Achievements and Challenges towards a Sustainable Conservation and Use of ‘Galega vulgar’ Olea europaea Variety
Abstract
:1. Introduction
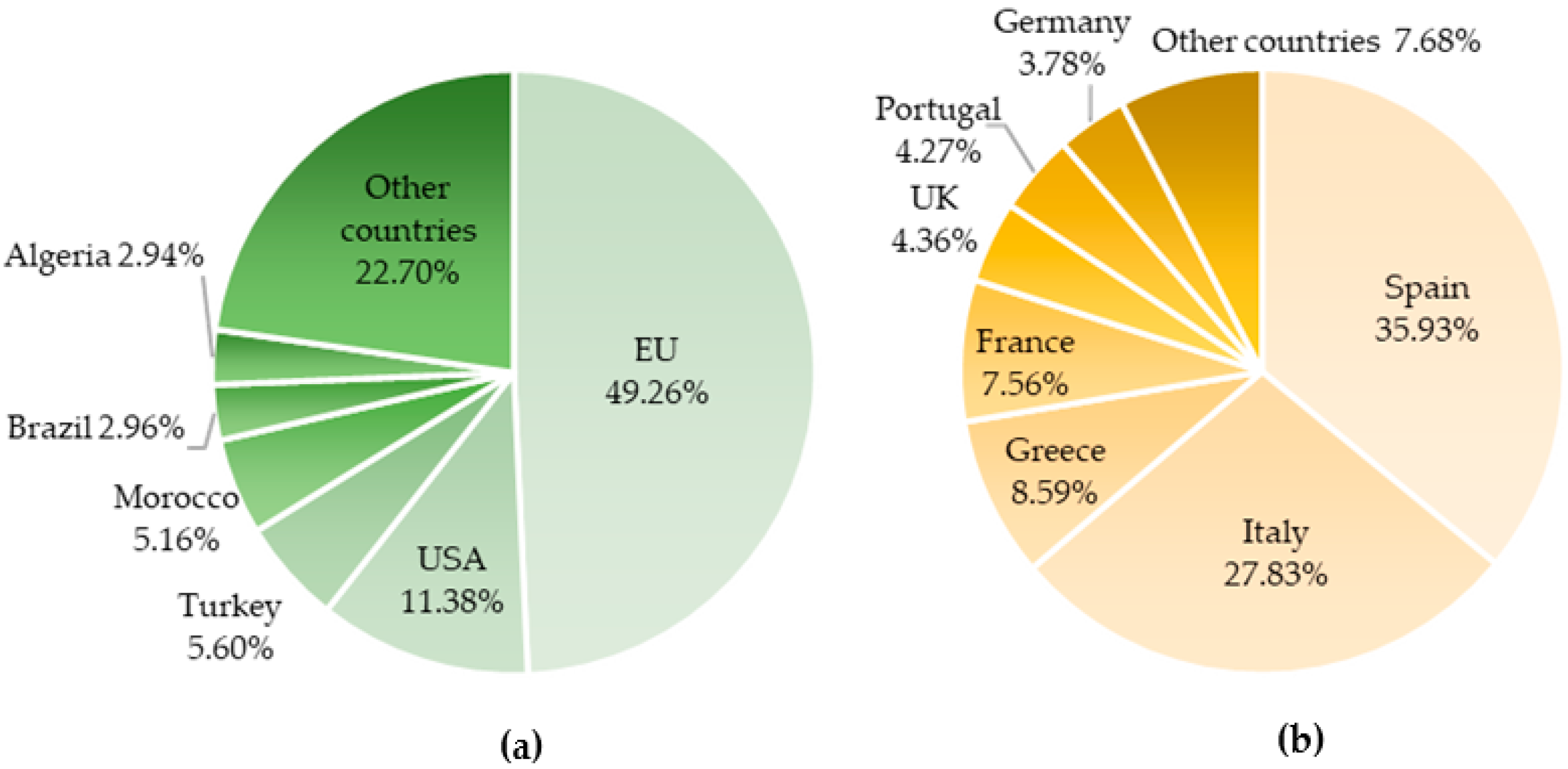
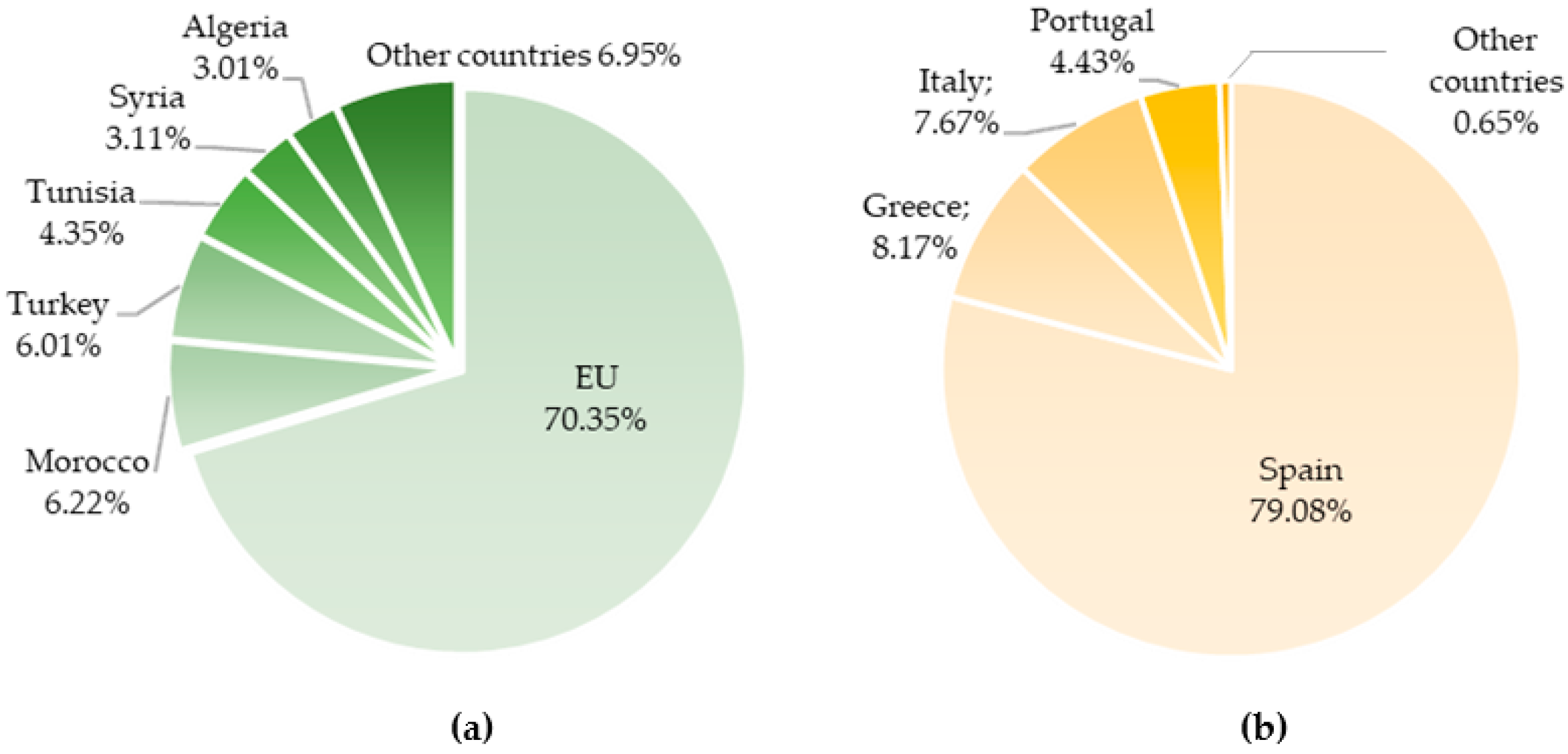
2. Olive Tree: A Millenary Crop with a Massive Economic Impact Worldwide
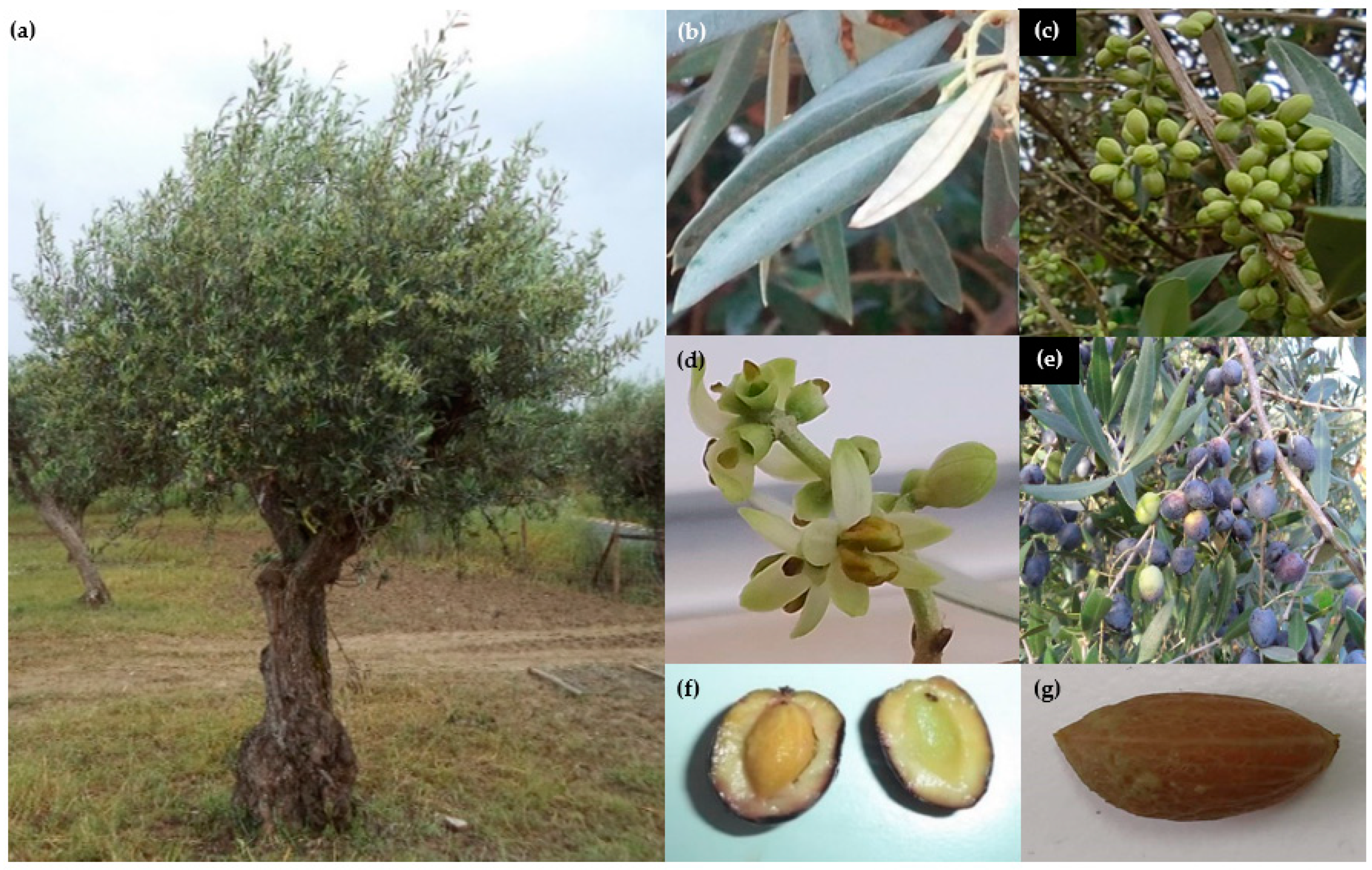
2.1. ‘Galega Vulgar’, the Most Important Portuguese Olive Variety
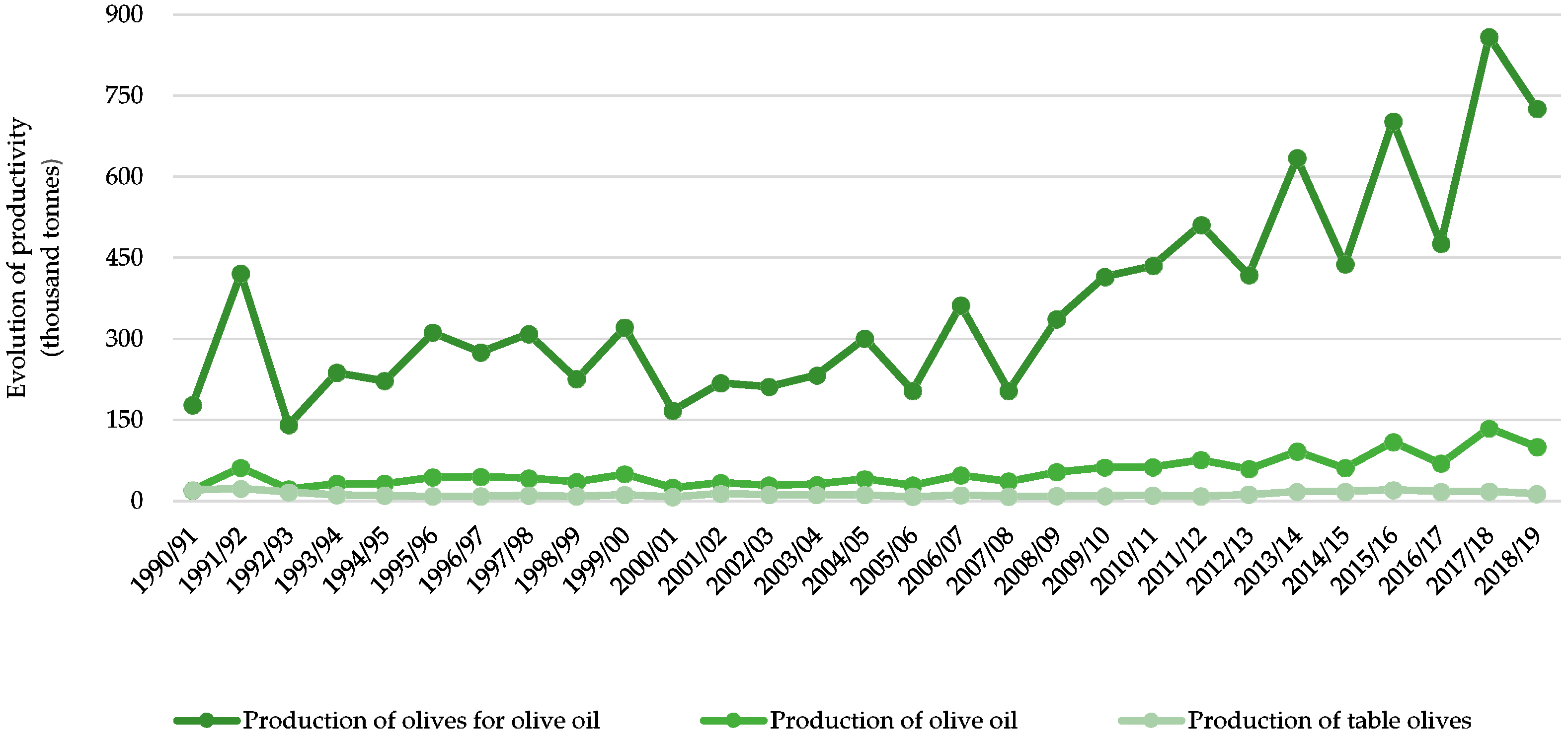
2.2. Recent Changes on Olive Production Systems and the Importance of a Life Cycle Assessment
3. Olea europaea Diversity Evolution
3.1. Domestication and Ancestral Origin of Olive Varieties
3.2. The Use of Molecular Markers on Olive Diversity Characterization
3.3. The Importance of Olea Germplasm Conservation
4. Olive Breeding Worldwide
4.1. From Conventional to Precision Olive Breeding
4.2. Marker Assisted Selection in Olive Breeding
4.3. Genotype to Phenotype (G2P) Prediction Models: The Growing Importance of Integrating Environmental Sensitivities and Crop Growth Models Information
5. Future Prospects for a Sustainable Conservation and Use of the ‘Galega’ Variety
Author Contributions
Funding
Conflicts of Interest
References
- Rugini, E.; Baldoni, L.; Muleo, R.; Sebastiani, L. Preface. In The Olive Tree Genome; Rugini, E., Baldoni, L., Muleo, R., Sebastiani, L., Eds.; Springer: Cham, Switzerland, 2016; pp. v–vi. [Google Scholar] [CrossRef]
- Besnard, G.; Khadari, B.; Navascués, M.; Fernández-Mazuecos, M.; El Bakkali, A.; Arrigo, N.; Baali-Cherif, D.; Brunini-Bronzini de Caraffa, V.; Santoni, S.; Vargas, P.; et al. The complex history of the olive tree: From Late Quaternary diversification of Mediterranean lineages to primary domestication in the northern Levant. Proc. R. Soc. B 2013, 280, 20122833. [Google Scholar] [CrossRef] [Green Version]
- Böhm, J. Domesticação da oliveira na antiguidade na bacia mediterrânica. In O Grande Livro da Oliveira e do Azeite—Portugal Oleícola; Böhm, J., Ed.; Dinalivro Editora: Lisboa, Portugal, 2013; pp. 52–65. ISBN 978-972-576-620-0. [Google Scholar]
- Trujillo, I.; Ojeda, M.A.; Urdiroz, N.M.; Potter, D.; Barranco, D.; Rallo, L.; Diez, C.M. Identification of the Worldwide Olive Germplasm Bank of Córdoba (Spain) using SSR and morphological markers. Tree Genet. Genomes 2014, 10, 141–155. [Google Scholar] [CrossRef]
- Rallo, L.; Barranco, D.; Castro-García, S.; Connor, D.J.; Gómez del Campo, M.; Rallo, P. High-density olive plantations. Hortic. Rev. 2013, 41, 303–383. [Google Scholar] [CrossRef]
- FAO; Plan Bleu. State of Mediterranean Forests 2018; Food and Agriculture Organization of the United Nations: Rome, Italy; Plan Bleu: Marseille, France, 2018; Available online: https://ec.europa.eu/knowledge4policy/publication/state-mediterranean-forests-2018_en (accessed on 20 June 2020).
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional uses, phytochemistry, and pharmacology of Olea europaea (olive). Evid. Based Complement. Altern. Med. 2015, 2015, 541591. [Google Scholar] [CrossRef] [Green Version]
- Saldanha, M.H. Benefícios do Azeite na Saúde Humana. Coleção Estudos e Análises 7; Direção-Geral de Desenvolvimento Rural: Lisboa, Portugal, 1999. Available online: https://www.dgadr.gov.pt/component/jdownloads/send/3-agricultura-e-desenvolvimento-rural/12-beneficios-do-azeite-na-saude-humana (accessed on 20 June 2020).
- Khadari, B.; Breton, C.; Moutier, N.; Roger, J.; Besnard, G.; Bervillé, A.; Dosba, F. The use of molecular markers for germplasm management in a French olive collection. Theor. Appl. Genet. 2003, 106, 521–529. [Google Scholar] [CrossRef]
- International Olive Council (IOC). Economic Affairs & Promotion Unit—Figures. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/#figures (accessed on 20 June 2020).
- CONSULAI; Juan-Vilar, Consultores Estratégicos. Alentejo: A Liderar a Olivicultura Moderna Internacional. Olivum. 2019. Available online: https://13b249f8-94d9-4c10-832b9c2323cab575.filesusr.com/ugd/a303d9_5993f29b65054e46a54accff8c90cf7f.pdf (accessed on 20 June 2020).
- Reis, P. O Olival em Portugal. Dinâmicas, Tecnologias e Relação com o Desenvolvimento Rural; Animar—Associação Portuguesa para o Desenvolvimento Local: Lisboa, Portugal, 2014; ISBN 978-989-8748-06-5. [Google Scholar]
- Instituto Nacional de Estatística (INE). Estatísticas Agrícolas. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_publicacoes&PUBLICACOESpagenumber=1&PUBLICACOEScoleccao=107660&PUBLICACOEStipo=ea&selTab=tab0 (accessed on 20 June 2020).
- Cordeiro, A.M.; Inês, C.; Morais, N. Aspetos gerais da cultura da oliveira: Principais cultivares de oliveira existentes em Portugal. In Boas Práticas no Olival e no Lagar; INIAV, I.P., Ed.; SIG—Sociedade Industrial Gráfica: Lisboa, Portugal, 2014; pp. 37–54. ISBN 978-972-579-041-0. [Google Scholar]
- Leitão, F.; Pontes, M.F.; Calado, M.L.; Almeida, F.J. Descrição de 22 Variedades de Oliveira Cultivadas em Portugal; Ministério da Agricultura, Pescas e Alimentação, Direcção-Geral do Planeamento e Agricultura: Lisboa, Portugal, 1986. Available online: https://www.dgadr.gov.pt/component/jdownloads/send/10-diversos/26-descricao-de-22-variedades-de-oliveira-cultivadas-em-portugal (accessed on 20 June 2020).
- Arias-Calderón, R.; Carvalho, M.T.; Cordeiro, A. A Importância dos Progenitores no Programa de Melhoramento da Oliveira por Hibridação; Instituto Nacional de Investigação Agrária e Veterinária, I.P. (INIAV): Lisboa, Portugal, 2017; Available online: http://www.iniav.pt/fotos/editor2/a_importancia_dos_progenitores_oliveiras.pdf (accessed on 15 July 2020).
- Gouveia, J.; Saldanha, J.; Martins, A.; Modesto, M.; Sobral, V. O Azeite em Portugal; Edições Inapa: Sintra, Portugal, 2002; ISBN 978-972-797-024-7. [Google Scholar]
- Costa, R. Caracterização do Perfil Fenólico e da Ultraestrutura do Pólen de Cultivares de Olea europaea L. como Contributo para uma Classificação Varietal. Master’s Thesis, University of Coimbra, Coimbra, Portugal, 2011. Available online: http://hdl.handle.net/10316/20275 (accessed on 21 June 2020).
- Cordeiro, A.M.; Calado, M.L.; Morais, N.; Miranda, A.; Carvalho, M.T. Variedades de Oliveira: ‘Galega Vulgar’; Direção Regional de Agricultura e Pescas do Centro: Castelo Branco, Portugal, 2010. Available online: https://www.drapc.gov.pt/base/documentos/018_variedades_oliveira_galega_vulgar_nov10.pdf (accessed on 20 June 2020).
- Del Río, C.; Caballero, J.M. Variability and classification of olive cultivars by fruit weight, flesh/stone ratio and oil percentage. Acta Hortic. 2008, 791, 39–44. [Google Scholar] [CrossRef]
- Albuquerque, T.G.; Costa, H.S.; Oliveira, M.B.P.P. An overview of Portuguese olive oils and table olives with protected designation of origin. Eur. J. Lipid Sci. Tech. 2019, 121, 1800129. [Google Scholar] [CrossRef]
- Gemas, V.J.; Almadanim, M.C.; Tenreiro, R.; Martins, A.; Fevereiro, P. Genetic diversity in the olive tree (Olea europaea L. subsp. europaea) cultivated in Portugal revealed by RAPD and ISSR markers. Genet. Resour. Crop Evol. 2004, 51, 501–511. [Google Scholar] [CrossRef]
- Serrano, M.C.F.; Amaral, L. Irrigation effects on some fructification parameters of olive cvs. Olea europaea L. ‘Galega vulgar’, ‘Cordovil de Serpa’ and ‘Verdeal Alentejana’ in Alentejo. Acta Hortic. 2008, 791, 325–331. [Google Scholar] [CrossRef]
- Coelho, R.; Sousa, A.; Rato, A.; Vaz, M. Response to salinity in young olive trees of three Iberian varieties. In Proceedings of the 12th Portuguese-Spanish Symposium on Plant Water Relations, Évora, Portugal, 30 September–3 October 2014; pp. 99–104. [Google Scholar]
- Peixe, A.; Antunes, A.; Hegewald, H.; Costa, C.; Pinto, A.P. Preliminary results on the evaluation of auxin endogenous levels and oxidative enzymes activity, among the rooting of two olive (Olea europaea L.) cultivars ‘Galega vulgar’ and ‘Cobrançosa’. In Atas Portuguesas de Horticultura 14, Proceedings of the 5th Simpósio Nacional de Olivicultura, Santarém, Portugal, 24–26 September 2009; Associação Portuguesa de Horticultura: Lisboa, Portugal, 2011; pp. 13–22. [Google Scholar]
- Lopes, J.P.P.S. Polinização em Oliveira cvs. Galega e Cobrançosa: Avaliação do Vingamento em Ensaios de Polinização Controlada e do Efeito da Aplicação de um Bioestimulante. Master’s Thesis, Instituto Superior de Agronomia, University of Lisboa, Lisboa, Portugal, 2011. Available online: http://hdl.handle.net/10400.5/4122 (accessed on 29 June 2020).
- Azevedo, A.; Bernardes, P. Influência das condições ecológicas no crescimento do olival em regime superintensivo. Reposta da cultivar Galega vulgar à fertilização azotada. In Proceedings of the 1st Congresso Nacional das Escolas Superiores Agrárias, Bragança, Portugal, 2–3 December 2015. [Google Scholar]
- Dias, M.C.; Pinto, D.C.G.A.; Freitas, H.; Santos, C.; Silva, A.M.S. The antioxidant system in Olea europaea to enhanced UV-B radiation also depends on flavonoids and secoiridoids. Phytochemistry 2020, 170, 112199. [Google Scholar] [CrossRef]
- Maia, F.B. Contribuição para o Estudo da Fenologia de Quatro Variedades de Oliveira (Olea europaea L.): ‘Arbequina’, ‘Cobrançosa’, ‘Galega Vulgar’ e ‘Picual’. Master’s Thesis, Instituto Superior de Agronomia, University of Lisboa, Lisboa, Portugal, 2010. Available online: http://hdl.handle.net/10400.5/15199 (accessed on 29 June 2020).
- Cordeiro, A.M.; Martins, P.C.S.; Ramos, A.; Sequeira, P. Characterization of olive cultivars fruit set in self-pollination. In Atas Portuguesas de Horticultura 14, Proceedings of the 5th Simpósio Nacional de Olivicultura, Santarém, Portugal, 24–26 September 2009; Associação Portuguesa de Horticultura: Lisboa, Portugal, 2011; pp. 23–30. [Google Scholar]
- Jimenez-Lopez, J.C.; Morales, S.; Castro, A.J.; Volkmann, D.; Rodríguez-García, M.I.; Alche, J.D. Characterization of profilin polymorphism in pollen with a focus on multifunctionality. PLoS ONE 2012, 7, e30878. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, H.; Calado, L.; Cunha, M.; Abreu, I. Evaluation of pollen viability and germination from 20 varieties of Olea europaea L. grown in Portugal. In Atas Portuguesas de Horticultura 14, Proceedings of the 5th Simpósio Nacional de Olivicultura, Santarém, Portugal, 24–26 September 2009; Associação Portuguesa de Horticultura: Lisboa, Portugal, 2011; pp. 39–46. [Google Scholar]
- Velada, I.; Grzebelus, D.; Lousa, D.; Soares, C.M.; Macedo, E.S.; Peixe, A.; Arnholdt-Schmitt, B.; Cardoso, H.G. AOX1-subfamily gene members in Olea europaea cv. “Galega Vulgar”—gene characterization and expression of transcripts during IBA-induced in vitro adventitious rooting. Int. J. Mol. Sci. 2018, 19, 597. [Google Scholar] [CrossRef] [Green Version]
- Velada, I.; Cardoso, H.; Porfirio, S.; Peixe, A. Expression profile of PIN-formed auxin efflux carrier genes during IBA-induced in vitro adventitious rooting in Olea europaea L. Plants 2020, 9, 185. [Google Scholar] [CrossRef] [Green Version]
- Macedo, E.; Vieira, C.; Carrizo, D.; Porfirio, S.; Hegewald, H.; Arnholdt-Schmitt, B.; Calado, M.L.; Peixe, A. Adventitious root formation in olive (Olea europaea L.) microshoots: Anatomical evaluation and associated biochemical changes in peroxidase and polyphenol oxidase activities. J. Hortic. Sci. Biotech. 2013, 88, 53–59. [Google Scholar] [CrossRef]
- Porfirio, S.; Calado, M.L.; Noceda, C.; Cabrita, M.J.; Silva, M.G.; Azadi, P.; Peixe, A. Tracking biochemical changes during adventitious root formation in olive (Olea europaea). Sci. Hortic. 2016, 204, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.V.A.P. Simplificação do Processo de Multiplicação in vitro da Oliveira “Olea europaea L.”. Master’s Thesis, University of Évora, Évora, Portugal, 2016. Available online: http://hdl.handle.net/10174/20702 (accessed on 29 June 2020).
- Pires, R.N. Ensaios com Vista à Indução de Embriogénese Somática em Oliveira (Olea europaea L.). Master’s Thesis, University of Évora, Évora, Portugal, 2018. Available online: http://hdl.handle.net/10174/23981 (accessed on 29 June 2020).
- Rallo, P.; Dorado, G.; Martin, A. Development of simple sequence repeats in olive tree (Olea europaea L.). Theor. Appl. Genet. 2000, 101, 984–989. [Google Scholar] [CrossRef]
- Díaz, A.; De la Rosa, R.; Martín, A.; Rallo, P. Development, characterization and inheritance of new microsatellites in olive (Olea europaea L.) and evaluation of their usefulness in cultivar identification and genetic relationship studies. Tree Genet. Genomes 2006, 2, 165–175. [Google Scholar] [CrossRef]
- Martins-Lopes, P.; Lima-Brito, J.; Gomes, S.; Meirinhos, J.; Santos, L.; Guedes-Pinto, H. RAPD and ISSR molecular markers in Olea europaea L.: Genetic variability and molecular cultivar identification. Genet. Resour. Crop Evol. 2007, 54, 117–128. [Google Scholar] [CrossRef]
- Gomes, S.; Martins-Lopes, P.; Lopes, J.; Guedes-Pinto, H. Assessing genetic diversity in Olea europaea L. using ISSR and SSR markers. Plant Mol. Biol. Rep. 2009, 27, 365–373. [Google Scholar] [CrossRef]
- Mariotti, R.; Cultrera, N.G.M.; Díez, C.M.; Baldoni, L.; Rubini, A. Identification of new polymorphic regions and differentiation of cultivated olives (Olea europaea L.) through plastome sequence comparison. BMC Plant Biol. 2010, 10, 211. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-García, M.C.; Belaj, A.; De la Rosa, R.; Šatović, Z.; Heller-Uszynska, K.; Kilian, A.; Martín, A.; Atienza, S.G. Development of DArT markers in olive (Olea europaea L.) and usefulness in variability studies and genome mapping. Sci. Hortic. 2012, 136, 50–60. [Google Scholar] [CrossRef]
- Parra-Lobato, M.C.; Delgado-Martinez, F.J.; Gomez-Jimenez, M.C. Morphological traits and RAPD markers for characterization and identification of minor Spanish olive cultivars from the Extremadura region. Genet. Mol. Res. 2012, 11, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Lazović, B.; Adakalić, M.; Pucci, C.; Perović, T.; Bandelj, D.; Belaj, A.; Mariotti, R.; Baldoni, L. Characterizing ancient and local olive germplasm from Montenegro. Sci. Hortic. 2016, 209, 117–123. [Google Scholar] [CrossRef]
- Veloso, M.; Simões-Costa, M.C.; Carneiro, L.C.; Guimarães, J.B.; Mateus, C.; Fevereiro, P.; Pinto-Ricardo, C. Olive tree (Olea europaea L.) diversity in traditional small farms of Ficalho, Portugal. Diversity 2018, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Lopes, M.S.; Mendonça, D.; Sefc, K.M.; Gil, F.S.; Da Câmara Machado, A. Genetic evidence of intra-cultivar variability within Iberian olive cultivars. HortScience 2004, 39, 1562–1565. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, A.I.; Sanchez-Sevilla, J.F.; Alvarez-Tinaut, M.C.; Gomez-Jimenez, M.C. Genetic diversity assessment in Portugal accessions of Olea europaea by RAPD markers. Biol. Plant. 2008, 52, 642–647. [Google Scholar] [CrossRef]
- Fevereiro, P.; Leitão, F.; Potes, F.; Gemas, V.; Alves, M.; Favoretto, P. The Portuguese olive (Olea europaea subsp. europaea) germplasm. Acta Hortic. 2011, 924, 291–298. [Google Scholar] [CrossRef]
- Figueiredo, E.; Canhoto, J.; Ribeiro, M.M. Fingerprinting and genetic diversity of Olea europaea L. ssp. europaea accessions from the cultivar Galega using RAPD markers. Sci. Hortic. 2013, 156, 24–28. [Google Scholar] [CrossRef]
- Cultrera, N.G.M.; Sarri, V.; Lucentini, L.; Ceccarelli, M.; Alagna, F.; Mariotti, R.; Mousavi, S.; Ruiz, C.G.; Baldoni, L. High levels of variation within gene sequences of Olea europaea L. Front. Plant Sci. 2019, 9, 1932. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.R. Diversidade Genómica e Genética Intra-Varietal em Oliveira ‘Galega Vulgar’. Master’s Thesis, Instituto Superior de Agronomia, University of Lisboa, Lisboa, Portugal, 2019. Available online: http://hdl.handle.net/10400.5/18359 (accessed on 29 June 2020).
- Dias, A.B.; Pimentel, R.S.; Pinheiro, A.; Peça, J.O. Resultados da avaliação da poda com máquina de discos num olival intensivo da variedade “Galega vulgar”. In Actas de Horticultura 73, Proceedings of the 1st Congreso Ibérico de Olivicultura, 5th Jornadas Nacionales del Grupo de Olivicultura de la Sociedad Española de Ciencias Hortícolas (SECH) and 7th Simpósio Nacional de Olivicultura da Associação Portuguesa de Horticultura (APH), Badajoz-Elvas, Spain, Portugal, 13–15 April 2016; Sociedad Española de Ciencias Hortícolas: Córdoba, Spain, 2018; pp. 194–201. [Google Scholar]
- Goldental-Cohen, S.; Biton, I.; Many, Y.; Ben-Sason, S.; Zemach, H.; Avidan, B.; Ben-Ari, G. Green olive browning differ between cultivars. Front. Plant Sci. 2019, 10, 1260. [Google Scholar] [CrossRef]
- Landum, M.C.; Félix, M.R.; Alho, J.; Garcia, R.; Cabrita, M.J.; Rei, F.; Varanda, C.M.R. Antagonistic activity of fungi of Olea europaea L. against Colletotrichum acutatum. Microbiol. Res. 2016, 183, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Materatski, P.; Varanda, C.; Carvalho, T.; Dias, A.B.; Campos, M.D.; Rei, F.; Félix, M.R. Spatial and temporal variation of fungal endophytic richness and diversity associated to the phyllosphere of olive cultivars. Fungal Biol. 2019, 123, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Varanda, C.M.R.; Materatski, P.; Landum, M.; Campos, M.D.; Félix, M.R. Fungal communities associated with peacock and cercospora leaf spots in olive. Plants 2019, 8, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, M.D.; Zellama, M.S.; Varanda, C.; Materatski, P.; Peixe, A.; Chaouachi, M.; Félix, M.R. Establishment of a sensitive qPCR methodology for detection of the olive-infecting viruses in Portuguese and Tunisian orchards. Front. Plant Sci. 2019, 10, 694. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Martins-Lopes, P.; Carvalho, T.; Barros, A.; Gomes, S. Impact of Colletotrichum acutatum pathogen on olive phenylpropanoid metabolism. Agriculture 2019, 9, 173. [Google Scholar] [CrossRef] [Green Version]
- Materatski, P.; Varanda, C.; Carvalho, T.; Dias, A.B.; Campos, M.D.; Gomes, L.; Nobre, T.; Rei, F.; Félix, M.R. Effect of long-term fungicide applications on virulence and diversity of Colletotrichum spp. associated to olive anthracnose. Plants 2019, 8, 311. [Google Scholar] [CrossRef] [Green Version]
- Azevedo-Nogueira, F.; Gomes, S.; Carvalho, T.; Martins-Lopes, P. Development of high-throughput real-time PCR assays for the Colletotrichum acutatum detection on infected olive fruits and olive oils. Food Chem. 2020, 317, 126417. [Google Scholar] [CrossRef]
- Alves, E.; Melo, T.; Barros, M.P.; Domingues, M.R.M.; Domingues, P. Lipidomic profiling of the olive (Olea europaea L.) fruit towards its valorisation as a functional food: In-depth identification of triacylglycerols and polar lipids in Portuguese olives. Molecules 2019, 24, 2555. [Google Scholar] [CrossRef] [Green Version]
- Ferro, M.D.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F. Chromatographic separation of phenolic compounds from extra virgin olive oil: Development and validation of a new method based on a biphenyl HPLC column. Int. J. Mol. Sci. 2019, 20, 201. [Google Scholar] [CrossRef] [Green Version]
- Peres, M.F.; Henriques, L.R.; Simões-Lopes, P.; Pinheiro-Alves, M.C. Azeites da ‘Galega Vulgar’—efeito do loteamento e do armazenamento. In Atas Portuguesas de Horticultura 13, Proceedings of the 3rd Simpósio Nacional de Olivicultura, Castelo Branco, Porttugal, 29–31 October 2003; Associação Portuguesa de Horticultura: Lisboa, Portugal, 2009; pp. 186–191. [Google Scholar]
- Marcelo, M.E.; Lopes, J.I.; Soares, F.M.; Centeno, M.S.L.; Cordeiro, A.M.; Vasconcelos, P.M. Effect of fertilization on polyphenols, sterols and waxes contents of the olive oil from six cultivars in Portugal. In Atas Portuguesas de Horticultura 14, Proceedings of the 5th Simpósio Nacional de Olivicultura, Santarém, Portugal, 24–26 September 2009; Associação Portuguesa de Horticultura: Lisboa, Portugal, 2011; pp. 141–150. [Google Scholar]
- Pita, D.; Vitorino, M.C.; Gouveia, C.; Peres, F. Extraction of monovarietal olive oils with natural microtalc. In Atas Portuguesas de Horticultura 14, Proceedings of the 5th Simpósio Nacional de Olivicultura, Santarém, Portugal, 24–26 September 2009; Associação Portuguesa de Horticultura: Lisboa, Portugal, 2011; pp. 151–157. [Google Scholar]
- Quintero-Flórez, A.; Nieva, L.S.; Sánchez-Ortíz, A.; Beltrán, G.; Perona, J.S. The fatty acid composition of virgin olive oil from different cultivars is determinant for foam cell formation by macrophages. J. Agric. Food Chem. 2015, 63, 6731–6738. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Domínguez-Perles, R.; Machado, N.; Carvalho, T.; Matos, C.; Barros, A.I.R.N.A. Effect of agro-environmental factors on the mineral content of olive oils: Categorization of the three major Portuguese cultivars. J. Am. Oil Chem. Soc. 2016, 93, 813–822. [Google Scholar] [CrossRef]
- Pires-Cabral, P.; Barros, T.; Nunes, P.; Quintas, C. Physicochemical, nutritional and microbiological characteristics of traditional table olives from Southern Portugal. Emir. J. Food Agric. 2018, 30, 611–620. [Google Scholar] [CrossRef]
- Saramago, I.S.L. Olival em Modo de Produção Biológico. Master’s Thesis, Escola Superior Agrária, Instituto Politécnico de Beja, Beja, Portugal, 2009. Available online: http://hdl.handle.net/10400.26/3979 (accessed on 15 September 2020).
- Tous, J.; Romero, A.; Hermoso, J.F.; Mallén, N. Sistemas de producción del olivo en seto. Experiencias en Cataluña. Agricultura 2007, 896, 360–367. [Google Scholar]
- Guerrero Maldonado, N.; López, M.J.; Caudullo, G.; de Rigo, D. Olea europaea in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publ. Off. EU: Luxembourg, 2016; p. e01534b+. [Google Scholar]
- Beghè, D.; Ferrarini, A.; Ganino, T.; Fabbri, A. Molecular characterization and identification of a group of local Olea europaea L. varieties. Tree Genet. Genomes 2011, 7, 1185–1198. [Google Scholar] [CrossRef]
- Marra, F.P.; Caruso, T.; Costa, F.; Di Vaio, C.; Mafrica, R.; Marchese, A. Genetic relationships, structure and parentage simulation among the olive tree (Olea europaea L. subsp. europaea) cultivated in Southern Italy revealed by SSR markers. Tree Genet. Genomes 2013, 9, 961–973. [Google Scholar] [CrossRef]
- Belaj, A.; Veral, M.G.; Sikaoui, H.; Moukhli, A.; Khadari, B.; Mariotti, R.; Baldoni, L. Olive genetic resources. In The Olive Tree Genome; Rugini, E., Baldoni, L., Muleo, R., Sebastiani, L., Eds.; Springer: Cham, Switzerland, 2016; pp. 27–54. [Google Scholar] [CrossRef]
- Lavee, S. Evaluation of the need and present potential of olive breeding indicating the nature of the available genetic resources involved. Sci. Hortic. 2013, 161, 333–339. [Google Scholar] [CrossRef]
- Belaj, A.; Muñoz-Diez, C.; Baldoni, L.; Šatović, Z.; Barranco, D. Genetic diversity and relationships of wild and cultivated olives at regional level in Spain. Sci. Hortic. 2010, 124, 323–330. [Google Scholar] [CrossRef]
- Maxted, N.; Guarino, L. Genetic erosion and genetic pollution of crop wild relatives. In Proceedings of the PGR Forum Workshop 5, Genetic Erosion and Pollution Assessment Methodologies, Terceira Island, Autonomous Region of the Azores, Portugal, 8–11 September 2004; pp. 35–45. [Google Scholar]
- Brown, A.H.D.; Hodgkin, T. Indicators of Genetic Diversity, Genetic Erosion, and Genetic Vulnerability for Plant Genetic Resources. In Genetic Diversity and Erosion in Plants. Sustainable Development and Biodiversity; Ahuja, M., Jain, S., Eds.; Springer: Cham, Switerzland, 2015; Volume 7, pp. 25–53. [Google Scholar] [CrossRef]
- Salomone, R.; Cappelletti, G.M.; Malandrino, O.; Mistretta, M.; Neri, E.; Nicoletti, G.; Notarnicola, B.; Pattara, C.; Russo, C.; Saija, G. Life cycle assessment in the olive oil sector. In Life Cycle Assessment in the Agri-Food Sector; Notarnicola, B., Salomone, R., Petti, L., Renzulli, P., Roma, R., Cerutti, A., Eds.; Springer: Cham, Switzerland, 2015; pp. 57–121. [Google Scholar] [CrossRef]
- Espadas-Aldana, G.; Vialle, C.; Belaud, J.P.; Vaca-Garcia, C.; Sablayrolles, C. Analysis and trends for Life Cycle Assessment of olive oil production. Sustain. Prod. Consum. 2019, 19, 216–230. [Google Scholar] [CrossRef] [Green Version]
- Salomone, R.; Ioppolo, G. Environmental impacts of olive oil production: A life cycle assessment case study in the province of Messina (Sicily). J. Clean. Prod. 2012, 28, 88–100. [Google Scholar] [CrossRef]
- Rajaeifar, M.A.; Akram, A.; Ghobadian, B.; Rafiee, S.; Heidari, M.D. Energy economic life cycle assessment (LCA) and greenhouse gas emissions analysis of olive oil production in Iran. Energy 2014, 66, 139–149. [Google Scholar] [CrossRef]
- Proietti, S.; Sdringola, P.; Regni, L.; Evangelisti, N.; Brunori, A.; Ilarioni, L.; Nasini, L.; Proietti, P. Extra virgin olive oil as carbon negative product: Experimental analysis and validation of results. J. Clean. Prod. 2017, 166, 550–562. [Google Scholar] [CrossRef]
- Tsarouhas, P.; Achillas, C.; Aidonis, D.; Folinas, D.; Maslis, V. Life cycle assessment of olive oil production in Greece. J. Clean. Prod. 2015, 93, 75–83. [Google Scholar] [CrossRef]
- El Hanandeh, A.; Gharaibeh, M.A. Environmental efficiency of olive oil production by small and micro-scale farmers in northern Jordan: Life cycle assessment. Agric. Syst. 2016, 148, 169–177. [Google Scholar] [CrossRef]
- Pattara, C.; Salomone, R.; Cichelli, A. Carbon footprint of extra virgin olive oil: A comparative and driver analysis of different production processes in Centre Italy. J. Clean. Prod. 2016, 127, 533–547. [Google Scholar] [CrossRef]
- Accorsi, R.; Cascini, A.; Ferrari, E.; Manzini, R.; Pareschi, A.; Versari, L. Life cycle assessment of an extra-virgin olive oil supply chain. In Proceedings of the 18th Summer School “Francesco Turco”—Industrial Mechanical Plants, Senigallia, Italy, 11–13 September 2013; pp. 172–178. [Google Scholar]
- Guiso, A.; Parenti, A.; Masella, P.; Guerrini, L.; Baldi, F.; Spugnoli, P. Environmental impact assessment of three packages for high-quality extra-virgin olive oil. Agric. Eng. 2016, 47, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Navarro, A.; Puig, R.; Martí, E.; Bala, A.; Fullana-i Palmer, P. Tackling the relevance of packaging in life cycle assessment of virgin olive oil and the environmental consequences of regulation. Environ. Manag. 2018, 62, 277–294. [Google Scholar] [CrossRef]
- Chatzisymeon, E.; Foteinis, S.; Mantzavinos, D.; Tsoutsos, T. Life cycle assessment of advanced oxidation processes for olive mill wastewater treatment. J. Clean. Prod. 2013, 54, 229–234. [Google Scholar] [CrossRef] [Green Version]
- El Hanandeh, A. Energy recovery alternatives for the sustainable management of olive oil industry waste in Australia: Life cycle assessment. J. Clean. Prod. 2015, 91, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Christoforou, E.A.; Fokaides, P.A. Life cycle assessment (LCA) of olive husk torrefaction. Renew. Energy 2016, 90, 257–266. [Google Scholar] [CrossRef]
- Parascanu, M.M.; Gamero, M.P.; Sánchez, P.; Soreanu, G.; Valverde, J.L.; Sanchez-Silva, L. Life cycle assessment of olive pomace valorisation through pyrolysis. Renew. Energy 2018, 122, 589–601. [Google Scholar] [CrossRef]
- Parascanu, M.M.; Sánchez, P.; Soreanu, G.; Valverde, J.L.; Sanchez-Silva, L. Environmental assessment of olive pomace valorization through two different thermochemical processes for energy production. J. Clean. Prod. 2018, 186, 771–781. [Google Scholar] [CrossRef]
- Mohamad, R.S.; Verrastro, V.; Cardone, G.; Bteich, M.R.; Favia, M.; Moretti, M.; Roma, R. Optimization of organic and conventional olive agricultural practices from a life cycle assessment and life cycle costing perspectives. J. Clean. Prod. 2014, 70, 78–89. [Google Scholar] [CrossRef]
- De Luca, A.I.; Falcone, G.; Stillitano, T.; Iofrida, N.; Strano, A.; Gulisano, G. Evaluation of sustainable innovations in olive growing systems: A life cycle sustainability assessment case study in southern Italy. J. Clean. Prod. 2018, 171, 1187–1202. [Google Scholar] [CrossRef]
- Romero-Gámez, M.; Castro-Rodríguez, J.; Suárez-Rey, E.M. Optimization of olive growing practices in Spain from a life cycle assessment perspective. J. Clean. Prod. 2017, 149, 25–37. [Google Scholar] [CrossRef]
- Bernardi, B.; Falcone, G.; Stillitano, T.; Benalia, S.; Strano, A.; Bacenetti, J.; De Luca, A.I. Harvesting system sustainability in Mediterranean olive cultivation. Sci. Total Environ. 2018, 625, 1446–1458. [Google Scholar] [CrossRef]
- Kaniewski, D.; Van Campo, E.; Boiy, T.; Terral, J.F.; Khadari, B.; Besnard, G. Primary domestication and early uses of the emblematic olive tree: Palaeobotanical, historical and molecular evidences from the Middle East. Biol. Rev. 2012, 87, 885–899. [Google Scholar] [CrossRef] [Green Version]
- Besnard, G.; Terral, J.; Cornille, A. On the origins and domestication of the olive: A review and perspectives. Ann. Bot. 2018, 121, 385–403. [Google Scholar] [CrossRef] [Green Version]
- Díez, C.M.; Moral, J.; Barranco, D.; Rallo, L. Genetic diversity and conservation of olive genetic resources. In Genetic Diversity and Erosion in Plants. Sustainable Development and Biodiversity; Ahuja, M., Jain, S., Eds.; Springer: Cham, Switerzland, 2015; Volume 8, pp. 337–356. [Google Scholar] [CrossRef]
- Besnard, G.; El Bakkali, A.; Haouane, H.; Baali-Cherif, D.; Moukhli, A.; Khadari, B. Population genetics of Mediterranean and Saharan olives: Geographic patterns of differentiation and evidence for early-generations of admixture. Ann. Bot. 2013, 112, 1293–1302. [Google Scholar] [CrossRef] [Green Version]
- Díez, C.M.; Trujillo, I.; Martinez-Urdiroz, N.; Barranco, D.; Rallo, L.; Marfil, P.; Gaut, B.S. Olive domestication and diversification in the Mediterranean Basin. New Phytol. 2015, 206, 436–447. [Google Scholar] [CrossRef]
- Besnard, G.; Rubio de Casas, R.; Christin, P.A.; Vargas, P. Phylogenetics of Olea (Oleaceae) based on plastid and nuclear ribosomal DNA sequences: Tertiary climatic shifts and lineage differentiation times. Ann. Bot. 2009, 104, 143–160. [Google Scholar] [CrossRef] [Green Version]
- Ladisa, G.; Calabrese, E.; Perrino, E.V. The origin and distribution of olive tree and olive crop. In Study on Biodiversity in Century-Old Olive Groves; Calabrese, G., Tartaglini, N., Ladisa, G., Eds.; CIHEAM—Mediterranean Agronomic Institute of Bari: Bari, Italy, 2012; pp. 1–3. [Google Scholar]
- Fuller, D.Q. Long and attenuated: Comparative trends in the domestication of tree fruits. Veg. Hist. Archaeobotany 2018, 27, 165–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belaj, A.; Dominguez-García, M.C.; Atienza, S.G.; Urdíroz, N.M.; De la Rosa, R.; Šatović, Z.; Martín, A.; Kilian, A.; Trujillo, I.; Valpuesta, V.; et al. Developing a core collection of olive (Olea europaea L.) based on molecular markers (DArTs, SSRs, SNPs) and agronomic traits. Tree Genet. Genomes 2012, 8, 365–378. [Google Scholar] [CrossRef]
- Fabbri, A.; Lambardi, M.; Ozden-Tokatli, Y. Olive Breeding. In Breeding Plantation Tree Crops: Tropical Species; Jain, S.M., Priyadarshan, P.M., Eds.; Springer: New York, NY, USA, 2009; pp. 423–465. ISBN 978-038-771-199-7. [Google Scholar]
- Doveri, S.; Baldoni, L. Olive. In Genome Mapping and Molecular Breeding in Plants—Fruits and Nuts; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 4, pp. 253–256. ISBN 978-354-034-531-2. [Google Scholar]
- Alves, M.L.V. Caracterização e Estrutura Genéticas da Cultivar de Oliveira ‘Cobrançosa’ e Sua Relação com o Zambujeiro. Master’s Thesis, University of Lisboa, Lisboa, Portugal, 2007. Available online: http://hdl.handle.net/10451/1358 (accessed on 30 June 2020).
- Belaj, A.; León, L.; Šatović, Z.; De la Rosa, R. Variability of wild olives (Olea europaea subsp. europaea var. sylvestris) analyzed by agro-morphological traits and SSR markers. Sci. Hortic. 2011, 129, 561–569. [Google Scholar] [CrossRef]
- Bouhadida, M.; Casas, A.M.; Moreno, M.A.; Gogorcena, Y. Molecular characterization of Miraflores peach variety and relatives using SSRs. Sci. Hortic. 2007, 111, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Wünsch, A.; Hormaza, J.I. Cultivar identification and genetic fingerprinting of temperate fruit tree species using DNA markers. Euphytica 2002, 125, 59–67. [Google Scholar] [CrossRef]
- Hammer, K.; Arrowsmith, N.; Gladis, T. Agrobiodiversity with emphasis on plant genetic resources. Naturwissenschaften 2003, 90, 241–250. [Google Scholar] [CrossRef]
- Pasqualone, A.; Montemurro, C.; Di Rienzo, V.; Summo, C.; Paradiso, V.M.; Caponio, F. Evolution and perspectives of cultivar identification and traceability from tree to oil and table olives by means of DNA markers. J. Sci. Food Agric. 2016, 96, 3642–3657. [Google Scholar] [CrossRef]
- Lazović, B.; Bosković, R.; James, C.; Tobutt, K.R.; Gasic, K. Genetic diversity of olives grown along the coast of Montenegro. Acta Hortic. 2002, 586, 167–170. [Google Scholar] [CrossRef]
- Besnard, G.; Baradat, P.; Chevalier, D.; Tagmount, A.; Bervillé, A. Genetic differentiation in the olive complex (Olea europaea L.) revealed by RAPDs and RFLPs in the rRNA genes. Genet. Resour. Crop Evol. 2001, 48, 165–182. [Google Scholar] [CrossRef]
- De la Rosa, R.; Angiolillo, A.; Rallo, L.; Guerrero, C.; Pellegrini, M.; Besnard, G.; Bervillé, A.; Martin, A.; Baldoni, L. A first linkage map of olive (Olea europaea L.) cultivars using RAPD, AFLP, RFLP and SSR markers. Theor. Appl. Genet. 2003, 106, 1273–1282. [Google Scholar] [CrossRef]
- Belaj, A.; Šatović, Z.; Trujillo, I.; Rallo, L. Genetic relationships of Spanish olive cultivars using RAPD markers. HortScience 2004, 39, 948–951. [Google Scholar] [CrossRef]
- Brake, M.; Migdadi, H.; Al-Gharaibeh, M.; Ayoub, S.; Haddad, N.; El Oqlah, A. Characterization of Jordanian olive cultivars (Olea europaea L.) using RAPD and ISSR molecular markers. Sci. Hortic. 2014, 176, 282–289. [Google Scholar] [CrossRef]
- Linos, A.; Nikoloudakis, N.; Katsiotis, A.; Hagidimitriou, M. Genetic structure of the Greek olive germplasm revealed by RAPD, ISSR and SSR markers. Sci. Hortic. 2014, 175, 33–43. [Google Scholar] [CrossRef]
- Besnard, G.; Bervillé, A. Multiple origins for Mediterranean olive (Olea europaea L. ssp europaea) based upon mitochondrial DNA polymorphisms. Comptes Rendus Acad. Sci. 2000, 323, 173–181. [Google Scholar] [CrossRef]
- Wu, S.B.; Collins, G.; Sedgley, M. A molecular linkage map of olive (Olea europaea L.) based on RAPD, microsatellite, and SCAR markers. Genome 2004, 47, 26–35. [Google Scholar] [CrossRef]
- Charafi, J.; Zine El Aabidine, A.; Grout, C.; Rahioui, B.; El Meziane, A.; Moukhli, A.; Boulouha, B.; El Modafar, C.; Khadari, B. A genetic linkage map of Olea europaea L. using a pseudo-test cross- mapping strategy based on SSR, AFLP, ISSR, RAPD and SCAR markers. Acta Hortic. 2009, 814, 609–614. [Google Scholar] [CrossRef]
- Terzopoulos, P.J.; Kolano, B.; Bebeli, P.J.; Kaltsikes, P.J.; Metzidakis, I. Identification of Olea europaea L. cultivars using inter-simple sequence repeat markers. Sci. Hortic. 2005, 105, 45–51. [Google Scholar] [CrossRef]
- Essadki, M.; Ouazzani, N.; Lumaret, R.; Moumni, M. ISSR variation in olive-tree cultivars from Morocco and other western countries of the Mediterranean basin. Genet. Resour. Crop Evol. 2006, 53, 475–482. [Google Scholar] [CrossRef]
- Owen, C.A.; Bita, E.C.; Banilas, G.; Hajjar, S.E.; Sellianakis, V.; Aksoy, U.; Hepaksoy, S.; Chamoun, R.; Talhook, S.N.; Metzidakis, I.; et al. AFLP reveals structural details of genetic diversity within cultivated olive germplasm from the Eastern Mediterranean. Theor. Appl. Genet. 2005, 110, 1169–1176. [Google Scholar] [CrossRef]
- Ercisli, S.; Barut, E.; Ipek, A. Molecular characterization of olive cultivars using amplified fragment length polymorphism markers. Genet. Mol. Res. 2009, 8, 414–419. [Google Scholar] [CrossRef]
- Ipek, M.; Seker, M.; Ipek, A.; Gul, M.K. Identification of molecular markers associated with fruit traits in olive and assessment of olive core collection with AFLP markers and fruit traits. Genet. Mol. Res. 2015, 14, 2762–2774. [Google Scholar] [CrossRef] [PubMed]
- El Aabidine, A.Z.; Charafi, J.; Grout, C.; Doligez, A.; Santoni, S.; Moukhli, A.; Jay-Allemand, C.; El Modafar, C.; Khadari, B. Construction of a genetic linkage map for the olive based on AFLP and SSR markers. Crop Sci. 2010, 50, 2291–2302. [Google Scholar] [CrossRef]
- Khadari, B.; El Aabidine, A.Z.; Grout, C.; Ben Sadok, I.; Doligez, A.; Moutier, N.; Santoni, S.; Costes, E. A genetic linkage map of olive based on amplified fragment length polymorphism, intersimple sequence repeat and simple sequence repeat markers. J. Am. Soc. Hortic. Sci. 2010, 135, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ayed, R.; Ennouri, K.; Ben-Hassen, H.; Triki, M.A.; Rebaï, A. Comparison between DNA-based, pomological and chemical markers accomplished by bioinformatic tools to distinguish within Tunisian olive cultivars. J. Fundam. Appl. Sci. 2015, 7, 408–421. [Google Scholar] [CrossRef]
- Morgante, M.; Olivieri, A.M. PCR-amplified microsatellites as markers in plant genetics. Plant J. 1993, 3, 175–182. [Google Scholar] [CrossRef]
- De la Rosa, R.; James, C.M.; Tobutt, K.R. Isolation and characterization of polymorphic microsatellites in olive (Olea europaea L.) and their transferability to other genera in the Oleaceae. Mol. Ecol. Notes 2002, 2, 265–267. [Google Scholar] [CrossRef]
- Rallo, P.; Tenzer, I.; Gessler, C.; Baldoni, L.; Dorado, G.; Martín, A. Transferability of olive microsatellite loci across the genus Olea. Theor. Appl. Genet. 2003, 107, 940–946. [Google Scholar] [CrossRef]
- Belaj, A.; Cipriani, G.; Testolin, R.; Rallo, L.; Trujillo, I. Characterization and identification of the main Spanish and Italian olive cultivars by simple-sequence-repeat markers. HortScience 2004, 39, 1557–1561. [Google Scholar] [CrossRef]
- Salimonti, A.; Simeone, V.; Cesari, G.; Lamaj, F.; Cattivelli, L.; Perri, E.; Desiderio, F.; Fanizzi, F.P.; Del Coco, L.; Zelasco, S. A first molecular investigation of monumental olive trees in Apulia region. Sci. Hortic. 2013, 162, 204–212. [Google Scholar] [CrossRef]
- Xu, Y. Molecular Plant Breeding; CAB International: Oxfordshire, UK, 2010; pp. 195–247. ISBN 978-184-593-392-0. [Google Scholar]
- Reale, S.; Doveri, S.; Díaz, A.; Angiolillo, A.; Lucentini, L.; Pilla, F.; Martín, A.; Donini, P.; Lee, D. SNP-based markers for discriminating olive (Olea europaea L.) cultivars. Genome 2006, 49, 1193–1205. [Google Scholar] [CrossRef]
- Carriero, F.; Fontanazza, G.; Cellini, F.; Giorio, G. Identification of simple sequence repeats (SSRs) in olive (Olea europaea L.). Theor. Appl. Genet. 2002, 104, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Baldoni, L.; Cultrera, N.G.; Mariotti, R.; Riccioloni, C.; Arcioni, S.; Vendramin, G.G.; Buonamici, A.; Porceddu, A.; Sarri, V.; Ojeda, M.A.; et al. A consensus list of microsatellite markers for olive genotyping. Mol. Breed. 2009, 24, 213–231. [Google Scholar] [CrossRef]
- Belaj, A.; Muñoz-Diez, C.; Baldoni, L.; Porceddu, A.; Barranco, D.; Šatović, Z. Genetic diversity and population structure of wild olives from the North-western Mediterranean by SSR markers. Ann. Bot. 2007, 100, 449–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ipek, A.; Barut, E.; Gulen, H.; Ipek, M. Assessment of inter-and intra-cultivar variations in olive using SSR markers. Sci. Agric. 2012, 69, 327–335. [Google Scholar] [CrossRef]
- Caruso, T.; Marra, F.P.; Costa, F.; Campisi, G.; Macaluso, L.; Marchese, A. Genetic diversity and clonal variation within the main Sicilian olive cultivars based on morphological traits and microsatellite markers. Sci. Hortic. 2014, 180, 130–138. [Google Scholar] [CrossRef]
- Abdessemed, S.; Muzzalupo, I.; Benbouza, H. Assessment of genetic diversity among Algerian olive (Olea europaea L.) cultivars using SSR marker. Sci. Hortic. 2015, 192, 10–20. [Google Scholar] [CrossRef]
- Lazović, B.; Klepo, T.; Adakalić, M.; Šatović, Z.; Arbeiter, A.B.; Hladnik, M.; Strikić, F.; Liber, Z.; Bandelj, D. Intra-varietal variability and genetic relationships among the homonymic East Adriatic olive (Olea europaea L.) varieties. Sci. Hortic. 2018, 236, 175–185. [Google Scholar] [CrossRef]
- Ben Sadok, I.; Celton, J.M.; Essalouh, L.; El Aabidine, A.Z.; Garcia, G.; Martinez, S.; Grati-Kamoun, N.; Rebai, A.; Costes, E.; Khadari, B. QTL mapping of flowering and fruiting traits in olive. PLoS ONE 2013, 8, e62831. [Google Scholar] [CrossRef] [Green Version]
- Mookerjee, S.; Guerin, J.; Collins, G.; Ford, C.; Sedgley, M. Paternity analysis using microsatellite markers to identify pollen donors in an olive grove. Theor. Appl. Genet. 2005, 111, 1174–1182. [Google Scholar] [CrossRef]
- De la Rosa, R.; Belaj, A.; Muñoz-Mérida, A.; Trelles, O.; Ortíz-Martín, I.; González-Plaza, J.J.; Valpuesta, V.; Beuzón, C.R. Development of EST-derived SSR markers with long-core repeat in olive and their use for paternity testing. J. Am. Soc. Hortic. Sci. 2013, 138, 290–296. [Google Scholar] [CrossRef]
- Hakim, I.R.; Grati-Kammoun, N.; Makhloufi, E.; Rebaï, A. Discovery and potential of SNP markers in characterization of Tunisian olive germplasm. Diversity 2010, 2, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-García, M.C.; Laib, M.; De La Rosa, R.; Belaj, A. Characterisation and identification of olive cultivars from North-eastern Algeria using molecular markers. J. Hortic. Sci. Biotechnol. 2012, 87, 95–100. [Google Scholar] [CrossRef]
- Biton, I.; Doron-Faigenboim, A.; Jamwal, M.; Mani, Y.; Eshed, R.; Rosen, A.; Sherman, A.; Ophir, R.; Lavee, S.; Avidan, B.; et al. Development of a large set of SNP markers for assessing phylogenetic relationships between the olive cultivars composing the Israeli olive germplasm collection. Mol. Breed. 2015, 35, 107–120. [Google Scholar] [CrossRef]
- Zhu, S.; Niu, E.; Shi, A.; Mou, B. Genetic diversity analysis of olive germplasm (Olea europaea L.) with Genotyping-by-Sequencing Technology. Front. Genet. 2019, 10, 755. [Google Scholar] [CrossRef] [Green Version]
- Baldoni, L.; Khadari, B.; De la Rosa, R. Genetic mapping and detection of Quantitative Trait Loci. In The Olive Tree Genome; Rugini, E., Baldoni, L., Muleo, R., Sebastiani, L., Eds.; Springer: Cham, Switzerland, 2016; pp. 65–74. [Google Scholar] [CrossRef]
- İpek, A.; Yılmaz, K.; Sıkıcı, P.; Tangu, N.A.; Öz, A.T.; Bayraktar, M.; İpek, M.; Gülen, H. SNP discovery by GBS in olive and the construction of a high-density genetic linkage map. Biochem. Genet. 2016, 54, 313–325. [Google Scholar] [CrossRef]
- Marchese, A.; Marra, F.P.; Caruso, T.; Mhelembe, K.; Costa, F.; Fretto, S.; Sargent, D.J. The first high-density sequence characterized SNP-based linkage map of olive (Olea europaea L. subsp. europaea) developed using genotyping by sequencing. Aust. J. Crop Sci. 2016, 10, 857. [Google Scholar] [CrossRef]
- El Bakkali, A.; Essalouh, L.; Tollon, C.; Rivallan, R.; Mournet, P.; Moukhli, A.; Zaher, H.; Mekkaoui, A.; Hadidou, A.; Sikaoui, L.; et al. Characterization of worldwide olive germplasm banks of Marrakech (Morocco) and Córdoba (Spain): Towards management and use of olive germplasm in breeding programs. PLoS ONE 2019, 14, e0223716. [Google Scholar] [CrossRef] [Green Version]
- FAO. International Treaty on Plant Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2001; Available online: http://www.fao.org/plant-treaty/overview/en/ (accessed on 15 July 2020).
- Sardaro, R.; Bozzo, F.; Petrontino, A.; Fucilli, V. Community preferences in support of a conservation programme for olive landraces in the Mediterranean area. Acta Hortic. 2018, 1199, 183–188. [Google Scholar] [CrossRef]
- Rallo, L.; Barranco, D.; Díez, C.M.; Rallo, P.; Suárez, M.P.; Trapero, C.; Pliego-Alfaro, F. Strategies for olive (Olea europaea L.) breeding: Cultivated genetic resources and crossbreeding. In Advances in Plant Breeding Strategies: Fruits; Al-Khayri, J., Jain, S.M., Johnson, D.V., Eds.; Springer: Cham, Switzerland, 2018; Volume 3, pp. 536–600. [Google Scholar]
- Baenziger, P.S.; Russell, W.K.; Graef, G.L.; Campbell, B.T. Improving lives: 50 years of crop breeding, genetics, and cytology (C-1). Crop Sci. 2006, 46, 2230–2244. [Google Scholar] [CrossRef]
- Rallo, L.; Caruso, T.; Díez, C.M.; Campisi, G. Olive growing in a time of change: From empiricism to genomics. In The Olive Tree Genome; Rugini, E., Baldoni, L., Muleo, R., Sebastiani, L., Eds.; Springer: Cham, Switzerland, 2016; pp. 55–64. [Google Scholar] [CrossRef]
- Baldoni, L.; Belaj, A. Olive. In Oil Crops. Handbook of Plant Breeding; Vollmann, J., Rajcan, I., Eds.; Springer: New York, NY, USA, 2009; Volume 4, pp. 397–421. [Google Scholar] [CrossRef]
- Rallo, L. Breeding oil and table olives for mechanical harvesting in Spain. Hortech 2014, 24, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Arias-Calderón, R.; Rodríguez-Jurado, D.; León, L.; Bejarano-Alcázar, J.; De la Rosa, R.; Belaj, A. Pre-breeding for resistance to Verticillium wilt in olive: Fishing in the wild relative gene pool. Crop Prot. 2015, 75, 25–33. [Google Scholar] [CrossRef]
- Trapero, C.; Rallo, L.; López-Escudero, F.J.; Barranco, D.; Díez, C.M. Variability and selection of verticillium wilt resistant genotypes in cultivated olive and in the Olea genus. Plant Pathol. 2015, 64, 890–900. [Google Scholar] [CrossRef]
- Klepo, T.; Toumi, A.; De la Rosa, R.; León, L.; Belaj, A. Agronomic evaluation of seedlings from crosses between the main Spanish olive cultivar ‘Picual’ and two wild olive trees. J. Hortic. Sci. Biotechnol. 2014, 89, 508–512. [Google Scholar] [CrossRef]
- Caceres, M.E.; Ceccarelli, M.; Pupilli, F.; Sarri, V.; Mencuccini, M. Obtainment of inter-subspecific hybribs in olive (Olea europaea L.). Euphytica 2015, 201, 307–319. [Google Scholar] [CrossRef]
- Rugini, E.; Cristofori, V.; Silvestri, C. Genetic improvement of olive (Olea europaea L.) by conventional and in vitro biotechnology methods. Biotechnol. Adv. 2016, 34, 687–696. [Google Scholar] [CrossRef]
- Rugini, E.; De Pace, C. Olive breeding with classical and modern approaches. In The Olive Tree Genome; Rugini, E., Baldoni, L., Muleo, R., Sebastiani, L., Eds.; Springer: Cham, Switzerland, 2016; pp. 163–193. [Google Scholar] [CrossRef]
- Leva, A.R. Innovative protocol for “ex vitro rooting” on olive micropropagation. Cent. Eur. J. Biol. 2011, 6, 352–358. [Google Scholar] [CrossRef]
- Benelli, C.; De Carlo, A.; Englmen, F. Recent advances in the cryopreservation of shoot-derived germplasm of economically important fruit trees of Actinidia, Diospyros, Malus, Olea, Prunus, Pyrus and Vitis. Biotechnol. Adv. 2013, 31, 175–185. [Google Scholar] [CrossRef]
- Rugini, E.; Silvestri, C.; Ceccarelli, M.; Muleo, R.; Cristofori, V. Mutagenesis and biotechnology techniques as tools for selecting new stable diploid and tetraploid olive genotypes and their dwarfing agronomical characterization. HortScience 2016, 51, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Capelo, A.M.; Silva, S.; Brito, G.; Santos, C. Somatic embryogenesis induction in leaves and petioles of a mature wild olive. Plant Cell Tissue Org. 2010, 103, 237–242. [Google Scholar] [CrossRef]
- Toufik, I.; Guenoun, F.; Belkoura, I. Embryogenesis expression from somatic explants of olive (Olea europaea L.) cv Picual. Moroc. J. Biol. 2014, 11, 17–25. [Google Scholar]
- Torreblanca, R.; Cerezo, S.; Palomo-Ríos, E.; Mercado, J.A.; Pliego-Alfaro, F. Development of a high throughput system for genetic transformation of olive (Olea europaea L.) plants. Plant Cell Tissue Org. 2010, 103, 61–69. [Google Scholar] [CrossRef]
- Titouh, K.; Khelifi, L.; Moussa, K.T.; Cerezo-Medina, S.; Mercado, J.A.; Pliego-Alfaro, F. Evaluation of the effect of phosphinothricin, as selection agent, on the growth of olive somatic embryos. Acta Hortic. 2014, 1057, 533–542. [Google Scholar] [CrossRef]
- Ozgenturk, N.O.; Oruç, F.; Sezerman, U.; Kuçukural, A.; Korkut, S.V.; Toksoz, F.; Un, C. Generation and analysis of expressed sequence tags from Olea europaea L. Comp. Funct. Genom. 2010, 2010, 757512. [Google Scholar] [CrossRef]
- Bazakos, C.; Manioudaki, M.E.; Therios, I.; Voyiatzis, D.; Kafetzopoulos, D.; Awada, T.; Kalaitzis, P. Comparative transcriptome analysis of two olive cultivars in response to NaCl-stress. PLoS ONE 2012, 7, e42931. [Google Scholar] [CrossRef] [PubMed]
- Parra, R.; Paredes, M.A.; Sanchez-Calle, I.M.; Gomez-Jimenez, M.C. Comparative transcriptional profiling analysis of olive ripe-fruit pericarp and abscission zone tissues shows expression differences and distinct patterns of transcriptional regulation. BMC Genom. 2013, 14, 866. [Google Scholar] [CrossRef] [Green Version]
- Yanik, H.; Turktas, M.; Dundar, E.; Hernandez, P.; Dorado, G.; Unver, T. Genome-wide identification of alternate bearing-associated microRNAs (miRNAs) in olive (Olea europaea L.). BMC Plant Biol. 2013, 13, 10. [Google Scholar] [CrossRef] [Green Version]
- Cabanás, C.G.; Schilirò, E.; Valverde-Corredor, A.; Mercado-Blanco, J. Systemic responses in a tolerant olive (Olea europaea L.) cultivar upon root colonization by the vascular pathogen Verticillium dahliae. Front. Microbiol. 2015, 6, 928. [Google Scholar] [CrossRef] [Green Version]
- Carmona, R.; Zafra, A.; Seoane, P.; Castro, A.J.; Guerrero-Fernández, D.; Castillo-Castillo, T.; Medina-García, A.; Cánovas, F.M.; Aldana-Montes, J.F.; Navas-Delgado, I.; et al. ReprOlive: A database with linked data for the olive tree (Olea europaea L.) reproductive transcriptome. Front. Plant Sci. 2015, 6, 625. [Google Scholar] [CrossRef] [Green Version]
- Leyva-Pérez, M.O.; Valverde-Corredor, A.; Valderrama, R.; Jiménez-Ruiz, J.; Muñoz-Merida, A.; Trelles, O.; Barroso, J.B.; Mercado-Blanco, J.; Luque, F. Early and delayed long-term transcriptional changes and short-term transient responses during cold acclimation in olive leaves. DNA Res. 2015, 22, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Giampetruzzi, A.; Morelli, M.; Saponari, M.; Loconsole, G.; Chiumenti, M.; Boscia, D.; Saldarelli, P. Transcriptome profiling of two olive cultivars in response to infection by the OQDS strain of Xylella fastidiosa ssp. pauca. BMC Genom. 2016, 17, 1. [Google Scholar] [CrossRef] [Green Version]
- González-Plaza, J.J.; Ortiz-Martín, I.; Muñoz-Mérida, A.; García-López, C.; Sánchez-Sevilla, J.F.; Luque, F.; Trelles, O.; Bejarano, E.R.; De la Rosa, R.; Valpuesta, V.; et al. Transcriptomic analysis using olive varieties and breeding progenies identifies candidate genes involved in plant architecture. Front. Plant Sci. 2016, 7, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarah, G.; Homa, F.; Pointet, S.; Contreras, S.; Sabot, F.; Nabholz, B.; Santoni, S.; Sauné, L.; Ardisson, M.; Chantret, N.; et al. A large set of 26 new reference transcriptomes dedicated to comparative population genomics in crops and wild relatives. Mol. Ecol. Resour. 2017, 17, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Zafra, A.; Carmona, R.; Traverso, J.A.; Hancock, J.T.; Goldman, M.H.S.; Claros, M.G.; Hiscock, S.J.; Alche, J.D. Identification and functional annotation of genes differentially expressed in the reproductive tissues of the olive tree (Olea europaea L.) through the generation of subtractive libraries. Front. Plant Sci. 2017, 8, 1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guodong, R.; Jianguo, Z.; Xiaoxia, L.; Ying, L. Identification of putative genes for polyphenol biosynthesis in olive fruits and leaves using full-length transcriptome sequencing. Food Chem. 2019, 300, 125246. [Google Scholar] [CrossRef]
- Besnard, G.; Hernández, P.; Khadari, B.; Dorado, G.; Savolainen, V. Genomic profiling of plastid DNA variation in the Mediterranean olive tree. BMC Plant Biol. 2011, 11, 80. [Google Scholar] [CrossRef] [Green Version]
- Kaya, E.; Vatansever, R.; Filiz, E. Assessment of the genetic relationship of Turkish olives (Olea europaea subsp. europaea) cultivars based on cpDNA trnL-F regions. Acta Bot. Croat. 2018, 77, 88–92. [Google Scholar] [CrossRef]
- Rugini, E.; De Pace, C.; Gutiérrez-Pesce, P.; Muleo, R. Olea. In Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 79–144. [Google Scholar] [CrossRef] [Green Version]
- Kaya, H.B.; Cetin, O.; Kaya, H.S.; Sahin, M.; Sefer, F.; Tanyolac, B. Association mapping in Turkish olive cultivars revealed significant markers related to some important agronomic traits. Biochem. Genet. 2016, 54, 506–533. [Google Scholar] [CrossRef]
- Atienza, S.G.; De La Rosa, R.; León, L.; Martín, A.; Belaj, A. Identification of QTL for agronomic traits of importance for olive breeding. Mol. Breed. 2014, 34, 725–737. [Google Scholar] [CrossRef]
- Ates, D. Identification of QTLs controlling genes of ripening time, flesh detachment from stone and firmness in olive (Olea europaea L.). In Proceedings of the 24th Plant and Animal Genome Conference, San Diego, CA, USA, 9–13 January 2016; p. P1163. [Google Scholar]
- Mauricio, R. Mapping quantitative trait loci in plants: Uses and caveats for evolutionary biology. Nat. Rev. Genet. 2001, 2, 370–381. [Google Scholar] [CrossRef]
- Morrell, P.L.; Buckler, E.S.; Ross-Ibarra, J. Crop genomics: Advances and applications. Nat. Rev. Genet. 2012, 13, 85–96. [Google Scholar] [CrossRef]
- Yu, J.; Buckler, E.S. Genetic association mapping and genome organization of maize. Curr. Opin. Biotechnol. 2006, 17, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.B.; Akdemir, D.; Lozano, R.; Cetin, O.; Kaya, H.S.; Sahin, M.; Smith, J.L.; Tanyolac, B.; Jannink, J.L. Genome wide association study of 5 agronomic traits in olive (Olea europaea L.). Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Salimonti, A.; Carbone, F.; Romano, E.; Pellegrino, M.; Benincasa, C.; Micali, S.; Tondelli, A.; Conforti, F.L.; Perri, E.; Ienco, A.; et al. Association study of the 5′ UTR intron of the FAD2-2 gene with oleic and linoleic acid content in Olea europaea L. Front. Plant Sci. 2020, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Van Eeuwijk, F.A.; Bustos-Korts, D.; Millet, E.J.; Boer, M.P.; Kruijer, W.; Thompson, A.; Malosetti, M.; Iwata, H.; Quiroz, R.; Kuppe, C.; et al. Modelling strategies for assessing and increasing the effectiveness of new phenotyping techniques in plant breeding. Plant Sci. 2019, 282, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Podlich, D.W.; Winkler, C.R.; Cooper, M. Mapping as you go. An effective approach for marker assisted selection of complex traits. J. Crop Sci. 2004, 44, 1560–1571. [Google Scholar] [CrossRef] [Green Version]
- Malosetti, M.; Ribaut, J.M.; Van Eeuwijk, F.A. The statistical analysis of multi-environment data: Modeling genotype-by-environment interaction and its genetic basis. Front. Physiol. 2013, 4, 44. [Google Scholar] [CrossRef] [Green Version]
- Hammer, G.; Messina, C.; Van Oosterom, E.; Chapman, S.; Singh, V.; Borrell, A.; Jordan, D.; Cooper, M. Molecular breeding for complex adaptive traits: How integrating crop ecophysiology and modelling can enhance efficiency. In Crop Systems Biology; Yin, X., Struik, P., Eds.; Springer: Cham, Switzerland, 2016; pp. 147–162. [Google Scholar] [CrossRef]
- Van Eeuwijk, F.A.; Bink, M.C.A.M.; Chenu, K.; Chapman, S.C. Detection and use of QTL for complex traits in multiple environments. Curr. Opin. Plant Biol. 2010, 13, 193–205. [Google Scholar] [CrossRef]
- Bustos-Korts, D.; Malosetti, M.; Chapman, S.; Van Eeuwijk, F. Modelling of genotype by environment interaction and prediction of complex traits across multiple environments as a synthesis of crop growth modelling, genetics and statistics. In Crop Systems Biology; Yin, X., Struik, P., Eds.; Springer: Cham, Switzerland, 2016; pp. 55–82. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.B.; Cullis, B.R.; Thompson, R. The analysis of crop cultivar breeding and evaluation trials: An overview of current mixed model approaches. J. Agric. Sci. 2005, 143, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Technow, F.; Messina, C.D.; Totir, L.R.; Cooper, M. Integrating crop growth models with whole genome prediction through approximate bayesian computation. PLoS ONE 2015, 10, e0130855. [Google Scholar] [CrossRef] [Green Version]
- López-Bernal, Á.; Morales, A.; García-Tejera, O.; Testi, L.; Orgaz, F.; De Melo-Abreu, J.P.; Villalobos, F.J. OliveCan: A process-based model of development, growth and yield of olive orchards. Front. Plant Sci. 2018, 9, 632. [Google Scholar] [CrossRef] [Green Version]
- Cruz, F.; Julca, I.; Gómez-Garrido, J.; Loska, D.; Marcet-Houben, M.; Cano, E.; Galán, B.; Frias, L.; Ribeca, P.; Derdak, S.; et al. Genome sequence of the olive tree, Olea europaea. GigaScience 2016, 5, 29. [Google Scholar] [CrossRef] [PubMed]


| Area | Applications | References |
|---|---|---|
| Ecophysiology | Water use efficiency; salinity stress | [23,24] |
| Rooting | [25] | |
| Effect of chemical and natural compounds application; influence UV-B radiation | [26,27,28] | |
| Phenology studies | [29] | |
| Different types of pollination; profilin polymorphism | [26,30,31] | |
| Pollen viability and germination | [32] | |
| In vitro culture | Gene characterization and expression | [33,34] |
| Adventitious root formation | [35,36] | |
| Micropropagation | [37,38] | |
| Genetic resources | Olive varieties identification and genetic relationships | [39,40,41,42,43,44,45,46,47] |
| Genetic variability; genotyping and genetic mapping | [22,44,48,49,50,51,52,53] | |
| Improvement in production systems and cultural techniques | Mechanical pruning and harvesting; effect of rejuvenation pruning | [54,55] |
| Phytosanitary protection | Richness and diversity of fungal communities | [56,57,58] |
| Susceptibility to fungal diseases; response to pathogens attack | [59,60,61,62] | |
| Technology, quality and markets | Analytical characterization of olive oil compounds; certification and olive oil traceability | [63,64] |
| Effect of blending and storage time of olive oil | [65] | |
| Effect of fertilization on olive oil polyphenol, sterol and wax content | [66] | |
| Extraction of monovarietal olive oils with natural compounds | [67] | |
| Physicochemical, nutritional and microbiological characterization; health effects; agro-environmental factors on the olive oil mineral content | [68,69,70] |
| Aim of the Application of LCA Methodology | FU 1 | Country | References |
|---|---|---|---|
| olive oil production | 1 ton of olives | Italy | [83] |
| 1 ha | Iran | [84] | |
| 1 L EVOO 2 | Italy | [85] | |
| 1 L EVOO | Greece | [86] | |
| 1 kg of olive oil | Jordan | [87] | |
| 5 L EVOO | Italy | [88] | |
| distribution processes of olive oil supply chain and packaging | 1 L EVOO | Italy | [89] |
| 1 L of bottling capacity | Italy | [90] | |
| 0.5 L bottle of VOO 3 | Spain | [91] | |
| olive oil industry waste treatments | 1 L olive mill waste | Greece | [92] |
| 1 mg of olive solid waste | Australia | [93] | |
| 1 ton of torrefied olive husk | Cyprus | [94] | |
| 100 kg olive pomace | Spain | [95] | |
| 1MJ of energy production | Spain | [96] | |
| field agricultural practices | 1 ha | Italy | [97,98] |
| 1 ton of olives | Spain | [99] | |
| olive-harvesting practices | 1 ha; 1 kg | Italy | [100] |
| Method | Applications | References |
|---|---|---|
| Isoenzymes | Olive varieties identification; genetic diversity; genetic relationships | [118] |
| RFLP (Restriction Fragment Length Polymorphism) | Genetic distance/genetic relationships | [119] |
| Genetic linkage map | [120] | |
| RAPD (Randomly Amplified Polymorphic DNA) | Olive varieties identification; genetic diversity; genetic relationships | [22,41,45,49,51,53,118,121,122,123] |
| Genetic distance estimation between wild and cultivated olive genotypes | [119,124] | |
| Genetic linkage map | [120,125,126] | |
| ISSRs (InterSimple Sequence Repeats) | Olive varieties identification; genetic diversity; genetic relationships | [22,41,42,53,122,123,127,128] |
| AFLPs (Amplified Fragment Length Polymorphism) | Olive varieties identification; genetic diversity; genetic relationships | [129,130,131] |
| Genetic linkage map | [120,126,132,133] |
| Method | Applications | References |
|---|---|---|
| SSRs | Identification/characterization of markers | [39,40,136,142,143] |
| Olive varieties identification; genetic diversity; genetic relationships | [4,40,42,46,47,48,50,75,105,112,123,138,144,145,146,147,148] | |
| Genetic linkage map | [120,125,126,132,133,149] | |
| Paternity analysis | [150,151] | |
| SNPs | Olive varieties identification; genetic diversity; genetic relationships | [52,141,152,153] |
| Genetic linkage map | [154,155,156,157,158] |
| Area | References |
|---|---|
| In vitro techniques for supporting unconventional methods of genetic improvement | [33,34,35,37,38,173,174,175] |
| Plant regeneration from in vitro cultured tissues | [176,177] |
| Genetic transformation and plant recovery | [178,179] |
| Transcriptome analysis | [180,181,182,183,184,185,186,187,188,189,190,191] |
| Plastome analysis | [2,43,192,193] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales, H.; Nunes, J.; Vaz Patto, M.C. Achievements and Challenges towards a Sustainable Conservation and Use of ‘Galega vulgar’ Olea europaea Variety. Agronomy 2020, 10, 1467. https://doi.org/10.3390/agronomy10101467
Sales H, Nunes J, Vaz Patto MC. Achievements and Challenges towards a Sustainable Conservation and Use of ‘Galega vulgar’ Olea europaea Variety. Agronomy. 2020; 10(10):1467. https://doi.org/10.3390/agronomy10101467
Chicago/Turabian StyleSales, Hélia, João Nunes, and Maria Carlota Vaz Patto. 2020. "Achievements and Challenges towards a Sustainable Conservation and Use of ‘Galega vulgar’ Olea europaea Variety" Agronomy 10, no. 10: 1467. https://doi.org/10.3390/agronomy10101467
APA StyleSales, H., Nunes, J., & Vaz Patto, M. C. (2020). Achievements and Challenges towards a Sustainable Conservation and Use of ‘Galega vulgar’ Olea europaea Variety. Agronomy, 10(10), 1467. https://doi.org/10.3390/agronomy10101467





