Improving the Performances of Random Copolymer Based Organic Solar Cells by Adjusting the Film Features of Active Layers Using Mixed Solvents
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Synthesis
Compound 3
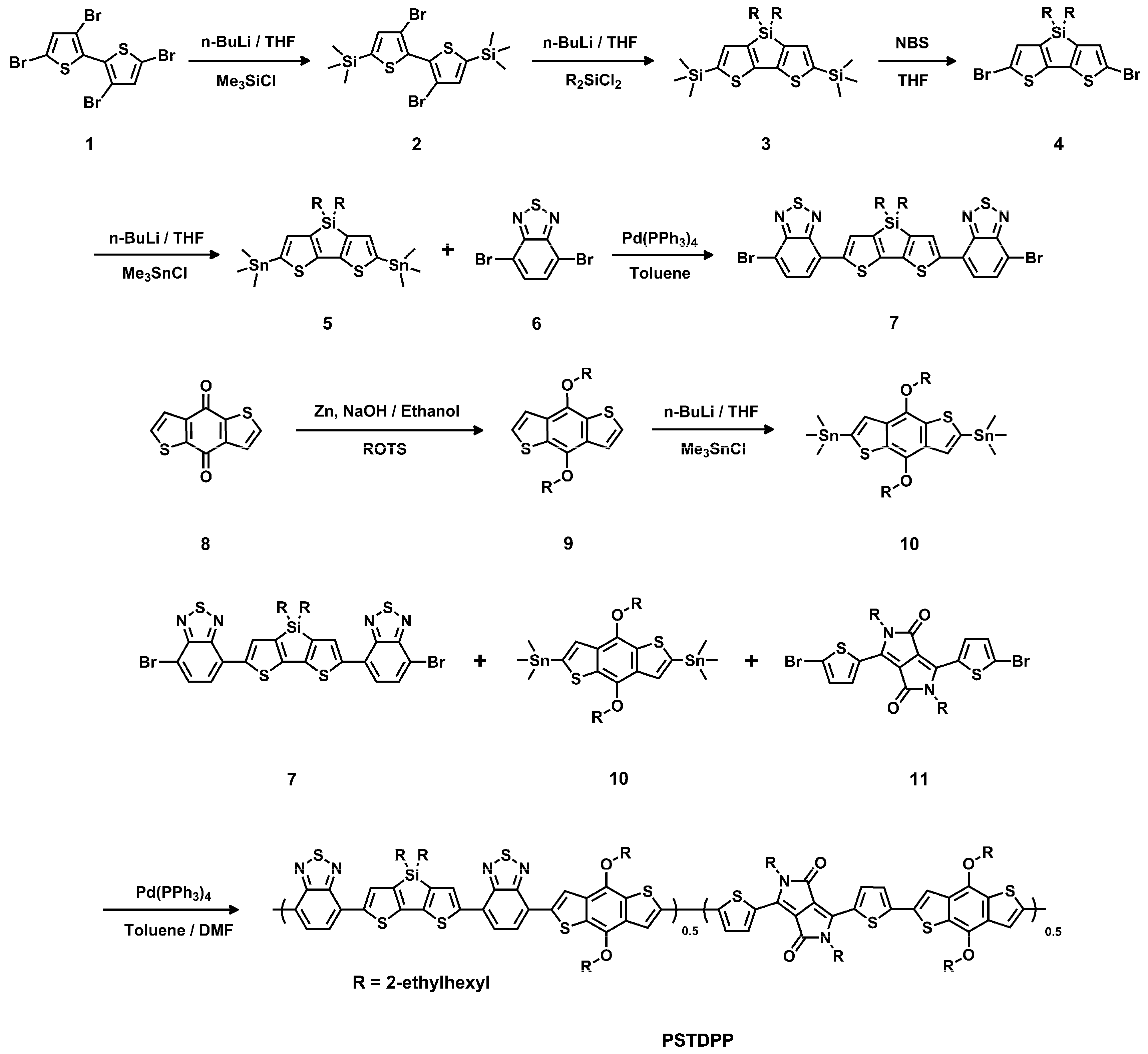
Compound 4
Compound 5
Compound 7 [34]
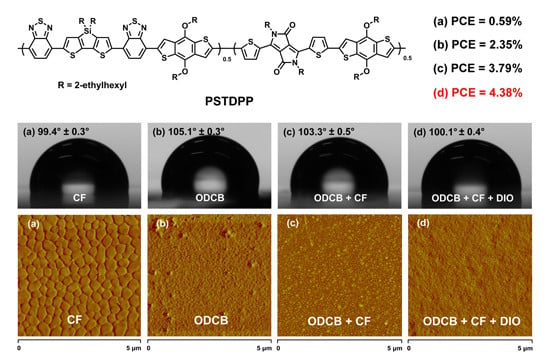
PSTDPP
2.2. Measurements
2.3. Organic Field-Effect Transistor (OFET) Devices Fabrication and Measurement
2.4. PSC Devices Fabrication and Measurement
3. Results and Discussion
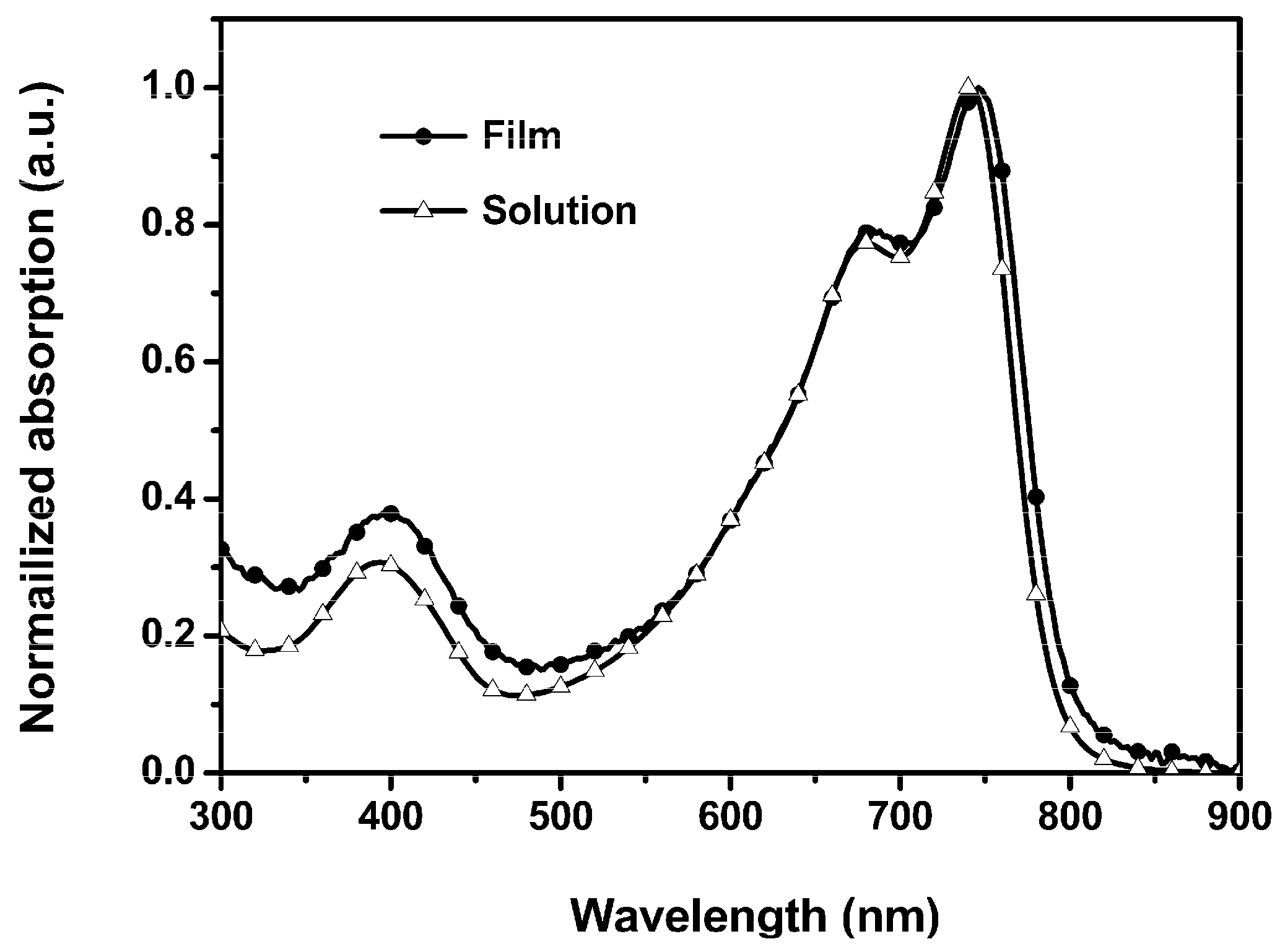
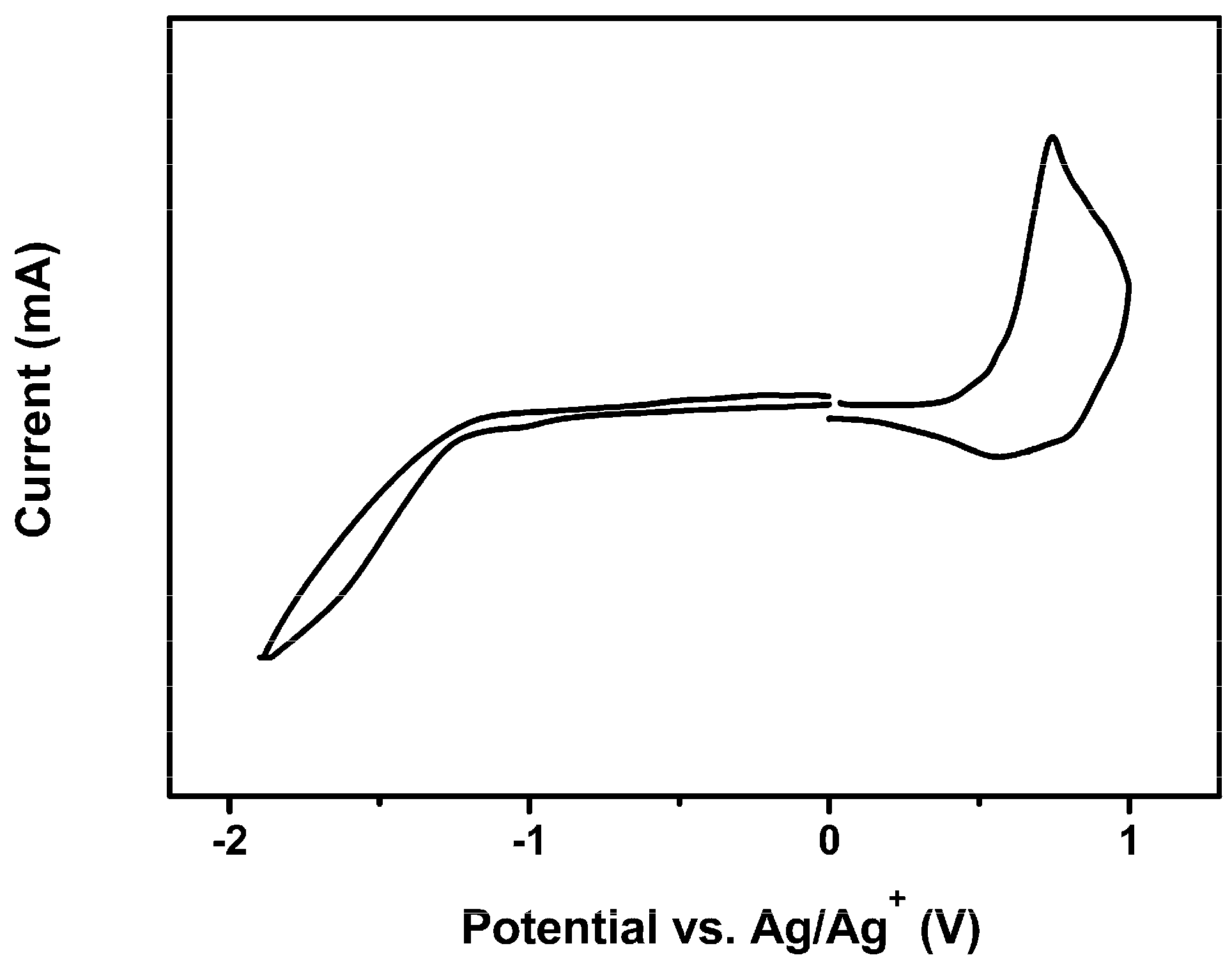
3.1. Optical and Electrochemical Properties
3.2. Crystallinity
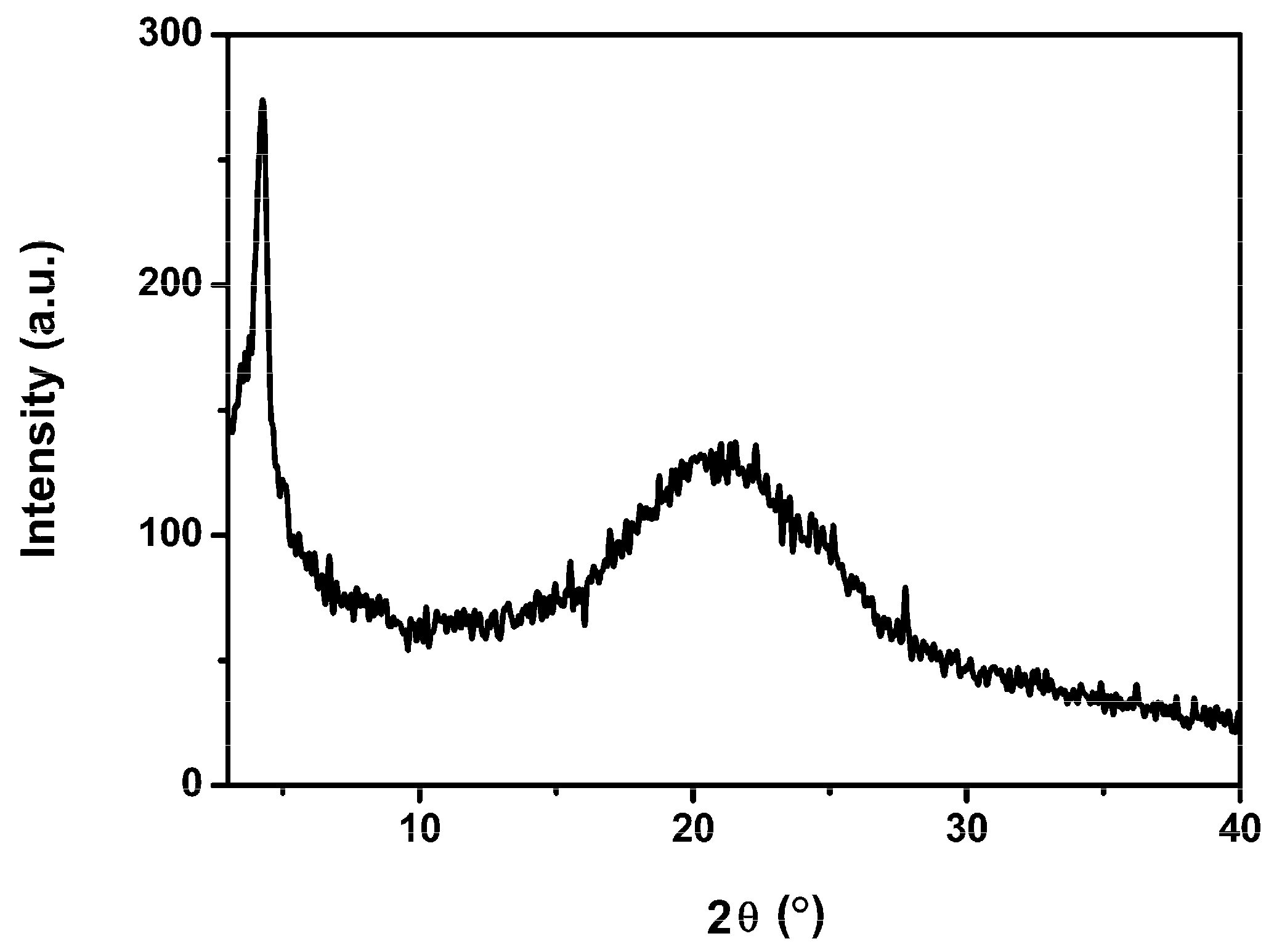
3.3. Hole Mobility
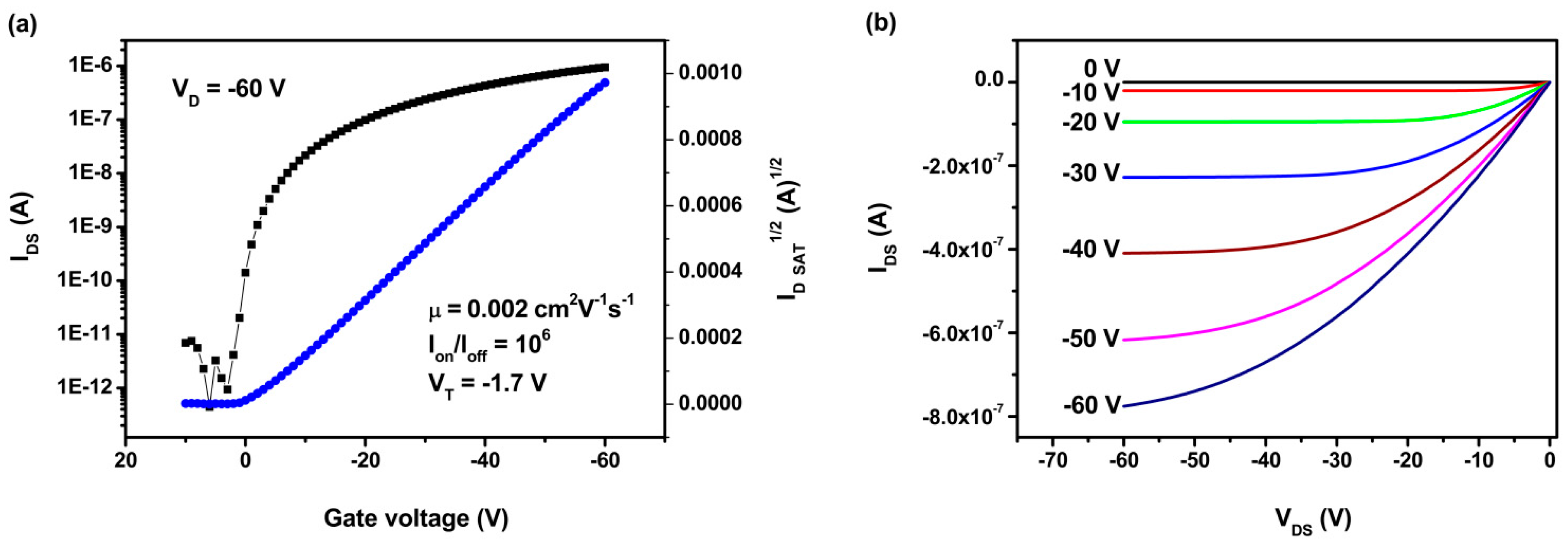
3.4. Photovoltaic Properties
| Solvent | D/A (w/w) | Voc (V) | Jsc (mA cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| ODCB | 1:1 | 0.51 | 5.53 | 34.8 | 0.98 |
| ODCB | 1:2 | 0.64 | 6.75 | 47.1 | 2.04 |
| ODCB | 1:3 | 0.63 | 9.89 | 37.9 | 2.35 |
| ODCB | 1:4 | 0.64 | 8.74 | 40.9 | 2.27 |
| CF | 1:3 | 0.69 | 1.68 | 50.9 | 0.59 |
| ODCB:CF (2:1) | 1:3 | 0.69 | 11.13 | 49.2 | 3.79 |
| ODCB:CF (2:1) with 2.5%DIO | 1:3 | 0.68 | 11.55 | 55.7 | 4.38 |

3.5. Water Contact Angle Characterizations
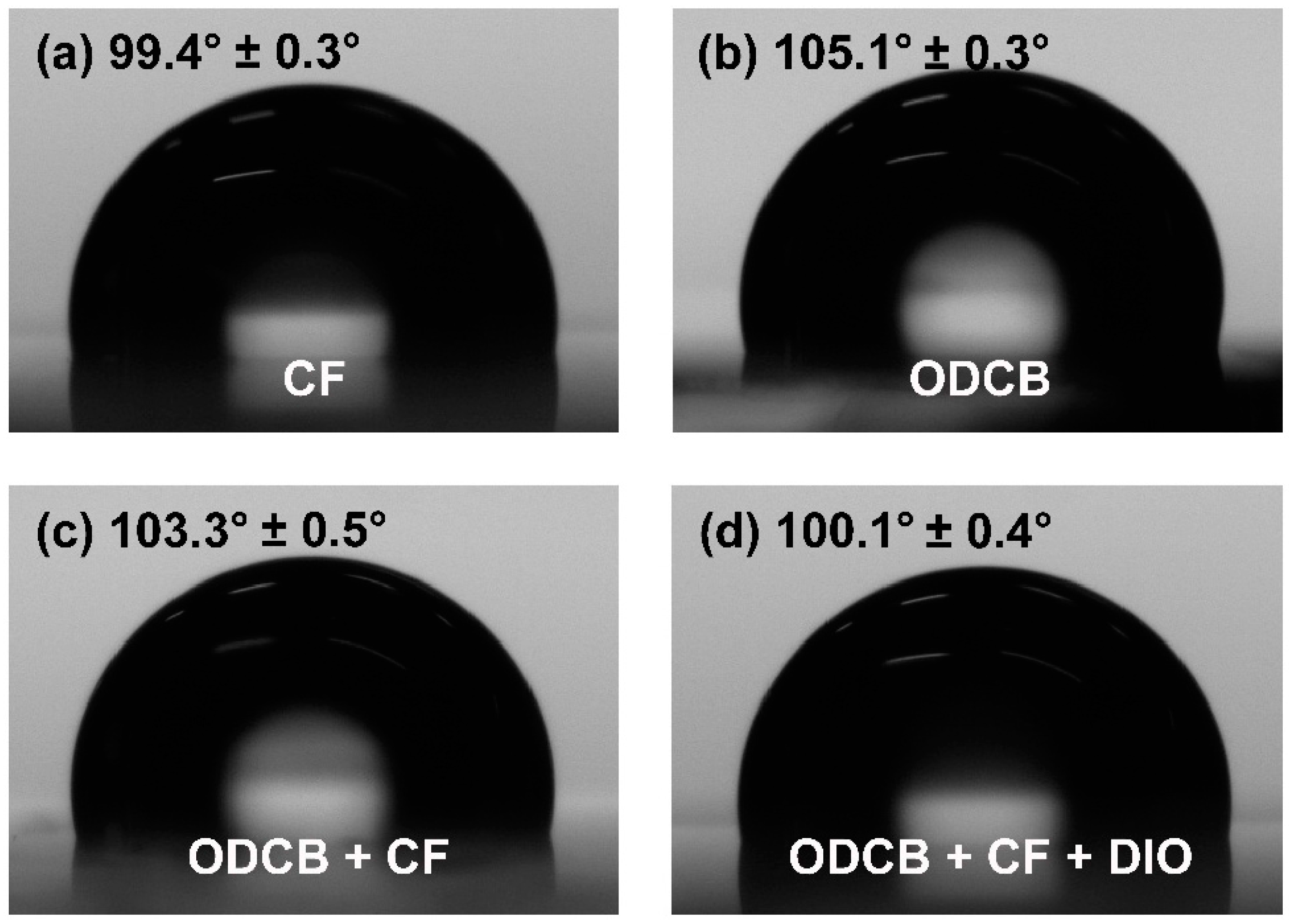
3.6. Film Morphologies
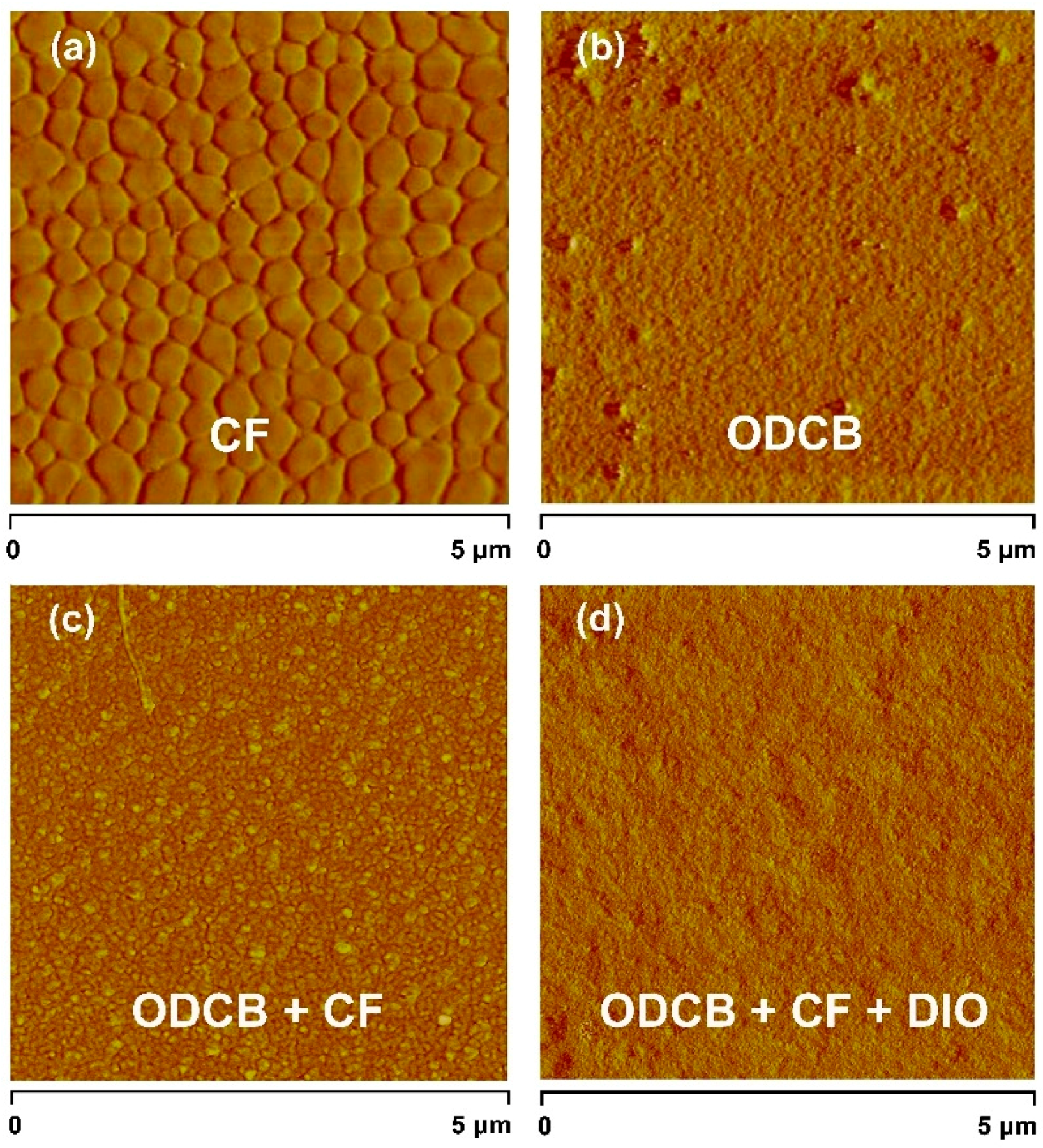
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Chen, J.; Cao, Y. Development of novel conjugated donor polymers for high-efficiency bulk-heterojunction photovoltaic devices. Acc. Chem. Res. 2009, 42, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- InganÄs, O.; Zhang, F.; Andersson, M.R. Alternating polyfluorenes collect solar light in polymer photovoltaics. Acc. Chem. Res. 2009, 42, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Molecular design of photovoltaic materials for polymer solar cells: Toward suitable electronic energy levels and broad absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xiao, B.; Liu, F.; Wu, H.; Yang, Y.; Xiao, S.; Wang, C.; Russell, T.P.; Cao, Y. Single-junction polymer solar cells with high efficiency and photovoltage. Nat. Photonics 2015, 9, 174–179. [Google Scholar] [CrossRef]
- Vohra, V.; Kawashima, K.; Kakara, T.; Koganezawa, T.; Osaka, I.; Takimiya, K.; Murata, H. Efficient inverted polymer solar cells employing favourable molecular orientation. Nat. Photonics 2015, 9, 403–408. [Google Scholar] [CrossRef]
- Dou, L.; Chen, C.-C.; Yoshimura, K.; Ohya, K.; Chang, W.-H.; Gao, J.; Liu, Y.; Richard, E.; Yang, Y. Synthesis of 5h-dithieno[3,2-b:2′,3′-d]pyran as an electron-rich building block for donor–acceptor type low-bandgap polymers. Macromolecules 2013, 46, 3384–3390. [Google Scholar] [CrossRef]
- Kroon, R.; Diaz de Zerio Mendaza, A.; Himmelberger, S.; Bergqvist, J.; Bäcke, O.; Faria, G.C.; Gao, F.; Obaid, A.; Zhuang, W.; Gedefaw, D.; et al. A new tetracyclic lactam building block for thick, broad-bandgap photovoltaics. J. Am. Chem. Soc. 2014, 136, 11578–11581. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, F.; Guo, X.; Zhang, W.; Gu, Y.; Zhang, J.; Han, C.C.; Russell, T.P.; Hou, J. Manipulating backbone structure to enhance low band gap polymer photovoltaic performance. Adv. Energy Mater. 2013, 3, 930–937. [Google Scholar] [CrossRef]
- Mei, J.; Bao, Z. Side chain engineering in solution-processable conjugated polymers. Chem. Mater. 2014, 26, 604–615. [Google Scholar] [CrossRef]
- Price, S.C.; Stuart, A.C.; Yang, L.; Zhou, H.; You, W. Fluorine substituted conjugated polymer of medium band gap yields 7% efficiency in polymer−fullerene solar cells. J. Am. Chem. Soc. 2011, 133, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.E.; Kim, K.-H.; Kim, B.J. Design of terpolymers as electron donors for highly efficient polymer solar cells. J. Mater. Chem. A 2014, 2, 15252–15267. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Ashraf, R.S.; Schroeder, B.C.; D′Angelo, P.; Watkins, S.E.; Song, K.; Anthopoulos, T.D.; McCulloch, I. Random benzotrithiophene-based donor-acceptor copolymers for efficient organic photovoltaic devices. Chem. Commun. 2012, 48, 5832–5834. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Liu, F.; Russell, T.P.; Jo, W.H. Semi-crystalline random conjugated copolymers with panchromatic absorption for highly efficient polymer solar cells. Energ. Environ Sci. 2013, 6, 3301–3307. [Google Scholar] [CrossRef]
- Kang, T.E.; Cho, H.-H.; Kim, H.j.; Lee, W.; Kang, H.; Kim, B.J. Importance of optimal composition in random terpolymer-based polymer solar cells. Macromolecules 2013, 46, 6806–6813. [Google Scholar] [CrossRef]
- Wang, L.; Shi, S.; Ma, D.; Chen, S.; Gao, C.; Wang, M.; Shi, K.; Li, Y.; Li, X.; Wang, H. Improved photovoltaic properties of donor–acceptor copolymers by introducing quinoxalino[2,3-b′]porphyrin as a light-harvesting unit. Macromolecules 2015, 48, 287–296. [Google Scholar] [CrossRef]
- Cho, N.; Yip, H.-L.; Jen, A.K.-Y. Morphology evolution by controlling solvent-solute interactions using a binary solvent in bulk heterojunction solar cells. Appl. Phys. Lett. 2013, 102, 233903. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, S.; Ma, W.; Fan, B.; Guo, X.; Huang, Y.; Ade, H.; Hou, J. From binary to ternary solvent: Morphology fine-tuning of D/A blends in PDPP3T-based polymer solar cells. Adv. Mater. 2012, 24, 6335–6341. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Jing, Y.; Guo, X.; Sun, H.; Zhang, S.; Zhang, M.; Huo, L.; Hou, J. Remove the residual additives toward enhanced efficiency with higher reproducibility in polymer solar cells. J. Phys. Chem. C 2013, 117, 14920–14928. [Google Scholar] [CrossRef]
- Son, S.K.; Lee, H.-S.; Ha, J.S.; Kim, K.H.; Son, H.J.; Ko, M.J.; Kim, H.; Lee, D.-K.; Kim, J.Y.; Lee, W.; et al. Effect of asymmetric solubility of diketopyrrolopyrrole-based polymers and PC71BMS in a binary solvent system on the performance of bulk heterojunction solar cells. Solar Energy Mater. Solar Cells 2014, 124, 232–240. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, F.; Li, L.; Yu, J.; Wang, J.; An, Q.; Tang, W. Tuning nanoscale morphology using mixed solvents and solvent vapor treatment for high performance polymer solar cells. RSC Adv. 2014, 4, 48724–48733. [Google Scholar] [CrossRef]
- Van Franeker, J.J.; Turbiez, M.; Li, W.; Wienk, M.M.; Janssen, R.A.J. A real-time study of the benefits of co-solvents in polymer solar cell processing. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Chen, H.-Y.; Zhang, S.; Li, G.; Yang, Y. Synthesis, characterization, and photovoltaic properties of a low band gap polymer based on silole-containing polythiophenes and 2,1,3-benzothiadiazole. J. Am. Chem. Soc. 2008, 130, 16144–16145. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.H.; Park, M.H.; Zhang, S.Q.; Yao, Y.; Chen, L.M.; Li, J.H.; Yang, Y. Bandgap and molecular energy level control of conjugated polymer photovoltaic materials based on benzo[1,2-b:4,5-b′]dithiophene. Macromolecules 2008, 41, 6012–6018. [Google Scholar] [CrossRef]
- Dhanabalan, A.; van Duren, J.K.J.; van Hal, P.A.; van Dongen, J.L.J.; Janssen, R.A.J. Synthesis and characterization of a low bandgap conjugated polymer for bulk heterojunction photovoltaic cells. Adv. Funct. Mater. 2001, 11, 255–262. [Google Scholar] [CrossRef]
- Wienk, M.M.; Turbiez, M.; Gilot, J.; Janssen, R.A.J. Narrow-bandgap diketo-pyrrolo-pyrrole polymer solar cells: The effect of processing on the performance. Adv. Mater. 2008, 20, 2556–2560. [Google Scholar] [CrossRef]
- Gupta, V.; Kyaw, A.K.K.; Wang, D.H.; Chand, S.; Bazan, G.C.; Heeger, A.J. Barium: An efficient cathode layer for bulk-heterojunction solar cells. Sci. Rep. 2013, 3, 1965. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, S.; Zhao, W.; Yao, H.; Hou, J. Highly efficient 2D-conjugated benzodithiophene-based photovoltaic polymer with linear alkylthio side chain. Chem. Mater. 2014, 26, 3603–3605. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Aggregation and morphology control enables multiple cases of high-efficiency polymer solar cells. Nat. Commun. 2014, 5, 5293. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Ko, S.-J.; Kim, T.; Morin, P.-O.; Walker, B.; Lee, B.H.; Leclerc, M.; Kim, J.Y.; Heeger, A.J. Small-bandgap polymer solar cells with unprecedented short-circuit current density and high fill factor. Adv. Mater. 2015, 27, 3318–3324. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Hou, J.; Chen, H.-Y.; Zhang, S.; Jiang, Y.; Chen, T.L.; Yang, Y. Bandgap and molecular level control of the low-bandgap polymers based on 3,6-dithiophen-2-yl-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione toward highly efficient polymer solar cells. Macromolecules 2009, 42, 6564–6571. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, Y.; Feng, D.; Tsai, S.-T.; Son, H.-J.; Li, G.; Yu, L. Development of new semiconducting polymers for high performance solar cells. J. Am. Chem. Soc. 2009, 131, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Bürgi, L.; Turbiez, M.; Pfeiffer, R.; Bienewald, F.; Kirner, H.-J.; Winnewisser, C. High-mobility ambipolar near-infrared light-emitting polymer field-effect transistors. Adv. Mater. 2008, 20, 2217–2224. [Google Scholar] [CrossRef]
- Takacs, C.J.; Sun, Y.; Welch, G.C.; Perez, L.A.; Liu, X.; Wen, W.; Bazan, G.C.; Heeger, A.J. Solar cell efficiency, self-assembly, and dipole–dipole interactions of isomorphic narrow-band-gap molecules. J. Am. Chem. Soc. 2012, 134, 16597–16606. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, H.; Yang, C.; Li, Y. Synthesis and electroluminescence of novel copolymers containing crown ether spacers. J. Mater. Chem. 2003, 13, 800–806. [Google Scholar] [CrossRef]
- Lu, K.; Fang, J.; Yu, Z.; Yan, H.; Zhu, X.; Zhang, Y.; He, C.; Wei, Z. Improving the performance of polymer solar cells by altering polymer side chains and optimizing film morphologies. Organic Electronics 2012, 13, 3234–3243. [Google Scholar] [CrossRef]
- He, Y.; Wu, W.; Zhao, G.; Liu, Y.; Li, Y. Poly(3,6-dihexyl-thieno[3,2-b]thiophene vinylene): Synthesis, field-effect transistors, and photovoltaic properties. Macromolecules 2008, 41, 9760–9766. [Google Scholar] [CrossRef]
- Lee, J.K.; Ma, W.L.; Brabec, C.J.; Yuen, J.; Moon, J.S.; Kim, J.Y.; Lee, K.; Bazan, G.C.; Heeger, A.J. Processing additives for improved efficiency from bulk heterojunction solar cells. J. Am. Chem. Soc. 2008, 130, 3619–3623. [Google Scholar] [CrossRef] [PubMed]
- Kiel, J.W.; Kirby, B.J.; Majkrzak, C.F.; Maranville, B.B.; Mackay, M.E. Nanoparticle concentration profile in polymer-based solar cells. Soft Matter. 2010, 6, 641–646. [Google Scholar] [CrossRef]
- Staniec, P.A.; Parnell, A.J.; Dunbar, A.D.F.; Yi, H.; Pearson, A.J.; Wang, T.; Hopkinson, P.E.; Kinane, C.; Dalgliesh, R.M.; Donald, A.M.; et al. The nanoscale morphology of a PCDTBT:PCBM photovoltaic blend. Adv. Energy Mater. 2011, 1, 499–504. [Google Scholar] [CrossRef]
- Chung, H.-S.; Lee, W.-H.; Song, C.E.; Shin, Y.; Kim, J.; Lee, S.K.; Shin, W.S.; Moon, S.-J.; Kang, I.-N. Highly conjugated side-chain-substituted benzo[1,2-b:4,5-b′]dithiophene-based conjugated polymers for use in polymer solar cells. Macromolecules 2013, 47, 97–105. [Google Scholar] [CrossRef]
- Guo, X.; Cui, C.H.; Zhang, M.J.; Huo, L.J.; Huang, Y.; Hou, J.H.; Li, Y. High efficiency polymer solar cells based on poly(3-hexylthiophene)/indene-C70 bisadduct with solvent additive. Energ. Environ Sci. 2012, 5, 7943–7949. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Lu, K.; Xia, B.; Fang, J.; Zhao, Y.; Zhao, T.; Wei, Z.; Jiang, L. Improving the Performances of Random Copolymer Based Organic Solar Cells by Adjusting the Film Features of Active Layers Using Mixed Solvents. Polymers 2016, 8, 4. https://doi.org/10.3390/polym8010004
Zhu X, Lu K, Xia B, Fang J, Zhao Y, Zhao T, Wei Z, Jiang L. Improving the Performances of Random Copolymer Based Organic Solar Cells by Adjusting the Film Features of Active Layers Using Mixed Solvents. Polymers. 2016; 8(1):4. https://doi.org/10.3390/polym8010004
Chicago/Turabian StyleZhu, Xiangwei, Kun Lu, Benzheng Xia, Jin Fang, Yifan Zhao, Tianyi Zhao, Zhixiang Wei, and Lei Jiang. 2016. "Improving the Performances of Random Copolymer Based Organic Solar Cells by Adjusting the Film Features of Active Layers Using Mixed Solvents" Polymers 8, no. 1: 4. https://doi.org/10.3390/polym8010004
APA StyleZhu, X., Lu, K., Xia, B., Fang, J., Zhao, Y., Zhao, T., Wei, Z., & Jiang, L. (2016). Improving the Performances of Random Copolymer Based Organic Solar Cells by Adjusting the Film Features of Active Layers Using Mixed Solvents. Polymers, 8(1), 4. https://doi.org/10.3390/polym8010004