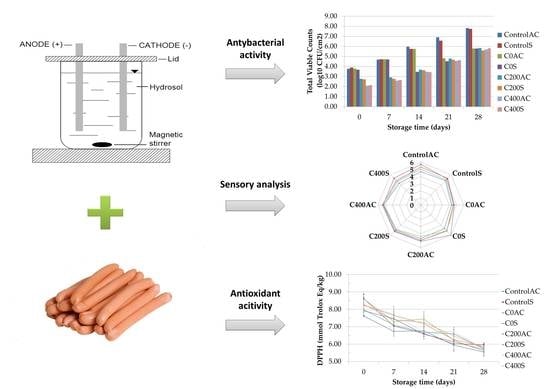
The Effects of Using Sodium Alginate Hydrosols Treated with Direct Electric Current as Coatings for Sausages
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Electrical Treatment of Sodium Alginate Hydrosols
2.3. Surface Treatment of Commercial Sausages with the Experimental Material
2.4. Pork Sausage Quality Characterization
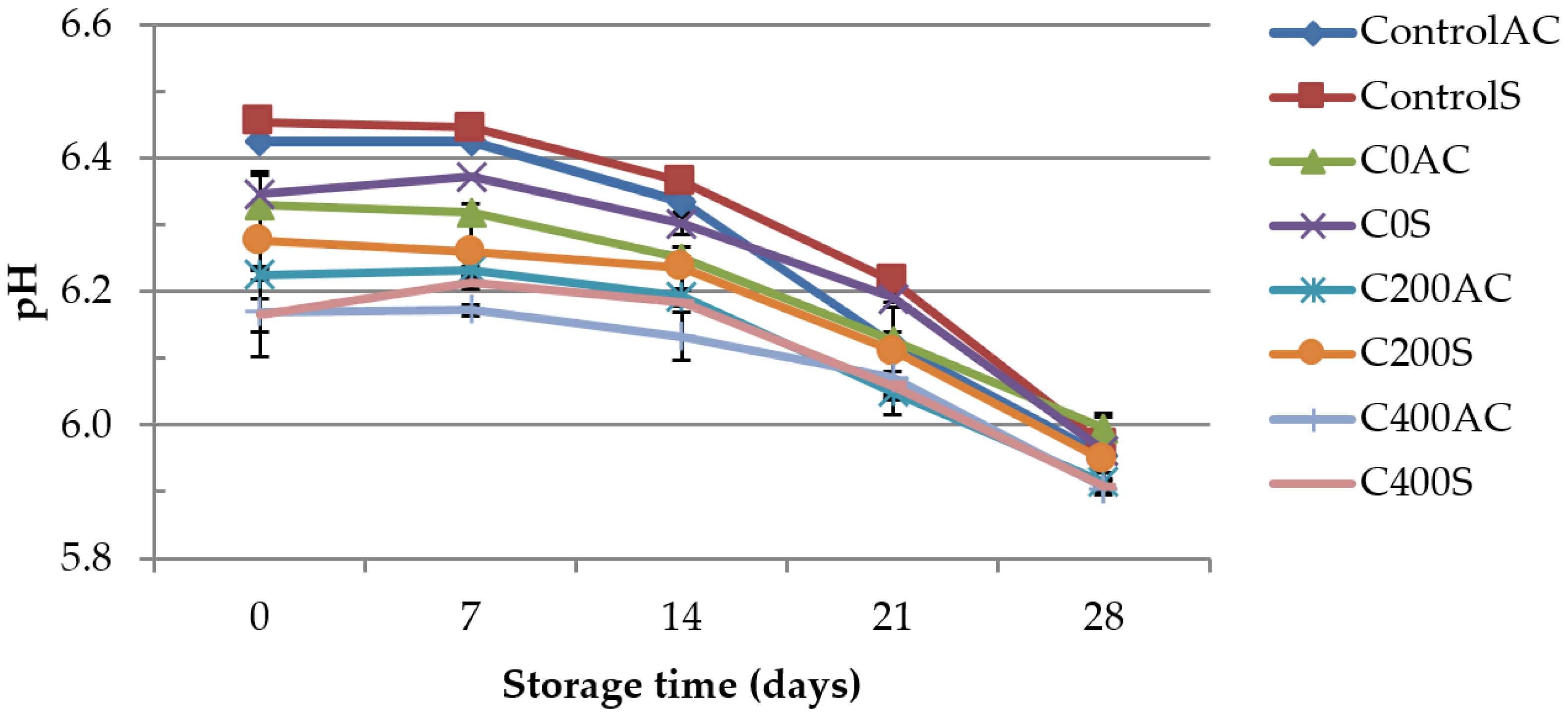
2.4.1. pH Measurement
2.4.2. Microbiological Analysis
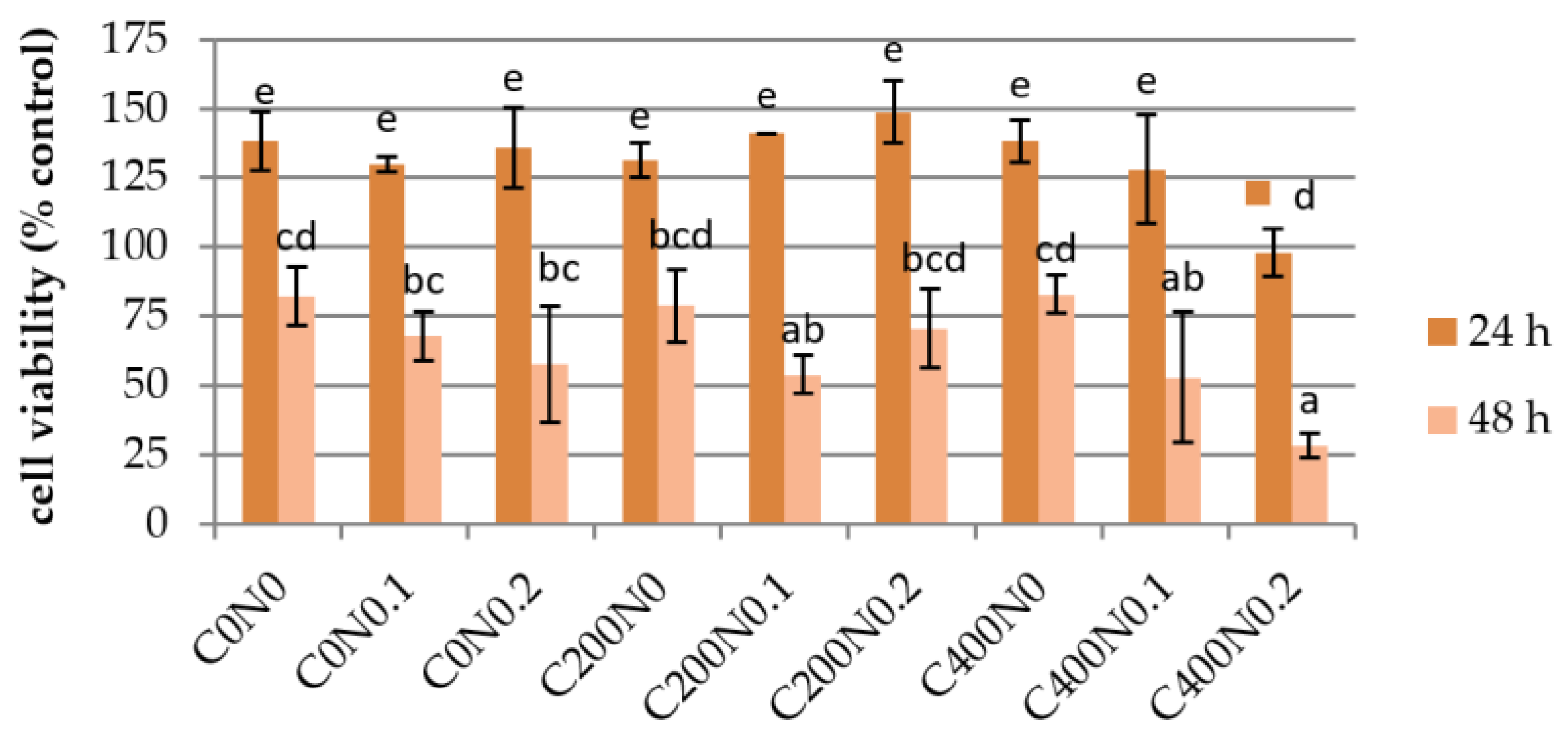
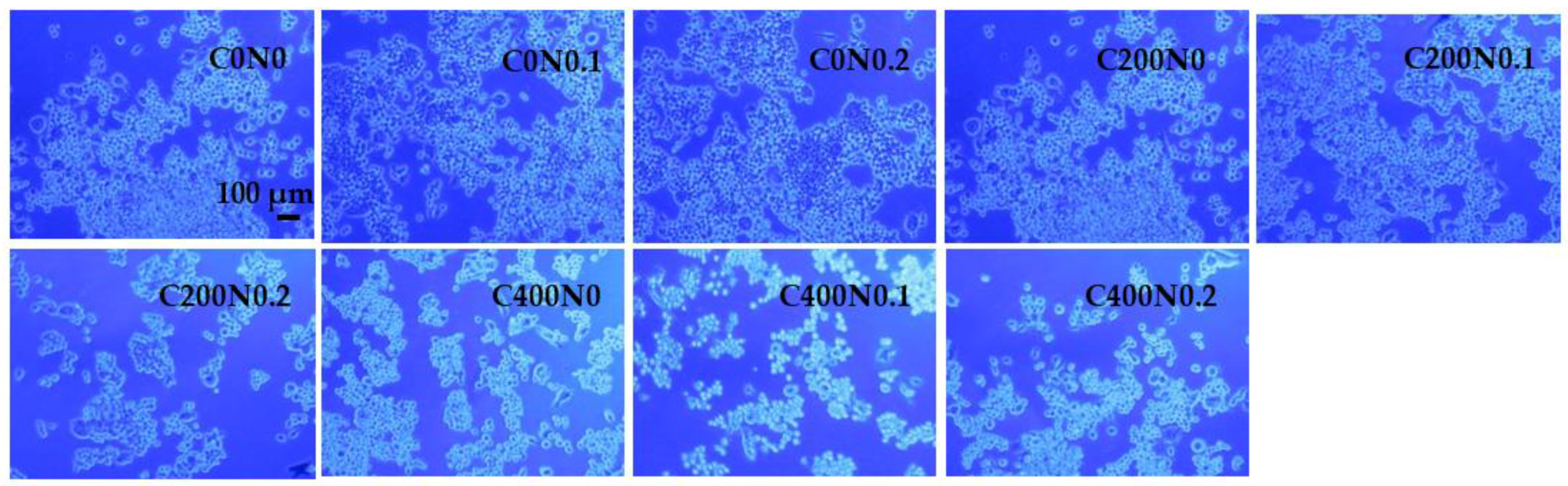
2.4.3. In Vitro Assessment of Sodium Alginate Hydrogels Treated with DC on Cytotoxicity to Mouse RAW 264.7 Macrophages and L929 Fibroblastic Cell Lines
Cell Culture
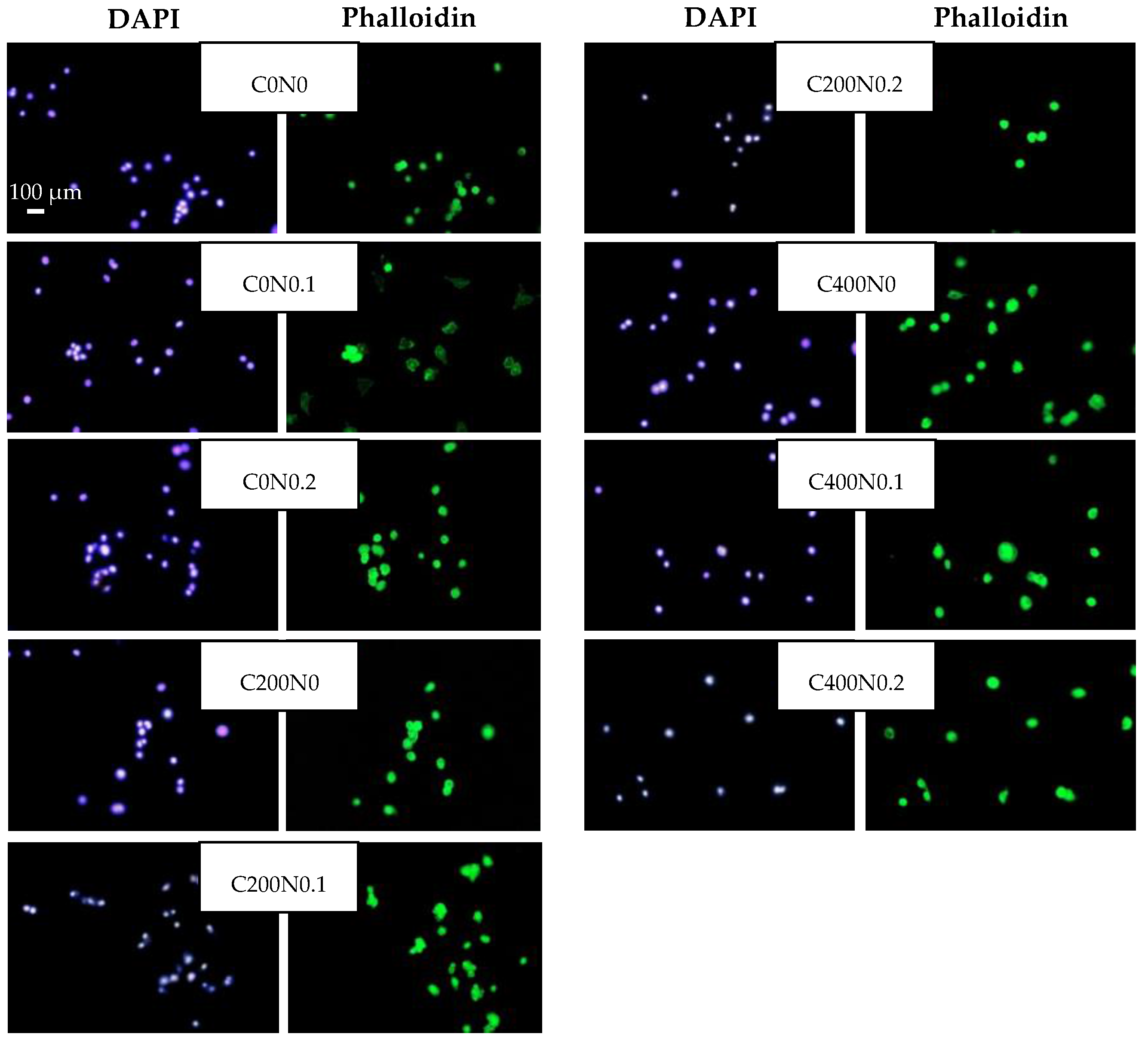
Cell Proliferation Assay
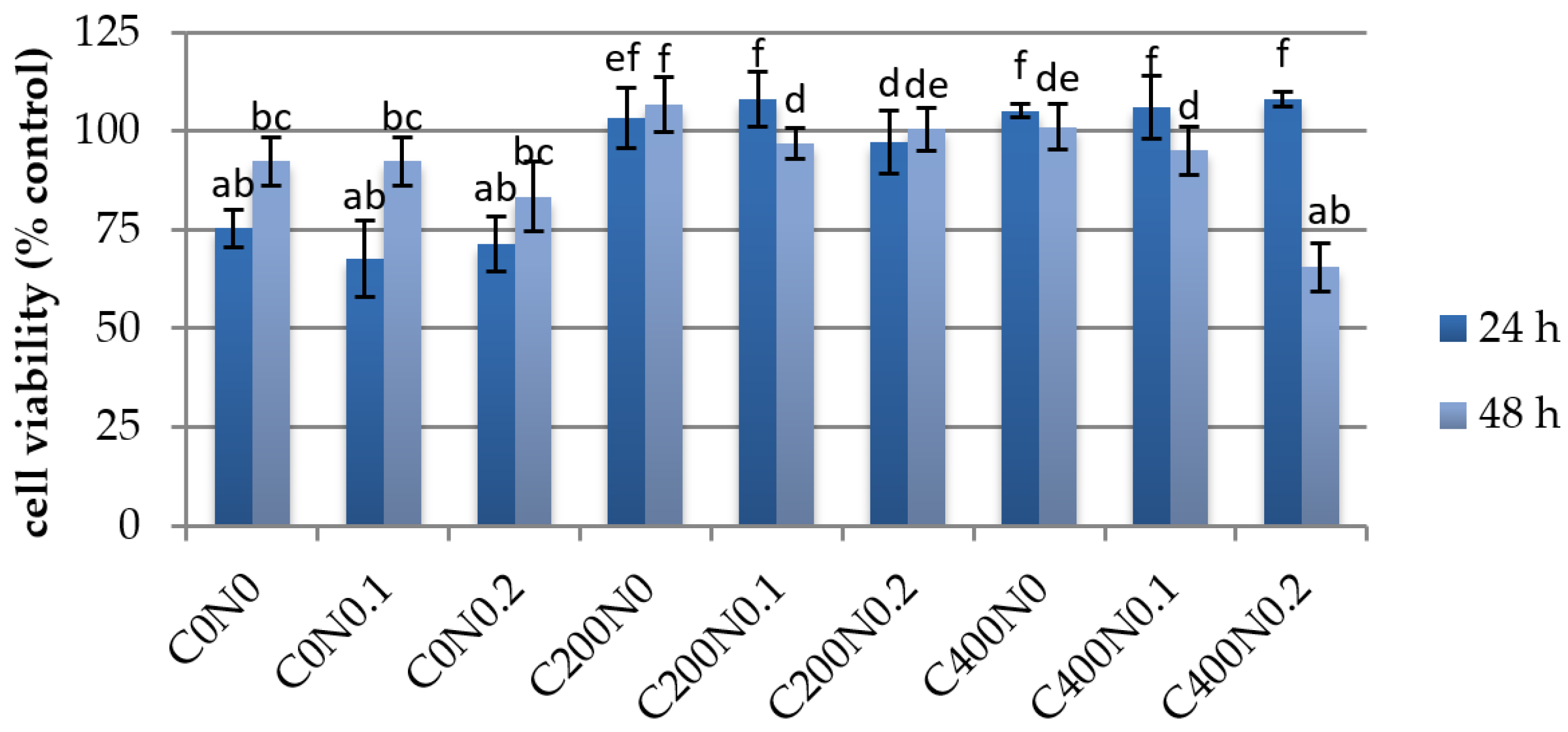
Cell Viability
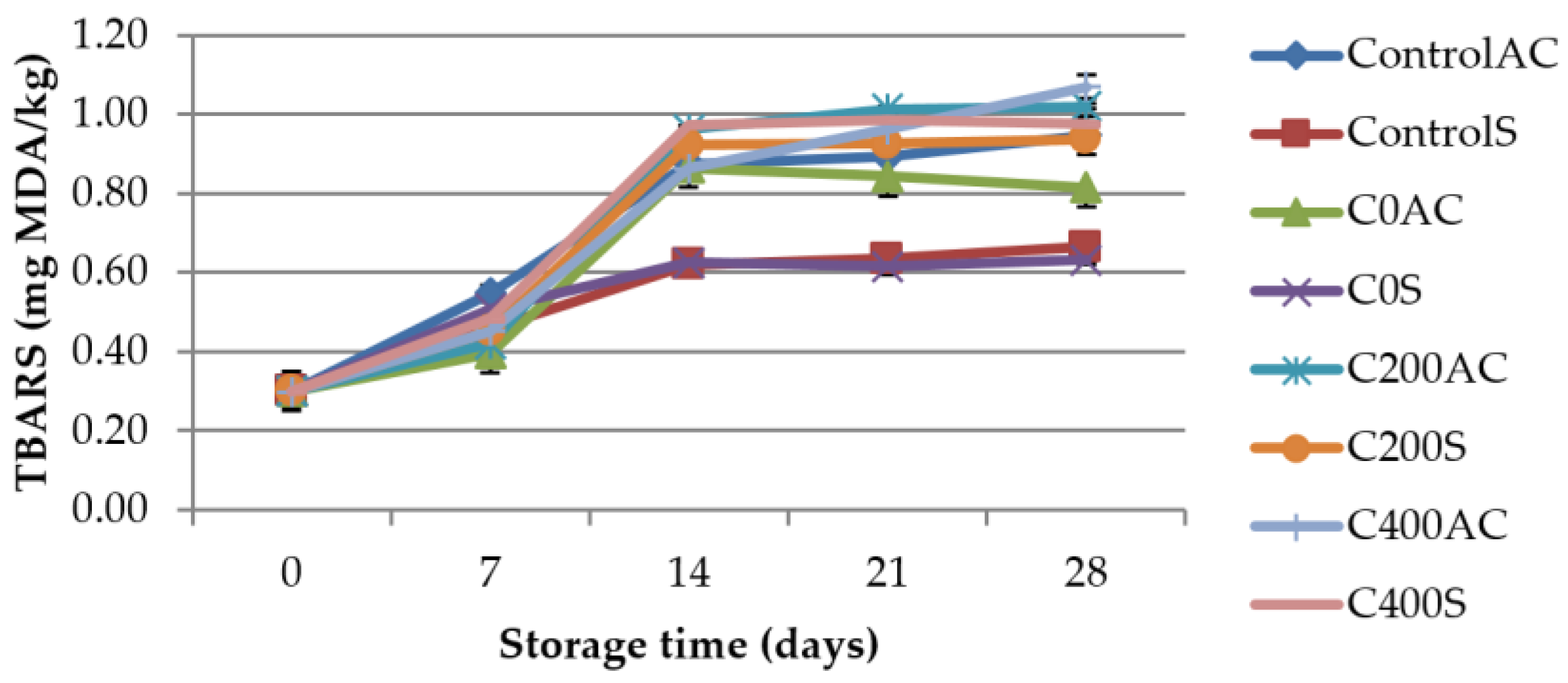
2.4.4. Determination of Thiobarbituric Acid-Reactive Substances (TBARS)
2.4.5. Total Antioxidant Capacity (TAC) Measurements
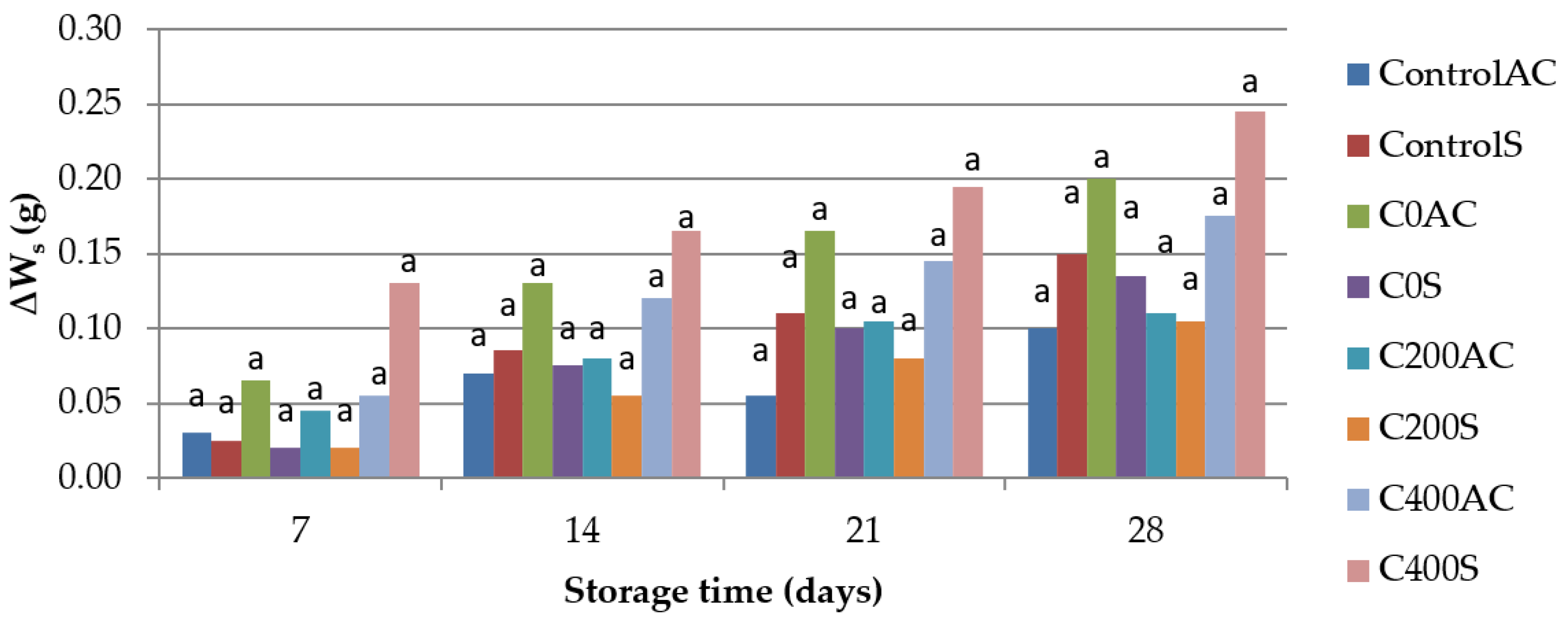
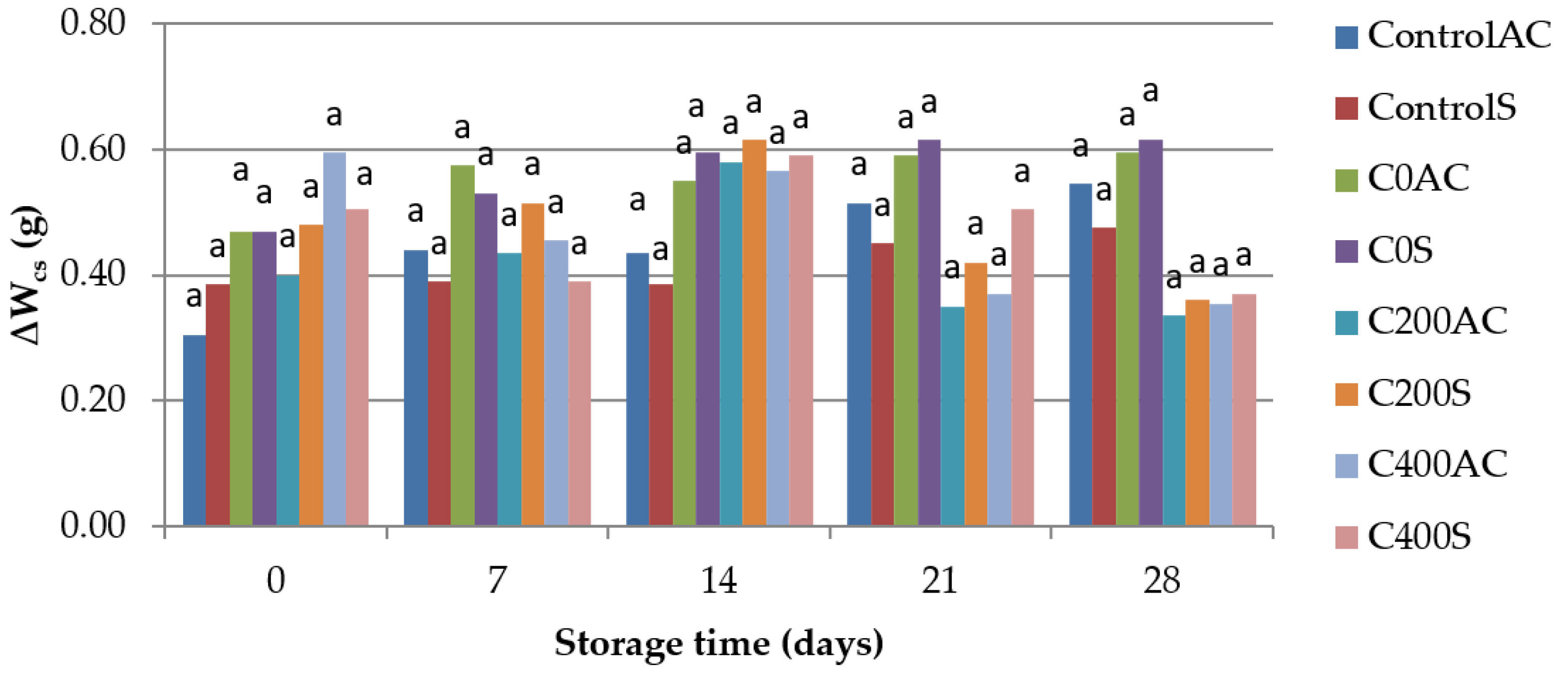
2.4.6. Weight Loss
- Storage losses of weight of sausages (∆Ws), which was calculated using the following equation:where W0 is the weight of a sausage on the first day of storage and Wst is the weight at a certain time of storage.
- Cooking losses of weight of sausages (∆Wcs), which was calculated using the equation of:where Wuc is the weight of uncooked sausage and Wc is the weight of sausage after cooking for 3 min.
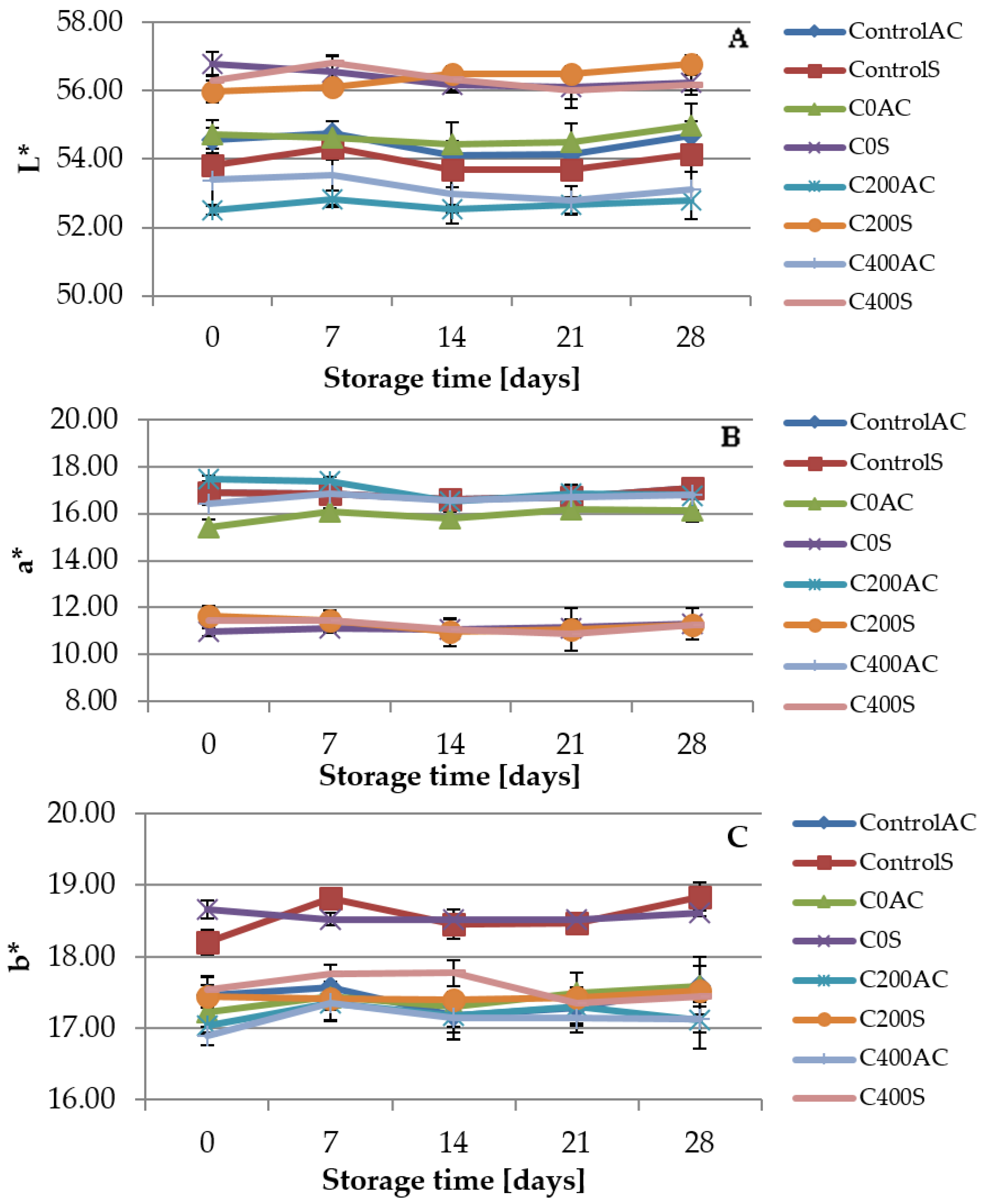
2.4.7. Color Measurement
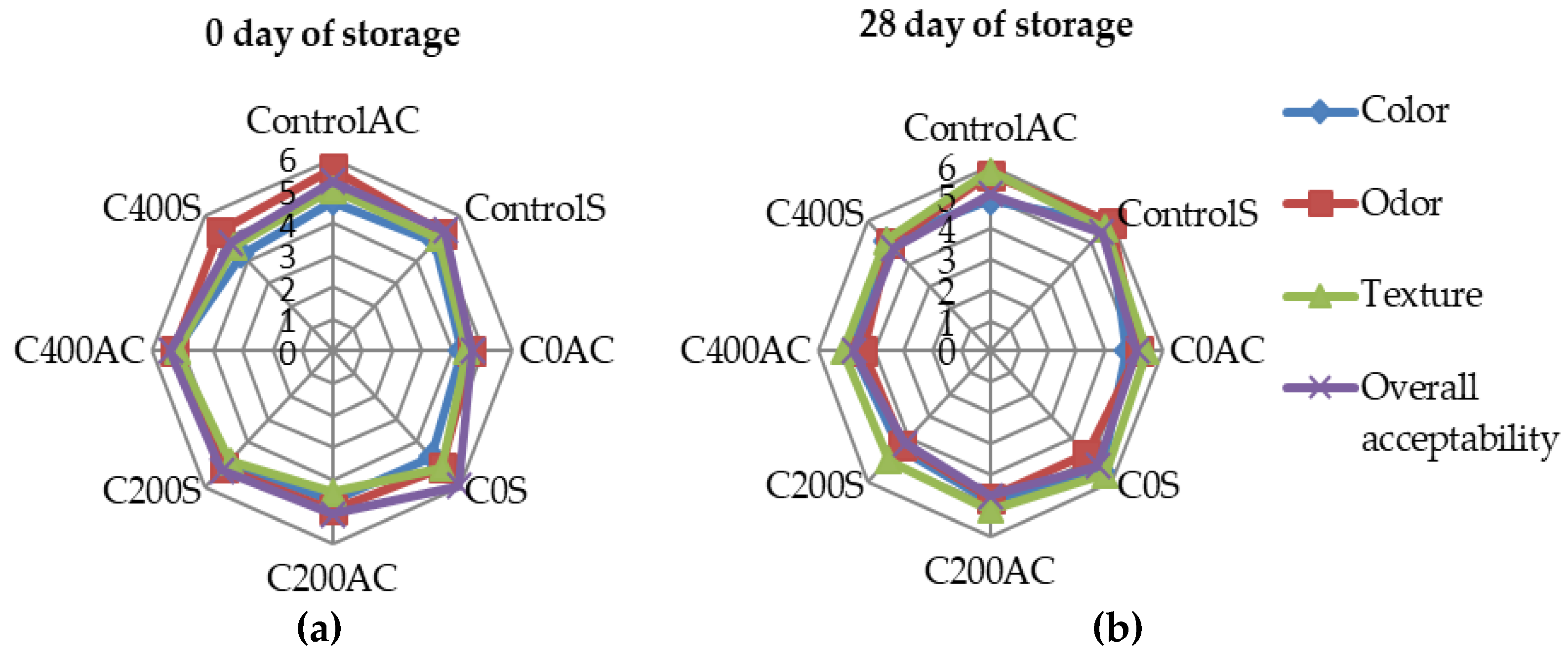
2.4.8. Sensory Evaluations
2.5. Statistical Analysis
3. Results
3.1. pH Measurement
3.2. Microbiological Analysis
3.3. In Vitro Assessment of Sodium Alginate Hydrogels Treated with DC on Cytotoxicity to Mouse RAW 264.7 Macrophages and L929 Fibroblastic Cell Lines
3.4. Determination of Thiobarbituric Acid-Reactive Substances (TBARS)
3.5. Total Antioxidant Capacity (TAC) Measurements
3.6. Weight Measurement
3.7. Color Measurement
3.8. Sensory Evaluations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coma, V. Bioactive packaging technologies for extended shelf life of meat based products. Meat Sci. 2008, 79, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Philips, T.W.; Williams, J.B.; Smith, B.S.; Schilling, M.W. Developing food-grade coatings for dry-cured hams to protect against ham mite infestation. Meat Sci. 2016, 113, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Woraprayote, W.; Yuwares, M.; Sorapukdee, S.; Swetwiwathana, A.; Banjakul, S.; Visessanguan, W. Bacteriocins from lactic acid bacteria and their application in meat and meat products. Meat Sci. 2016, 120, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, D.D.; Jo, C. Essential oils as potential antimicrobial agents in meat products: A review. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- Issa-Zacharia, A.; Kamitani, Y.; Tiisekwa, A.; Morita, K.; Koichi, I. In Vitro inactivation of Escherichia coli, Staphylococcus aureus and Salmonella spp. using slightly acidic electrolyzed water. J. Biosci. Bioeng. 2010, 110, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Skurtys, O.; Acevedo, C.; Pedreschi, F.; Enrione, J.; Osorio, F.; Aguliera, J.M. Food hydrocolloids edible films and coatings. In Food Hydrocolloids: Characteristics, Properties and Structures; Hollingworth, C.S., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; pp. 41–80. [Google Scholar]
- Adzaly, N.Z.; Jackson, A.; Villabos-Carvajal, R.; Kang, I.; Almenar, E. Development of a novel sausage casing. J. Food Eng. 2015, 152, 24–31. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Gao, Y.; Ye, C.; Wang, H. Effect of collagen-lysozyme coating on fresh-salmon fillets preservation. LWT Food Sci. Technol. 2017, 75, 59–64. [Google Scholar] [CrossRef]
- Song, Z.; Li, F.; Guan, H.; Xu, Y.; Li, D. Combination of nisin and polylysine with chitosan coating inhibits the white blush of fresh-cut carrots. Food Control 2017, 74, 34–44. [Google Scholar] [CrossRef]
- Narsaiah, K.; Wilson, R.A.; Gokul, K.; Mandge, H.M.; Jha, S.N.; Bhadwal, S.; Anurag, R.K.; Malik, R.K.; Vij, S. Effect of bacteriocin-incorporated alginate coating on shelf-life of minimally processed papaya (Carica papaya L.). Postharvest Biol. Technol. 2015, 100, 212–218. [Google Scholar] [CrossRef]
- Raesi, M.; Tabaraei, A.; Hashemi, M.; Behnampour, N. Effect of sodium alginate coating incorporated with nisin, Cinamonum zeylanicum, and rosemary essential oils on microbial quality of chicken meat and fate of Listeria monocytogenes during refrigeration. Int. J. Food Microbiol. 2016, 238, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ulbin-Figlewicz, N.; Zimoch-Korzycka, A.; Jarmoluk, A. Antibacterial activity and physical properties of edible chitosan films exposed to low-pressure plasma. Food Bioprocess Technol. 2014, 7, 3646–3654. [Google Scholar] [CrossRef]
- Brychcy, E.; Król, Ż.; Kulig, D.; Jarmoluk, A. The effect of carrageenan and gelatine hydosols incorporated with acidic electrolysed water on surface microbiota and quality changes on pork meat. Int. J. Food Sci. Technol. 2016, 51, 1618–1629. [Google Scholar] [CrossRef]
- Król, Ż.; Malik, M.; Marycz, K.; Jarmoluk, A. Physicochemical properties of biopolymer hydrogels treated by direct electric current. Polymers 2016, 8, 248. [Google Scholar] [CrossRef]
- Król, Ż.; Malik, M.; Marycz, K.; Jarmoluk, A. Characteristic of gelatin, carrageenan and sodium alginate hydrosols treated by direct electric current. Polymers 2016, 8, 275. [Google Scholar] [CrossRef]
- Król, Ż.; Marycz, M.; Kulig, D.; Marędziak, M.; Jarmoluk, A. Cytotocixity, bactericidal, and antioxidant activity of sodium alginate hydrosols treated with direct electric Current. Int. J. Mol. Sci. 2017, 18, 678. [Google Scholar] [CrossRef] [PubMed]
- Comaposada, J.; Gou, P.; Marcos, B.; Arnau, J. Physical properties of sodium alginate solutions and edible wet calcium alginate coatings. LWT Food Sci. Technol. 2015, 64, 212–219. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Macros, B.; Gou, P.; Arnau, J.; Comaposada, J. Influence of processing conditions on the properties of alginate solutions ad wet edible calcium alginate coating. LWT Food Sci. Technol. 2016, 74, 271–279. [Google Scholar]
- Kulig, D.; Zimoch-Korzycka, A.; Król, Ż.; Oziembłowski, M.; Jarmoluk, A. Effect of film-forming alginate/chitosan polyelectrolyte complex on the storage quality of pork. Molecules 2017, 22, 98. [Google Scholar] [CrossRef] [PubMed]
- ISO 18593:2004. Microbiology of food and animal feeding stuffs. Horizontal methods for sampling techniques from surfaces using contact plates and swabs.
- ISO 17604:2003. Microbiology of food and animal feeding stuffs. Carcass sampling for microbiological analysis.
- ISO 2293:1988. Meat and meat products. Enumeration of microorganisms—Colony count technique at 30 °C.
- ISO 17410:2001. Microbiology of food and animal feeding stuffs. Horizontal method for the enumeration of psychrotrophic microorganisms.
- ISO 21527-1:2008. Microbiology of food and animal feeding stuffs - Horizontal method for the enumeration of yeasts and moulds - Part 1: Colony count technique in products with water activity greater than 0.95.
- ISO 13721:1995. Meat and meat products. Enumeration of lactic acid bacteria—Colony-count technique at 30 °C.
- ISO 5552:2005. Meat and meat products. Detection and enumeration of Enterobacteriaceae without resuscitation—MPN technique and colony count technique.
- Grzesiak, J.; Marycz, K.; Wrzeszcz, K. Isolation and morphological characterization of ovine adipose-derived mesenchymal stem cells in culture. Int. J. Stem Cells 2011, 4, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.; Marycz, K.; Poniewierska-Baran, A. Very small embryonic-like stem cells as a novel development concept and the hierarchy of the stem cell compartment. Adv. Med. Sci. 2014, 59, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Jiang, M.; Rui, X.; Li, W.; Chen, X.H.; Dong, M.S. Effect of rose polyphenols on oxidation, biogenic amines and microbial diversity in naturally dry fermented sausages. Food Control 2017, 78, 324–330. [Google Scholar] [CrossRef]
- Slima, S.B.; Ktari, N.; Trabelski, I.; Triki, M.; Tounski, M.F.; Moussa, H.; Makni, I.; Herrero, A.; Jimenez-Colmenere, F.; Perez, C.R.-C. Effect of partial replacement of nitrite with novel probiotic Lactobacillus plantarum TN8 on color, physico-chemical, texture and microbiological properties of beed sausages. LWT Food Sci. Technol. 2017, 86, 219–226. [Google Scholar] [CrossRef]
- Huang, Y.R.; Hung, Y.-C.; Hsu, S.Y.; Huang, Y.W.; Hwang, D.F. Application of electrolyzed water in the food industry. Food Control 2008, 19, 329–345. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, B.D. Storage Quality and Shelf Life of Functional Restructured Spent Hen Meat Blocks in Vacuum Packaging at Refrigerated Storage (4 ± 1 °C). Agric. Res. 2016, 5, 391–397. [Google Scholar] [CrossRef]
- Sharma, H.; Mendiratta, S.K.; Agrawal, R.K.; Gurunathan, K.; Kumar, S.; Singh, T.P. Use of various essential oils as bio preservatives and their effect on the quality of vacuum packed fresh chicken sausages and frozen condition. LWT Food Sci. Technol. 2017, 81, 118–127. [Google Scholar] [CrossRef]
- Mathenjwa, S.A.; Hugo, C.J.; Bothma, C.; Hugo, A. Effect of alternative preservation on the microbial quality, lipid stability and sensory evaluation of beorewors. Meat Sci. 2012, 91, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Garriga, M.; Grebol, N.; Aymerich, M.T.; Monfort, J.M.; Hugas, M. Microbial inactivation after high-pressure processing at 600 MPa in commercial meat products over its shelf life. Innov. Food Sci. Emerg. Technol. 2004, 5, 451–457. [Google Scholar] [CrossRef]
- Vercammen, A.; Vanoirbeek, K.G.A.; Lurquin, I.; Steen, L.; Geomaere, O.; Szczepaniak, S.; Paelinck, H.; Hendrickx, M.E.G.; Michiels, C.W. Shelf-life extension of cooked ham model products by high hydrostatic pressure and natural preservatives. Innov. Food Sci. Emerg. Technol. 2011, 12, 407–415. [Google Scholar] [CrossRef]
- Zimoch-Korzycka, A.; Jarmoluk, A. Polysaccharide-based edible coating containing cellulase for improved preservation of meat quality during storage. Molecules 2017, 22, 390. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, L.; Devlieghere, F.; Graef, V.; Debevere, J. Un vitro and in situ growth characteristic and behavior of spoilage organisms associated with anaerobically stored cooked meat products. J. Appl. Microbiol. 2005, 98, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Drees, K.P.; Abaszadegan, M.; Maier, R.M. Comparative electrochemical inactivation of bacteria and bacteriophage. Water Res. 2003, 37, 2291–2300. [Google Scholar] [CrossRef]
- Luo, K.; Kim, S.Y.; Wang, J.; Oh, D.-H. A combined hurdle approach of slightly acidic electrolyzed water simultaneous with ultrasound to inactivate Bacillus cereus on potato. LWT Food Sci. Technol. 2016, 73, 615–621. [Google Scholar] [CrossRef]
- Ohshima, T.; Tanino, T.; Kameda, T.; Harashima, H. Engineering of operation condition in milk pasteurization PEF treatment. Food Control 2016, 68, 297–302. [Google Scholar] [CrossRef]
- She, P.; Song, B.; Xing, X.-H.; Loosdrecht, M.; Liu, Z. Electrolytic stimulation of bacteria Enterobacter dissolvensby a direct current. Biochem. Eng. J. 2006, 28, 23–29. [Google Scholar] [CrossRef]
- Jackman, S.A.; Maini, G.; Sharman, A.K.; Knowles, C.J. The effects of direct electric current on the viability and metabolism of acidophilic bacteria. Enzyme Microb. Technol. 1999, 24, 316–324. [Google Scholar] [CrossRef]
- Król, Ż.; Jarmoluk, A. The effects of using a direct electric current on the chemical properties of gelatine gels and bacterial growth. J. Food Eng. 2015, 170, 1–7. [Google Scholar] [CrossRef]
- Al-Qadiri, H.M.; Al-Holy, M.A.; Shiroodi, S.G.; Ovissipour, M.; Govindan, B.N.; Al-Alami, N.; Sablani, S.S.; Rasco, B. Effect of acidic electrolyzed water-induced bacterial inhibition and injury in live clam (Venerupis philippinarum) and mussel (Mytilus edulis). Int. J. Food Microbiol. 2016, 231, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Tirawat, D.; Phongpaichit, S.; Benjakul, S.; Sumpavapol, P. Microbial load reduction of sweet basil using acidic electrolyzed water and lactic acid in combination with mild heat. Food Control 2016, 64, 29–36. [Google Scholar] [CrossRef]
- Marita, C.; Nishida, T.; Ito, K. Biological toxicity of acid electrolyzed functional water: Effect of oral administration on mouse digestive tract and changes in body weight. Arch. Oral Biol. 2011, 56, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Kubota, A.; Nose, K.; Yonekura, T.; Kosumi, T.; Yamauchi, K.; Oyanagi, H. Effects of electrolyzed strong acid water on peritoneal irrigation of experimental perforated peritonitis. Surg. Today 2009, 39, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Mokudai, T.; Kanno, T.; Niwano, Y. Involvement of reactive oxygen species in the cytotoxic effect ofaid-electrolyzed water. J. Toxicol. Sci. 2015, 40, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H. Electrolyzed water as a disinfectant for fresh-cut vegetables. J. Food Sci. 1999, 64, 536–539. [Google Scholar] [CrossRef]
- Shimamura, Y.; Shinke, M.; Hiraishi, M.; Tsuchiya, Y.; Masuda, S. The application of alkaline and acidic electrolyzed water in the sterilization of chicken breasts and beef liver. Food Sci. Nutr. 2015, 4, 431–440. [Google Scholar] [CrossRef] [PubMed]
- El-Nashi, H.B.; Fattah, A.; Rahman, N.R.A.; El-Razik, M.M. Quality characteristic of beef sausage containing pomegranate peels during refrigerated storage. Ann. Agric. Sci. 2015, 60, 403–412. [Google Scholar] [CrossRef]
- Kouziunis, D.; Lazaridou, A.; Katsandis, E. Partial replacement of animal fat by oleogels structured with monoglycerides and phytosterols in frankfurters sausages. Meat Sci. 2017, 130, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Marchiore, N.G.; Manso, I.J.; Kaufmann, K.C.; Lemes, G.F.; Oliveira Pizolli, A.P.; Droval, A.A.; Bracht, L.; Gonealves, O.H.; Leimann, F.V. Migration evaluation of silver nanoparticles from antimicrobial edible coating to sausages. LWT Food Sci. Technol. 2017, 76, 203–208. [Google Scholar] [CrossRef]
- Suman, S.P.; Mancin, R.A.; Joseph, P.; Ramanathan, R.; Konda, M.K.R.; Dady, G. Packaging-specific influence of chitosan on colour stability and lipid oxidation in refrigerated ground beef. Meat Sci. 2010, 86, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Kubow, S. Routes of formation and toxic consequences of lipid oxidation products in foods. Free Radic. Biol. Med. 1992, 12, 63–81. [Google Scholar] [CrossRef]
- Miks-Krajnik, M.; Feng, L.X.J.; Bang, W.S.; Yuk, H.-G. Inactivation of Listeria monocytogenes and natural microbiota on raw salmon fillets using acidic electrolyzed water, ultraviolet light or/and ultrasound. Food Control 2017, 74, 54–60. [Google Scholar] [CrossRef]
- Xuan, X.-T.; Ding, T.; Li, J.; Ahn, J.-H.; Zhao, Y.; Chen, S.-G.; Ye, X.-Q.; Liu, D.-H. Estimation of growth parameters of Listeria monocytogenes after sublethal heat and slightly acidic electrolyzed water (SAEW) treatment. Food Control 2017, 71, 17–25. [Google Scholar] [CrossRef]
- Rahman, S.M.E.; Park, J.; Song, K.B.; Al-Harbi, N.A.; Oh, D.H. Effects of slightly acidic low concentration electrolyzed water on microbiological, physicochemical, and sensory quality of fresh chicken breast meat. J. Food Sci. 2012, 77, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.S.; Imran, A.; Nadeem, M.T.; Sohaib, M.; Saeed, F.; Anjum, F.M.; Kwon, J.-H.; Hussain, S. Enhancing the quality and lipid stability of chicken nuggets using natural oxidants. Lipids Health Dis. 2017, 16, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinez, J.; Nieto, G.; Ros, G. Total antioxidant capacity of meat and meat products consumed in a reference ‘Spanish standard diet’. Food Sci. Technol. 2014, 22, 1–9. [Google Scholar] [CrossRef]
- Antonini, F.M.; Petruzzi, E.; Pinzani, P.; Orlando, C.; Poggesi, M.; Serio, M. The meat in the diet of aged subjects and the antioxidant effect of carnosine. Arch. Gerontol. Geriatr. 2002, 8, 7–14. [Google Scholar] [CrossRef]
- Sohaib, M.; Anjum, S.M.; Arshad, M.S.; Imran, M.; Imran, A.; Hussain, S. Oxidative stability and lipid oxidation flavoring volatiles in antioxidants treated chicken meat patties during storage. Lipids Health Dis. 2017, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Nussinovitch, A. Biopolymer films and composite coatings. In Modern Biopolymer Science: Bridging the Divide between Fundamental Treatise and Industrial Application; Kasapis, S., Norton, I.T., Ubbink, J.B., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 295–326. [Google Scholar]
- Zając, M.; Kulawik, P.; Tkaczewska, J.; Migdał, W.; Filipczak-Fiutak, M.; Fiutak, G. The effect of hyaluronic acid addition on the properties of smoked homogenised sausages. J. Food Sci. Agric. 2016, 97, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A. Cross-linked alginate/chitosan polyelectrolytes as carrier of active compound and beef color stabilizer. Meat Sci. 2017, 123, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Athayde, D.R.; Flores, S.R.M.; Soares da Silva, J.; Gomes Genro, A.L.; Stafanello Silva, M.; Klein, B.; Mello, R.; Bastinello Campagnol, P.C.; Wagner, R.; Ragagnin de Menezes, C.; et al. Application of electrolyzed water for improving pork meat quality. Food Res. Int. 2017, 100, 757–763. [Google Scholar] [CrossRef] [PubMed]
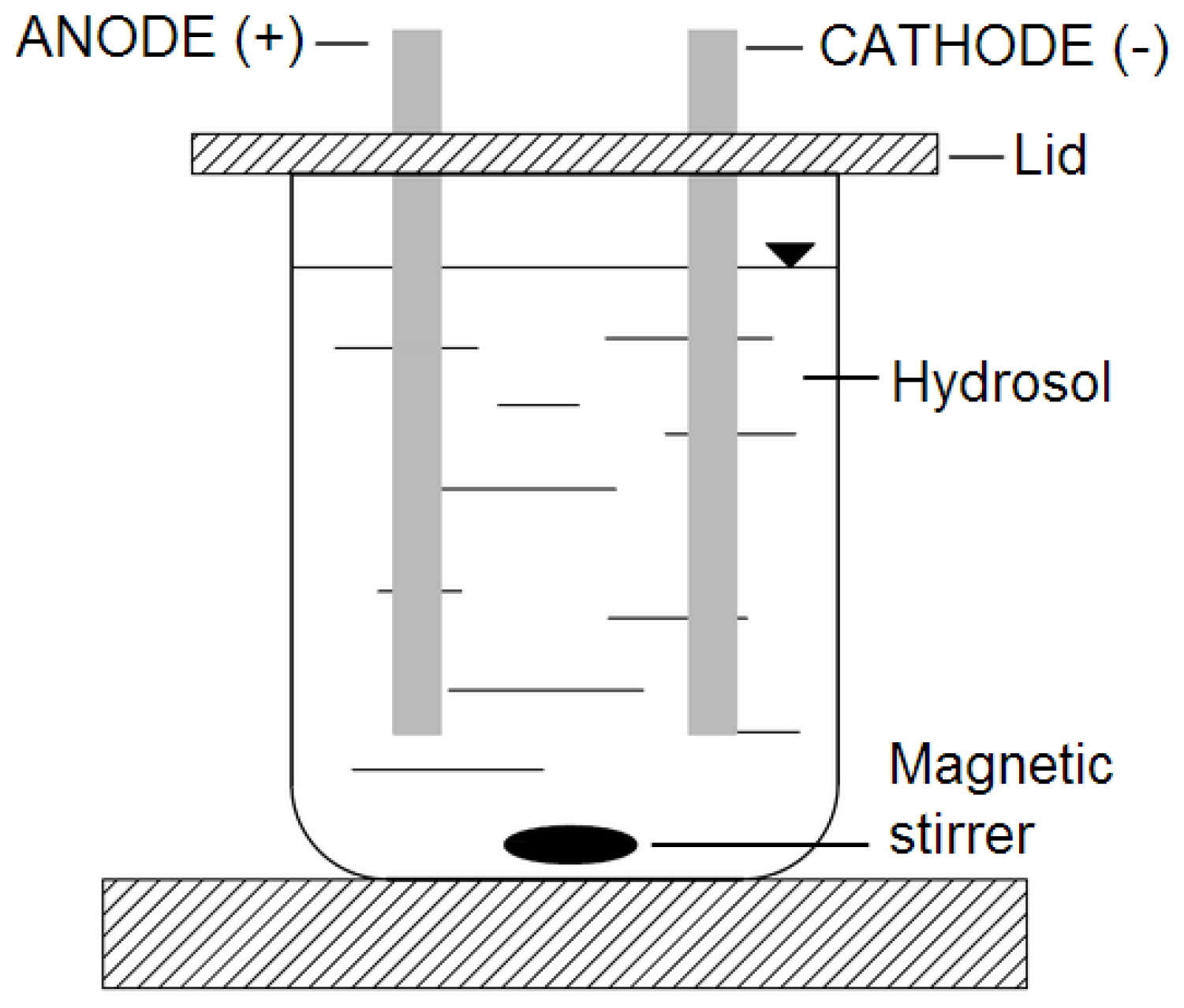


| Run Code Letters | Current (C) (mA) | Type of Sausage (AC/S) |
|---|---|---|
| ControlAC * | 0 | AC |
| ControlS * | S | |
| C0AC | 0 | AC |
| C0S | S | |
| C200AC | 200 | AC |
| C200S | S | |
| C400AC | 400 | AC |
| C400S | S |
| Run Code Letters | Current (C) (mA) | NaCl (N) (%) |
|---|---|---|
| C0N0 | 0 | 0.0 |
| C0N0.1 | 0.1 | |
| C0N0.2 | 0.2 | |
| C200N0 | 200 | 0.0 |
| C200N0.1 | 0.1 | |
| C200N0.2 | 0.2 | |
| C400N0 | 400 | 0.0 |
| C400N0.1 | 0.1 | |
| C400N0.2 | 0.2 |
| Total Viable Counts | |||||
|---|---|---|---|---|---|
| Days of storage | 0 | 7 | 14 | 21 | 28 |
| ControlAC | 3.77 ± 0.04 c,d | 4.68 ± 0.01 d | 5.96 ± 0.00 e | 6.97 ± 0.08 d | 7.80 ± 0.02 b |
| ControlS | 3.91 ± 0.09 d | 4.72 ± 0.01 d | 5.76 ± 0.06 d | 6.58 ± 0.11 c | 7.71 ± 0.06 b |
| C0AC | 3.79 ± 0.01 c,d | 4.73 ± 0.06 d | 5.76 ± 0.01 d | 6.71 ± 0.09 c | 7.60 ± 0.20 b |
| C0S | 3.68 ± 0.16 c | 4.69 ± 0.01 d | 5.74 ± 0.09 d | 6.73 ± 0.06 c | 7.74 ± 0.11 b |
| C200AC | 2.78 ± 0.02 b | 2.92 ± 0.00 c | 3.69 ± 0.09 c | 4.80 ± 0.04 b | 5.83 ± 0.06 a |
| C200S | 2.73 ± 0.05 b | 2.78 ± 0.00 b | 3.64 ± 0.09 b,c | 4.66 ± 0.09 a,b | 5.60 ± 0.23 a |
| C400AC | 2.09 ± 0.12 a | 2.61 ± 0.11 a | 3.49 ± 0.02 a,b | 4.56 ± 0.05 a | 5.69 ± 0.13 a |
| C400S | 2.13 ± 0.03 a | 2.65 ± 0.11 a,b | 3.43 ± 0.10 a | 4.64 ± 0.04 ab | 5.81 ± 0.09 a |
| Enterobacteriaceae | |||||
| Days of storage | 0 | 7 | 14 | 21 | 28 |
| ControlAC | ND a | ND a | ND a | ND a | ND a |
| ControlS | |||||
| C0AC | |||||
| C0S | |||||
| C200AC | |||||
| C200S | |||||
| C400AC | |||||
| C400S | |||||
| Yeast and molds | |||||
| Days of storage | 0 | 7 | 14 | 21 | 28 |
| ControlAC | ND a | ND a | ND a | ND a | 1.67 ± 0.14 b |
| ControlS | 1.75 ± 0.10 b | ||||
| C0AC | 1.39 ± 0.27 b | ||||
| C0S | 1.78 ± 0.11 b | ||||
| C200AC | ND a | ||||
| C200S | |||||
| C400AC | |||||
| C400S | |||||
| Psychrotrophs | |||||
| Days of storage | 0 | 7 | 14 | 21 | 28 |
| ControlAC | 2.83 ± 0.03 cd | 3.80 ± 0.14 b | 4.75 ± 0.11 b | 5.72 ± 0.07 c | 6.75 ± 0.01 c |
| ControlS | 2.93 ± 0.05 d | 3.66 ± 0.02 b | 4.68 ± 0.14 b | 5.76 ± 0.10 c | 6 65 ± 0.01 c |
| C0AC | 2.82 ± 0.02 c | 3.61 ± 0.08 b | 4.66 ± 0.04 b | 5.69 ± 0.04 c | 6.76 ± 0.04 c |
| C0S | 2.75 ± 0.00 c | 3.72 ± 0.18 b | 4.72 ± 0.10 b | 5.71 ± 0.05 b | 6.71 ± 0.13 c |
| C200AC | 2.55 ± 0.08 b | 2.71 ± 0.11 a | 2.90 ± 0.09 a | 3.82 ± 0.01 b | 4.76 ± 0.10 b |
| C200S | 2.61 ± 0.04 b | 2.63 ± 0.23 a | 2.87 ± 0.03 a | 3.71 ± 0.07 a | 4.70 ± 0.04 b |
| C400AC | 2.29 ± 0.04 a | 2.47 ± 0.07 a | 2.85 ± 0.14 a | 3.45 ± 0.08 a | 4.64 ± 0.02 b |
| C400S | 2.32 ± 0.05 a | 2.50 ± 0.03 a | 2.78 ± 0.12 a | 3.50 ± 0.15 a | 4.38 ± 0.13 a |
| Lactic acid bacteria (LAB) | |||||
| Days of storage | 0 | 7 | 14 | 21 | 28 |
| ControlAC | ND a | 1.97 ± 0.04 c | 4.83 ± 0.03 c | 5.82 ± 0.05 d | 7.52 ± 0.06 e |
| ControlS | 1.74 ± 0.04 b | 4.93 ± 0.05 c | 5.81 ± 0.06 d | 7.34 ± 0.12 e | |
| C0AC | 1.41 ± 0.13 a | 4.75 ± 0.03 c | 5.76 ± 0.14 d | 6.82 ± 0.06 d | |
| C0S | 1.24 ± 0.09 a | 4.75 ± 0.02 c | 5.75 ± 0.07 d | 6.75 ± 0.07 d | |
| C200AC | ND a | 1.97 ± 0.00 b | 2.77 ± 0.06 c | 3.80 ± 0.00 c | |
| C200S | 1.74 ± 0.04 a | 2.66 ± 0.18 ab | 3.66 ± 0.06 bc | ||
| C400AC | 1.66 ± 0.16 a | 2.49 ± 0.03 a | 3.50 ± 0.16 ab | ||
| C400S | 1.59 ± 0.10 a | 2.59 ± 0.06 a,b | 3.41 ± 0.06 a | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król, Ż.; Kulig, D.; Marycz, K.; Zimoch-Korzycka, A.; Jarmoluk, A. The Effects of Using Sodium Alginate Hydrosols Treated with Direct Electric Current as Coatings for Sausages. Polymers 2017, 9, 602. https://doi.org/10.3390/polym9110602
Król Ż, Kulig D, Marycz K, Zimoch-Korzycka A, Jarmoluk A. The Effects of Using Sodium Alginate Hydrosols Treated with Direct Electric Current as Coatings for Sausages. Polymers. 2017; 9(11):602. https://doi.org/10.3390/polym9110602
Chicago/Turabian StyleKról, Żaneta, Dominika Kulig, Krzysztof Marycz, Anna Zimoch-Korzycka, and Andrzej Jarmoluk. 2017. "The Effects of Using Sodium Alginate Hydrosols Treated with Direct Electric Current as Coatings for Sausages" Polymers 9, no. 11: 602. https://doi.org/10.3390/polym9110602
APA StyleKról, Ż., Kulig, D., Marycz, K., Zimoch-Korzycka, A., & Jarmoluk, A. (2017). The Effects of Using Sodium Alginate Hydrosols Treated with Direct Electric Current as Coatings for Sausages. Polymers, 9(11), 602. https://doi.org/10.3390/polym9110602