Transcriptomic Analysis Reveals Salt Tolerance Mechanisms Present in Date-Plum Persimmon Rootstock (Diospyros lotus L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and RNA Extraction
2.2. Plant Phenotyping
2.3. RNA Extraction
2.4. RNA Sequencing
2.5. Pre-Processing of RNA-Seq Data
2.6. Transcriptome de Novo Assembly and Annotation
2.7. Differential Expression Analyses
3. Results
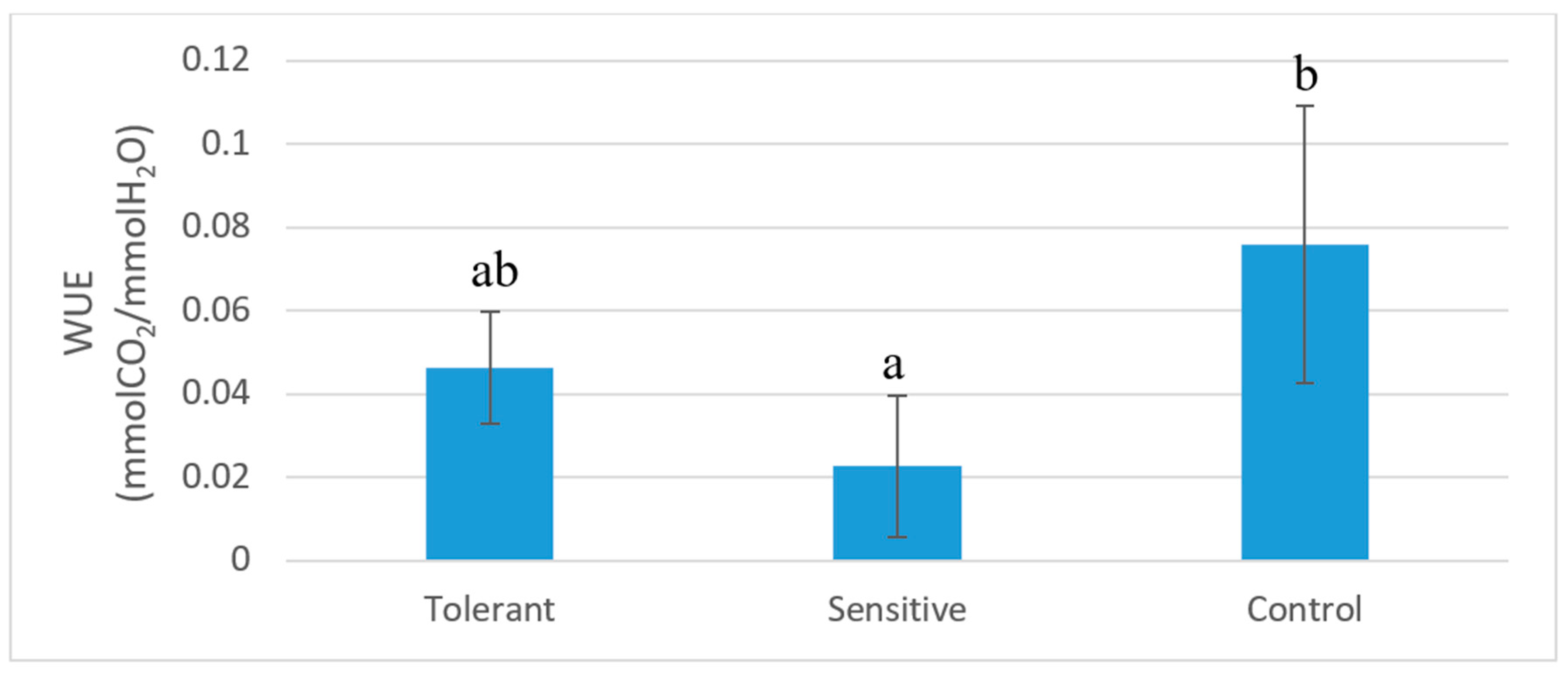
3.1. Plant Physiological Responses Associated to Salt Stress Tolerance
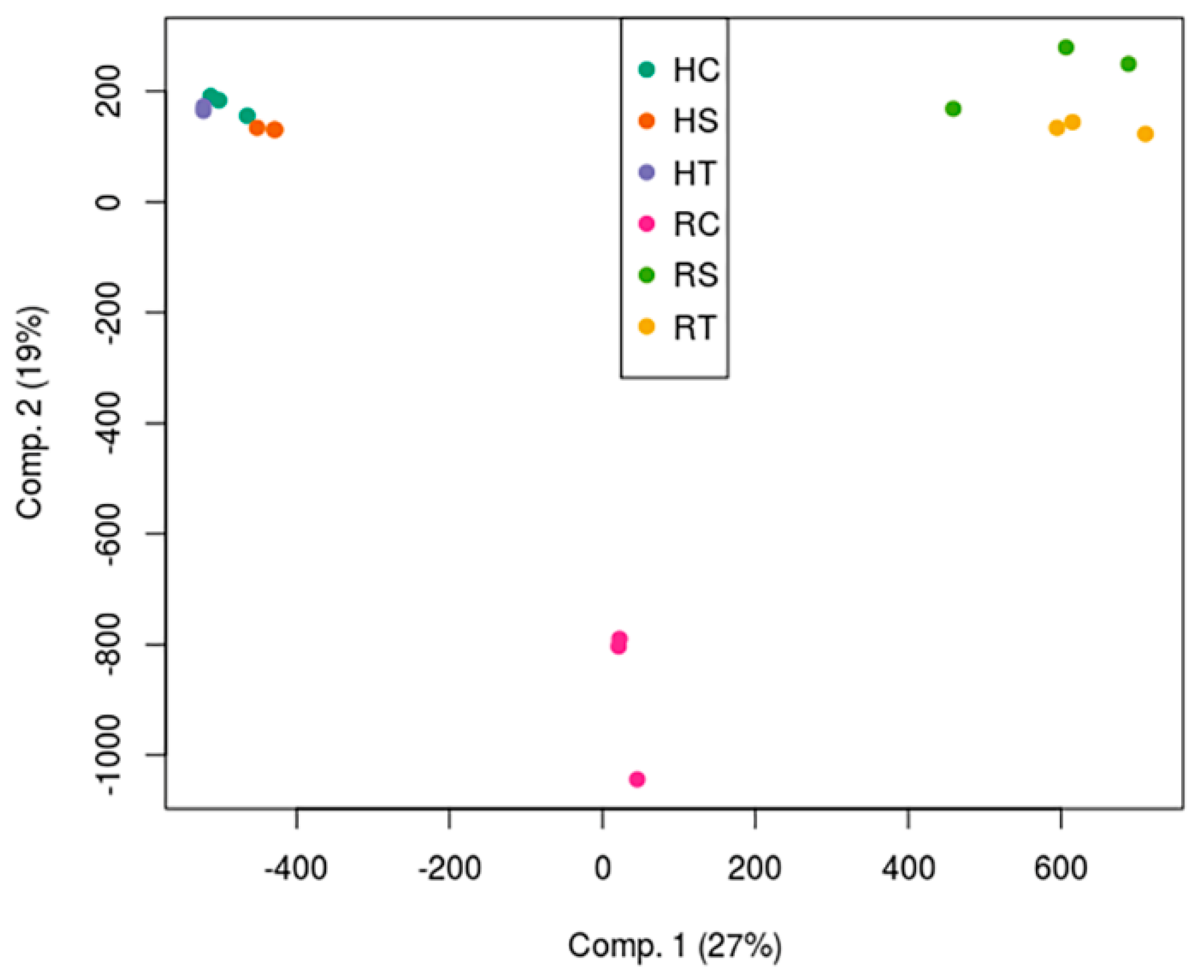
3.2. Gene Expression Changes in Response to Salinity
4. Discussion
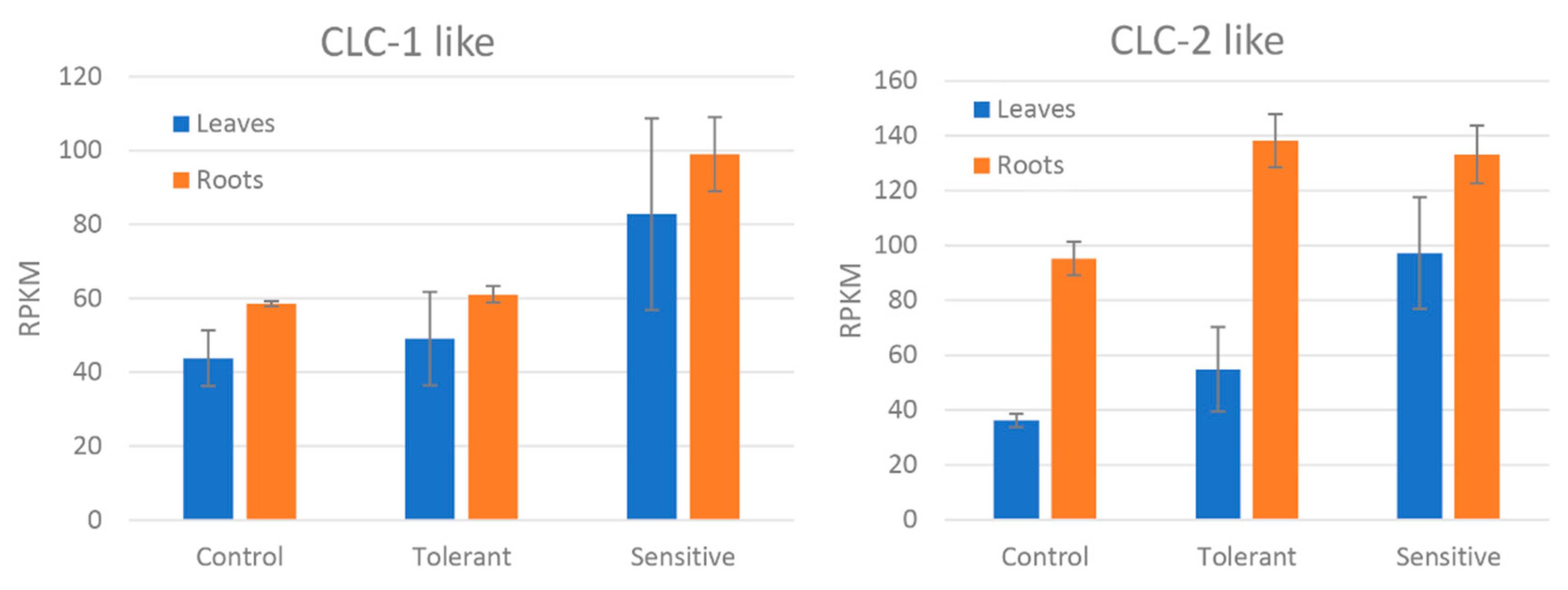
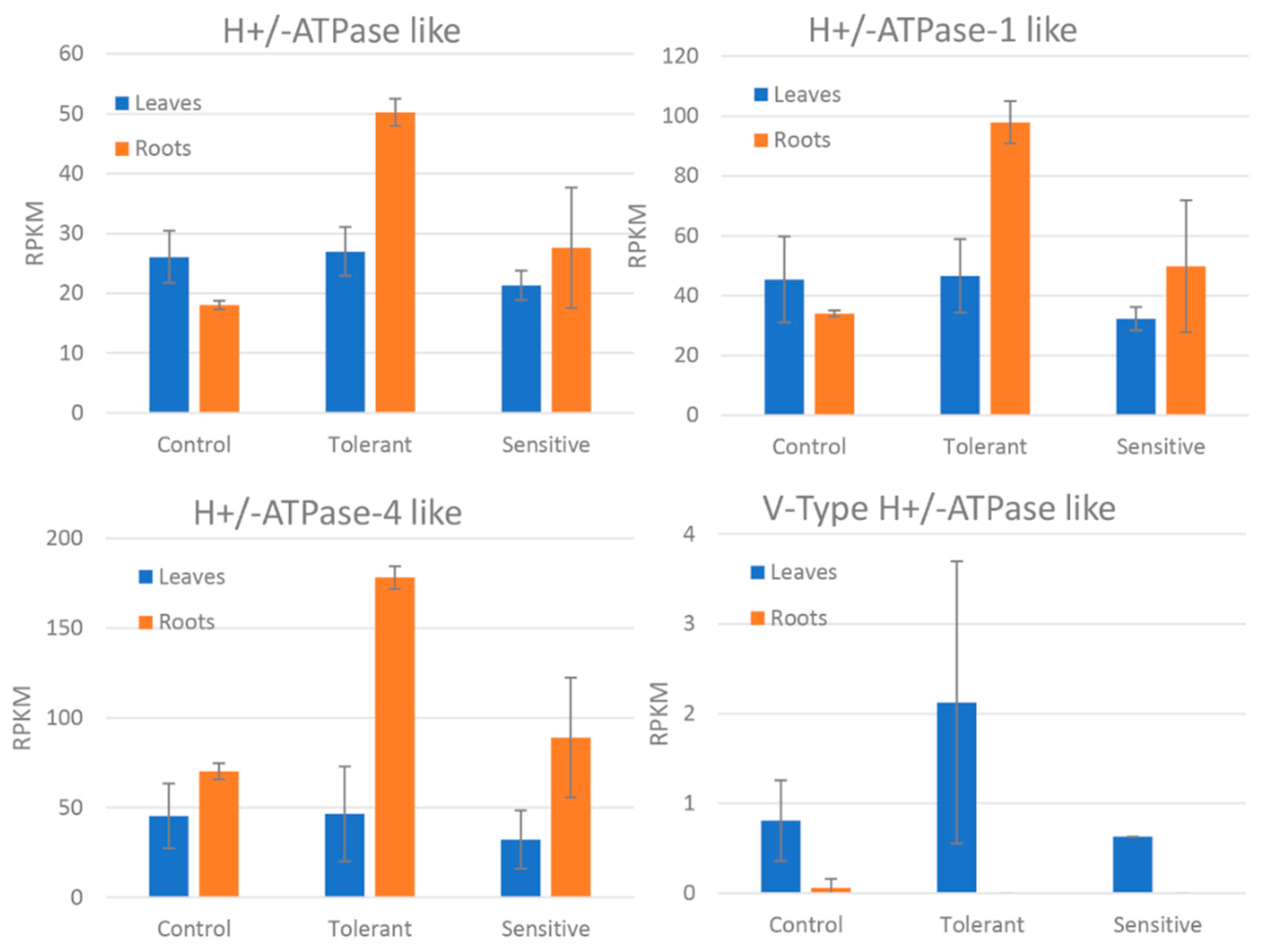
4.1. Ion Transport
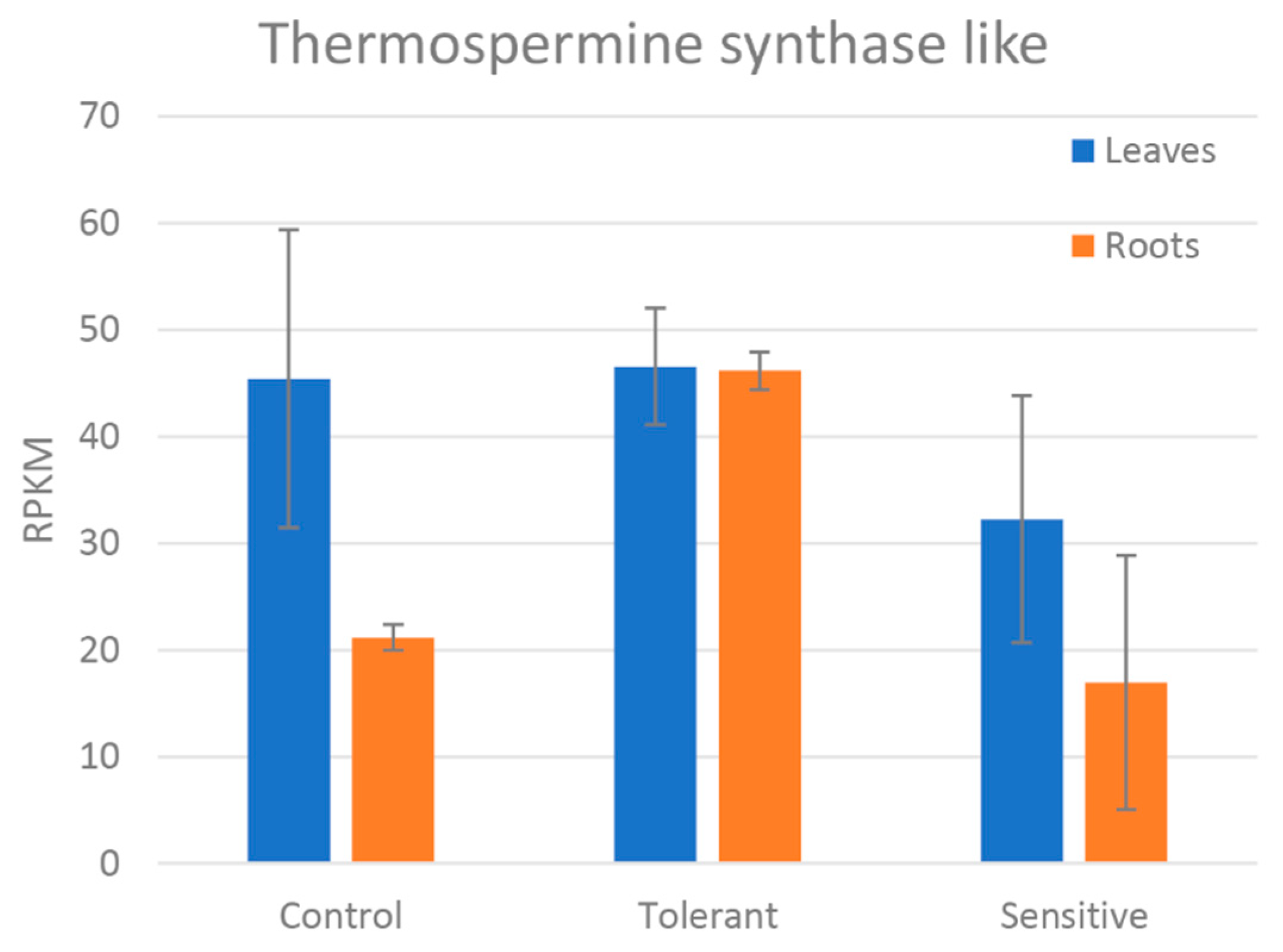
4.2. Photosynthesis, Respiration Systems, and ROS Signaling
4.3. Root Architecture Involvement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef]
- Libutti, A.; Cammerino, A.R.B.; Monteleone, M. Risk assessment of soil salinization due to tomato cultivation in mediterranean climate conditions. Water 2018, 10, 1503. [Google Scholar] [CrossRef]
- Ashrai, M.; McNelly, T. Improvement of Salt Tolerance in Maize by Selection and Breeding. Plant Breed. 1990, 104, 101–107. [Google Scholar] [CrossRef]
- Saranga, Y.; Cahaner, A.; Zamir, D.; Marani, A.; Rudich, J. Breeding tomatoes for salt tolerance: Inheritance of salt tolerance and related traits in interspecific populations. Appl. Genet. 1992, 84, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, J.; Bolarín, M.C.; Asíns, M.J.; Moreno, V. Increasing salt tolerance in the tomato. J. Exp. Bot. 2006, 57, 1045–1058. [Google Scholar] [CrossRef]
- Forner-Giner, M.A.; Ancillo, G. Breeding salinity tolerance in citrus using rootstocks. In Salt Stress in Plants: Signalling, Omics and Adaptations; Springer: New York, NY, USA, 2013; Volume 9781461461, pp. 355–376. ISBN 9781461461081. [Google Scholar]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Simón-Grao, S.; Alfosea-Simón, M.; Cámara-Zapata, J.M.; Mattson, N.S.; Garcia-Sanchez, F. Rootstocks influence the salt tolerance of Kinnow mandarin trees by altering the antioxidant defense system, osmolyte concentration, and toxic ion accumulation. Sci. Hortic. 2019, 250, 1–11. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate–Stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef]
- Tester, M.; Davenport, R. Na+tolerance and Na+transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Guo, Y.; Jagendorf, A.T.; Zhu, J.K. Biochemical characterization of the Arabidopsis protein kinase SOS2 that functions in salt tolerance. Plant Physiol. 2002, 130, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.L.; Wang, L.Y.; Wang, S.Y.; Lin, C.H.; Ho, K.C.; Shi, F.K.; Chang, I.F. Functional phosphoproteomic profiling of phosphorylation sites in membrane fractions of salt-stressed Arabidopsis thaliana. Proteome Sci. 2009, 7, 42. [Google Scholar] [CrossRef]
- Martínez-Atienza, J.; Jiang, X.; Garciadeblas, B.; Mendoza, I.; Zhu, J.K.; Pardo, J.M.; Quintero, F.J. Conservation of the salt overly sensitive pathway in rice. Plant Physiol. 2007, 143, 1001–1012. [Google Scholar] [CrossRef]
- Volkov, V. Salinity tolerance in plants. Quantitative approach to ion transport starting from halophytes and stepping to genetic and protein engineering for manipulating ion fluxes. Front. Plant Sci. 2015, 6, 873. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.L.; Liu, L.N.; Xie, Q.; Sui, N. Photosynthetic Regulation Under Salt Stress and Salt-Tolerance Mechanism of Sweet Sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar] [CrossRef]
- Chen, S.; Wu, F.; Li, Y.; Qian, Y.; Pan, X.; Li, F.; Wang, Y.; Wu, Z.; Fu, C.; Lin, H.; et al. NTMYB4 and NTCHS1 are critical factors in the regulation of flavonoid biosynthesis and are involved in salinity responsiveness. Front. Plant Sci. 2019, 10, 178. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Sako, K.; Matsui, A.; Suzuki, Y.; Mostofa, M.G.; Van Ha, C.; Tanaka, M.; Tran, L.S.P.; Habu, Y.; Seki, M. Ethanol enhances high-salinity stress tolerance by detoxifying reactive oxygen species in arabidopsis thaliana and rice. Front. Plant Sci. 2017, 8, 1001. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.J.; Chen, H.W.; Ma, B.; Zhang, W.K.; Chen, S.Y.; Zhang, J.S. The role of ethylene in plants under salinity stress. Front. Plant Sci. 2015, 6, 1059. [Google Scholar] [CrossRef]
- Suzuki, N.; Bassil, E.; Hamilton, J.S.; Inupakutika, M.A.; Zandalinas, S.I.; Tripathy, D.; Luo, Y.; Dion, E.; Fukui, G.; Kumazaki, A.; et al. ABA is required for plant acclimation to a combination of salt and heat stress. PLoS ONE 2016, 11, e0147625. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, X.; Zhang, L. Structural and functional dynamics of dehydrins: A plant protector protein under abiotic stress. Int. J. Mol. Sci. 2018, 19, 3420. [Google Scholar] [CrossRef]
- Hanin, M.; Brini, F.; Ebel, C.; Toda, Y.; Takeda, S.; Masmoudi, K. Plant dehydrins and stress tolerance: Versatile proteins for complex mechanisms. Plant Signal. Behav. 2011, 6, 1503–1509. [Google Scholar] [CrossRef]
- Kapilan, R.; Vaziri, M.; Zwiazek, J.J. Regulation of aquaporins in plants under stress. Biol. Res. 2018, 51. [Google Scholar] [CrossRef]
- Boudsocq, M.; Laurière, C. Osmotic signaling in plants. Multiple pathways mediated by emerging kinase families. Plant Physiol. 2005, 138, 1185–1194. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 2007, 19, 1415–1431. [Google Scholar] [CrossRef]
- Luo, Y.; Reid, R.; Freese, D.; Li, C.; Watkins, J.; Shi, H.; Zhang, H.; Loraine, A.; Song, B.H. Salt tolerance response revealed by RNA-Seq in a diploid halophytic wild relative of sweet potato. Sci. Rep. 2017, 7, 9624. [Google Scholar] [CrossRef]
- Shafi, A.; Zahoor, I. Plant Survival and Tolerance Under High Salinity: Primary and Secondary Cell Wall-Sensing Mechanism. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution; Springer: Singapore, 2019; pp. 129–146. [Google Scholar]
- Moustafa, K.; AbuQamar, S.; Jarrar, M.; Al-Rajab, A.J.; Trémouillaux-Guiller, J. MAPK cascades and major abiotic stresses. Plant Cell Rep. 2014, 33, 1217–1225. [Google Scholar] [CrossRef]
- Sinha, A.K.; Jaggi, M.; Raghuram, B.; Tuteja, N. Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal. Behav. 2011, 6, 196–203. [Google Scholar] [CrossRef]
- Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA 2000, 97, 3730–3734. [Google Scholar] [CrossRef]
- Mahajan, S.; Pandey, G.K.; Tuteja, N. Calcium- and salt-stress signaling in plants: Shedding light on SOS pathway. Arch. Biochem. Biophys. 2008, 471, 146–158. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, F.; Han, X.; Xia, X.; Yin, W. The calcium sensor PeCBL1, interacting with PeCIPK24/25 and PeCIPK26, regulates Na+/K+ homeostasis in Populus euphratica. Plant Cell Rep. 2013, 32, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Q.; Zhang, F.; Wang, B.; Wang, J.; Zheng, J. ZmCIPK21, a Maize CBL-Interacting Kinase, Enhances Salt Stress Tolerance in Arabidopsis Thaliana. Int. J. Mol. Sci. 2014, 15, 14819–14834. [Google Scholar] [CrossRef]
- Li, R.; Zhang, J.; Wu, G.; Wang, H.; Chen, Y.; Wei, J. HbCIPK2, a novel CBL-interacting protein kinase from halophyte Hordeum brevisubulatum, confers salt and osmotic stress tolerance. Plant Cell Environ. 2012. [Google Scholar] [CrossRef]
- Gil-Muñoz, F.; Pérez-Pérez, J.G.; Quiñones, A.; Primo-Capella, A.; Cebolla, J.; Ángeles Forner-Giner, M.; Badenes, M.L.; del Mar Naval, M. A cross population between D. Kaki and D. Virginiana shows high variability for saline tolerance and improved salt stress tolerance. PLoS ONE 2020, 15, e0229023. [Google Scholar] [CrossRef]
- Garciadeblás, B.; Senn, M.E.; Bañuelos, M.A.; Rodríguez-Navarro, A. Sodium transport and HKT transporters: The rice model. Plant J. 2003, 34, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Spielmeyer, W.; Lagudah, E.S.; James, R.A.; Platten, J.D.; Dennis, E.S.; Munns, R. A sodium transporter (HKT7) is a candidate for Nax1, a gene for salt tolerance in durum wheat. Plant Physiol. 2006, 142, 1718–1727. [Google Scholar] [CrossRef]
- Horie, T.; Costa, A.; Kim, T.H.; Han, M.J.; Horie, R.; Leung, H.Y.; Miyao, A.; Hirochika, H.; An, G.; Schroeder, J.I. Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J. 2007, 26, 3003–3014. [Google Scholar] [CrossRef]
- Almeida, P.; Katschnig, D.; de Boer, A.H. HKT transporters-state of the art. Int. J. Mol. Sci. 2013, 14, 20359–20385. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Besada, C.; Gil, R.; Bonet, L.; Quiñones, A.; Intrigliolo, D.; Salvador, A. Chloride stress triggers maturation and negatively affects the postharvest quality of persimmon fruit. Involvement of calyx ethylene production. Plant Physiol. Biochem. 2016, 100, 105–112. [Google Scholar] [CrossRef]
- Formentin, E.; Sudiro, C.; Perin, G.; Riccadonna, S.; Barizza, E.; Baldoni, E.; Lavezzo, E.; Stevanato, P.; Sacchi, G.A.; Fontana, P.; et al. Transcriptome and cell physiological analyses in different rice cultivars provide new insights into adaptive and salinity stress responses. Front. Plant Sci. 2018, 9, 204. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Z.; Zhang, Q.; Ci, H.; Wang, P.; Yu, L.; Jia, G. Genome-wide transcriptome analysis of the salt stress tolerance mechanism in Rosa chinensis. PLoS ONE 2018, 13, e0200938. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, J.; Zhang, Y.; Fan, F.; Li, W.; Wang, F.; Zhong, W.; Wang, C.; Yang, J. Comparative transcriptome analysis reveals molecular response to salinity stress of salt-tolerant and sensitive genotypes of indica rice at seedling stage. Sci. Rep. 2018, 8, 2085. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Chen, P.; Korth, K.L.; Ping, J.; Thomas, J.; Wu, C.; Srivastava, S.; Pereira, A.; Hancock, F.; Brye, K.; et al. RNA sequencing analysis of salt tolerance in soybean (Glycine max). Genomics 2019, 111, 629–635. [Google Scholar] [CrossRef]
- Amirbakhtiar, N.; Ismaili, A.; Ghaffari, M.R.; Firouzabadi, F.N.; Shobbar, Z.S. Transcriptome response of roots to salt stress in a salinity-tolerant bread wheat cultivar. PLoS ONE 2019, 14, e0213305. [Google Scholar] [CrossRef]
- Gilliam, J.W. Rapid Measurement of Chlorine in Plant Materials1. Soil Sci. Soc. Am. J. 1971, 35, 512. [Google Scholar] [CrossRef]
- Gambino, G.; Perrone, I.; Gribaudo, I. A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem. Anal. 2008, 19, 520–525. [Google Scholar] [CrossRef]
- Kopylova, E.; Noé, L.; Touzet, H. SortMeRNA: Fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 2012. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013. [Google Scholar] [CrossRef]
- Kurtzer, G.M.; Sochat, V.; Bauer, M.W. Singularity: Scientific containers for mobility of compute. PLoS ONE 2017. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009. [Google Scholar] [CrossRef]
- Suzek, B.E.; Wang, Y.; Huang, H.; McGarvey, P.B.; Wu, C.H. UniRef clusters: A comprehensive and scalable alternative for improving sequence similarity searches. Bioinformatics 2015. [Google Scholar] [CrossRef]
- Bryant, D.M.; Johnson, K.; DiTommaso, T.; Tickle, T.; Couger, M.B.; Payzin-Dogru, D.; Lee, T.J.; Leigh, N.D.; Kuo, T.H.; Davis, F.G.; et al. A Tissue-Mapped Axolotl De Novo Transcriptome Enables Identification of Limb Regeneration Factors. Cell Rep. 2017. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019. [Google Scholar] [CrossRef]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016. [Google Scholar] [CrossRef]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Anders, S.; Huber, W. Moderated estimation offold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Palmgren, M.G. Plant Plasma Membrane H+/-ATPases: Powerhouses for Nutrient Uptake. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 817–845. [Google Scholar] [CrossRef]
- Niu, X.; Narasimhan, M.L.; Salzman, R.A.; Bressan, R.A.; Hasegawa, P.M. NaCl regulation of plasma membrane H+-ATPase gene expression in a glycophyte and a halophyte. Plant Physiol. 1993. [Google Scholar] [CrossRef] [PubMed]
- Vera-Estrella, R.; Barkla, B.J.; Higgins, V.J.; Blumwald, E. Plant defense response to fungal pathogens. Activation of host-plasma membrane H+-ATPase by elicitor-induced enzyme dephosphorylation. Plant Physiol. 1994. [Google Scholar] [CrossRef]
- Chen, Z.; Pottosin, I.I.; Cuin, T.A.; Fuglsang, A.T.; Tester, M.; Jha, D.; Zepeda-Jazo, I.; Zhou, M.; Palmgren, M.G.; Newman, I.A.; et al. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol. 2007, 145, 1714–1725. [Google Scholar] [CrossRef]
- Sahu, B.B.; Shaw, B.P. Salt-inducible isoform of plasma membrane H+ATPase gene in rice remains constitutively expressed in natural halophyte, Suaeda maritima. J. Plant Physiol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Gévaudant, F.; Duby, G.; Von Stedingk, E.; Zhao, R.; Morsomme, P.; Boutry, M. Expression of a constitutively activated plasma membrane H +-ATPase alters plant development and increases salt tolerance. Plant Physiol. 2007. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Wang, R.; Jing, W.; Zhang, W. Rice Phospholipase Dα is Involved in Salt Tolerance by the Mediation of H+-ATPase Activity and Transcription. J. Integr. Plant Biol. 2011. [Google Scholar] [CrossRef]
- de Paz, J.M.; Visconti, F.; Chiaravalle, M.; Quiñones, A. Determination of persimmon leaf chloride contents using near-infrared spectroscopy (NIRS). Anal. Bioanal. Chem. 2016, 408, 3537–3545. [Google Scholar] [CrossRef]
- Gil-Muñoz, F.; Pérez-Pérez, J.G.; Quiñones, A.; Naval, M.d.M.; Badenes, M.L. Intra and Inter-specific Variability of Salt Tolerance Mechanisms in Diospyros Genus. Front. Plant Sci. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef]
- Baetz, U.; Eisenach, C.; Tohge, T.; Martinoia, E.; De Angeli, A. Vacuolar chloride fluxes impact ion content and distribution during early salinity stress. Plant Physiol. 2016, 172, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Yoshimoto, K.; Kakehi, J.-I.; Motose, H.; Niitsu, M.; Takahashi, T. Thermospermine modulates expression of auxin-related genes in Arabidopsis. Front. Plant Sci. 2014, 5, 94. [Google Scholar] [CrossRef]
- Osakabe, Y.; Arinaga, N.; Umezawa, T.; Katsura, S.; Nagamachi, K.; Tanaka, H.; Ohiraki, H.; Yamada, K.; Seo, S.U.; Abo, M.; et al. Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 2013, 25, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Julkowska, M.M.; Hoefsloot, H.C.J.; Mol, S.; Feron, R.; De Boer, G.J.; Haring, M.A.; Testerink, C. Capturing arabidopsis root architecture dynamics with root-fit reveals diversity in responses to salinity. Plant Physiol. 2014, 166, 1387–1402. [Google Scholar] [CrossRef]
- Julkowska, M.M.; Koevoets, I.T.; Mol, S.; Hoefsloot, H.; Feron, R.; Tester, M.A.; Keurentjes, J.J.B.; Korte, A.; Haring, M.A.; De Boer, G.J.; et al. Genetic components of root architecture remodeling in response to salt stress. Plant Cell 2017, 29, 3198–3213. [Google Scholar] [CrossRef]
- Incesu, M.; Cimen, B.; Yesiloglu, T.; Yilmaz, B. Growth and photosynthetic response of two persimmon rootstocks (Diospyros kaki and D. virginiana) under different salinity levels. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 386–391. [Google Scholar] [CrossRef][Green Version]
- Intrigliolo, D.S.; Visconti, F.; Bonet, L.; Parra, M.; Besada, C.; Abrisqueta, I.; Rubio, J.S.; De Paz, J.M. Persimmon (Diospyros kaki) trees responses to restrictions in water amount and quality. In Water Scarcity and Sustainable Agriculture in Semiarid Environment: Tools, Strategies, and Challenges for Woody Crops; Elsevier: Amsterdam, The Netherlands, 2018; pp. 149–177. ISBN 9780128131640. [Google Scholar]
- Visconti, F.; Intrigliolo, D.S.; Quiñones, A.; Tudela, L.; Bonet, L.; de Paz, J.M. Differences in specific chloride toxicity to Diospyros kaki cv. “Rojo Brillante” grafted on D. lotus and D. virginiana. Sci. Hortic. 2017, 214, 83–90. [Google Scholar] [CrossRef]
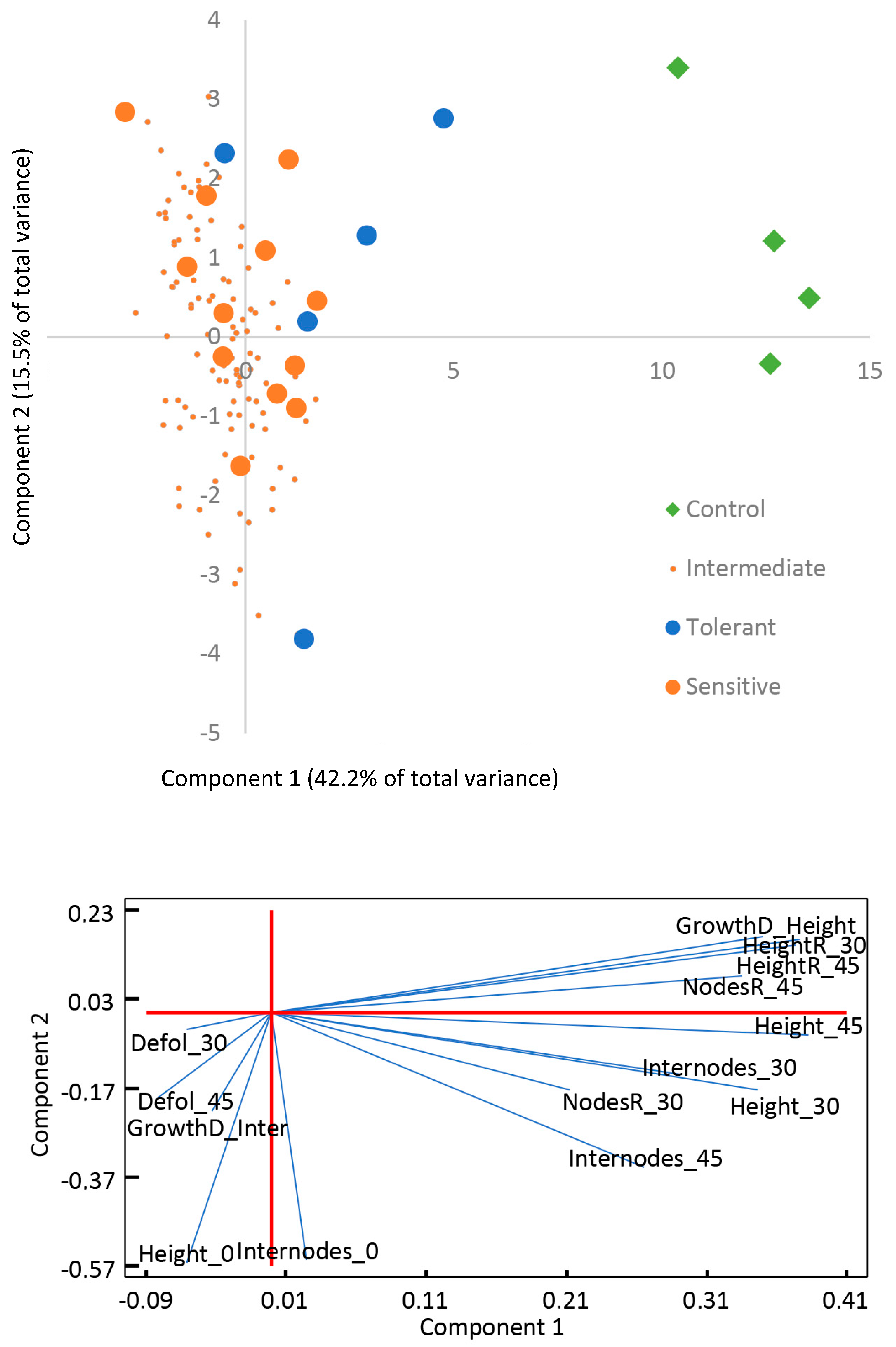

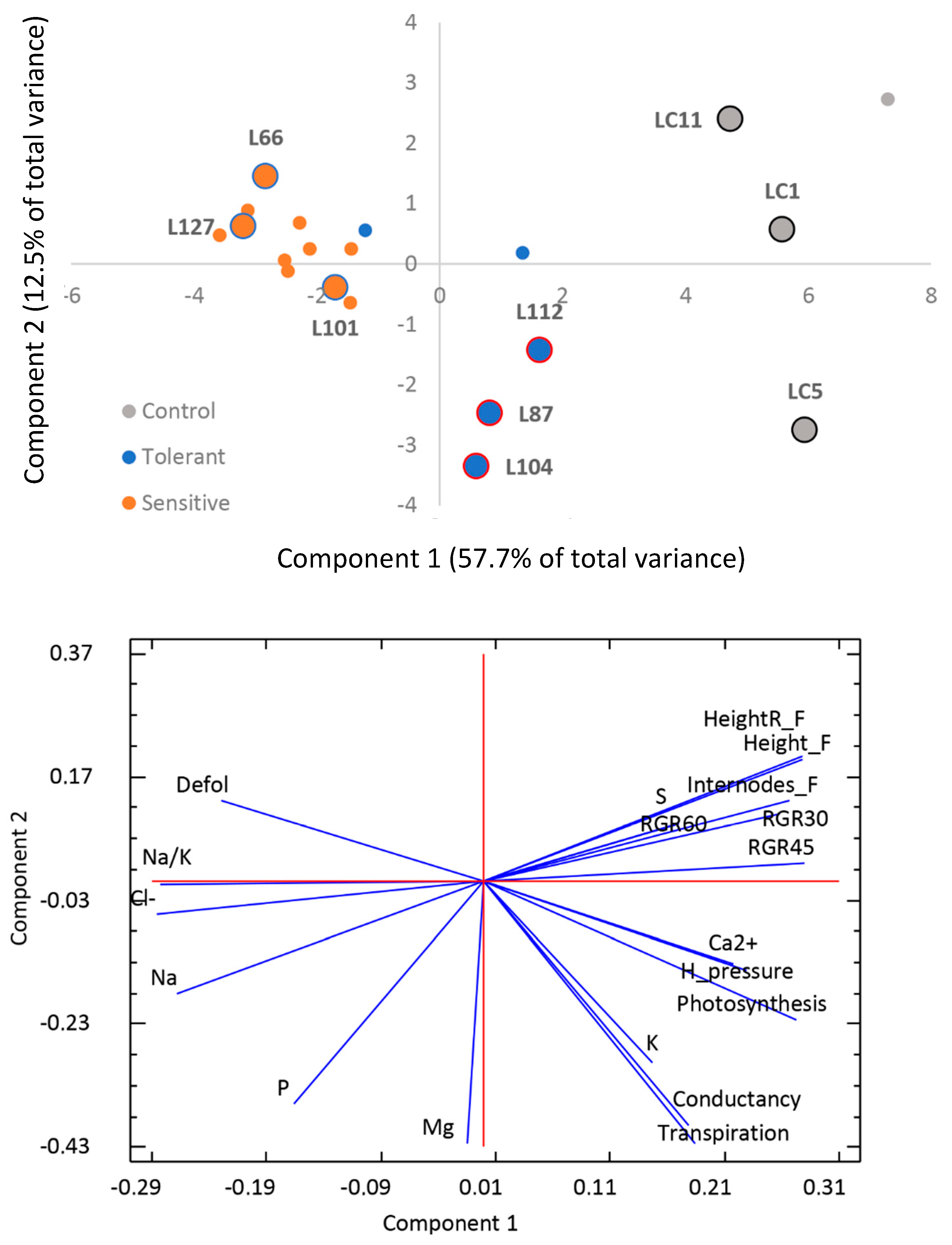
| Days | Variable | Tolerant | Sensitive | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | Height (cm) | 14 | ± | 3 | ns | 14.3 | ± | 1.3 | ns | 11.8 | ± | 1.3 | ns |
| 0 | Nodes | 5.4 | ± | 0.9 | ns | 5.5 | ± | 0.5 | ns | 4.5 | ± | 0.6 | ns |
| 0 | Internode length (cm) | 2.6 | ± | 0.7 | ns | 2.6 | ± | 0.4 | ns | 2.6 | ± | 0.5 | ns |
| 30 | Height (cm) | 27 | ± | 6 | a | 25 | ± | 4 | a | 42 | ± | 6 | b |
| 30 | Nodes | 13 | ± | 2 | ns | 13.6 | ± | 1.0 | ns | 15.5 | ± | 0.6 | ns |
| 30 | Internode length (cm) | 2.0 | ± | 0.3 | ab | 1.8 | ± | 0.2 | a | 2.7 | ± | 0.3 | b |
| 30 | Symptoms | 1.4 | ± | 0.9 | ab | 2.4 | ± | 0.5 | b | 0.0 | ± | 0.0 | a |
| 30 | Defoliation (%) | 0.0 | ± | 0.0 | ns | 0.0 | ± | 0.0 | ns | 0.0 | ± | 0.0 | ns |
| 45 | Height (cm) | 33 | ± | 7 | a | 26 | ± | 4 | a | 60 | ± | 6 | b |
| 45 | Nodes | 19 | ± | 5 | ab | 17 | ± | 2 | a | 25.3 | ± | 1.9 | b |
| 45 | Internode length (cm) | 1.8 | ± | 0.1 | b | 1.6 | ± | 0.2 | b | 2.4 | ± | 0.1 | a |
| 45 | Symptoms | 2.0 | ± | 0.7 | b | 3.0 | ± | 0.0 | c | 0.0 | ± | 0.0 | a |
| 45 | Defoliation (%) | 0.1 | ± | 0.1 | ns | 0.1 | ± | 0.1 | ns | 0.0 | ± | 0.0 | ns |
| 60 | Height (cm) | 41 | ± | 7 | b | 28 | ± | 4 | a | 80 | ± | 20 | c |
| 60 | Nodes | 26 | ± | 4 | b | 19.8 | ± | 1.6 | a | 37 | ± | 6 | b |
| 60 | Internode length (cm) | 1.59 | ± | 0.12 | a | 1.40 | ± | 0.14 | a | 2.1 | ± | 0.4 | b |
| 60 | Symptoms | 1.8 | ± | 1.1 | a | 3.3 | ± | 0.5 | b | 0.0 | ± | 0.0 | a |
| 60 | Defoliation (%) | 0.19 | ± | 0.09 | ab | 0.4 | ± | 0.2 | b | 0.0 | ± | 0.0 | a |
| 30 | Relative height | 1.9 | ± | 0.4 | a | 1.7 | ± | 0.2 | a | 3.6 | ± | 0.2 | b |
| 45 | Relative height | 2.4 | ± | 0.6 | ab | 1.8 | ± | 0.2 | a | 5.1 | ± | 0.2 | b |
| 60 | Relative height | 3.1 | ± | 0.8 | b | 1.9 | ± | 0.2 | a | 6.7 | ± | 1.7 | c |
| Variable | Tolerant | Sensitive | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
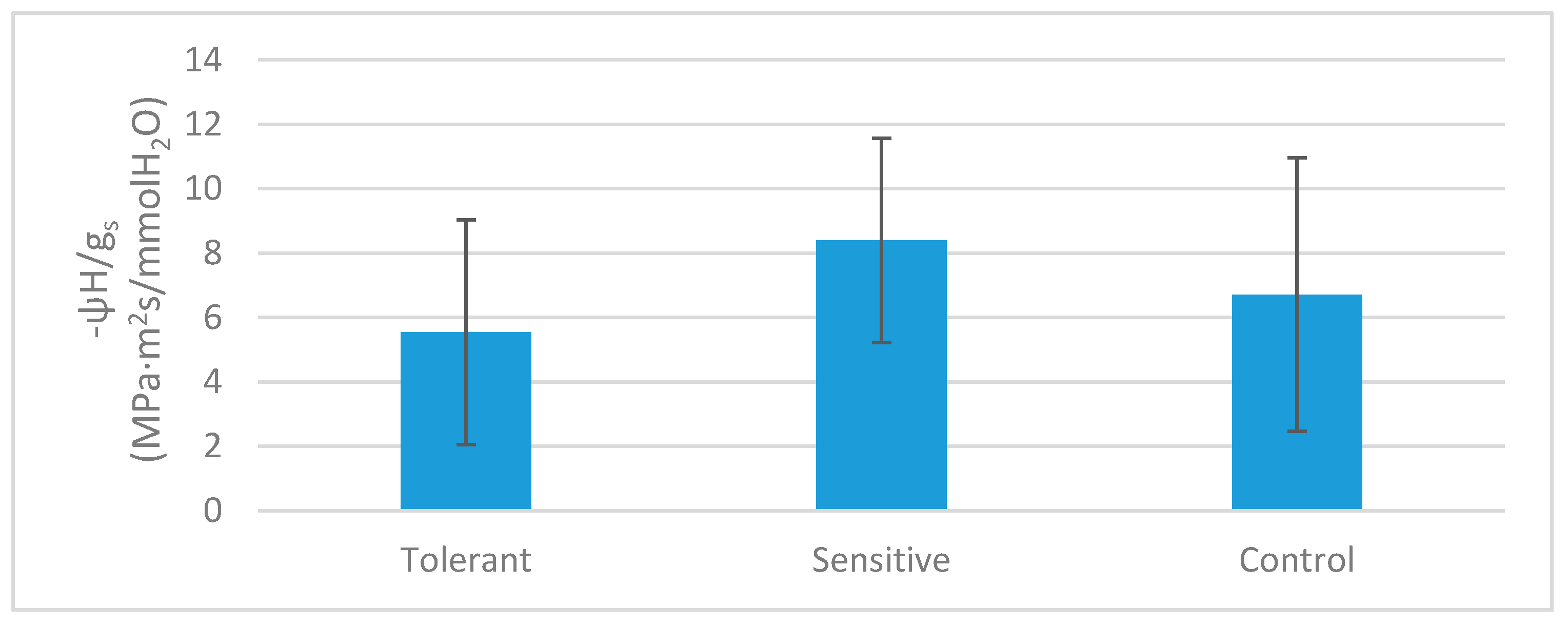
| ψH (MPa) | −0.77 | ± | 0.17 | b | −0.35 | ± | 0.11 | a | −0.81 | ± | 0.13 | b |
| ACO2 (µmol CO2/m2s) | 8 | ± | 2 | b | 1.2 | ± | 0.9 | a | 11 | ± | 3 | b |
| gs (mmol H2O/m2s) | 180 | ± | 100 | b | 40 | ± | 30 | a | 200 | ± | 200 | b |
| Ci (µmol CO2/mol) | 300 | ± | 20 | a | 350 | ± | 30 | b | 250 | ± | 50 | ab |
| Cl− (mg/100 mg d.w.) | 4.3 | ± | 0.6 | b | 5.8 | ± | 0.4 | c | 0.50 | ± | 0.08 | a |
| Ca2+ (mg/100 mg d.w.) | 0.40 | ± | 0.07 | ab | 0.34 | ± | 0.09 | a | 0.56 | ± | 0.14 | b |
| K+ (mg/100 mg d.w.) | 1.3 | ± | 0.4 | ns | 1.0 | ± | 0.3 | ns | 1.4 | ± | 0.4 | ns |
| Mg2+ (mg/100 mg d.w.) | 0.19 | ± | 0.04 | ns | 0.181 | ± | 0.019 | ns | 0.177 | ± | 0.012 | ns |
| Na+ (mg/100 mg d.w.) | 4.7 | ± | 1.1 | b | 5.2 | ± | 0.5 | b | 0.21 | ± | 0.06 | a |
| P (mg/100 mg d.w.) | 0.13 | ± | 0.03 | b | 0.108 | ± | 0.017 | b | 0.061 | ± | 0.014 | a |
| S (mg/100 mg d.w.) | 0.065 | ± | 0.016 | a | 0.07 | ± | 0.03 | a | 0.11 | ± | 0.03 | b |
| Na+/K+ | 3.8 | ± | 0.7 | b | 5.4 | ± | 1.6 | b | 0.15 | ± | 0.05 | a |
| Na+/Ca2+ | 11.7 | ± | 1.2 | b | 15.9 | ± | 3.8 | b | 0.40 | ± | 0.16 | a |
| Sequence Description | log2 FC | p-Value (adj) | Tissue |
|---|---|---|---|
| CALCIUM | |||
| Mitochondrial calcium uptake protein 1-like | 0.93 | 1.1 × 10−8 | L |
| Cation/calcium exchanger 3-like | 0.75 | 5.7 × 10−3 | |
| Extracellular calcium sensing receptor-like | 0.85 | 1.0 × 10−4 | L |
| Calcium-transporting ATPase-like 1 | 1.25 | 3.0 × 10−4 | |
| Calcium-transporting ATPase-like 2 | 1.28 | 5.3 × 10−7 | |
| Calcium-transporting ATPase-like 3 | 1.30 | 1.6 × 10−5 | |
| Calcium uniporter protein-like | −1.50 | 1.2 × 10−3 | L |
| SODIUM | |||
| Sodium/hydrogen exchanger 2-like-1 | 0.60 | 1.5 × 10−5 | |
| Sodium/hydrogen exchanger 2-like-2 | −0.74 | 3.6 × 10−16 | |
| POTASSIUM | |||
| Chloroplastic K+ efflux antiporter 3, -like | 0.95 | 2.1 × 10−3 | |
| Potassium channel AKT2/3-like 1 | 1.83 | 1.3 × 10−14 | |
| Potassium channel AKT2/3-like 2 | 1.90 | 3.5 × 10−11 | |
| Probable potassium transporter-like | −2.21 | 2.2 × 10−14 | |
| Potassium channel KAT3-like | 1.44 | 3.5 × 10−3 | |
| Potassium channel SKOR-like 1 | 3.16 | 6.9 × 10−4 | L |
| Potassium channel SKOR-like 2 | 4.13 | 5.3 × 10−4 | L |
| CATIONS | |||
| Mechanosensitive ion channel-like | 1.10 | 3.0 × 10−12 | |
| Chloroplastic mechanosensitive ion channel 2-like | 0.70 | 2.0 × 10−4 | |
| Cation/H(+) antiporter like-1 | 1.75 | 1.1 × 10−3 | |
| Cation/H(+) antiporter 18-like | −1.07 | 7.2 × 10−3 | |
| Cation/H(+) antiporter like-2 | −1.82 | 8.3 × 10−3 | |
| Cation/H(+) antiporter 14-like | −1.64 | 4.4 × 10−3 | |
| Vacuolar cation/proton exchanger-like | −2.98 | 2.5 × 10−4 | L |
| Vacuolar cation/proton exchanger 3-like 1 | −2.83 | 7.6 × 10−4 | L |
| Vacuolar cation/proton exchanger 3-like 2 | −2.71 | 2.6 × 10−3 | L |
| ANIONS | |||
| S-type anion channel SLAH1-like | −1.70 | 1.0 × 10−3 | |
| Voltage-dependent anion-selective channel 2-like | 5.32 | 5.8 × 10−4 | L |
| Chloride channel protein-like 1 | −0.88 | 3.4 × 10−9 | |
| Chloride channel protein-like 2 | −0.64 | 5.5 × 10−3 | L |
| Voltage dependent anion channel 1-like | 1.24 | 5.8 × 10−6 | L |
| Aluminum-activated malate transporter-like | 3.58 | 2.3 × 10−10 | L |
| OTHER NUTRIENTS | |||
| Ammonium transporter-like | 1.91 | 2.3 × 10−9 | L |
| Ammonium transporter 1 member 1-like | 1.94 | 8.9 × 10−4 | L |
| Magnesium/proton exchanger-like | 1.12 | 2.8 × 10−8 | L |
| Boron transporter 1-like | 1.56 | 5.3 × 10−4 | |
| Zinc transporter-like | 2.79 | 1.8 × 10−5 | L |
| Phosphate transporter PHO1-like | 3.82 | 1.1 × 10−18 | |
| H+ ATPases | |||
| Plasma membrane H+-ATPase-like | 0.85 | 1.5 × 10−3 | |
| Plasmalemma H+-ATPase 1-like | 0.92 | 4.2 × 10−3 | |
| Plasma membrane ATPase 4-like | 1.17 | 1.9 × 10−5 | |
| V-type proton ATPase subunit G-like | 7.33 | 2.9 × 10−4 | L |
| Sequence Description | log2 FC | p-Value (adj) | Tissue |
|---|---|---|---|
| PHOTOSYNTHESIS AND RESPIRATION | |||
| PSI reaction center subunit N-like-1 | 2.32 | 2.5 × 10−7 | L |
| PSI reaction center subunit N-like-2 | 2.13 | 1.5 × 10−8 | L |
| PSI reaction center subunit III-like | 2.26 | 1.8 × 10−8 | L |
| PSI reaction center subunit V-like | 1.86 | 3.2 × 10−6 | L |
| PSII reaction center W-like | 1.66 | 1.2 × 10−6 | L |
| PSII PsbY-like | 1.75 | 1.5 × 10−6 | L |
| PSII repair protein PSB27-H1-like | 0.93 | 4.6 × 10−3 | L |
| Cytochrome c oxidase subunit 5A-like | 6.86 | 5.4 × 10−3 | L |
| Sequence Description | Log2 FC | p-Value (adj) | Tissue |
|---|---|---|---|
| ROS DETOXIFICATION | |||
| Peroxiredoxin 1-like | 4.49 | 8.4 × 10−4 | L |
| Type II Peroxiredoxin 2-like | 1.18 | 7.3 × 10−8 | L |
| Peroxidase-like | −1.03 | 1.1 × 10−3 | L |
| Peroxidase 12-like 1 | 3.89 | 4.0 × 10−18 | L |
| Peroxidase 12-like 2 | 5.09 | 6.2 × 10−3 | L |
| Spermidine synthase-like | 1.95 | 8.7 × 10−5 | L |
| Thermospermine synthase ACAULIS5-like | 2.12 | 2.4 × 10−11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Muñoz, F.; Delhomme, N.; Quiñones, A.; Naval, M.d.M.; Badenes, M.L.; García-Gil, M.R. Transcriptomic Analysis Reveals Salt Tolerance Mechanisms Present in Date-Plum Persimmon Rootstock (Diospyros lotus L.). Agronomy 2020, 10, 1703. https://doi.org/10.3390/agronomy10111703
Gil-Muñoz F, Delhomme N, Quiñones A, Naval MdM, Badenes ML, García-Gil MR. Transcriptomic Analysis Reveals Salt Tolerance Mechanisms Present in Date-Plum Persimmon Rootstock (Diospyros lotus L.). Agronomy. 2020; 10(11):1703. https://doi.org/10.3390/agronomy10111703
Chicago/Turabian StyleGil-Muñoz, Francisco, Nicolas Delhomme, Ana Quiñones, Maria del Mar Naval, Maria Luisa Badenes, and M. Rosario García-Gil. 2020. "Transcriptomic Analysis Reveals Salt Tolerance Mechanisms Present in Date-Plum Persimmon Rootstock (Diospyros lotus L.)" Agronomy 10, no. 11: 1703. https://doi.org/10.3390/agronomy10111703
APA StyleGil-Muñoz, F., Delhomme, N., Quiñones, A., Naval, M. d. M., Badenes, M. L., & García-Gil, M. R. (2020). Transcriptomic Analysis Reveals Salt Tolerance Mechanisms Present in Date-Plum Persimmon Rootstock (Diospyros lotus L.). Agronomy, 10(11), 1703. https://doi.org/10.3390/agronomy10111703