, 1066–1070. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Veselov, S.U.; Melentiev, A.I.; Martynenko, E.V.; Kudoyarova, G.R. Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 2005, 272, 201–209. [Google Scholar] [CrossRef]
- Ping, L.; Boland, M. Signals from the underground: Bacterial volatiles promote growth in Arabidopsis. Trends Plant Sci. 2004, 9, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Sakakibara, H.; Taniguchi, M.; Sugiyama, T. Nitrogen-dependent accumulation of cytokinins in root and the translocation to leaf: Implication of cytokinin species that induces gene expression of maize response regulator. Plant Cell Physiol. 2001, 42, 85–93. [Google Scholar] [CrossRef] [PubMed]
- García de Salamone, I.E.; Hynes, R.K.; Nelson, L.M. Cytokinin production by plant growth promoting rhizobacteria and selected mutants. Can. J. Microbiol. 2001, 47, 404–411. [Google Scholar] [CrossRef]
- Vavrina, C.S. An Introduction to the Production of Containerized Vegetable Transplants; University of Florida, Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 1996; Volume 302. [Google Scholar]
- Gurska, J.; Wang, W.; Gerhardt, K.E.; Khalid, A.M.; Isherwood, D.M.; Huang, X.-D.; Glick, B.R.; Greenberg, B.M. Three Year Field Test of a Plant Growth Promoting Rhizobacteria Enhanced Phytoremediation System at a Land Farm for Treatment of Hydrocarbon Waste. Env. Sci. Technol. 2009, 43, 4472–4479. [Google Scholar] [CrossRef]
- Stefan, M.; Munteanu, N.; Stoleru, V.; Mihasan, M. Effects of inoculation with plant growth promoting rhizobacteria on photosynthesis, antioxidant status and yield of runner bean. Rom. Biotechnol. Lett. 2013, 18, 8132–8143. [Google Scholar]
- Stefan, M.; Munteanu, N.; Stoleru, V.; Mihasan, M.; Hritcu, L. Seed inoculation with plant growth promoting rhizobacteria enhances photosynthesis and yield of runner bean (Phaseolus coccineus L.). Sci. Hortic. 2013, 151, 22–29. [Google Scholar] [CrossRef]
- Han, H.S.; Lee, K.D. Plant growth promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil salinity. Res. J. Agric Biol. Sci. 2005, 1, 210–215. [Google Scholar]
- Baset, M.; Shamsuddin, Z.H.; Wahab, Z.; Marziah, M. Effect of plant growth promoting rhizobacterial (PGPR) inoculation on growth and nitrogen incorporation of tissue-cultured‘musa’plantlets under nitrogen-free hydroponics condition. Aust. J. Crop Sci. 2010, 4, 85. [Google Scholar]
- Ihl, M.; Shene, C.; Scheuermann, E.; Bifani, V. Correlation for pigment content through colour determination using tristimulus values in a green leafy vegetable, swiss chard. J. Sci. Food Agric. 1994, 66, 527–531. [Google Scholar] [CrossRef]
- Madeira, A.C.; Ferreira, A.; De Varennes, A.; Vieira, M.I. SPAD Meter Versus Tristimulus Colorimeter to Estimate Chlorophyll Content and Leaf Color in Sweet Pepper. Commun. Soil Sci. Plant Anal. 2003, 34, 2461–2470. [Google Scholar] [CrossRef]
- Ciardi, J.A.; Vavrina, C.S.; Orzolek, M.D. Evaluation of tomato transplant production methods for improving establishment rates. HortScience 1998, 33, 229–232. [Google Scholar]
- European Commission Commission regulation (EU) No 1258/2011 of 2 December 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for nitrates in foodstuffs. Off. J. Eur. Union 2011, L 320/15, 15–17.
- Balanza, V.; Martinez, J.; Conesa, E.; Egea-Gilabert, C.; Niñirola, D.; López-Marín, J.; Gonzalez, A.; Fernández, J.A. Effect of PGPR application and nitrogen doses on baby leaf lettuce grown in a floating system. Acta Hortic. 2012, 952, 679–687. [Google Scholar] [CrossRef]
- Song, X.; Liu, M.; Wu, D.; Griffiths, B.S.; Jiao, J.; Li, H.; Hu, F. Interaction matters: Synergy between vermicompost and PGPR agents improves soil quality, crop quality and crop yield in the field. Appl. Soil Ecol. 2015, 89, 25–34. [Google Scholar] [CrossRef]
- Kavino, M.; Harish, S.; Kumar, N.; Saravanakumar, D.; Samiyappan, R. Effect of chitinolytic PGPR on growth, yield and physiological attributes of banana (Musa spp.) under field conditions. Appl. Soil Ecol. 2010, 45, 71–77. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, M.; Zhang, Y.; Ji, Y.; Zhao, M.; Wu, Z. Effect of different plant growth regulator added in nutrient solution on growth and development of summer tomato seedling. North Hortic 2017, 6, 8–13. [Google Scholar]
- Khan, N.A. Effect of gibberellic acid on carbonic anhydrase, photosynthesis, growth and yield of mustard. Boil. Plant. 1996, 38, 145. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Sabatino, L.; Vetrano, F. Effect of Gibberellic Acid on Growth, Yield, and Quality of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy 2019, 9, 382. [Google Scholar] [CrossRef]
- Khan, N.A.; Mir, R.; Javid, S. Samiullah Effects of gibberellic acid spray on nitrogen yield efficiency of mustard grown with different nitrogen levels. Plant Growth Regul. 2002, 38, 243–247. [Google Scholar] [CrossRef]
- Miceli, A.; Vetrano, F.; Sabatino, L.; D’Anna, F.; Moncada, A. Influence of Preharvest Gibberellic Acid Treatments on Postharvest Quality of Minimally Processed Leaf Lettuce and Rocket. Horticulturae 2019, 5, 63. [Google Scholar] [CrossRef]
- Vetrano, F.; Moncada, A.; Miceli, A. Use of Gibberellic Acid to Increase the Salt Tolerance of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy 2020, 10, 505. [Google Scholar] [CrossRef]
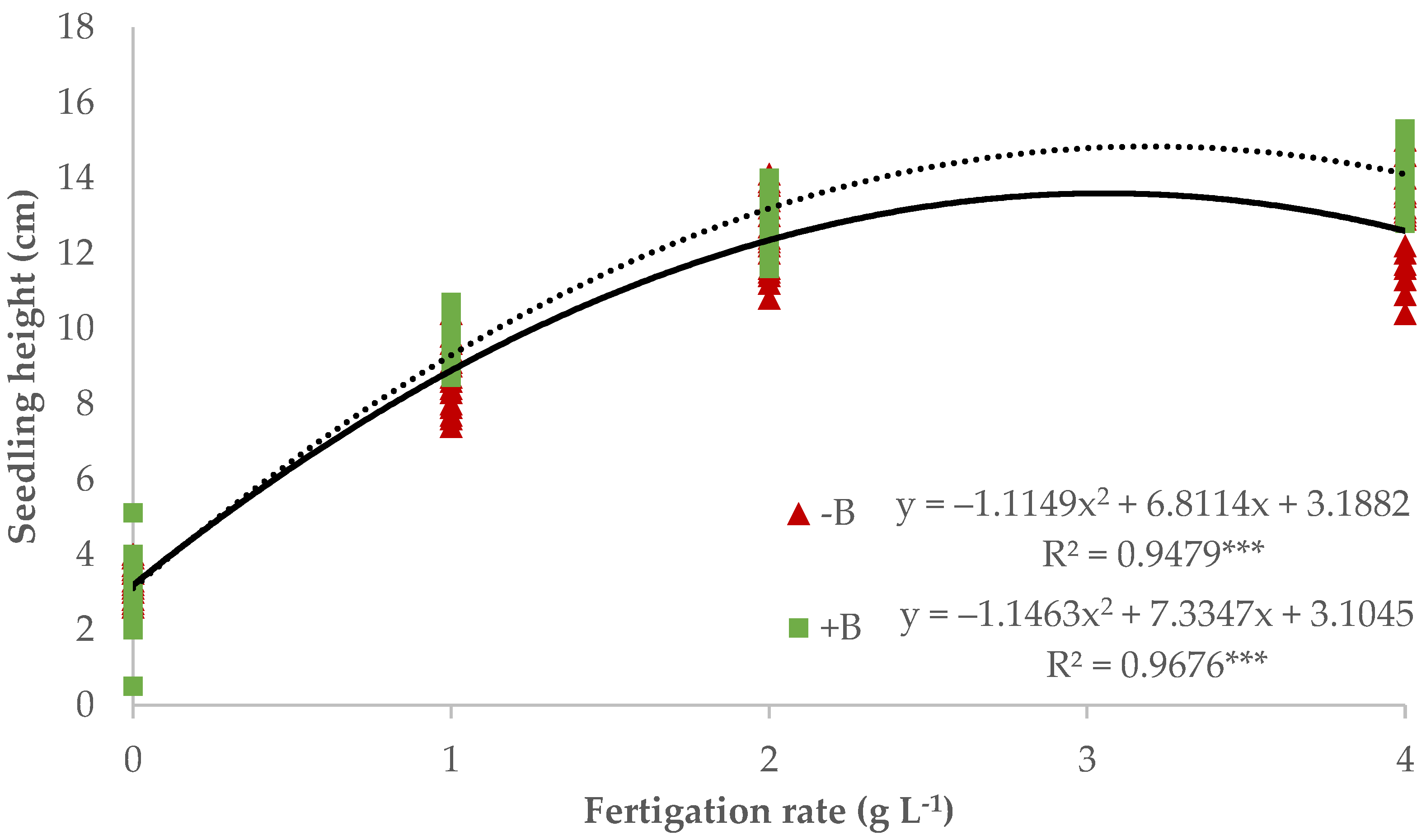

| Source of Variance | Plant Height (cm) | Stem Diameter (mm) | Fresh Weight (g plant−1) | Dry Weight (mg plant−1) | Dry Matter (%) | WUE (g DW L−1 H2O) | NUE (g DW g−1 N) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Shoot (S) | Root (R) | S/R | Total | Shoot (S) | Root (R) | S/R | |||||||
| Bacteria | ||||||||||||||
| +B | z 9.9 | 1.6a | 1.35 | 1.24 | 0.11a | 11.4 | 72.2 | 63.0a | 9.2b | 7.7 | 6.6 | 2.6 | 9.6 | |
| -B | 9.3 | 1.5a | 1.21 | 1.11 | 0.10b | 11.4 | 68.7 | 56.4b | 12.3a | 5.4 | 6.7 | 2.5 | 9.9 | |
| Fertigation (g L−1) | ||||||||||||||
| 0 | 3.1 | 1.1d | 0.25 | 0.19 | 0.06c | 3.2a | 34.9c | 21.9d | 13.0a | 2.0 | 11.8a | 1.6c | 20.2a | |
| 1 | 9.2 | 1.7c | 1.04 | 0.91 | 0.13a | 6.8b | 63.4b | 51.8c | 11.7ab | 4.9 | 5.7b | 2.3b | 8.6b | |
| 2 | 12.7 | 1.7b | 1.81 | 1.68 | 0.13a | 13.5c | 86.4a | 76.8b | 9.7ab | 8.2 | 4.6c | 3.0a | 6.6c | |
| 4 | 13.4 | 1.6a | 2.02 | 1.93 | 0.09b | 22.1d | 97.1a | 88.4a | 8.7b | 11.0 | 4.6c | 3.2a | 3.7d | |
| Bacteria × Fertigation | ||||||||||||||
| +B | 0 | 3.0e | 1.0c | 0.26f | 0.20f | 0.06 | 3.4 | 32.8 | 22.8 | 10.0 | 2.5c | 11.7 | 1.6 | 19.0 |
| 1 | 9.7c | 1.9a | 1.18d | 1.03d | 0.15 | 6.8 | 65.3 | 55.3 | 10.0 | 6.0bc | 5.4 | 2.5 | 9.1 | |
| 2 | 12.9b | 1.6ab | 1.85bc | 1.72bc | 0.13 | 13.0 | 89.5 | 79.5 | 10.0 | 8.1b | 4.6 | 3.2 | 7.0 | |
| 4 | 14.2a | 1.6ab | 2.12a | 2.03a | 0.09 | 22.4 | 101.2 | 94.5 | 6.7 | 14.1a | 4.7 | 3.0 | 3.6 | |
| -B | 0 | 3.3e | 1.1bc | 0.24f | 0.18f | 0.06 | 3.0 | 37.0 | 21.0 | 16.0 | 1.5c | 11.8 | 1.5 | 21.4 |
| 1 | 8.7d | 1.5b | 0.90e | 0.79e | 0.12 | 6.9 | 61.6 | 48.3 | 13.3 | 3.7c | 6.1 | 2.1 | 8.2 | |
| 2 | 12.5b | 1.8ab | 1.77c | 1.65c | 0.12 | 14.1 | 83.3 | 74.0 | 9.3 | 8.3b | 4.5 | 2.9 | 6.3 | |
| 4 | 12.6b | 1.6ab | 1.92b | 1.84b | 0.08 | 21.9 | 92.9 | 82.3 | 10.7 | 8.0b | 4.5 | 3.4 | 3.8 | |
| Significance x | ||||||||||||||
| Bacteria | *** | ns | ** | *** | * | ns | ns | ** | ** | *** | ns | ns | ns | |
| Fertigation | *** | *** | *** | *** | *** | *** | *** | *** | * | *** | *** | *** | *** | |
| Bacteria × Fertigation | *** | * | ** | * | ns | ns | ns | ns | ns | ** | ns | ns | ns | |
| Source of Variance | Number of Leaves | Leaf Area (cm2 Seedling−1) | Leaf Area (cm2 Leaf−1) | SLA (cm2 g DW−1) | Stomatal Conductance (mmol m2 s−1) | L* | a* | b* | Chroma | Hue° | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | |||||||||||
| +B | z 5.1 | 55.2a | 9.7 | 848.7 | 345.3a | 56.5 | −20.9 | 38.1 | 43.5 | 118.8 | |
| -B | 5.0 | 50.1b | 9.1 | 849.2 | 275.5b | 56.2 | −21.1 | 37.5 | 43.0 | 119.3 | |
| Fertigation (g L−1) | |||||||||||
| 0 | 3.1c | 6.6c | 2.2c | 393.2c | 253.0b | 59.2a | −19.3a | 37.3b | 42.0c | 117.3b | |
| 1 | 5.1b | 44.5b | 8.8b | 930.1b | 273.8b | 56.5b | −21.8b | 38.5a | 44.2a | 119.5a | |
| 2 | 5.8a | 77.6a | 13.3a | 1068.6a | 349.7ab | 55.2bc | −21.4b | 38.1ab | 43.7ab | 119.4a | |
| 4 | 6.2a | 81.7a | 13.2a | 1003.8ab | 365.2a | 54.3c | −21.5b | 37.2b | 43.0bc | 120.0a | |
| Bacteria × Fertigation | |||||||||||
| +B | 0 | 3.2 | 6.3 | 2.0 | 370.1 | 317.8 | 59.9 | −18.9 | 37.7 | 42.2 | 116.7 |
| 1 | 5.3 | 49.9 | 9.5 | 980.3 | 285.2 | 56.6 | −21.8 | 39.0 | 44.7 | 119.2 | |
| 2 | 6.0 | 79.4 | 13.3 | 1051.0 | 388.4 | 55.6 | −21.3 | 38.3 | 43.9 | 119.1 | |
| 4 | 6.1 | 85.0 | 13.9 | 993.5 | 389.8 | 53.8 | −21.7 | 37.2 | 43.0 | 120.3 | |
| -B | 0 | 3.0 | 6.9 | 2.3 | 416.4 | 188.2 | 58.6 | −19.6 | 37.0 | 41.9 | 118.0 |
| 1 | 4.9 | 39.0 | 8.0 | 880.0 | 262.3 | 56.4 | −21.8 | 37.9 | 43.8 | 119.8 | |
| 2 | 5.7 | 75.9 | 13.4 | 1086.1 | 310.9 | 54.9 | −21.6 | 37.9 | 43.6 | 119.7 | |
| 4 | 6.3 | 78.4 | 12.6 | 1014.2 | 340.6 | 54.7 | −21.3 | 37.2 | 42.9 | 119.8 | |
| Significance x | |||||||||||
| Bacteria | ns | ** | ns | ns | * | ns | ns | ns | ns | ns | |
| Fertigation | *** | *** | *** | *** | * | *** | *** | * | *** | *** | |
| Bacteria × Fertigation | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| Source of Variance | Yield (kg m−2) | Fresh Weight (g plant−1) | Dry Weight (g plant−1) | Dry Matter (%) | Stem Diameter (mm) | NO3− (mg kg−1 FW) | |
|---|---|---|---|---|---|---|---|
| Bacteria | |||||||
| +B | z 3.6a | 322.9a | 13.0a | 4.1 | 26.5 | 1853.0b | |
| -B | 3.2b | 286.2b | 11.0b | 3.9 | 25.9 | 2571.7a | |
| Fertigation (g L−1) | |||||||
| 0 | 2.7c | 243.2c | 10.5c | 4.3a | 24.2b | 2093.6b | |
| 1 | 3.1bc | 286.9bc | 11.7b | 4.1ab | 25.8ab | 2102.0b | |
| 2 | 3.7ab | 330.7ab | 12.5ab | 3.8b | 27.4a | 2155.9b | |
| 4 | 4.0a | 357.3a | 13.3a | 3.7b | 27.4a | 2497.8a | |
| Bacteria × Fertigation | |||||||
| +B | 0 | 2.9 | 267.8 | 11.2 | 4.1 | 24.1 | 1819.9 |
| 1 | 3.1 | 281.6 | 12.8 | 4.6 | 25.9 | 1787.2 | |
| 2 | 3.9 | 346.6 | 13.2 | 3.8 | 28.6 | 1733.9 | |
| 4 | 4.4 | 395.5 | 14.7 | 3.7 | 27.4 | 2070.8 | |
| -B | 0 | 2.4 | 218.5 | 9.9 | 4.5 | 24.4 | 2367.3 |
| 1 | 3.1 | 292.3 | 10.5 | 3.6 | 25.8 | 2416.8 | |
| 2 | 3.5 | 314.7 | 11.8 | 3.8 | 26.2 | 2577.9 | |
| 4 | 3.5 | 319.2 | 11.9 | 3.7 | 27.4 | 2924.7 | |
| Significance x | |||||||
| Bacteria | * | * | * | ns | ns | ** | |
| Fertigation | *** | *** | *** | *** | ** | *** | |
| Bacteria × Fertigation | ns | ns | ns | ns | ns | ns | |
| Source of Variance | Leaf Number | L* | a* | b* | Chroma | Hue° | |
|---|---|---|---|---|---|---|---|
| Bacteria | |||||||
| +B | z 48.1 | 47.6 | −19.7a | 29.3 | 35.4 | 124.0 | |
| -B | 48.0 | 47.6 | −20.0b | 29.6 | 35.8 | 124.1 | |
| Fertigation (g L−1) | |||||||
| 0 | 43.0b | 47.1 | −19.8a | 28.6b | 34.8b | 124.7a | |
| 1 | 47.8a | 48.5 | −19.5a | 29.0b | 35.0b | 124.1a | |
| 2 | 49.0a | 47.3 | −19.7a | 29.0b | 35.1b | 124.2a | |
| 4 | 52.4a | 47.6 | −20.5b | 31.4a | 37.5a | 123.2b | |
| Bacteria × Fertigation | |||||||
| +B | 0 | 45.0 | 47.6 | −19.8 | 28.7 | 34.9 | 124.7 |
| 1 | 46.2 | 47.9 | −19.4 | 28.8 | 34.7 | 124.0 | |
| 2 | 49.5 | 46.9 | −19.3 | 28.1 | 34.1 | 124.5 | |
| 4 | 51.8 | 48.2 | −20.4 | 31.7 | 37.7 | 122.8 | |
| -B | 0 | 41.0 | 46.6 | −19.7 | 28.6 | 34.7 | 124.6 |
| 1 | 49.5 | 49.1 | −19.7 | 29.1 | 35.2 | 124.2 | |
| 2 | 48.5 | 47.7 | −20.1 | 29.9 | 36.1 | 123.9 | |
| 4 | 53.0 | 47.0 | −20.6 | 31.0 | 37.2 | 123.6 | |
| Significance x | |||||||
| Bacteria | ns | ns | * | ns | ns | ns | |
| Fertigation | *** | ns | *** | *** | *** | *** | |
| Bacteria × Fertigation | ns | ns | ns | ns | ns | ns | |
| Variable | PC1 | PC2 | PC3 | PC4 | PC5 |
|---|---|---|---|---|---|
| Lettuce seedlings | |||||
| Height | 0.990 | 0.078 | 0.055 | 0.079 | 0.004 |
| Stem diameter | 0.771 | 0.532 | 0.198 | 0.012 | 0.249 |
| Total fresh weight | 0.986 | −0.041 | 0.000 | 0.150 | 0.023 |
| Shoot fresh weight | 0.983 | −0.080 | 0.001 | 0.153 | 0.022 |
| Root fresh weight | 0.476 | 0.875 | 0.039 | −0.057 | −0.012 |
| Shoot/Root FW | 0.901 | −0.407 | 0.062 | 0.095 | 0.068 |
| Total dry weight | 0.987 | −0.060 | 0.039 | 0.125 | 0.008 |
| Shoot dry weight | 0.992 | −0.065 | −0.017 | 0.093 | 0.019 |
| Root dry weight | -0.724 | 0.083 | 0.538 | 0.236 | −0.114 |
| Shoot/Root DW | 0.923 | −0.191 | −0.218 | −0.070 | 0.205 |
| Dry matter % | −0.942 | −0.280 | −0.175 | −0.017 | 0.038 |
| WUE | 0.943 | 0.003 | 0.034 | 0.298 | −0.053 |
| NUE | −0.971 | −0.092 | −0.162 | 0.086 | 0.071 |
| Leaf number | 0.978 | 0.119 | 0.095 | 0.095 | −0.040 |
| Leaf area | 0.984 | 0.023 | 0.026 | 0.158 | −0.003 |
| SLA | 0.919 | 0.332 | 0.189 | 0.066 | −0.011 |
| Stomatal conductance | 0.779 | −0.157 | −0.533 | 0.026 | −0.248 |
| L* | −0.960 | 0.033 | −0.219 | −0.020 | −0.095 |
| Chroma | 0.517 | 0.840 | 0.040 | −0.114 | −0.007 |
| Hue° | 0.852 | 0.111 | 0.421 | −0.131 | 0.109 |
| Lettuce plants | |||||
| Yield | 0.902 | −0.163 | −0.358 | −0.095 | −0.092 |
| Fresh weight | 0.916 | −0.155 | −0.299 | −0.146 | −0.114 |
| Dry weight | 0.791 | 0.018 | −0.553 | −0.053 | 0.233 |
| Dry matter % | −0.623 | 0.343 | −0.178 | 0.227 | 0.628 |
| Stem diameter | 0.888 | 0.081 | −0.091 | 0.320 | −0.212 |
| Leaf number | 0.897 | −0.172 | 0.112 | −0.134 | −0.281 |
| L* | 0.205 | 0.253 | 0.149 | −0.922 | −0.141 |
| Chroma | 0.614 | −0.643 | 0.222 | −0.264 | 0.275 |
| Hue° | −0.783 | 0.318 | −0.079 | 0.311 | −0.413 |
| Nitrate content | 0.163 | −0.437 | 0.862 | 0.101 | −0.007 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vetrano, F.; Miceli, C.; Angileri, V.; Frangipane, B.; Moncada, A.; Miceli, A. Effect of Bacterial Inoculum and Fertigation Management on Nursery and Field Production of Lettuce Plants. Agronomy 2020, 10, 1477. https://doi.org/10.3390/agronomy10101477
Vetrano F, Miceli C, Angileri V, Frangipane B, Moncada A, Miceli A. Effect of Bacterial Inoculum and Fertigation Management on Nursery and Field Production of Lettuce Plants. Agronomy. 2020; 10(10):1477. https://doi.org/10.3390/agronomy10101477
Chicago/Turabian StyleVetrano, Filippo, Claudia Miceli, Vincenzo Angileri, Benedetto Frangipane, Alessandra Moncada, and Alessandro Miceli. 2020. "Effect of Bacterial Inoculum and Fertigation Management on Nursery and Field Production of Lettuce Plants" Agronomy 10, no. 10: 1477. https://doi.org/10.3390/agronomy10101477
APA StyleVetrano, F., Miceli, C., Angileri, V., Frangipane, B., Moncada, A., & Miceli, A. (2020). Effect of Bacterial Inoculum and Fertigation Management on Nursery and Field Production of Lettuce Plants. Agronomy, 10(10), 1477. https://doi.org/10.3390/agronomy10101477





