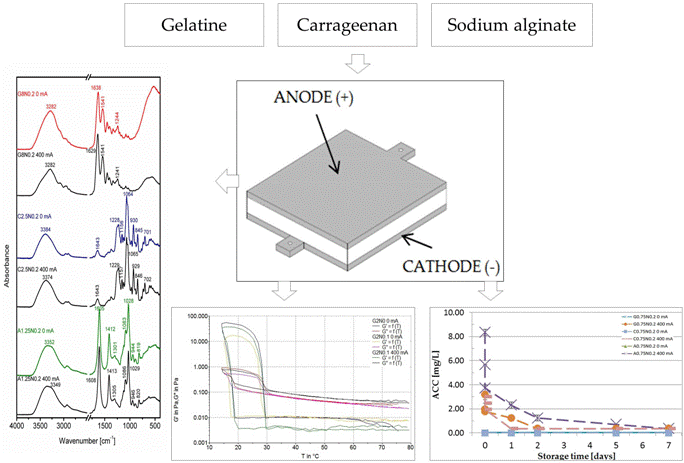
Characteristic of Gelatine, Carrageenan and Sodium Alginate Hydrosols Treated by Direct Electric Current
Abstract
:1. Introduction
2. Experimental Section
2.1. Apparatus
2.2. Materials
2.3. Preparation of Hydrosols
2.4. Hydrosols Characterization
2.4.1. Physiochemical Properties
2.4.2. Rheological Measurements
Flow Properties
Gelation and Flow Temperatures
2.4.3. Fourier Transform Infrared Spectroscopy
2.4.4. Scanning Electron Microscopy
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physiochemical Properties of Hydrosols
3.2. Rheological Measurements
3.2.1. Flow Properties
3.2.2. Gelation and Flow Temperatures
3.3. Fourier Transform Infrared Spectroscopy
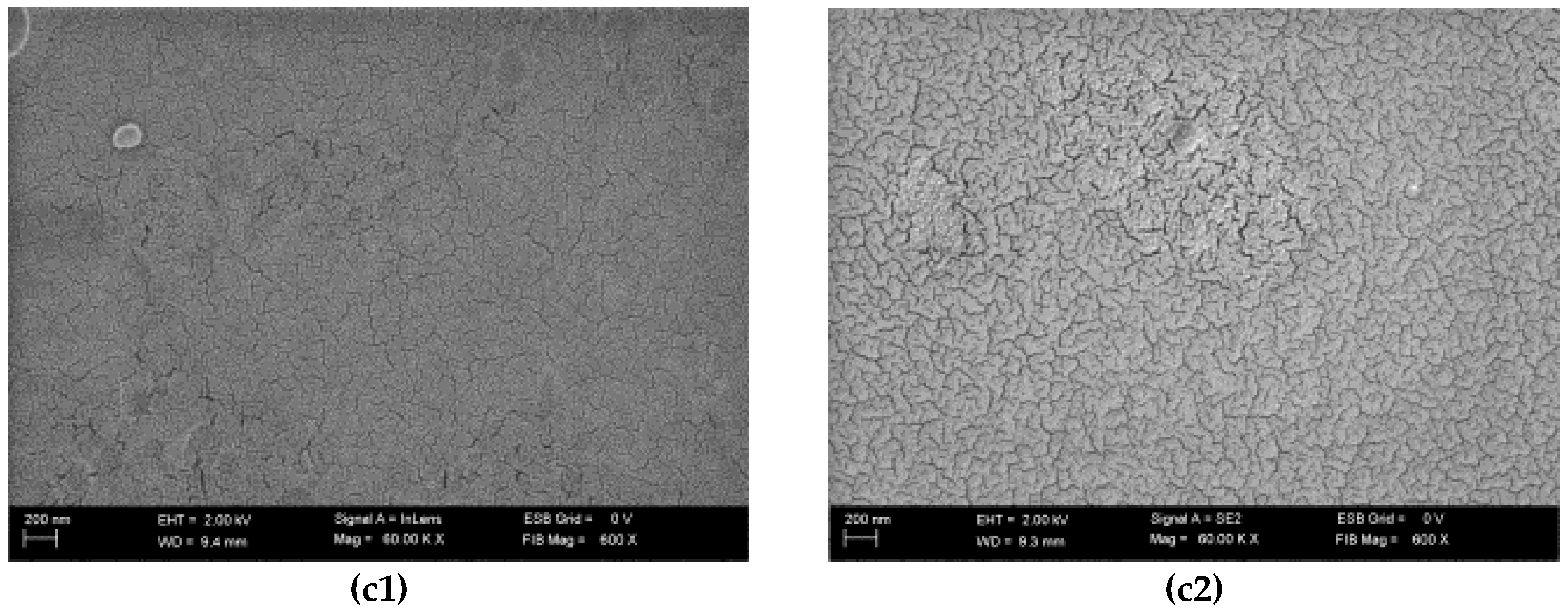
3.4. Scanning Electron Microscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Williams, P.; Philips, G. Handbook of Hydrocolloids, 2nd ed.; CRC Press: Cambridge, UK, 2000; pp. 1–19. [Google Scholar]
- Li, J.-M.; Nie, S.-P. The functional and nutritional aspects of hydrocolloids in foods. Food Hydrocoll. 2016, 53, 46–61. [Google Scholar] [CrossRef]
- Osorio, F.A.; Molina, P.; Matiacevich, S.; Enrione, J.; Skurtys, O. Characteristic of hydroxyl propyl methyl cellulose (HPMC) based edible film developed for blueberry coatings. Procedia Food Sci. 2011, 1, 287–293. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S. Irradiation of Food Commodities: Techniques, Applications, Detection, Legislation, Safety and Consumer Opinion; Elsevier: London, UK, 2010. [Google Scholar]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C.; Aktas, N.; Turk, M. Biocompatible and biodegradable poly(Tannic Acid) hydrogel with antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2016, 82, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Zimoch-Korzycka, A.; Kulig, D.; Jarmoluk, A.; Marycz, K.; Matuszczak, W. Study of Enzymatically Treated Alginate/Chitosan Hydrosols in Sponges Formation Process. Polymers 2016, 8, 8. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Darget, K.I.; Braek, G.S.; Smidsrod, O. Alginic acid gels: The effect of alginate chemical composition and molecular weight. Carbohydr. Polym. 1994, 25, 31–38. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar]
- Tavassoli-Kafrani, E.; Shekarchzadeh, H.; Behabadi-Masoudpour, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.S.G.; Bandeira, S.F.; Pinto, L.A.A. Characteristics and chemical composition of skins gelatin from cobia (Rachycentron canadum). LWT Food Sci. Technol. 2014, 57, 580–585. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Chen, S.; Shi, J.; Xu, X.; Ding, J.; Zhong, W.; Zhang, L.; Xing, M.; Zhang, L. Study of stiffness effects of poly(amidoamine)-poly(n-isopropyl acrylamide) hydrogen on wound healing. Colloids Surf. B Biointerfaces 2016, 140, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Vulpe, R.; Popa, M.; Picton, L.; Balan, V.; Dulong, V.; Butnaru, M.; Verestiuc, L. Crosslinked hydrogels based on biological marcomolecules with potential use in skin tissue engineering. Int. J. Biol. Macromol. 2016, 84, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Gelfuso, G.M.; Barros, M.A.; Delgado-Charro, M.B.; Guy, R.H.; Lopez, R.F.V. Ionthophoresis of minoxidil suphate loaded microparticles, a strategy for follicular drug treating. Colloids Surf. B Biointerfaces 2015, 134, 408–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, J.G.; Diaz, K.; Silva, S.A.M.; Rezende, L.C.D.; Rocha, E.M.; Emery, F.S.; Lopez, R.F.V. Transcorneal iontophoresis of dendrimers: PAMAM corneal penetration and dexamethasone delivery. J. Control. Release 2015, 200, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Cezares-Delgadillo, J.; Ganem-Rondero, A.; Merino, V.; Kalia, Y.N. Controlled transdermal iontophoresis for polypharmacotherapy: Simultaneous delivery of granisetron, metoclopramide and dexamethasone sodium phosphate in vitro and in vivo. Eur. J. Pharm. Sci. 2016, 85, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Cubayachi, C.; Oliveira do Couto, R.; Masetto de Gaitani, C.; Pedrazzi, V.; Freitas, O.; Lopez, R.F.V. Needle-Free buccal anesthesia using iontophoresis and amino amide salts combined in a mucoadhesive formulation. Colloids Surf. B Biointerfaces 2015, 136, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Song, B.; Xing, X.-H.; Loosdrecht, M.; Liu, Z. Electrolytic stimulation of bacteria Enterobacter dissolvens by a direct current. Biochem. Eng. J. 2006, 28, 23–29. [Google Scholar] [CrossRef]
- Jackman, S.A.; Maini, G.; Sharman, A.K.; Knowles, C.J. The effects of direct electric current on the viability and metabolism of acidophilic bacteria. Enzym. Microb. Technol. 1999, 24, 316–324. [Google Scholar] [CrossRef]
- Król, Ż.; Jarmoluk, A. The effects of using a direct electric current on the chemical properties of gelatine gels and bacterial growth. J. Food Eng. 2015, 170, 1–7. [Google Scholar] [CrossRef]
- Cao, W.; Zhu, Z.W.; Shi, Z.X.; Wang, C.Y.; Li, B.M. Efficiency of slightly acidic electrolyzed water for inactivation of Salmonella enteritisis and its contaminated shell eggs. Int. J. Food Microbiol. 2009, 130, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.E.; Park, J.H.; Wang, J.; Oh, D.-H. Stability of low concentration electrolyzed water and its sanitization potential against foodborne pathogens. J. Food Eng. 2012, 113, 538–553. [Google Scholar] [CrossRef]
- Król, Ż.; Malik, M.; Marycz, K.; Jarmoluk, A. Physicochemical properties of biopolymer hydrogels treated by direct electric current. Polymers 2016, 8, 248. [Google Scholar]
- Śmieszek, S.; Donesz-Sikorska, A.; Grzesiak, J. Biological effects of sol-gel derived ZrO2 and SiO2/ZrO2 coatings on stainless steel surface—In vitro model using mesenchymal stem cells. J. Biomater. Appl. 2014, 29, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.R.; Hung, Y.-C.; Hsu, S.Y.; Huang, Y.W.; Hwang, D.F. Application of electrolyzed water in the food industry. Food Control 2008, 19, 329–345. [Google Scholar] [CrossRef]
- Hsu, S.Y. Effects of flow rate, temperature and salt concentration on chemical and physical properties of electrolyzed oxidizing water. J. Food Eng. 2005, 66, 171–176. [Google Scholar] [CrossRef]
- Brychcy, E.; Malik, M.; Drożdżewski, E.; Król, Ż.; Jarmoluk, A. Antibacterial and Physicochemical Properties of Carrageenan and Gelatine Hydrosols and Hydrogels Incorporated with Acidic Electrolysed Water. Polymers 2015, 7, 2638–2649. [Google Scholar] [CrossRef]
- Cui, X.; Shang, Y.; Shi, Z.; Xin, H.; Cao, W. Physicochemical properties and bactericidal efficiency of neutral and acidic electrolyzed water under different storage conditions. J. Food Eng. 2009, 91, 582–586. [Google Scholar] [CrossRef]
- Al-Haq, M.; Sugiyama, J.; Isobe, S. Applications of electrolyzed water in agriculture & food industries. Food Sci. Technol. Res. 2005, 11, 135–150. [Google Scholar]
- Ding, T.; Ge, Z.; Shi, J.; Xu, Y.T.; Jones, C.L.; Liu, D.H. Impact of slightly acidic electrolyzed water (SAEW) and ultrasound on microbial loads and quality of fresh fruits. LWT Food Sci. Technol. 2015, 60, 1195–1199. [Google Scholar] [CrossRef]
- Ghoshal, K.; Das, S.; Aich, K.; Goswami, S.; Chowdhury, S.; Bhattacharyya, M. A novel sensor estimate the prevalence of hypochlorous (HOCl) toxicity in individuals with type 2 diabetes and dyslipidaemia. Clin. Chim. Acta 2016, 458, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Bruchet, A.; Duguet, J.P. Role of oxidants and disinfectants on the removal masking and generation of tastes and odours. Water Sci. Technol. 2014, 49, 297–306. [Google Scholar] [CrossRef]
- Izumi, H. Electrolyzed water as a disinfectant for fresh-cut vegetables. J. Food. Sci. 1999, 64, 536–539. [Google Scholar] [CrossRef]
- Mokudai, T.; Kanno, T.; Niwano, Y. Involvmet of reactive oxygen species in the effect of acid-electrolyzed water. J. Toxicol. Sci. 2015, 40, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Len, S.V.; Hung, Y.C.; Chung, D. Effects of storage conditions and pH on chlorine loss on electrolyzed oxidizing (EO) water. J. Agric. Food Chem. 2002, 50, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Junyi, M.; Lin, Y.; Chen, X.; Zhao, B.; Ji, Z. Flow behavior, thixotropy and dynamical viscoelasticity of sodium alginate aqueous solutions. Food Hydrocoll. 2014, 38, 119–128. [Google Scholar]
- Duggirala, S.; Deluca, P. Rheological characterization of cellulosic and alginate polymers. PDA J. Pharm. Sci. Technol. 1996, 50, 290–305. [Google Scholar] [PubMed]
- Hambleton, A.; Perpinan-Saiz, N.; Fabra, M.J.; Voilley, A.; Debeaufort, F. The schroeder paradox or how the state of water affects the moisture transfer through edible films. Food Chem. 2012, 132, 1671–1678. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Maheriya, P.M.; Jani, G.K.; Solanki, H.K. Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydr. Polym. 2014, 105, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Venegas, J.A.; Motohiro, T.; Takaomi, K. Ultrasound effect used as external stimulus for viscosity change of aqueous carrageenans. Ultrason. Sonochem. 2013, 20, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Deeth, H.; Spoade, P.; Sharma, R.; Bansal, N. Rheology, texture and microstructure of gelatin gels with and without milk proteins. Food Hydrocoll. 2014, 35, 484–493. [Google Scholar] [CrossRef]
- Xiao, H.; Lin, Q.; Xia, X. Rheological properties of sweet potato starch before and after denaturalization. J. Cent. South Univ. Technol. 2008, 15, 500–505. [Google Scholar] [CrossRef]
- Gomez-Diaz, D.; Navaza, J.M. Rheology of aqueous solutions of food additivesL effect of concentration, temperature and blending. J. Food. Eng. 2003, 56, 387–392. [Google Scholar] [CrossRef]
- Björn, A.; Segura de La Monja, P.; Karlsson, A.; Ejlertsson, J.; Svensson, B.H. Rheological Characterization, Biogas; Kumar, S., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Chatterjee, S.; Bohidar, H.B. Effects of Salt and Temperature on Viscoelasticity OD Gelatin Hydrogels. J. Surf. Sci. Technol. 2006, 22, 1–13. [Google Scholar]
- Cuvelier, G.; Launay, B.; Michon, C. Concentration dependence of the critical viscoelastic properties of gelatin at the gel point. Rheol. Acta 1993, 32, 94–103. [Google Scholar]
- Jones, G.M.J. Rheological Properties of Gelatin, Carrageenan and Locust Bean Gum Mixtures. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2004. [Google Scholar]
- Hoque, M.S.; Benjakul, S.; Prodpran, T. Effect of heat treatment of film-forming solution on the properties of film from cuttlefish (Sepia pharaonis) skin gelation. J. Food Eng. 2010, 96, 66–73. [Google Scholar] [CrossRef]
- Prado, F.J.; Rodriguez, V.J.A.; Tojo, E.; Andrade, J.M. Quantitation of κ, l- and λ-carrageenans by mid-infrared spectroscopy and PLS regression. Anal. Chim. Acta 2003, 480, 23–37. [Google Scholar] [CrossRef]
- Sen, M.; Erboz, E.N. Determination of critical gelation conditions of κ-carrageenan by viscosimetric and FT-IR analyses. Food Res. Int. 2010, 43, 1361–1364. [Google Scholar] [CrossRef]
- Chandia, N.P.; Matsuhiro, B.; Vasquez, A.E. Alginic acids in Lessonia trabeculata: Characterization by formic acid hydrolysis and FT-IR spectroscopy. Carbohydr. Polym. 2001, 46, 81–87. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on alginate-chitosan complex formed with different polymer ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef]
- Bandekar, J. Amide modes and protein conformation. Biochim. Biophys. Acta 1992, 1120, 123–143. [Google Scholar] [CrossRef]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Mohmood, A.; Kim, S.J.; Lee, K. Chlorine resistant binary complexed NaAlg/PVA composite membrane for nanofiltration. Sep. Purif. Technol. 2014, 137, 21–27. [Google Scholar] [CrossRef]
- Hsu, C.-S.; Block, L.H. Anionic Gels as Vehicles for Electrically-Modulated Drug Delivery. I. Solvent and Drug Transport Phenomena. Pharm. Res. 1996, 13, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Block, L.W. The use of chitosan gels as matrices for electrically-modulated drug delivery. J. Control. Release 2001, 70, 109–123. [Google Scholar] [CrossRef]
- Jin, X.; Kang, H.; Huang, Y.; Liu, H.; Hu, Y. Synthesis and Properties of Electrically-Sensitive Poly(acrylic acid-co-acetoacetocxy ethyl methacrylate) Gels. J. Appl. Polym. Sci. 2008, 110, 3690–3696. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Choubey, J. Design of gelatin nanoparticles as swelling controlled delivery system for chloroquine phosphate. J. Mater. Sci. Mater. Med. 2006, 17, 345–358. [Google Scholar] [CrossRef] [PubMed]
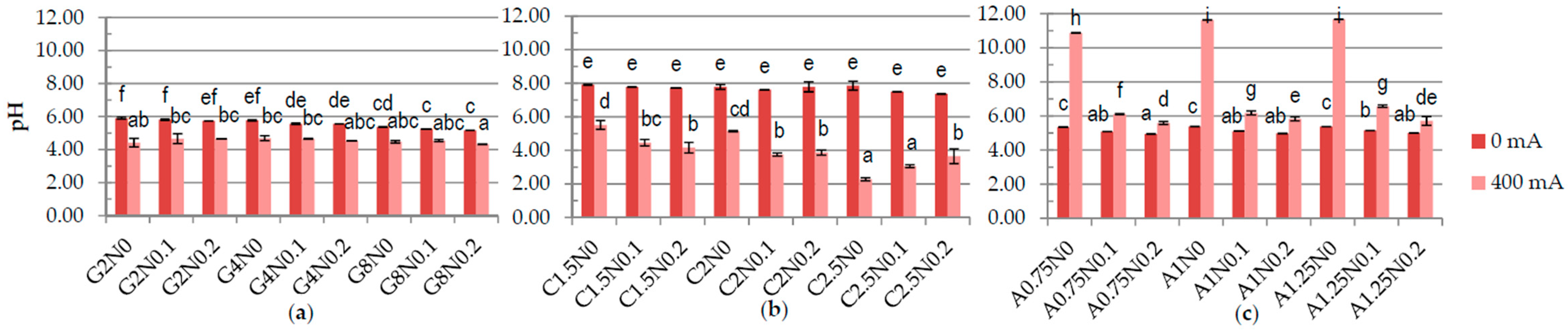
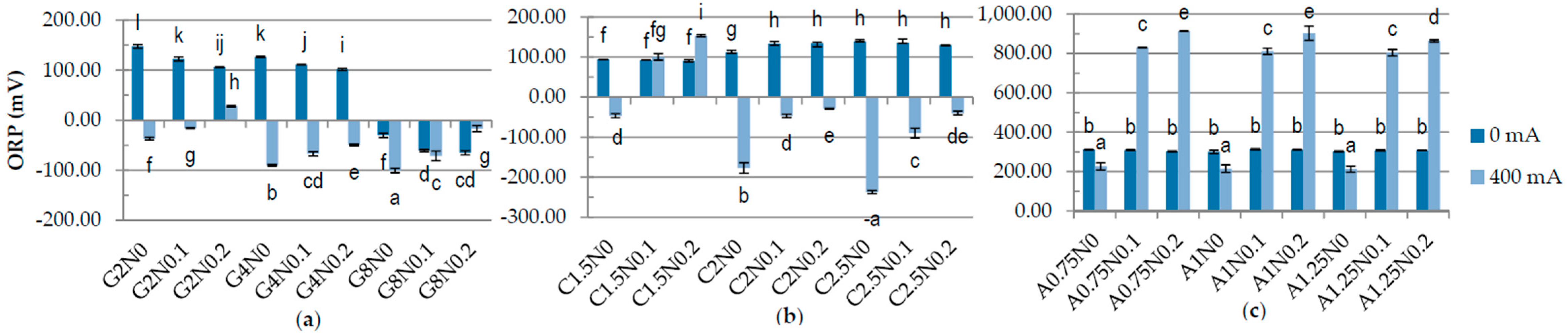

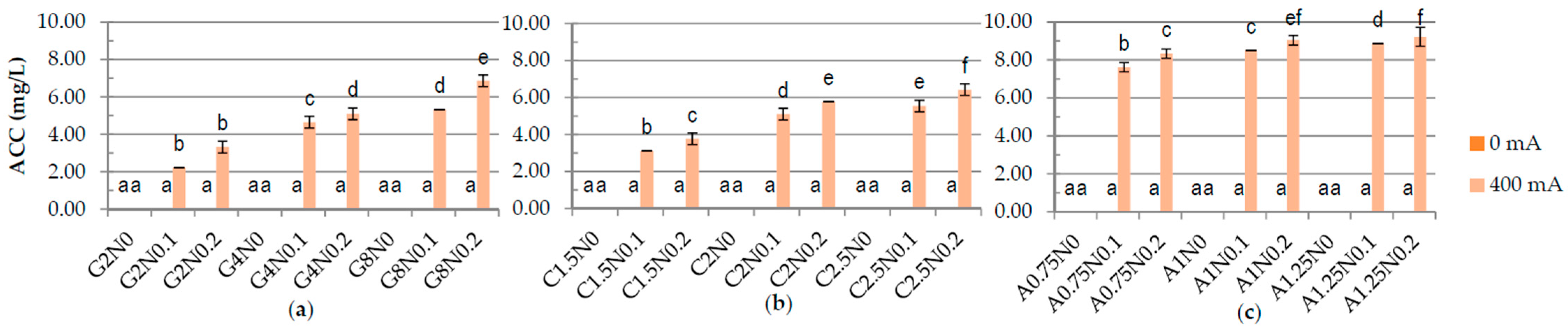
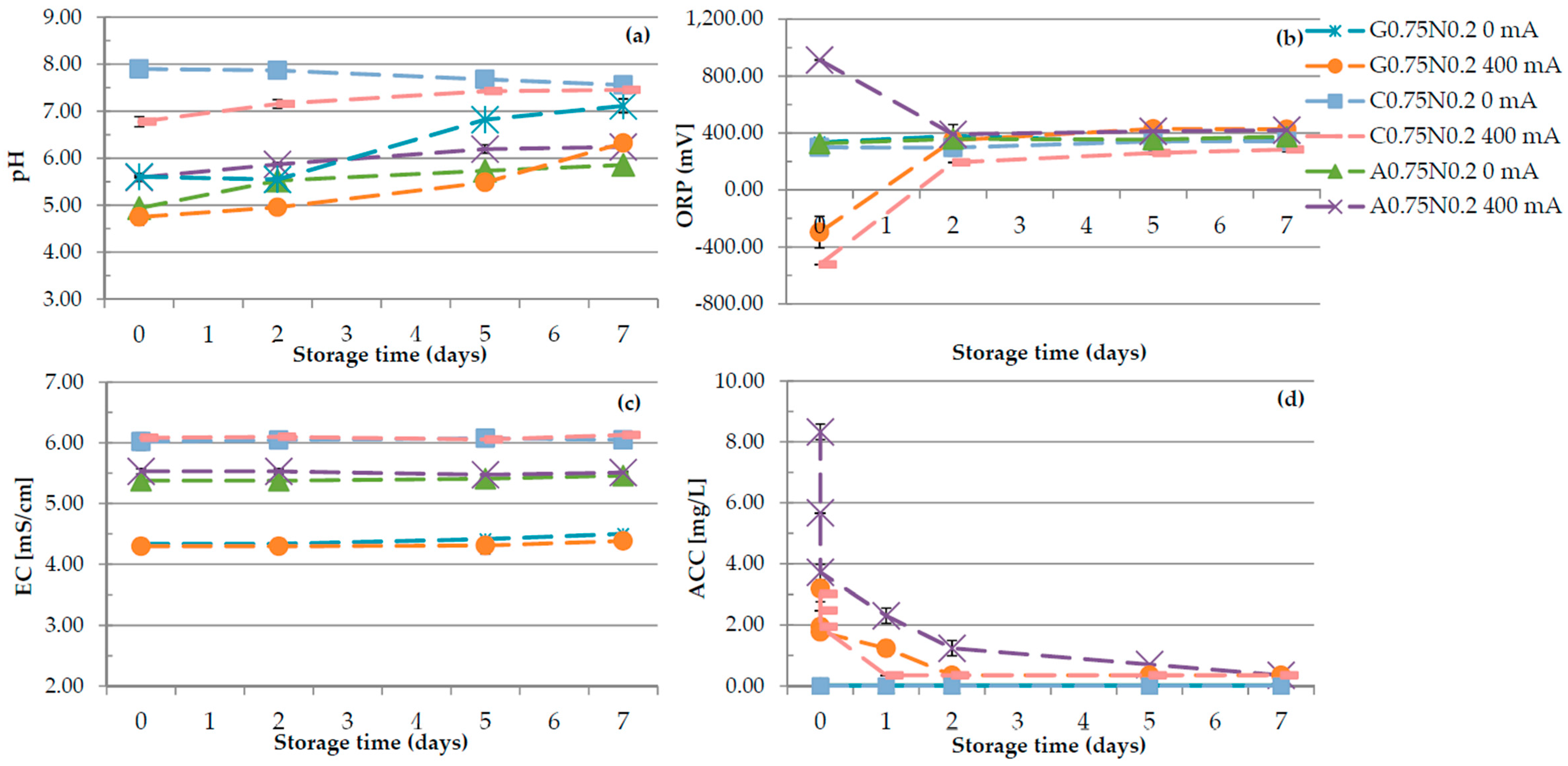

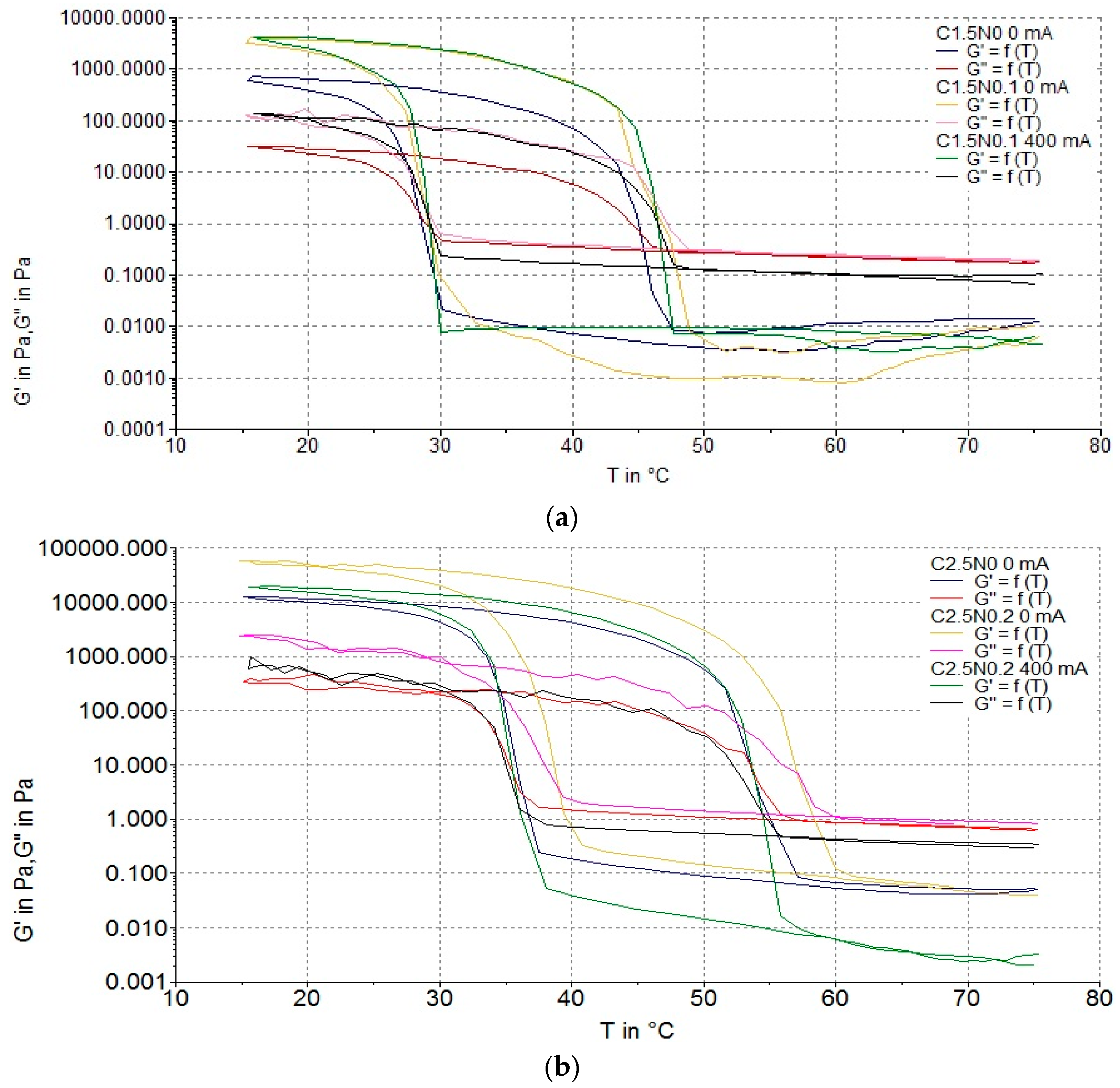
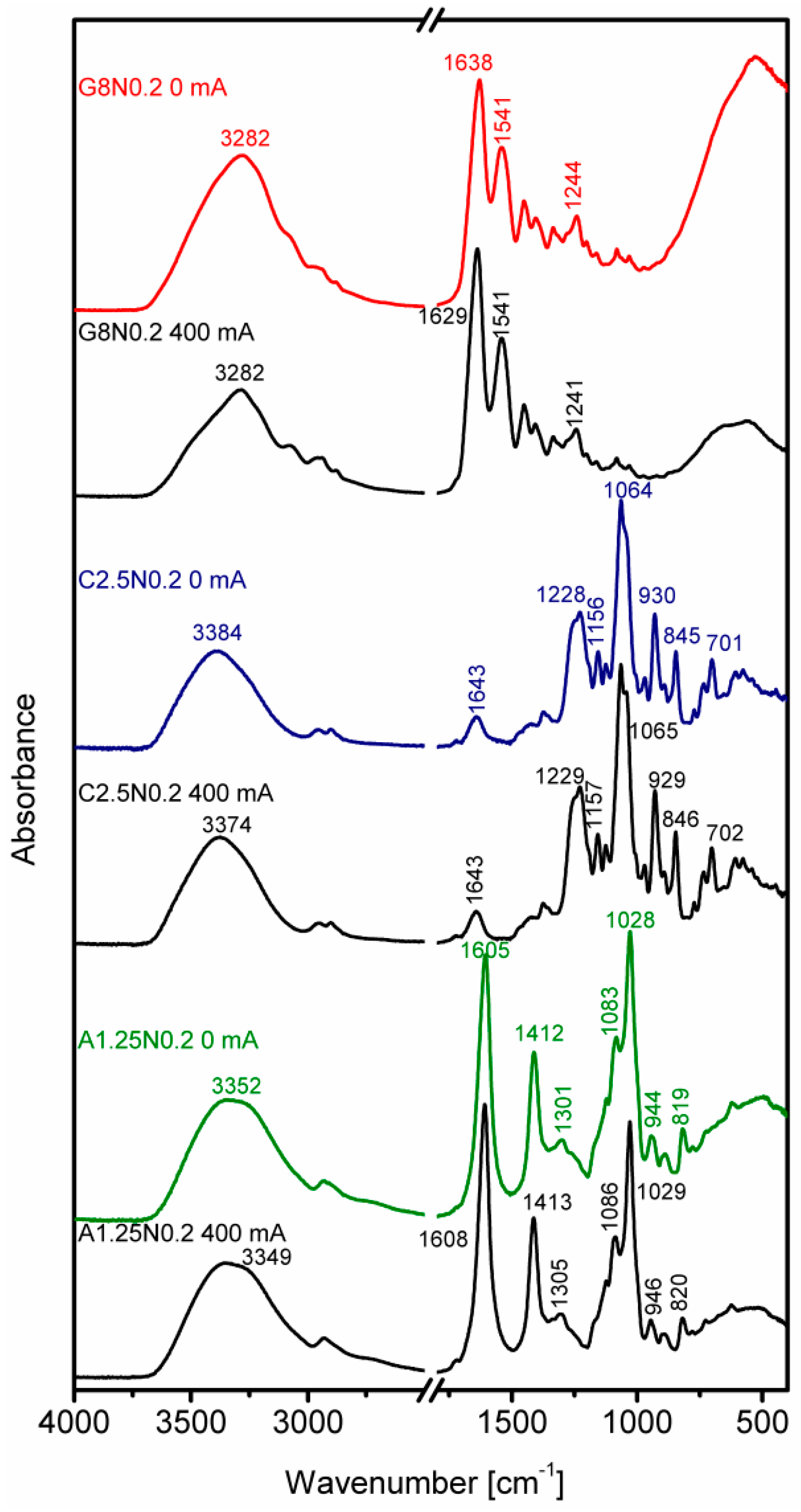
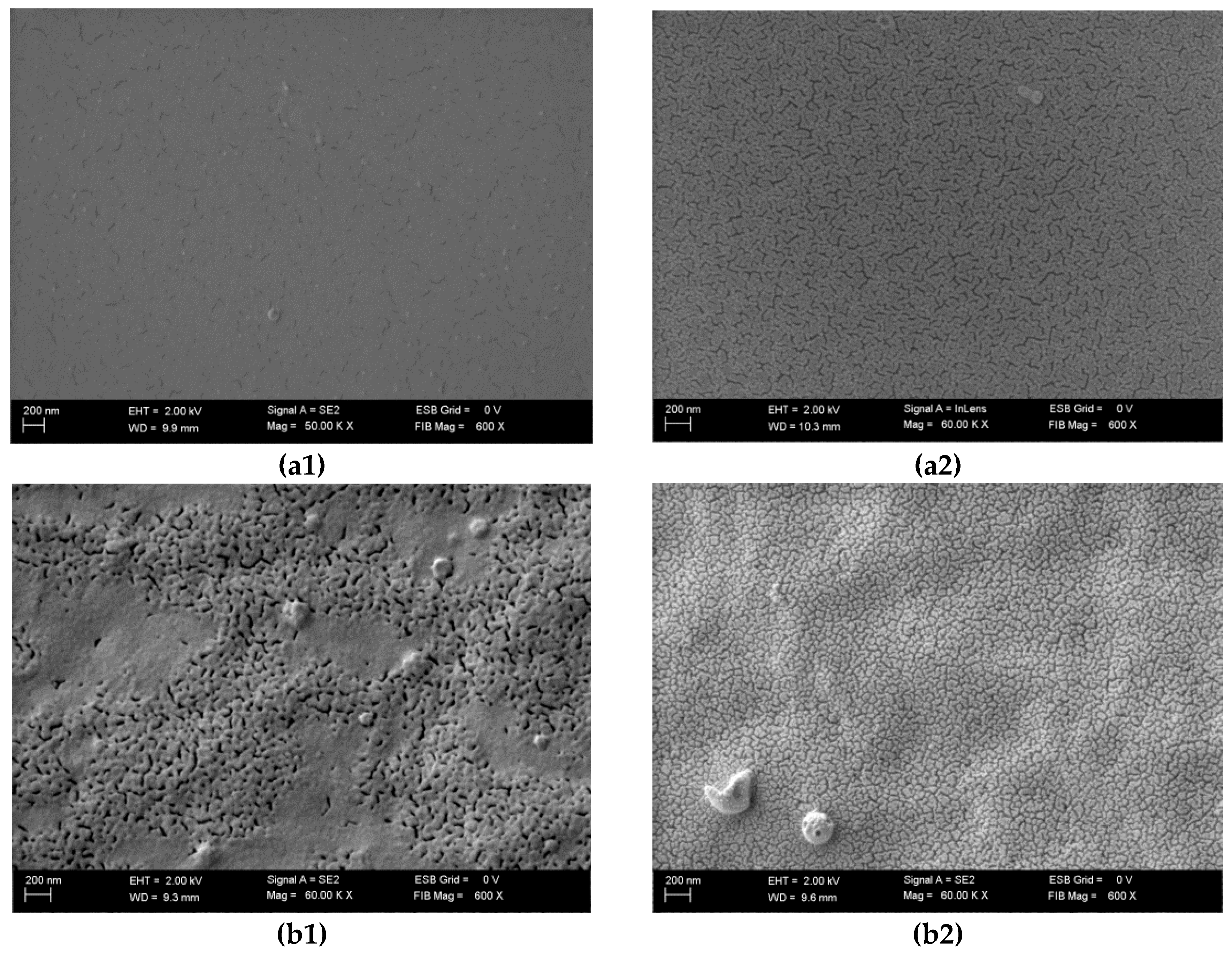
| Run code letters | Gelatine (G) (%) | Carrageenan (C) (%) | Sodium alginate (A) (%) | NaCl (N) (%) | ||
|---|---|---|---|---|---|---|
| G2N0 | C1.5N0 | A0.75N0 | 2.0 | 1.5 | 0.75 | 0.0 |
| G2N0.1 | C1.5N0.1 | A0.75N0.1 | 0.1 | |||
| G2N0.2 | C1.5N0.2 | A0.75N0.2 | 0.2 | |||
| G4N0 | C2N0 | A1N0 | 4.0 | 2.0 | 1.0 | 0.0 |
| G4N0.1 | C2N0.1 | A1N0.1 | 0.1 | |||
| G4N0.2 | C2N0.2 | A1N0.2 | 0.2 | |||
| G8N0 | C2.5N0 | A1.25N0 | 8.0 | 2.5 | 1.25 | 0.0 |
| G8N0.1 | C2.5N0.1 | A1.25N0.1 | 0.1 | |||
| G8N0.2 | C2.5N0.2 | A1.25N0.2 | 0.2 | |||
| Variants | Current (mA) | Ostwald de waele model | Herschel–Bulkley model | Shear stress τ0 (Pa) | Shear stress τ100 (Pa) | Apparent viscosity η0 (mPa·s) | Apparent viscosity η100 (mPa·s) | |
|---|---|---|---|---|---|---|---|---|
| Consistency index k (Pa·s) | Flow behavior index n (-) | Yield stress τ0 (Pa) | ||||||
| Gelatine | ||||||||
| G2N0 | 0 | 0.009 ± 0.001 b,c | 0.749 ± 0.05 a,b | 0.026 ± 0.004 b | 0.011 ± 0.001 a | 0.340 ± 0.007 c | 29.90 ± 0.42 f | 7.90 ± 0.07 e |
| G2N0 | 400 | 0.015 ± 0.001 d,e | 0.688 ± 0.03 a,b | 0.004 ± 0.000 a | 0.008 ± 0.000 a | 0.329 ± 0.007 c | 9.33 ± 0.09 b,c | 3.24 ± 0.16 c |
| G2N0.2 | 0 | 0.007 ± 0.001 a,b | 0.837 ± 0.00 b | 0.024 ± 0.002 b | 0.012 ± 0.001 a | 0.326 ± 0.013 c | 9.84 ± 0.11 b,c | 3.16 ± 0.01 c |
| G2N0.2 | 400 | 0.008 ± 0.000 b,c | 0.844 ± 0.06 b | 0.021 ± 0.002 b | 0.010 ± 0.000 a | 0.346 ± 0.007 c | 8.80 ± 0.17 b | 3.37 ± 0.06 c |
| G8N0 | 0 | 0.010 ± 0.001 c | 0.563 ± 0.23 a | 0.023 ± 0.000 c | 0.285 ± 0.330 a | 1.689 ± 0.017 e | 47.55 ± 0.35 g | 17.00 ± 0.28 f |
| G8N0 | 400 | 0.014 ± 0.001 d | 0.557 ± 0.05 a | 0.035 ± 0.002 d | 0.027 ± 0.018 a | 0.191 ± 0.058 b | 13.09 ± 0.16 d | 1.53 ± 0.04 b |
| G8N0.2 | 0 | 0.017 ± 0.003 e | 0.336 ± 0.08 c | 0.003 ± 0.000 a | 0.026 ± 0.000 a | 0.589 ± 0.010 d | 23.45 ± 0.35 e | 5.89 ± 0.10 d |
| G8N0.2 | 400 | 0.004 ± 0.000 a | 0.796 ± 0.02 b | 0.013 ± 0.000 b | 0.002 ± 0.000 a | 0.131 ± 0.016 a | 6.83 ± 0.06 a | 1.23 ± 0.04 a |
| Carrageenan | ||||||||
| C1.5N0 | 0 | 0.226 ± 0.019 a,b | 0.869 ± 0.02 a,b | 0.033 ± 0.000 a | 0.258 ± 0.011 b,c | 15.025 ± 1.407 c | 310.50 ± 0.71 f | 140.27 ± 0.38 e |
| C1.5N0 | 400 | 0.128 ± 0.003 a,b | 0.905 ± 0.01 b–d | 0.022 ± 0.001 a | 0.129 ± 0.006 a | 8.281 ± 0.064 a | 129.60 ± 0.71 b | 83.60 ± 0.42 a |
| C1.5N0.2 | 0 | 0.164 ± 0.006 a,b | 0.925 ± 0.01 c,d | 0.218 ± 0.308 a | 0.238 ± 0.016 b | 11.110 ± 0.735 b | 200.61 ± 0.86 c | 120.15 ± 0.21 d |
| C1.5N0.2 | 400 | 0.113 ± 0.001 a | 0.950 ± 0.00 d | 0.015 ± 0.000 a | 0.117 ± 0.006 a | 8.694 ± 0.103 a | 119.75 ± 0.35 a | 88.58 ± 1.24 b |
| C2.5N0 | 0 | 1.088 ± 0.258 c | 0.828 ± 0.05 b | 1.416 ± 0.143 b | 1.500 ± 0.028 f | 47.288 ± 2.850 e | 1,587.70 ± 4.95 h | 471.10 ± 1.98 g |
| C2.5N0 | 400 | 0.409 ± 0.013 b | 0.828 ± 0.01 b | 0.089 ± 0.004 a | 0.304 ± 0.002 d | 8.400 ± 0.028 a | 279.30 ± 0.99 e | 179.28 ± 1.03 f |
| C2.5N0.2 | 0 | 1.135 ± 0.191 c | 0.777 ± 0.03 a | 2.350 ± 0.049 c | 1.064 ± 0.034 e | 38.385 ± 0.990 d | 390.10 ± 7.07 g | 120.00 ± 1.70 d |
| C2.5N0.2 | 400 | 0.193 ± 0.008 a,b | 0.895 ± 0.01 b,c | 0.276 ± 0.010 a | 0.279 ± 0.045 c | 11.080 ± 0.813 b | 259.65 ± 0.49 d | 110.20 ± 0.28 c |
| Sodium alginate | ||||||||
| A0.75N0 | 0 | 0.095 ± 0.000 c | 0.966 ± 0.002 g | 0.127 ± 0.002 d | 0.130 ± 0.001 c | 8.104 ± 0.027 b | 119.75 ± 0.35 d | 80.31 ± 0.27 d |
| A0.75N0 | 400 | 0.131 ± 0.002 d | 0.877 ± 0.000 a | 0.023 ± 0.000 a | 0.121 ± 0.010b c | 7.444 ± 0.112 b | 110.51 ± 0.71 c | 73.35 ± 0.35 c |
| A0.75N0.2 | 0 | 0.080 ± 0.001 b | 0.947 ± 0.002 f | 0.113 ± 0.015 c | 0.108 ± 0.008 b | 6.284 ± 0.115 a | 93.80 ± 0.99 b | 62.15 ± 0.21 b |
| A0.75N0.2 | 400 | 0.054 ± 0.002 a | 0.978 ± 0.003 h | 0.076 ± 0.002 b | 0.086 ± 0.012 a | 5.622 ± 0.837 a | 71.15 ± 0.07 a | 49.95 ± 0.49 a |
| A1.25N0 | 0 | 0.351 ± 0.013 h | 0.925 ± 0.000 c | 0.435 ± 0.001 e | 0.444 ± 0.012 g | 24.590 ± 0.608 e | 421.10 ± 1.56 h | 239.60 ± 0.57 h |
| A1.25N0 | 400 | 0.196 ± 0.004 e | 0.930 ± 0.001d | 0.032 ± 0.000 a | 0.295 ± 0.005 e | 17.956 ± 0.517 c | 260.60 ± 0.85 f | 179.45 ± 0.78 f |
| A1.25N0.2 | 0 | 0.336 ± 0.001 g | 0.911 ± 0.000 b | 0.071 ± 0.000 b | 0.405 ± 0.005 f | 22.420 ± 0.042 d | 370.10 ± 0.14 g | 220.05 ± 0.07 g |
| A1.25N0.2 | 400 | 0.223 ± 0.000 f | 0.936 ± 0.001 e | 0.031 ± 0.000 a | 0.259 ± 0.011 d | 16.956 ± 0.418 c | 240.21 ± 0.30 e | 170.60 ± 0.85 e |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król, Ż.; Malik, M.; Marycz, K.; Jarmoluk, A. Characteristic of Gelatine, Carrageenan and Sodium Alginate Hydrosols Treated by Direct Electric Current. Polymers 2016, 8, 275. https://doi.org/10.3390/polym8080275
Król Ż, Malik M, Marycz K, Jarmoluk A. Characteristic of Gelatine, Carrageenan and Sodium Alginate Hydrosols Treated by Direct Electric Current. Polymers. 2016; 8(8):275. https://doi.org/10.3390/polym8080275
Chicago/Turabian StyleKról, Żaneta, Magdalena Malik, Krzysztof Marycz, and Andrzej Jarmoluk. 2016. "Characteristic of Gelatine, Carrageenan and Sodium Alginate Hydrosols Treated by Direct Electric Current" Polymers 8, no. 8: 275. https://doi.org/10.3390/polym8080275
APA StyleKról, Ż., Malik, M., Marycz, K., & Jarmoluk, A. (2016). Characteristic of Gelatine, Carrageenan and Sodium Alginate Hydrosols Treated by Direct Electric Current. Polymers, 8(8), 275. https://doi.org/10.3390/polym8080275