Effect of Weather Conditions on Yield and Health Status of Faba Bean Seeds in Poland
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Księżak, J.; Staniak, M.; Bojarszczuk, J. The regional differentiation of legumes cropping area in Poland between 2001 and 2007. Pol. J Agronom. 2009, 1, 25–31. [Google Scholar]
- Crépon, K.; Marget, P.; Peyronnet, C.; Carrouée, B.; Arese, P.; Duc, G. Nutritional value of faba bean (Vicia faba L.) seeds for feed and food. Field Crop Res. 2010, 115, 329–339. [Google Scholar] [CrossRef]
- Sahile, S.; Sakhuja, P.K.; Fininsa, C.; Ahmed, S. Potential antagonistic fungal species from Ethiopia for biological control of chocolate spot disease of faba bean. Afr. Crop Sci. J. 2011, 19, 213–225. [Google Scholar]
- Sillero, J.C.; Villegas-Fernández, A.M.; Thomas, J.; Rojas-Molina, M.M.; Emeran, A.A.; Fernández-Aparicio, M.; Rubiales, D. Faba bean breeding for disease resistance. Field Crops Res. 2010, 115, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Barłóg, P.; Grzebisz, W.; Łukowiak, R. Faba bean yield and growth dynamics in response to soil potassium availability and sulfur application. Field Crops Res. 2018, 219, 87–97. [Google Scholar] [CrossRef]
- Kulig, B.; Kołodziej, J.; Oleksy, A.; Kołodziejczyk, M.; Sajdak, A. Influence of the weather conditions on faba bean yielding. Ecol. Chem. Eng. A 2011, 18, 1–7. [Google Scholar]
- Horoszkiewicz-Janka, J.; Jajor, E.; Korbas, M. Potential risk of infection of pathogenic fungi to legumes (Fabales) and possibilities of their control. Prog. Plant Protect. 2013, 53, 762–767. [Google Scholar]
- Kaur, S.; Kimber, R.B.; Cogan, N.O.; Materne, M.; Forster, J.W.; Paull, J.G. SNP discovery and high-density genetic mapping in faba bean (Vicia faba L.) permits identification of QTLs for ascochyta blight resistance. Plant Sci. 2014, 217, 47–55. [Google Scholar] [CrossRef]
- Deneke, S. Review on Epidemiology and Management of Faba Bean (Vicia fabae) Chocolate Spot (Botrytis fabae), Root Rot (Fusarium solani) and Rust (Uromyces vicia fabae) in Ethiopia. Int. J. Sci. Res. Publ. 2018, 8, 105–111. [Google Scholar] [CrossRef]
- El-Ammari, A.S. Plant Fungal Diseases of Faba bean in Benghazi. ContROL 2017, 1, 1–5. [Google Scholar]
- Elwakil, M.A.; El-Refai, I.M.; Awadallah, O.A.; El-Metwally, M.A.; Mohammed, M.S. Seed-borne pathogens of faba bean in Egypt: detection and pathogencity. Plant Pathol. 2009, 8, 90–97. [Google Scholar] [CrossRef]
- Podleśny, J.; Podleśna, A.; Nędzi, M. Occurrence of fungal diseases caused by fungi on faba bean (Vicia faba L. var. minor Harz.) plants in different regions of Poland. Prog. Plant Protect. 2017, 57, 190–195. [Google Scholar]
- Sillero, J.C.; Rojas-Molina, M.M.; Avila, C.M.; Rubiales, D. Induction of systemic acquired resistance against rust, ascochyta blight and broomrape in faba bean by exogenous application of salicylic acid and benzothiadiazole. Crop Protect. 2012, 34, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Okorski, A.; Polak-Śliwińska, M.; Karpiesiuk, K.; Pszczółkowska, A.; Kozera, W. Real time PCR: a good tool to estimate mycotoxin contamination in pig diets. World Mycotoxin J. 2017, 10, 219–228. [Google Scholar] [CrossRef]
- Gleń, K.; Boligłowa, E.; Gospodarek, J. The fungal community colonizing broad bean seeds depending on the biological protection. J. Res. Appl. Agric. Eng. 2013, 58, 147–154. [Google Scholar]
- Gleń, K.; Gospodarek, J. Microflora of broad bean (Vicia faba L. ssp. maior) seeds in conditions of soil polluted with heavy metals. Prog. Plant Protect. 2009, 49, 1260–1263. [Google Scholar]
- Houghton, J.; Ding, Y.; Griggs, D.; Noguer, M.; Van Der Linden, P.; Dai, X.; Maskell, K.; Johnson, C. Climate Change: The Scientific Basis. Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]
- EL-Mansoury, M.; Saleh, S. Influence of Climatic Changes on Faba Bean (Vicia faba L.) Yield in North Nile Delta. J. Soil Sci. Agric. Eng. 2017, 8, 29–34. [Google Scholar] [CrossRef]
- Fordoński, G.; Pszczółkowska, A.; Krzebietke, S.; Olszewski, J.; Okorski, A. Yield and mineral composition of seeds of leguminous plants and grain of spring wheat as well as their residual effect on the yield and chemical composition of winter oilseed rape seeds. J. Elem. 2015, 20, 827–838. [Google Scholar]
- Ellis, M.B. Dematiaceous Hyphomycetes; Commonwealth Mycological Institute Kew: Surrey, UK, 1971. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing Professional: Ames, IA, USA, 2006. [Google Scholar]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Yadav, S.K.; Verma, N.; Singh, A.K.; Singh, N.; Rana, S.C.; Ranga, S.S.; Kumar, K. Diversity and development in Faba bean. Legume Res. 2016, 40, 618–623. [Google Scholar]
- Podleśny, J. Effect of amount and distribution of precipitation during vegetation on growth, development and yielding of determinate and traditional faba bean varieties. Acta Agroph. 2009, 14, 413–425. [Google Scholar]
- Karkanis, A.; Ntatsi, G.; Lepse, L.; Fernández, J.A.; Vågen, I.M.; Rewald, B.; Alsina, I.; Kronberga, A.; Balliu, A.; Olle, M.; et al. Faba Bean Cultivation—Revealing Novel Managing Practices for More Sustainable and Competitive European Cropping Systems. Front. Plant Sci. 2018, 9, 1115. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.Z.; Honda, Y.; Islam, S.Z.; Arase, S. Effect of metabolic inhibitors on red light induced resistance of broad bean (Vicia faba L.) against Botrytis cinerea. J. Phytopathol. 2002, 150, 463–468. [Google Scholar] [CrossRef]
- Tekalign, A.; Derera, J.; Sibiya, J.; Fikre, A. Participatory assessment of production threats, farmers’ desired traits and selection criteria of faba bean (Vicia faba L.) varieties: opportunities for faba bean breeding in Ethiopia. Indian J. Agric. Res. 2016, 50, 295–302. [Google Scholar]
- Sahile, S.; Fininsa, C.; Sakhuja, P.K.; Ahmed, S. Yield loss of faba bean (Vicia faba) due to chocolate spot (Botrytis fabae) in sole and mixed cropping systems in Ethiopia. Arch. Phytopathol. Plant Protect. 2010, 43, 1144–1159. [Google Scholar] [CrossRef]
- Saeed, M.F.; Baćanović, J.; Bruns, C.; Schmidt, H.; Finckh, M.R. Seed health of organic peas and faba beans and its effects on the health of the harvested grains. J. Plant Dis. Prot. 2017, 124, 331–337. [Google Scholar] [CrossRef]
- Marcenaro, D.; Valkonen, J.P.T. Seedborne Pathogenic Fungi in Common Bean (Phaseolus vulgaris cv. INTA Rojo) in Nicaragua. PLoS ONE 2016, 11, 0168662. [Google Scholar] [CrossRef] [Green Version]
- Marcinkowska, J. Fungi occurrence on seeds of field pea. Acta Mycol. 2008, 43, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Pszczółkowska, A.; Okorski, A.; Fordoński, G.; Prusiński, J.; Faligowska, A.; Borowska, M. Fungal colonization of seeds of three lupine species in different regions of Poland. Acta Agrobot. 2017, 70, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Pszczółkowska, A.; Okorski, A.; Fordoński, G.; Faligowska, A.; Kaszkowiak, E.; Olszewski, J.; Chareńska, A. The frequency of occurrence of pathogenic and saprotrophic fungi in pea seeds in different regions of Poland. Legume Res. 2019, 42, 270–276. [Google Scholar]
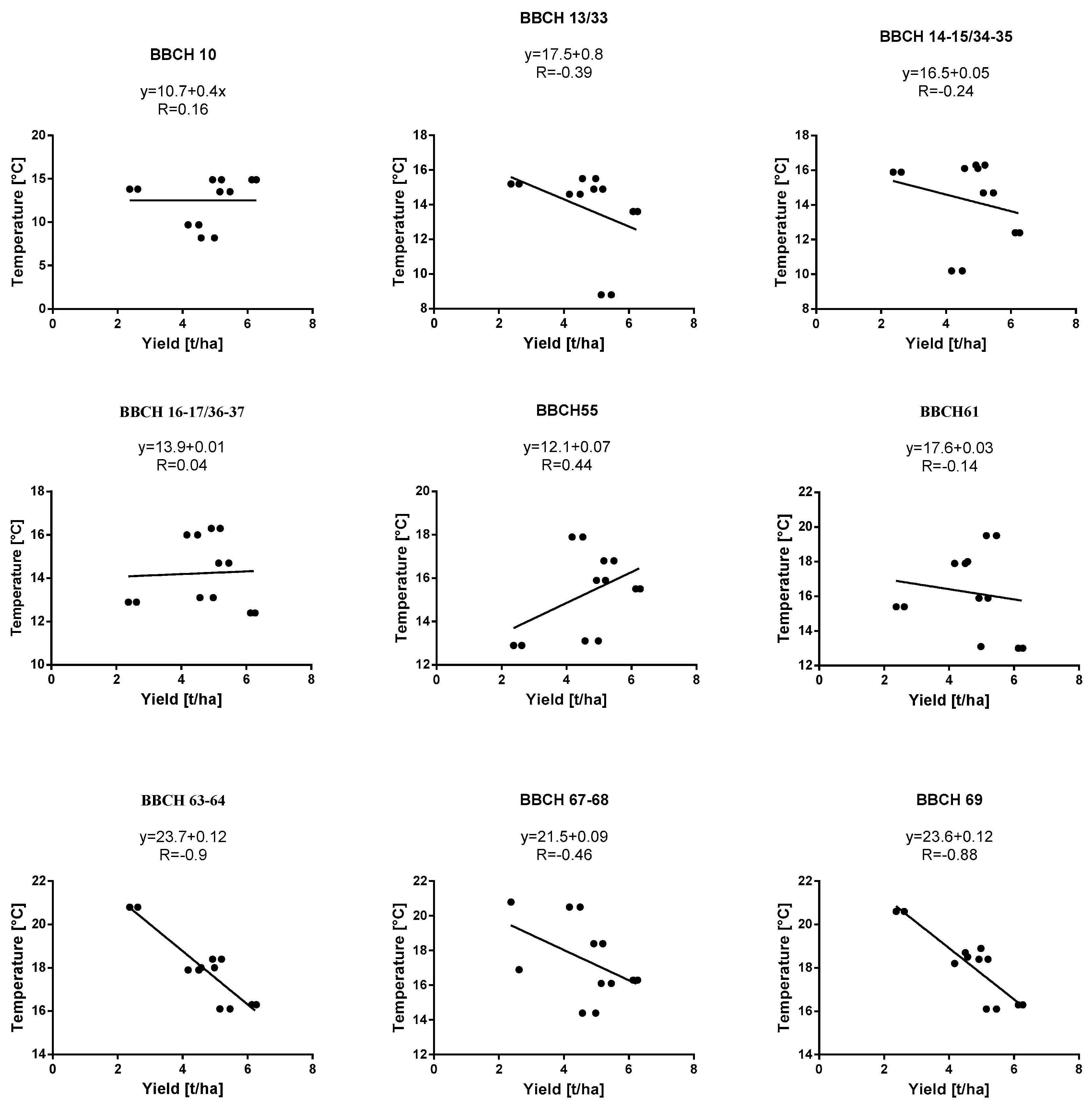
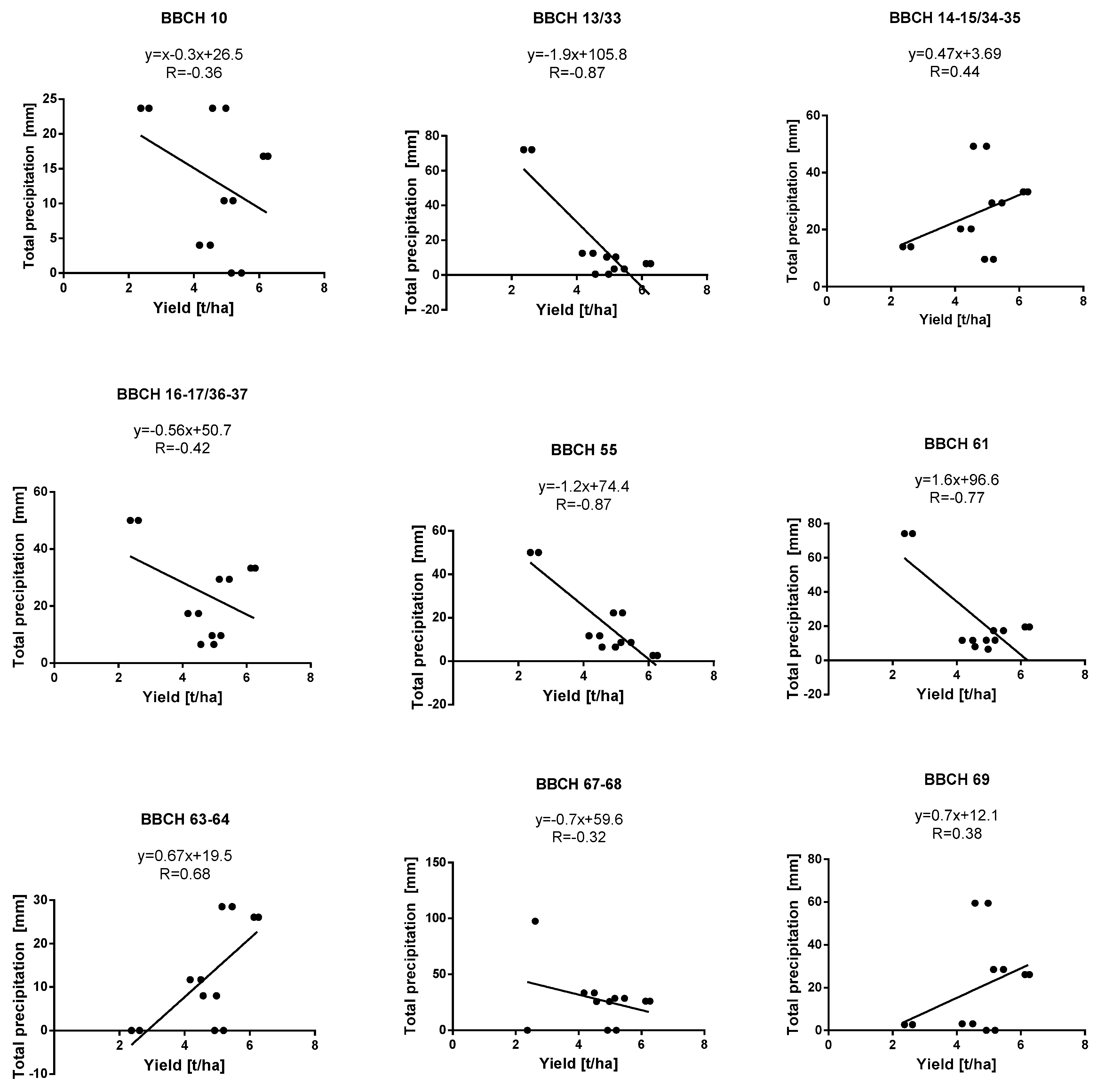

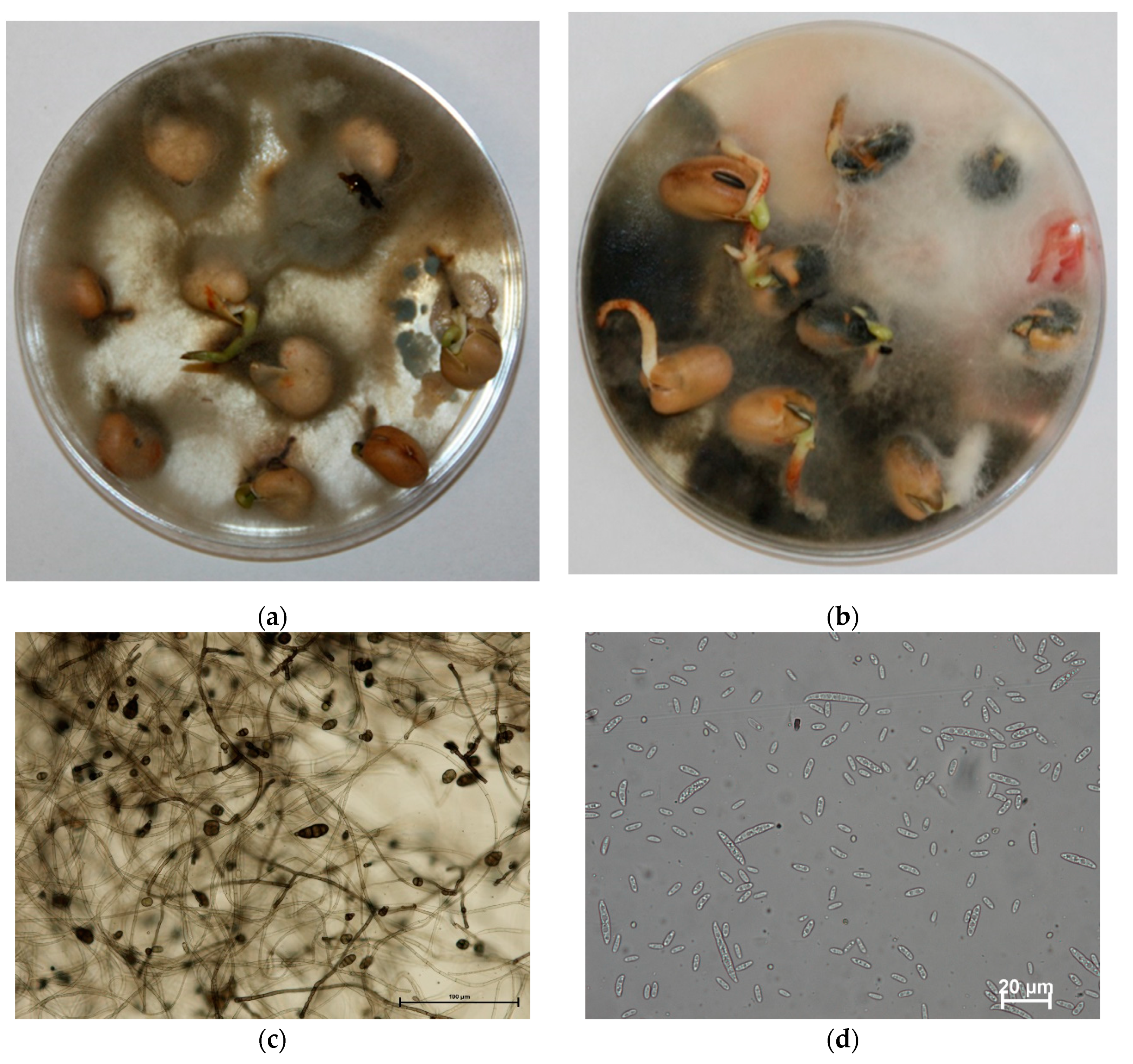
| Growth Stage | BBCH Key * | Warmia and Mazury (Bałcyny) | Lower Silesia Pawłowice (District of Wroclaw) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Olga | Granit | Olga | Granit | ||||||||||
| 2011 | 2012 | 2013 | 2011 | 2012 | 2013 | 2011 | 2012 | 2013 | 2011 | 2012 | 2013 | ||
| Emergence | 10 | 26 Apr | 28 Apr | 9 May | 26 Apr | 28 Apr | 9 May | 18 Apr | 19 Apr | 29 Apr | 18 Apr | 19 Apr | 29 Apr |
| 3 leaves unfolded | 13/33 | 5 May | 8 May | 19 May | 5 May | 8 May | 19 May | 27 Apr | 27 Apr | 10 May | 27 Apr | 27 Apr | 10 May |
| 4–5 leaves unfolded | 14–15/34–35 | 18 May | 15 May | 25 May | 18 May | 15 May | 24 Jun | 5 May | 4 May | 16 May | 5 May | 4 May | 16 May |
| 6–7 leaves unfolded | 16–17/36–37 | 21 May | 19 May | 29 May | 21 May | 19 May | 27 May | 16 May | 10 May | 25 May | 16 May | 10 May | 23 May |
| Inflorescence emergence | 55 | 24 May | 22 May | 5 Jun | 24 May | 21 May | 2 Jun | 23 May | 18 May | 31 May | 21 May | 16 May | 29 May |
| Beginning of flowering | 61 | 1 Jun | 28 May | 10 Jun | 1 Jun | 28 May | 8 Jun | 25 May | 23 May | 5 Jun | 24 May | 21 May | 3 Jun |
| 3–4 flowers open | 63–64 | 4 May | 3 Jun | 14 Jun | 4 Jun | 4 Jun | 12 Jun | 30 May | 28 May | 14 Jun | 29 May | 25 May | 12 Jun |
| 7–8 flowers open, pod development | 67–68 | 10 Jun | 16 Jun | 19 Jun | 8 Jun | 18 Jun | 17 Jun | 4 Jun | 8 Jun | 21 Jun | 2 Jun | 6 Jun | 18 Jun |
| End of flowering | 69 | 18 Jun | 26 May | 25 Jun | 17 Jun | 26 May | 23 Jun | 20 Jun | 21 Jun | 6 Jul | 16 Jun | 19 Jun | 2 Jul |
| Full ripeness | 89 | 17 Aug | 17 Aug | 12 Aug | 17 Aug | 18 Aug | 12 Aug | 10 Aug | 14 Aug | 10 Aug | 5 Aug | 9 Aug | 6 Aug |
| 2011 | Temperature (°C)/Daily Average | Precipitation (mm) | ||||||||
| Warmia and Mazury (Bałcyny) | ||||||||||
| Month | Days 1–10 | Days 11–20 | Days 21–30/31 | Month | Long-Term Average | Days 1–10 | Days 11–20 | Days 21–30/31 | Month | Long-Term Average |
| MAR | −2.4 | 3.8 | 4.5 | 2 | 1.4 | 0.3 | 5 | 3.3 | 8.6 | 28.5 |
| APR | 8.4 | 7.2 | 13.5 | 9.7 | 7 | 17.4 | 16.3 | 0 | 33.7 | 35.4 |
| MAY | 8.8 | 14.7 | 16.8 | 13.4 | 12.5 | 3.5 | 29.4 | 8.6 | 41.5 | 57.6 |
| JUN | 19.5 | 16.1 | 16.8 | 17.5 | 15.8 | 21 | 28.5 | 6.7 | 56.2 | 69.5 |
| JUL | 16.9 | 19.5 | 17.7 | 18 | 17.2 | 77.3 | 53.4 | 41.2 | 171.9 | 81.6 |
| AUG | 18.1 | 17.7 | 18.3 | 18 | 16.8 | 17 | 45 | 21.6 | 83.6 | 75.2 |
| Lower Silesia Pawłowice (District of Wroclaw) | ||||||||||
| MAR | 0.3 | 6 | 7.3 | 4.4 | 3.8 | 3.1 | 40.7 | 1.4 | 45.2 | 31.7 |
| APR | 11.4 | 9.7 | 14.6 | 11.9 | 8.3 | 10.5 | 4 | 12.5 | 27 | 30.5 |
| MAY | 10.2 | 16 | 17.9 | 14.8 | 14.1 | 20.3 | 17.4 | 11.7 | 49.4 | 51.3 |
| JUN | 20.5 | 18.7 | 18.2 | 19.1 | 16.9 | 33.4 | 3.1 | 59.2 | 95.5 | 59.5 |
| JUL | 18.1 | 20.3 | 16.4 | 18.2 | 18.7 | 54.7 | 34.7 | 81.5 | 170.9 | 78.9 |
| AUG | 19.3 | 19.4 | 19.1 | 19.3 | 17.9 | 14.1 | 34.9 | 29.9 | 78.9 | 61.7 |
| 2012 | Temperature (°C)/Daily Average | Precipitation (mm) | ||||||||
| Warmia and Mazury (Bałcyny) | ||||||||||
| Month | Days 1–10 | Days 11–20 | Days 21–30/31 | Month | Long-Term Average | Days 1–10 | Days 11–20 | Days 21–30/31 | Month | Long-Term Average |
| MAR | −0.2 | 4.6 | 5.8 | 3.4 | 1.4 | 3.5 | 4.2 | 13.6 | 21.3 | 28.5 |
| APR | 2.5 | 7.9 | 14.9 | 8.4 | 7 | 19.5 | 8.4 | 16.8 | 44.7 | 35.4 |
| MAY | 13.6 | 12.4 | 15.5 | 13.8 | 12.4 | 6.6 | 33.3 | 2.6 | 42.5 | 57.6 |
| JUN | 13 | 16.3 | 16.2 | 15.2 | 15.8 | 38 | 26.1 | 43.1 | 107.2 | 69.5 |
| JUL | 21.2 | 15.7 | 20 | 19 | 17.2 | 86.1 | 24.6 | 1.5 | 112.2 | 81.6 |
| AUG | 19.2 | 16.9 | 17.6 | 17.9 | 16.8 | 8.5 | 10.5 | 6.7 | 25.7 | 75.2 |
| Lower Silesia Pawłowice (District of Wroclaw) | ||||||||||
| Month | Days 1–10 | Days 11–20 | Days 21–30/31 | Month | Long-Term Average | Days 1–10 | Days 11–20 | Days 21–30/31 | Month | Long-Term Average |
| MAR | 1.9 | 7.8 | 8.4 | 6.1 | 3.8 | 4 | 1.8 | 7.9 | 13.7 | 31.7 |
| APR | 5.6 | 8.2 | 15.5 | 9.8 | 8.9 | 3.4 | 23.7 | 0.5 | 27.6 | 30.5 |
| MAY | 16.1 | 13.1 | 18 | 15.8 | 14.4 | 49.2 | 6.5 | 8 | 63.7 | 51.3 |
| JUN | 14.4 | 18.5 | 18.9 | 17.3 | 17.1 | 25.7 | 59.5 | 9.5 | 94.7 | 59.5 |
| JUL | 22.3 | 17.4 | 20.3 | 20 | 19.3 | 42.8 | 38.5 | 26.7 | 108 | 78.9 |
| AUG | 20.4 | 18.1 | 19.5 | 19.3 | 18.3 | 37.6 | 8.7 | 26.9 | 73.2 | 61.7 |
| 2013 | Temperature (°C)/Daily Average | Precipitation (mm) | ||||||||
| Warmia and Mazury (Bałcyny) | ||||||||||
| Month | Days 1–10 | Days 11–20 | Days 21–30/31 | Month | Long-Term Average | Days 1–10 | Days 11–20 | Days 21–30/31 | Month | Long-Term Average |
| MAR | 0.7 | −7.4 | −5.2 | −4 | 1.4 | 2.8 | 3.8 | 7.4 | 14 | 28.5 |
| APR | −0.4 | 9.2 | 10.2 | 6.3 | 7 | 11.8 | 7.4 | 3.3 | 22.5 | 35.4 |
| MAY | 14.9 | 16.3 | 13.8 | 15 | 12.4 | 10.4 | 9.6 | 26.2 | 46.2 | 57.6 |
| JUN | 15.9 | 18.4 | 17.8 | 17.4 | 15.8 | 22.2 | 0 | 23.2 | 45.4 | 69.5 |
| JUL | 18 | 16.4 | 19.2 | 17.9 | 17.2 | 13.3 | 123.5 | 27 | 163.8 | 81.6 |
| AUG | 21.3 | 17.4 | 15.6 | 18.1 | 16.8 | 16.2 | 8.6 | 0.5 | 25.3 | 75.2 |
| Lower Silesia Pawłowice (District of Wroclaw) | ||||||||||
| Month | Days 1–10 | Days 11–20 | Days 21–30/31 | Month | Long-Term Average | Days 1–10 | Days 11–20 | Days 21–30/31 | Month | Long-Term Average |
| MAR | 21.1 | −2.3 | −2.4 | −0.9 | 3.8 | 19.2 | 15.4 | 8.4 | 43 | 31.7 |
| APR | 1.5 | 12.1 | 13.8 | 9.2 | 8.9 | 5.8 | 13.2 | 23.7 | 42.7 | 30.5 |
| MAY | 15.2 | 15.9 | 12.9 | 14.6 | 14.4 | 72 | 14 | 50 | 136 | 51.3 |
| JUN | 15.4 | 20.8 | 16.9 | 17.7 | 7.1 | 74.1 | 0 | 97.6 | 171.7 | 59.5 |
| JUL | 20.6 | 18.7 | 22 | 20.5 | 19.3 | 2.7 | 16 | 17.6 | 36.3 | 78.9 |
| AUG | 23.1 | 17.9 | 16.3 | 19 | 18.3 | 45.8 | 16.5 | 5.9 | 68.2 | 61.7 |
| Year | Cultivar/Region (dt/ha) | |||
|---|---|---|---|---|
| Olga (WM) | Olga (LS) | Granit (WM) | Grant (LS) | |
| 2011 | 51.5 | 41.7 | 54.6 | 45.0 |
| 2012 | 61.3 | 49.8 | 62.7 | 45.7 |
| 2013 | 49.2 | 26.2 | 52.0 | 23.7 |
| Mean | 54.0 | 39.23 | 56.43 | 38.13 |
| No. | Fungal Species | Cultivar/Region | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indeterminate | Determinate | ||||||||||||||
| Olga (WM) | Olga (LS) | Total | Granit (WM) | Granit (LS) | Total | ||||||||||
| 2011 | 2012 | 2013 | 2011 | 2012 | 2013 | 2011 | 2012 | 2013 | 2011 | 2012 | 2013 | ||||
| 1. | Ascochyta fabae Speg. | 4 | 4 | ||||||||||||
| 2. | Botrytis cinerea Pers. ex Pers. | 2 | 2 | 2 | 6 | 10 | 2 | 12 | |||||||
| 3. | Botrytis fabae Sard | 12 | 1 | 13 | 19 | 19 | |||||||||
| 4. | Fusarium avenaceum (Corda ex Fr.) Sacc. | 2 | 1 | 3 | |||||||||||
| 5. | Fusarium poae (Peck) Wollenw. | 2 | 2 | ||||||||||||
| 6. | Fusarium sporotrichioides Scherb. | 3 | 3 | ||||||||||||
| 7. | Fusarium spp. | 2 | 2 | ||||||||||||
| 8. | Acremonium spp. | 5 | 5 | ||||||||||||
| 9. | Alternaria alternata (Fr.) Keissler | 61 | 10 | 3 | 46 | 4 | 21 | 145 | 59 | 22 | 32 | 16 | 42 | 171 | |
| 10. | Aspergillus spp. | 2 | 2 | 4 | 4 | ||||||||||
| 11. | Cladosporium cladospirioides (Fries.) de Vries | 6 | 14 | 5 | 2 | 2 | 29 | 20 | 12 | 4 | 2 | 2 | 10 | 50 | |
| 12. | Epicoccum nigrum Link Schol-Schwarz | 4 | 4 | ||||||||||||
| 13. | Mucor spp. | 4 | 2 | 6 | 4 | 2 | 6 | ||||||||
| 14. | Penicillium spp. | 25 | 2 | 3 | 2 | 7 | 39 | 46 | 21 | 2 | 20 | 6 | 95 | ||
| 15. | Rhizopus nigricans Ehernb. | 4 | 13 | 14 | 2 | 33 | 28 | 8 | 6 | 42 | |||||
| 16. | Trichoderma spp. | 6 | 6 | 4 | 6 | 10 | |||||||||
| Total isolates | 112 | 33 | 28 | 57 | 23 | 36 | 289 | 166 | 71 | 39 | 44 | 44 | 58 | 422 | |
| Percentage of pathogenic fungi (%) | 12.5 | 9.09 | 0 | 0 | 13.04 | 11.11 | 8.30 | 19.88 | 8.45 | 2.56 | 0 | 0 | 0 | 9.48 | |
| Percentage of saprophytic fungi (%) | 87.5 | 90.91 | 100 | 100 | 90.91 | 88.89 | 91.70 | 80.12 | 91.55 | 97.44 | 100 | 100 | 100 | 90.52 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pszczółkowska, A.; Okorski, A.; Fordoński, G.; Kotecki, A.; Kozak, M.; Dzienis, G. Effect of Weather Conditions on Yield and Health Status of Faba Bean Seeds in Poland. Agronomy 2020, 10, 48. https://doi.org/10.3390/agronomy10010048
Pszczółkowska A, Okorski A, Fordoński G, Kotecki A, Kozak M, Dzienis G. Effect of Weather Conditions on Yield and Health Status of Faba Bean Seeds in Poland. Agronomy. 2020; 10(1):48. https://doi.org/10.3390/agronomy10010048
Chicago/Turabian StylePszczółkowska, Agnieszka, Adam Okorski, Gabriel Fordoński, Andrzej Kotecki, Marcin Kozak, and Grzegorz Dzienis. 2020. "Effect of Weather Conditions on Yield and Health Status of Faba Bean Seeds in Poland" Agronomy 10, no. 1: 48. https://doi.org/10.3390/agronomy10010048
APA StylePszczółkowska, A., Okorski, A., Fordoński, G., Kotecki, A., Kozak, M., & Dzienis, G. (2020). Effect of Weather Conditions on Yield and Health Status of Faba Bean Seeds in Poland. Agronomy, 10(1), 48. https://doi.org/10.3390/agronomy10010048






